Elevational Variation in and Environmental Determinants of Fungal Diversity in Forest Ecosystems of Korean Peninsula
Abstract
:1. Introduction
2. Materials and Methods
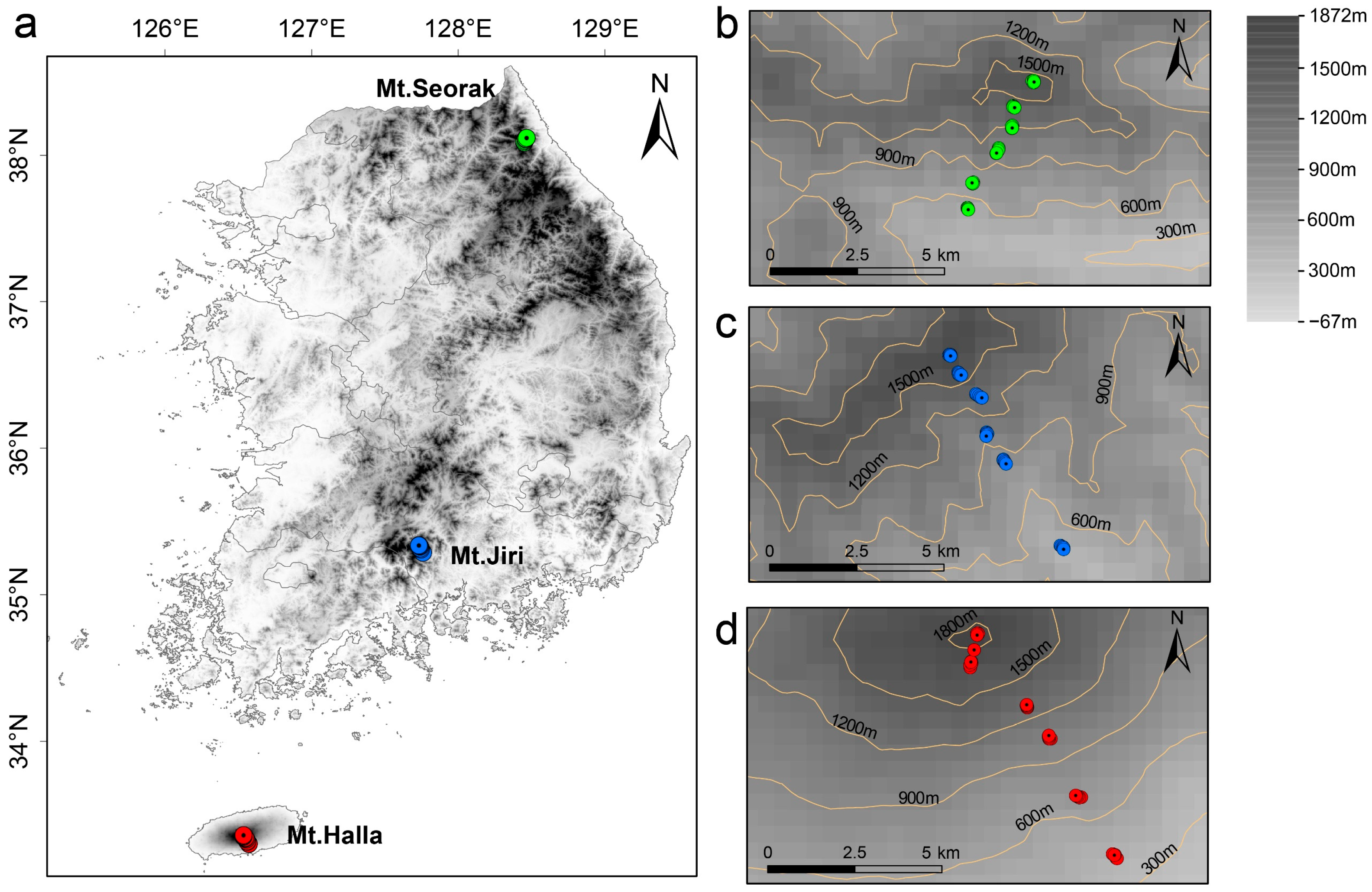
2.1. Sampling Site
2.2. Environmental Data Sources and Measurement
2.3. High-Throughput Sequencing and Amplicon Data Analysis
2.4. Statistical Analysis
3. Results
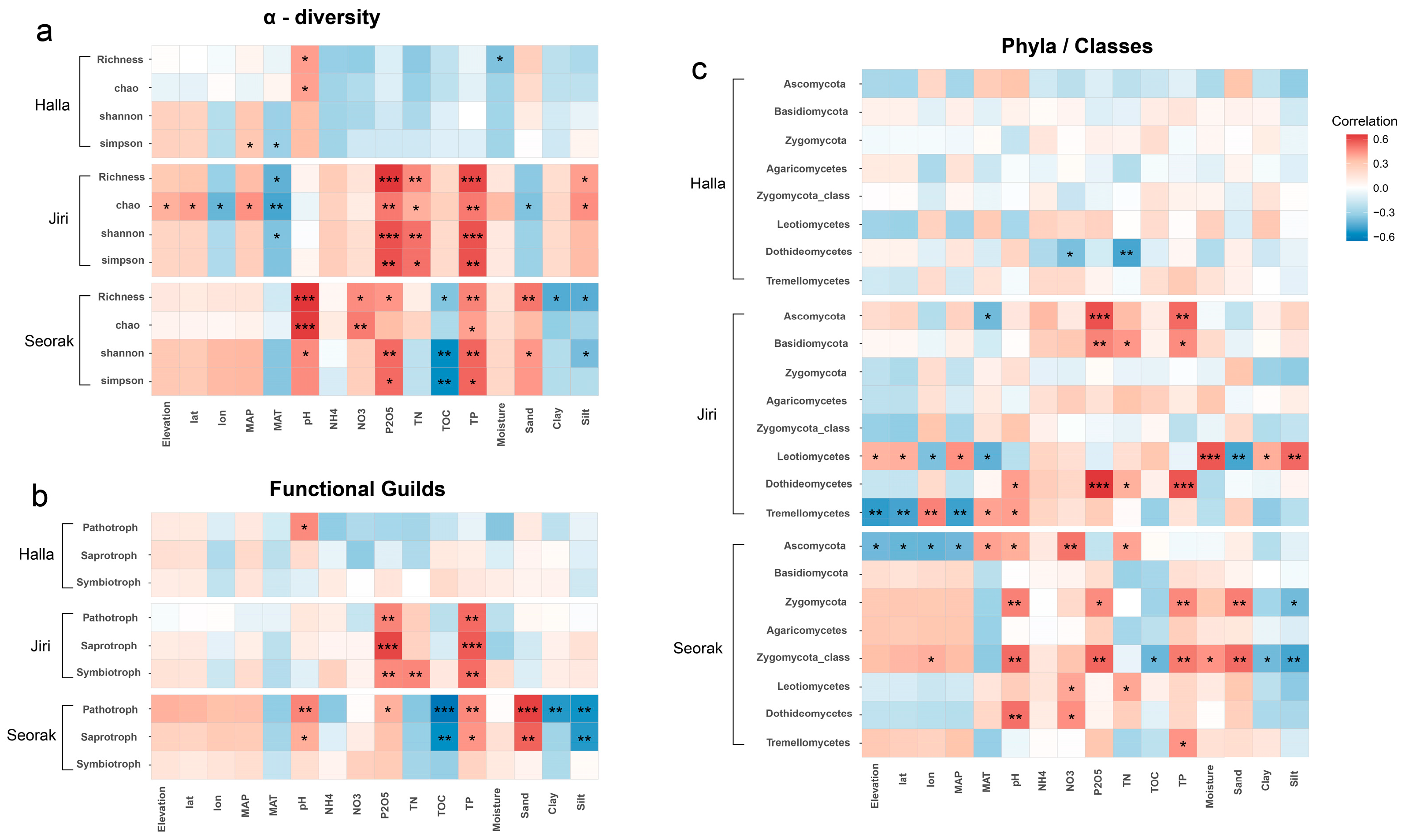
3.1. Alpha Diversity Patterns along Elevational Gradients
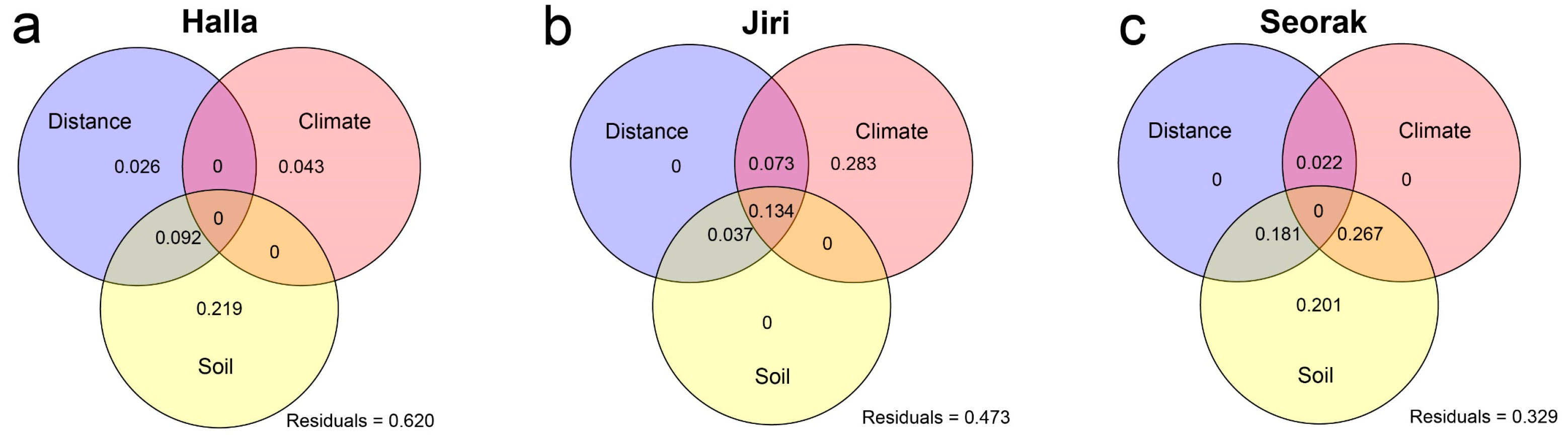
3.2. Impact of Environmental Factors on Elevational Patterns of Fungal Diversity
3.3. Environmental Drivers of Dominant Taxa and Functional Group Diversity along Elevational Gradients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 2006, 18, 200–205. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Costa, F.V.d.; Viana-Júnior, A.B.; Aguilar, R.; Silveira, F.A.O.; Cornelissen, T.G. Biodiversity and elevation gradients: Insights on sampling biases across worldwide mountains. J. Biogeogr. 2023, 50, 1879–1889. [Google Scholar] [CrossRef]
- Dong, K.; Moroenyane, I.; Tripathi, B.; Kerfahi, D.; Takahashi, K.; Yamamoto, N.; An, C.; Cho, H.; Adams, J. Soil nematodes show a mid-elevation diversity maximum and elevational zonation on Mt. Norikura, Japan. Sci. Rep. 2017, 7, 3028. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Kelt, D.A.; Sun, Z.; Liu, H.; Hu, L.; Ren, H.; Wen, J. Global variation in elevational diversity patterns. Sci. Rep. 2013, 3, 3007. [Google Scholar] [CrossRef]
- Werenkraut, V.; Ruggiero, A. Quality of basic data and method to identify shape affect richness-altitude relationships in meta-analysis. Ecology 2011, 92, 253–260. [Google Scholar] [CrossRef]
- Castro, F.S.d.; Da Silva, P.G.; Solar, R.; Fernandes, G.W.; Neves, F.d.S. Environmental drivers of taxonomic and functional diversity of ant communities in a tropical mountain. Insect Conserv. Divers. 2020, 13, 393–403. [Google Scholar] [CrossRef]
- Rahbek, C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 2004, 8, 224–239. [Google Scholar] [CrossRef]
- Kessler, M.; Kluge, J.; Hemp, A.; Ohlemüller, R. A global comparative analysis of elevational species richness patterns of ferns. Glob. Ecol. Biogeogr. 2011, 20, 868–880. [Google Scholar] [CrossRef]
- McCain, C.M. Elevational Gradients in Diversity of Small Mammals. Ecology 2005, 86, 366–372. [Google Scholar] [CrossRef]
- Sanders, N.J.; Moss, J.; Wagner, D. Patterns of ant species richness along elevational gradients in an arid ecosystem. Glob. Ecol. Biogeogr. 2003, 12, 93–102. [Google Scholar] [CrossRef]
