Metals Transfer in Mushroom Tricholoma matsutake from Regional High Geochemical Background Areas: Environmental Influences and Human Health Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pretreatment
2.2. Chemical Analysis
2.3. Bioaccumulation and Translocation Analysis
2.4. Health Risk Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Soil pH, OM and Metals Concentration
3.2. Metals Concentration and Distribution in T. matsutake
3.2.1. High Metals Concentration in T. matsutake
3.2.2. Metals Distribution in Cap and Stipe
3.3. Metals Bioaccumulation and Transfer in T. matsutake
3.4. Potential Risk to Human Health
3.4.1. Metals Daily Intake Estimate
3.4.2. Health Risk Assessment
3.5. Correlation between Soil Properties and T. matsutake Metals Accumulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brzezicha-Cirocka, J.; Grembecka, M.; Grochowska, I.; Falandysz, J.; Szefer, P. Elemental composition of selected species of mushrooms based on a chemometric evaluation. Ecotoxicol. Environ. Saf. 2019, 173, 353–365. [Google Scholar] [CrossRef]
- Wagner, D.; Heider, D.; Hattab, G. Mushroom data creation, curation, and simulation to support classification tasks. Sci. Rep. 2021, 11, 8134–8145. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P. Trace element contents in European species of wild growing edible mushrooms: A review for the period 2000–2009. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Li, J.; Wang, Y. Research progress on elements of wild edible mushrooms. J. Fungi 2022, 8, 964–991. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Fan, W.; Qiao, M.; Hao, H.; Wang, X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ. Monit. Assess. 2011, 179, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Huang, Q.; Cai, H.J.; Guo, X.; Wang, T.T.; Gui, M.Y. Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem. 2015, 188, 294–300. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017, 7, 46221–46231. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cadorin, M.; Liang, Y.J.; Yang, Z.L. DNA-based geographic typing of the gourmet mushroom Tricholoma matsutake traded in China. Mycoscience 2010, 51, 248–251. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.L.; Chen, C.; Li, S.H.; Huang, W.L.; Xiong, C.; Jin, X.; Zheng, L.Y. Analysis of bacterial diversity and communities associated with Tricholoma matsutake fruiting bodies by barcoded pyrosequencing in Sichuan Province, Southwest China. J. Microbiol. Biotechnol. 2016, 26, 89–98. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Li, W.H.; Li, X.L.; Huang, W.L.; Yang, H.; Zheng, L.Y. Chemical compositions and volatile compounds of Tricholoma matsutake from different geographical areas at different stages of maturity. Food Sci. Biotechnol. 2016, 25, 71–77. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhang, J.; Wu, L.H.; Zhao, Y.L.; Li, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Li, Y.; Liang, C.; Huang, H.; Wang, S. Available heavy metals concentrations in agricultural soils: Relationship with soil properties and total heavy metals concentrations in different industries. J. Hazard. Mater. 2024, 471, 134410. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Zhang, J.; Zhao, Y.; Liu, H. Trace element content of Boletus tomentipes mushroom collected from Yunnan, China. Food Chem. 2011, 127, 1828–1830. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Kocak, M.S.; Uren, M.C. Metal concentration and antioxidant activity of edible mushrooms from Turkey. Food Chem. 2015, 175, 549–555. [Google Scholar] [CrossRef]
- Szymańska, K.; Strumińska-Parulska, D.; Falandysz, J. Uranium (234U, 238U) and thorium (230Th, 232Th) in mushrooms of genus Leccinum and Leccinellum and the potential effective ionizing radiation dose assessment for human. Chemosphere 2020, 250, 126242. [Google Scholar] [CrossRef]
- George, T.S.; Richardson, A.E.; Simpson, R.J. Behaviour of plant-derived extracellular phytase upon addition to soil. Soil Biol. Biochem. 2005, 37, 977–988. [Google Scholar] [CrossRef]
- Melgar, M.J.; Alonso, J.; Garcia, M.A. Mercury in edible mushrooms and underlying soil: Bioconcentration factors and toxicological risk. Sci. Total Environ. 2009, 407, 5328–5334. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Luo, Z.; Bi, J.; Liu, C.; Liu, X. Transfer of heavy metals from soil to tea and the potential human health risk in a regional high geochemical background area in southwest China. Sci. Total Environ. 2024, 908, 168122–168134. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Chen, Y.; Li, Z.; Hedding, D.W.; Nel, W.; Ji, J.; Chen, J. Geochemical behavior and potential health risk of heavy metals in basalt-derived agricultural soil and crops: A case study from Xuyi County, eastern China. Sci. Total Environ. 2020, 729, 139058–139067. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.; Mitic, V.; Dordevic, D.; Popovic, G.; Krstic, N.; Nikolic, J.; Jovanovic, V.S. Macroelements versus toxic elements in selected wild edible mushrooms of the Russulacea family from Serbia. J. Serb. Chem. Soc. 2021, 86, 927–940. [Google Scholar] [CrossRef]
- Cui, Y.J.; Zhu, Y.G.; Zhai, R.H.; Chen, D.Y.; Huang, Y.Z.; Qiu, Y.; Liang, J.Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). 2021. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database (accessed on 11 August 2024).