- Singh, D.; Lee-Cruz, L.; Kim, W.-S.; Kerfahi, D.; Chun, J.-H.; Adams, J.M. Strong elevational trends in soil bacterial community composition on Mt. Halla, South Korea. Soil Biol. Biochem. 2014, 68, 140–149. [Google Scholar] [CrossRef]
- Wang, J.; Hu, A.; Meng, F.; Zhao, W.; Yang, Y.; Soininen, J.; Shen, J.; Zhou, J. Embracing mountain microbiome and ecosystem functions under global change. New Phytol. 2022, 234, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, A.T.; Fierer, N.; Turner, B.L.; Whitaker, J.; Ostle, N.J.; McNamara, N.P.; Bardgett, R.D.; Leff, J.W.; Salinas, N.; Silman, M.R.; et al. Microbes follow Humboldt: Temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology 2018, 99, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Deng, Y.; Ding, J.Z.; Hu, H.W.; Xu, T.L.; Li, F.; Yang, G.B.; Yang, Y.H. Distinct microbial communities in the active and permafrost layers on the Tibetan Plateau. Mol. Ecol. 2017, 26, 6608–6620. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Tong, Z.; Rong, D.; Zhou, Z.; Xu, X. Accurate thermal buckling analysis of functionally graded orthotropic cylindrical shells under the symplectic framework. Thin-Walled Struct. 2018, 129, 1–9. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Wang, X.; Liu, F.; Li, X.; Zhu, X. Soil properties and geography shape arbuscular mycorrhizal fungal communities in black land of China. Appl. Soil Ecol. 2021, 167, 104109. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, X.; Han, L.; Liu, Z.; Kang, S.; Zhao, Y. Fungal community diversity in soils along an elevation gradient in a Quercus aliena var. acuteserrata forest in Qinling Mountains, China. Appl. Soil Ecol. 2021, 167, 104104. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Takahashi, K.; Dong, K.; Song, H.K.; Moroenyane, I.; Waldman, B.; Adams, J.M. Fungal Elevational Rapoport pattern from a High Mountain in Japan. Sci. Rep. 2019, 9, 6570. [Google Scholar] [CrossRef]
- Yang, T.; Adams, J.M.; Shi, Y.; He, J.S.; Jing, X.; Chen, L.; Tedersoo, L.; Chu, H. Soil fungal diversity in natural grasslands of the Tibetan Plateau: Associations with plant diversity and productivity. New Phytol. 2017, 215, 756–765. [Google Scholar] [CrossRef]
- Shen, C.; Gunina, A.; Luo, Y.; Wang, J.; He, J.Z.; Kuzyakov, Y.; Hemp, A.; Classen, A.T.; Ge, Y. Contrasting patterns and drivers of soil bacterial and fungal diversity across a mountain gradient. Environ. Microbiol. 2020, 22, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Li, M.; Frey, B.; Dai, G.; Yang, L.; Li, M.H. Effect of elevation on composition and diversity of fungi in the rhizosphere of a population of Deyeuxia angustifolia on Changbai Mountain, northeastern China. Front. Microbiol. 2023, 14, 1087475. [Google Scholar] [CrossRef]
- Guerrero-Galan, C.; Calvo-Polanco, M.; Zimmermann, S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 2019, 29, 291–301. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Liu, X.; Gilbert, G.S.; Zheng, Y.; Luo, S.; Huang, F.; Yu, S. Adult trees cause density-dependent mortality in conspecific seedlings by regulating the frequency of pathogenic soil fungi. Ecol. Lett. 2016, 19, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.P.; Callaway, R.M.; Hart, M.M.; Pither, J.; Klironomos, J. Phylogenetic structure of arbuscular mycorrhizal fungal communities along an elevation gradient. Mycorrhiza 2017, 27, 273–282. [Google Scholar] [CrossRef]
- Ren, L.; Jeppesen, E.; He, D.; Wang, J.; Liboriussen, L.; Xing, P.; Wu, Q.L. pH influences the importance of niche-related and neutral processes in lacustrine bacterioplankton assembly. Appl. Environ. Microbiol. 2015, 81, 3104–3114. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.; Chen, L.; Wang, J.; Zhang, L. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef]
- Kerfahi, D.; Guo, Y.; Dong, K.; Wang, Q.; Adams, J.M. pH is the major predictor of soil microbial network complexity in Chinese forests along a latitudinal gradient. Catena 2024, 234, 107595. [Google Scholar] [CrossRef]
- Hu, Y.; Veresoglou, S.D.; Tedersoo, L.; Xu, T.; Ge, T.; Liu, L.; Chen, Y.; Hao, Z.; Su, Y.; Rillig, M.C.; et al. Contrasting latitudinal diversity and co-occurrence patterns of soil fungi and plants in forest ecosystems. Soil Biol. Biochem. 2019, 131, 100–110. [Google Scholar] [CrossRef]
- Lee, S.-C.; Choi, S.-H.; Kang, H.-M.; Cho, H.-S.; Cho, J.-W. The change and structure of altitudinal vegetation on the east side of Hallasan National Park. Korean J. Environ. Ecol. 2010, 24, 26–36. [Google Scholar]
- Yim, Y.; Kim, J. The Vegetation of Mt. Chiri National Park; The Chungang University Press: Seoul, Republic of Korea, 1992; 466p. (In Korean) [Google Scholar]
- Choi, S.-W.; An, J.-S. What we know and do not know about moth diversity from seven-year-monitoring in Mt. Jirisan National Park, South Korea. J. Asia-Pac. Entomol. 2013, 16, 401–409. [Google Scholar] [CrossRef]