- USEPA. A Review of the Reference Dose and Reference Concentration Processes; U.S. Environmental Protection Agency RAF: Washington, DC, USA, 2002. Available online: https://www.epa.gov/iris (accessed on 11 August 2024).
- Zhang, R.; Tao, C.; Zhang, Y.; Hou, Y.; Chang, Q. Health risk assessment of heavy metals in agricultural soils and identification of main influencing factors in a typical industrial park in northwest China. Chemosphere 2020, 252, 126591–126600. [Google Scholar] [CrossRef]
- Kalač, P.; Svoboda, L. A review of trace element concentrations in edible mushrooms. Food Chem. 2000, 69, 273–281. [Google Scholar] [CrossRef]
- García, M.Á.; Alonso, J.; Melgar, M.J. Lead in edible mushrooms: Levels and bioaccumulation factors. J. Hazard. Mater. 2009, 167, 777–783. [Google Scholar] [CrossRef]
- Qi, D.; Wieneke, X.; Tao, J.; Zhou, X.; Desilva, U. Soil pH is the primary factor correlating with soil microbiome in karst rocky desertification regions in the Wushan County, Chongqing, China. Front. Microbiol. 2018, 9, 1027–1038. [Google Scholar] [CrossRef]
- Tanikawa, T.; Fujii, S.; Sun, L.; Hirano, Y.; Matsuda, Y.; Miyatani, K.; Doi, R.; Mizoguchi, T.; Maie, N. Leachate from fine root litter is more acidic than leaf litter leachate: A 2.5-year laboratory incubation. Sci. Total Environ. 2018, 645, 179–191. [Google Scholar] [CrossRef]
- Rasalanavho, M.; Moodley, R.; Jonnalagadda, S.B. Elemental bioaccumulation and nutritional value of five species of wild growing mushrooms from South Africa. Food Chem. 2020, 319, 126596–126606. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzynska, S.; Szostek, M.; Kuczynska-Kippen, N.; Kalac, P.; Niedzielski, P.; Gasecka, M.; et al. Toxicological risks and nutritional value of wild edible mushroom species -a half-century monitoring study. Chemosphere 2021, 263, 128095–128106. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Andres, E.; Escalona, Y.; Oostenbrink, C.; Tunega, D.; Gerzabek, M.H. Soil organic matter stabilization at molecular scale: The role of metal cations and hydrogen bonds. Geoderma 2021, 401, 115237–115249. [Google Scholar] [CrossRef]
- Saqib Rashid, M.; Liu, G.; Yousaf, B.; Song, Y.; Ahmed, R.; Rehman, A.; Arif, M.; Irshad, S.; Cheema, A.I. Efficacy of rice husk biochar and compost amendments on the translocation, bioavailability, and heavy metals speciation in contaminated soil: Role of free radical production in maize (Zea mays L.). J. Clean. Prod. 2022, 330, 129805–129820. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, X.; Dong, L. Coal fly ash and straw immobilize Cu, Cd and Zn from mining wasteland. Environ. Chem. Lett. 2014, 12, 289–295. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Kwong, R.W.M.; Tang, W.; Yang, Y.; Zhong, H. Straw return enhances the risks of metals in soil? Ecotoxicol. Environ. Saf. 2021, 207, 111201–111210. [Google Scholar] [CrossRef]
- Hernandez-soriano, M.C.; Jimenez-lopez, J.C. Effects of soil water content and organic matter addition on the speciation and bioavailability of heavy metal. Sci. Total Environ. 2012, 423, 55–61. [Google Scholar] [CrossRef]
- Chen, H.X.; Chen, Y.; Li, S.; Zhang, W.; Zhang, Y.; Gao, S.; Li, N.; Tao, L.; Wang, Y. Trace elements determination and health risk assessment of Tricholoma matsutake from Yunnan Province, China. J. Consum. Prot. Food Saf. 2020, 15, 153–162. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Shen, T.; Shi, Y.D.; Yang, S.B.; Zhang, T.; Li, J.Q.; Wang, Y.Z.; Liu, H.G. Mineral element content in prized matsutake mushroom (Tricholoma matsutake) collected in China. Chem. Pap. 2013, 67, 672–676. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Kalac, P.; Siwulski, M.; Niedzielski, P. Family and species as determinants modulating mineral composition of selected wild-growing mushroom species. Environ. Sci. Pollut. Res. 2021, 28, 389–404. [Google Scholar] [CrossRef]
- Zsigmond, A.R.; Fejer, I.R.; Kantor, I.; May, Z.; Urak, I. Influence of the urban environment on four mushroom species in the light of their elemental composition. Chemosphere 2023, 335, 139052–139060. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fu, Y.; Shi, M.; Wang, H.; Guo, J. Pollution level and risk assessment of lead, cadmium, mercury, and arsenic in edible mushrooms from Jilin Province, China. J. Food Sci. 2021, 86, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Gucia, M.; Jarzynska, G.; Rafal, E.; Roszak, M.; Kojta, A.K.; Osiej, I.; Falandysz, J. Multivariate analysis of mineral constituents of edible Parasol Mushroom (Macrolepiota procera) and soils beneath fruiting bodies collected from Northern Poland. Environ. Sci. Pollut. Res. 2012, 19, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Tuzen, M.; Sesli, E.; Soylak, M. Trace element levels of mushroom species from East Black Sea region of Turkey. Food Control 2007, 18, 806–810. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.E.; Terlikowska, K.M.; Borawska, M.H.; Witkowska, A.M. Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the eastern territory of “Green Lungs of Poland”: Nutritional and toxicological implications. Int. J. Environ. Res. Public Health 2019, 16, 3614–3635. [Google Scholar] [CrossRef]