- Kim, J.; Lim, J.H.; Yun, C. Dynamics of Abies nephrolepis Seedlings in Relation to Environmental Factors in Seorak Mountain, South Korea. Forests 2019, 10, 702. [Google Scholar] [CrossRef]
- Lee, M.H.; Song, J.H.; Byeon, S.Y.; Lee, J.E.; Kim, H.J.; Chae, S.B.; Yun, C.W.; Kim, J.D. The species range-size patterns for vascular plants of Seorak Mountain (Korea): Relationship between group of life forms and phytogeography affinity along the elevational gradient. Ecol. Evol. 2021, 11, 12872–12881. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zou, S.; Li, N.; Kerfahi, D.; Lee, C.; Adams, J.; Kwak, H.J.; Kim, J.; Lee, S.S.; Dong, K. Elevation-related climatic factors dominate soil free-living nematode communities and their co-occurrence patterns on Mt. Halla, South Korea. Ecol. Evol. 2021, 11, 18540–18551. [Google Scholar] [CrossRef]
- Yu, Z.; Lee, C.; Kerfahi, D.; Li, N.; Yamamoto, N.; Yang, T.; Lee, H.; Zhen, G.; Song, Y.; Shi, L.; et al. Elevational dynamics in soil microbial co-occurrence: Disentangling biotic and abiotic influences on bacterial and fungal networks on Mt. Seorak. Soil Ecol. Lett. 2024, 6, 240246. [Google Scholar] [CrossRef]
- Zou, S.; Adams, J.; Yu, Z.; Li, N.; Kerfahi, D.; Tripathi, B.; Lee, C.; Yang, T.; Moroenyane, I.; Chen, X.; et al. Stochasticity dominates assembly processes of soil nematode metacommunities on three Asian mountains. Pedosphere 2023, 33, 331–342. [Google Scholar] [CrossRef]
- Rong, S.; Fu-Liang, Q.; Yi-Ting, C.; Fa-Ping, Z.; Wei, D.; Ya-Xian, L.; Zhi-Pang, H.; Xiao-Yan, Y.; Wen, X. Soil sampling methods for microbial study in montane regions. Glob. Ecol. Conserv. 2023, 47, e02679. [Google Scholar] [CrossRef]
- Dickie, I.A.; Boyer, S.; Buckley, H.L.; Duncan, R.P.; Gardner, P.P.; Hogg, I.D.; Holdaway, R.J.; Lear, G.; Makiola, A.; Morales, S.E.; et al. Towards robust and repeatable sampling methods in eDNA-based studies. Mol. Ecol. Resour. 2018, 18, 940–952. [Google Scholar] [CrossRef]
- Chun, J.H.; Lee, C.B. Partitioning the regional and local drivers of phylogenetic and functional diversity along temperate elevational gradients on an East Asian peninsula. Sci. Rep. 2018, 8, 2853. [Google Scholar] [CrossRef]
- Turenne, C.Y.; Sanche, S.E.; Hoban, D.J.; Karlowsky, J.A.; Kabani, A.M. Rapid identification of fungi by using the ITS2 genetic region and an automated fluorescent capillary electrophoresis system. J. Clin. Microbiol. 1999, 37, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Accuracy of taxonomy prediction for 16S rRNA and fungal ITS sequences. PeerJ 2018, 6, e4652. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Walters William, A.; Lennon Niall, J.; Bochicchio, J.; Krohn, A.; Caporaso, J.G.; Pennanen, T. Accurate Estimation of Fungal Diversity and Abundance through Improved Lineage-Specific Primers Optimized for Illumina Amplicon Sequencing. Appl. Environ. Microbiol. 2016, 82, 7217–7226. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, H.; Hildebrand, F.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.-H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi—Recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the Spatial Component of Ecological Variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Oksanen, J. Vegan: An Introduction to Ordination; University of Helsinki: Helsinki, Finland, 2008. [Google Scholar]
- Xu, S.; Dong, K.; Lee, S.; Ogwu, M.C.; Undrakhbold, S.; Singh, D.; Ariunzaya, D.; Enkhmandal, O.; Spence, L.A.; Sharkhuu, A.; et al. Metagenetics of fairy rings reveals complex and variable soil fungal communities. Pedosphere 2023, 33, 567–578. [Google Scholar] [CrossRef]
- Wang, M.; Shi, S.; Lin, F.; Jiang, P. Response of the soil fungal community to multi-factor environmental changes in a temperate forest. Appl. Soil Ecol. 2014, 81, 45–56. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Nakano, T.; Hattori, M.; Nara, K. The mid-domain effect in ectomycorrhizal fungi: Range overlap along an elevation gradient on Mount Fuji, Japan. ISME J. 2014, 8, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Geml, J.; Morgado, L.N.; Semenova-Nelsen, T.A.; Schilthuizen, M. Changes in richness and community composition of ectomycorrhizal fungi among altitudinal vegetation types on Mount Kinabalu in Borneo. New Phytol. 2017, 215, 454–468. [Google Scholar] [CrossRef]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Han, D.; Wang, N.; Hu, Y.; Mu, L.; Feng, F. Vertical zonation of soil fungal community structure in a Korean pine forest on Changbai Mountain, China. World J. Microbiol. Biotechnol. 2017, 33, 12. [Google Scholar] [CrossRef]
- Koranda, M.; Rinnan, R.; Michelsen, A. Close coupling of plant functional types with soil microbial community composition drives soil carbon and nutrient cycling in tundra heath. Plant Soil 2023, 488, 551–572. [Google Scholar] [CrossRef]
- Dasila, K.; Pandey, A.; Samant, S.S.; Pande, V. Endophytes associated with Himalayan silver birch (Betula utilis D. Don) roots in relation to season and soil parameters. Appl. Soil Ecol. 2020, 149, 103513. [Google Scholar] [CrossRef]
- Fu, F.; Li, J.; Li, S.; Chen, W.; Ding, H.; Xiao, S.; Li, Y. Elevational distribution patterns and drivers of soil microbial diversity in the Sygera Mountains, southeastern Tibet, China. Catena 2023, 221, 106738. [Google Scholar] [CrossRef]
- Wang, J.-T.; Cao, P.; Hu, H.-W.; Li, J.; Han, L.-L.; Zhang, L.-M.; Zheng, Y.-M.; He, J.-Z. Altitudinal Distribution Patterns of Soil Bacterial and Archaeal Communities along Mt. Shegyla on the Tibetan Plateau. Microb. Ecol. 2015, 69, 135–145. [Google Scholar] [CrossRef]
- Djukic, I.; Zehetner, F.; Mentler, A.; Gerzabek, M.H. Microbial community composition and activity in different Alpine vegetation zones. Soil Biol. Biochem. 2010, 42, 155–161. [Google Scholar] [CrossRef]
- Pan, J.; Guo, Q.; Li, H.; Luo, S.; Zhang, Y.; Yao, S.; Fan, X.; Sun, X.; Qi, Y. Dynamics of Soil Nutrients, Microbial Community Structure, Enzymatic Activity, and Their Relationships along a Chronosequence of Pinus massoniana Plantations. Forests 2021, 12, 376. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reith, F.; Dennis, P.G.; Hamonts, K.; Powell, J.R.; Young, A.; Singh, B.K.; Bissett, A. Ecological drivers of soil microbial diversity and soil biological networks in the Southern Hemisphere. Ecology 2018, 99, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Viscarra Rossel, R.A.; Yang, Y.; Bissett, A.; Behrens, T.; Dixon, K.; Nevil, P.; Li, S. Environmental controls of soil fungal abundance and diversity in Australia’s diverse ecosystems. Soil Biol. Biochem. 2022, 170, 108694. [Google Scholar] [CrossRef]
- Bahram, M.; Kõljalg, U.; Courty, P.E.; Diédhiou, A.G.; Kjøller, R.; Põlme, S.; Ryberg, M.; Veldre, V.; Tedersoo, L.; Wurzburger, N. The distance decay of similarity in communities of ectomycorrhizal fungi in different ecosystems and scales. J. Ecol. 2013, 101, 1335–1344. [Google Scholar] [CrossRef]
- Coince, A.; Cordier, T.; Lengelle, J.; Defossez, E.; Vacher, C.; Robin, C.; Buee, M.; Marcais, B. Leaf and root-associated fungal assemblages do not follow similar elevational diversity patterns. PLoS ONE 2014, 9, e100668. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.G.; Woodward, S.; Taylor, A.F.S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol. 2015, 206, 1145–1155. [Google Scholar] [CrossRef]
- Frey, S.D.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]
- Meng, H.; Li, K.; Nie, M.; Wan, J.R.; Quan, Z.X.; Fang, C.M.; Chen, J.K.; Gu, J.D.; Li, B. Responses of bacterial and fungal communities to an elevation gradient in a subtropical montane forest of China. Appl. Microbiol. Biotechnol. 2013, 97, 2219–2230. [Google Scholar] [CrossRef]
- Wang, J.-T.; Zheng, Y.-M.; Hu, H.-W.; Zhang, L.-M.; Li, J.; He, J.-Z. Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J. Soils Sediments 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Fungal and bacterial growth responses to N fertilization and pH in the 150-year ‘Park Grass’ UK grassland experiment. FEMS Microbiol. Ecol. 2011, 76, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, Y.; Wang, S.; Xu, D.; Yu, H.; Wu, L.; Lin, Q.; Hu, Y.; Li, X.; He, Z.; et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J. 2014, 8, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Y.; Zhang, J.; Niu, L.; Zhang, W.; Cai, W.; Zhu, X. Sediment bacterial communities in a eutrophic lake influenced by multiple inflow-rivers. Environ. Sci. Pollut. Res. Int. 2017, 24, 19795–19806. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Wu, J.H.; Sun, Y.X.; Zhang, Y.Y.; Zhao, Y.F.; Huang, Z.; Duan, W.H. Climate and geochemistry at different altitudes influence soil fungal community aggregation patterns in alpine grasslands. Sci. Total Environ. 2023, 881, 163375. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.; Van Der Heijden, M.G.; Weedon, J.T.; Kowalchuk, G.A.; Roling, W.F. Community assembly, species richness and nestedness of arbuscular mycorrhizal fungi in agricultural soils. Mol. Ecol. 2012, 21, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.; Barsoum, N.; Lilleskov, E.A.; Bidartondo, M.I. Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients. Ecol. Lett. 2010, 13, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, M.S.; Hamel, C.; Atul, N.; St-Arnaud, M. Long-Term Phosphorus Fertilization Impacts Soil Fungal and Bacterial Diversity but not AM Fungal Community in Alfalfa. Microb. Ecol. 2010, 59, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; van Dorst, J.; Ji, M.; Ferrari, B.C.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Bhalla, K.; Qu, X.; Kretschmer, M.; Kronstad, J.W. The phosphate language of fungi. Trends Microbiol. 2022, 30, 338–349. [Google Scholar] [CrossRef]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, L.; Li, J.; Shi, G.; Jiang, S.; Ma, X.; An, L.; Du, G.; Feng, H. Resource availability differentially drives community assemblages of plants and their root-associated arbuscular mycorrhizal fungi. Plant Soil 2014, 386, 341–355. [Google Scholar] [CrossRef]
- van der Gast, C.J.; Gosling, P.; Tiwari, B.; Bending, G.D. Spatial scaling of arbuscular mycorrhizal fungal diversity is affected by farming practice. Environ. Microbiol. 2011, 13, 241–249. [Google Scholar] [CrossRef]
- Caruso, T. Disentangling the factors shaping arbuscular mycorrhizal fungal communities across multiple spatial scales. New Phytol. 2018, 220, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Cajthaml, T.; Polme, S.; Hiiesalu, I.; Anslan, S.; Harend, H.; Buegger, F.; Pritsch, K.; Koricheva, J.; et al. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 2016, 10, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tian, J.; Bai, C.; Xiang, M.; Sun, J.; Liu, X. The biogeography of fungal communities in wetland sediments along the Changjiang River and other sites in China. ISME J. 2013, 7, 1299–1309. [Google Scholar] [CrossRef]
- Bayranvand, M.; Akbarinia, M.; Salehi Jouzani, G.; Gharechahi, J.; Kooch, Y.; Baldrian, P. Composition of soil bacterial and fungal communities in relation to vegetation composition and soil characteristics along an altitudinal gradient. FEMS Microbiol. Ecol. 2021, 97, fiaa201. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.-J.; Banerjee, S.; White, J.F.; Zhou, N.; Zhao, Z.-Y.; Zhang, K.; Hu, M.-F.; Kingsley, K.; Tian, C.-Y. Not by Salinity Alone: How Environmental Factors Shape Fungal Communities in Saline Soils. Soil. Sci. Soc. Am. J. 2019, 83, 1387–1398. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yu, Z.; Zhao, M.; Kerfahi, D.; Li, N.; Shi, L.; Qi, X.; Lee, C.-B.; Dong, K.; Lee, H.-I.; et al. Elevational Variation in and Environmental Determinants of Fungal Diversity in Forest Ecosystems of Korean Peninsula. J. Fungi 2024, 10, 556. https://doi.org/10.3390/jof10080556
Chen L, Yu Z, Zhao M, Kerfahi D, Li N, Shi L, Qi X, Lee C-B, Dong K, Lee H-I, et al. Elevational Variation in and Environmental Determinants of Fungal Diversity in Forest Ecosystems of Korean Peninsula. Journal of Fungi. 2024; 10(8):556. https://doi.org/10.3390/jof10080556
Chicago/Turabian StyleChen, Lei, Zhi Yu, Mengchen Zhao, Dorsaf Kerfahi, Nan Li, Lingling Shi, Xiwu Qi, Chang-Bae Lee, Ke Dong, Hae-In Lee, and et al. 2024. "Elevational Variation in and Environmental Determinants of Fungal Diversity in Forest Ecosystems of Korean Peninsula" Journal of Fungi 10, no. 8: 556. https://doi.org/10.3390/jof10080556