- Svoboda, L.; Zimmermannová, K.; Kalač, P. Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Sci. Total Environ. 2000, 246, 61–67. [Google Scholar] [CrossRef]
- Falandysz, J.; Hanć, A.; Barałkiewicz, D.; Zhang, J.; Treu, R. Metallic and metalloid elements in various developmental stages of Amanita muscaria (L.) Lam. Fungal Biol. 2020, 124, 174–182. [Google Scholar] [CrossRef]
- Demirbaş, A. Heavy metal bioaccumulation by mushrooms from artificially fortified soils. Food Chem. 2001, 74, 293–301. [Google Scholar] [CrossRef]
- Lalotra, P.; Gupta, D.; Yangdol, R.; Sharma, Y.P.; Gupta, S.K. Bioaccumulation of heavy metals in the sporocarps of some wild mushrooms. Curr. Res. Environ. Appl. Mycol. 2016, 6, 159–165. [Google Scholar] [CrossRef]
- Damodaran, D.; Vidya Shetty, K.; Raj Mohan, B. Uptake of certain heavy metals from contaminated soil by mushroom–Galerina vittiformis. Ecotoxicol. Environ. Saf. 2014, 104, 414–422. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Barea-Sepulveda, M.; Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Bouziane, H.; Lopez-Castillo, J.G.; Palma, M.F.; Barbero, G.F. Toxic elements and trace elements in Macrolepiota procera mushrooms from southern Spain and northern Morocco. J. Food Compos. Anal. 2022, 108, 104419–104430. [Google Scholar] [CrossRef]
- Gadd, G.M. Interactions of fungi with toxic metals. New Phytol. 1993, 124, 25–60. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Benítez-Rodríguez, A.; López-Castillo, J.G.; Palma, M.; Barbero, G.F. Metal concentrations in Lactarius mushroom species collected from Southern Spain and Northern Morocco: Evaluation of health risks and benefits. J. Food Compos. Anal. 2021, 99, 103859–103868. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Bouziane, H.; López-Castillo, J.G.; Palma, M.; Barbero, G.F. Exposure to Essential and Toxic Elements via Consumption of Agaricaceae, Amanitaceae, Boletaceae, and Russulaceae Mushrooms from Southern Spain and Northern Morocco. J. Fungi 2022, 8, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Keskin, F.; Sarikurkcu, C.; Akata, I.; Tepe, B. Metal concentrations of wild mushroom species collected from Belgrad forest (Istanbul, Turkey) with their health risk assessments. Environ. Sci. Pollut. Res. 2021, 28, 36193–36204. [Google Scholar] [CrossRef] [PubMed]
- Ziarati, P.; Rabizadeh, H. The Effect of Thermal and Non Thermal Food Processes and Cooking Method in some Essential Mineral Contents in Mushroom (Agaricus bisporus) in Iran. J. Novel Appl. Sci. 2013, 2, 954–959. [Google Scholar]
- Sarikurkcu, C.; Popović-Djordjević, J.; Solak, M.H. Wild edible mushrooms from Mediterranean region: Metal concentrations and health risk assessment. Ecotoxicol. Environ. Saf. 2020, 190, 110058–110064. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378–394. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Li, J.; Li, T.; Liu, H.; Wang, Y. Determination of mineral contents of wild Boletus edulis mushroom and its edible safety assessment. J. Environ. Sci. Health Part B 2018, 53, 454–463. [Google Scholar] [CrossRef]
- Proskura, N.; Podlasinska, J.; Skopicz-Radkiewicz, L. Chemical composition and bioaccumulation ability of Boletus badius (Fr.) Fr. collected in western Poland. Chemosphere 2017, 168, 106–111. [Google Scholar] [CrossRef] [PubMed]
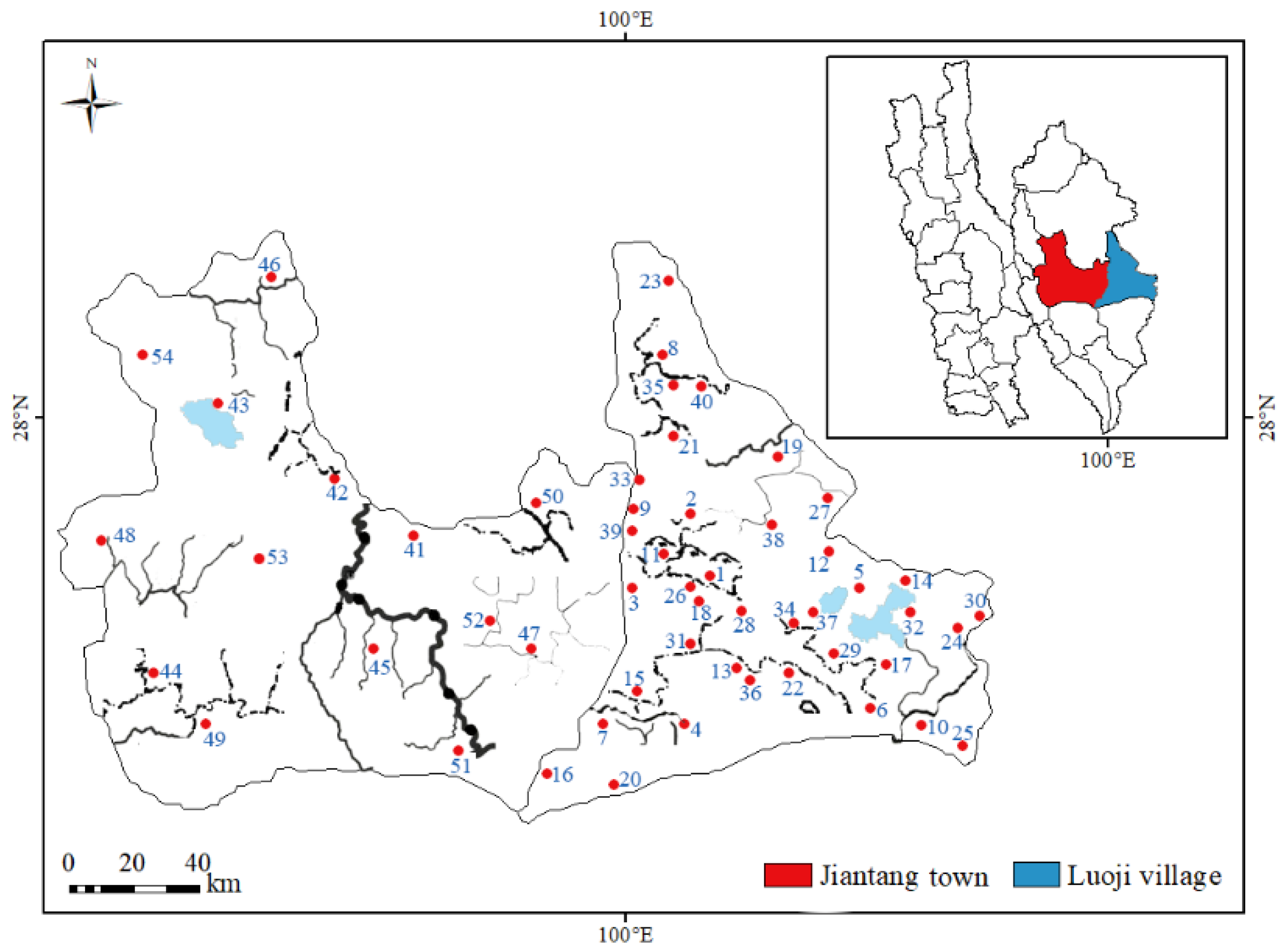
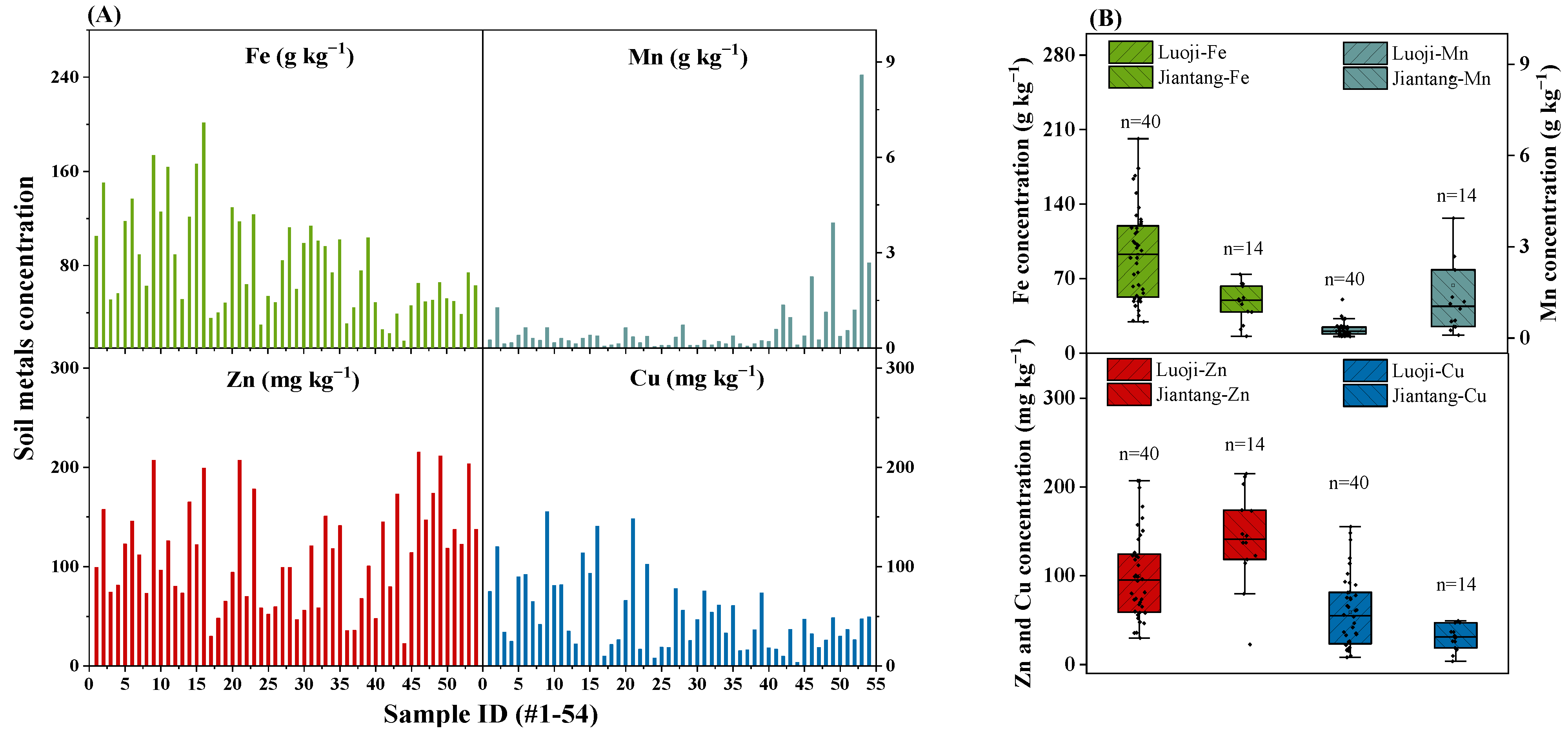


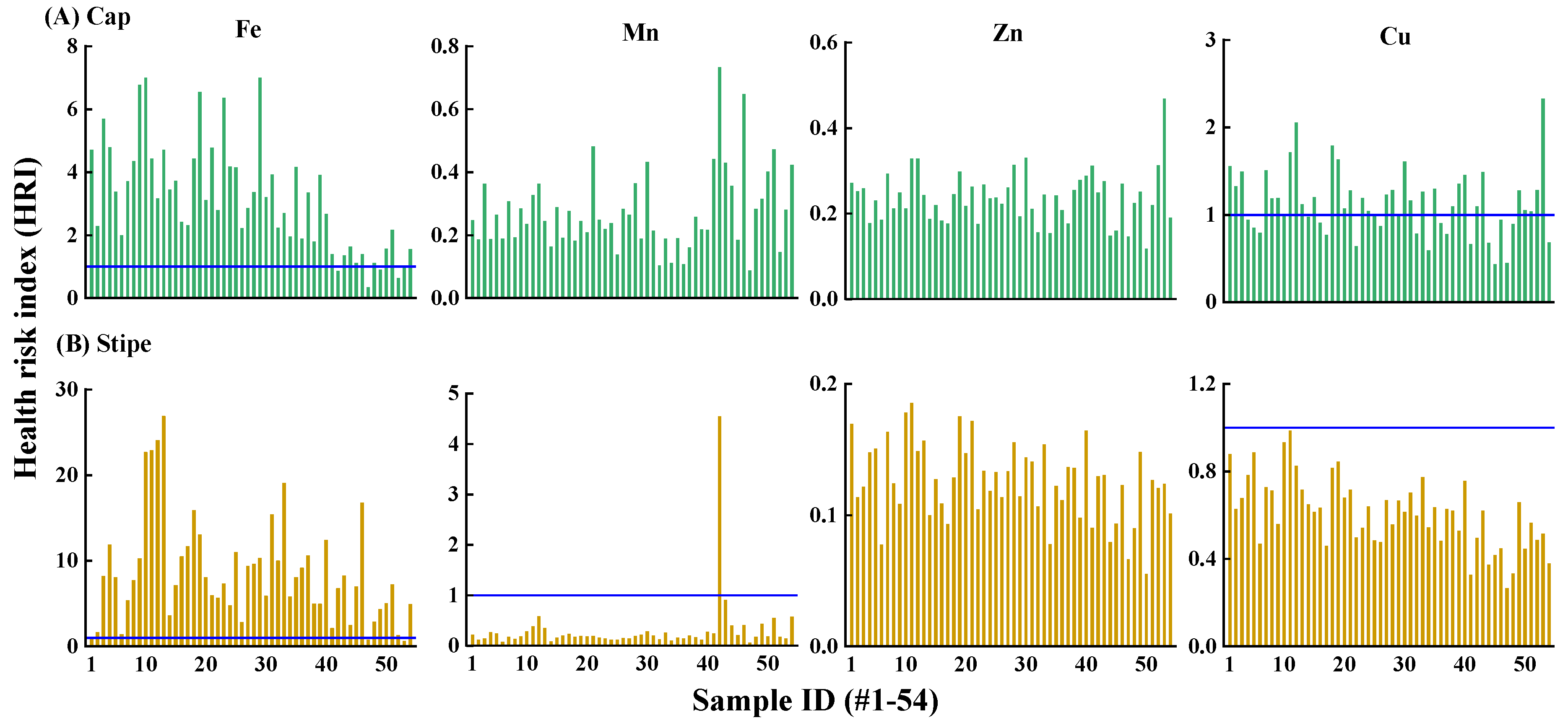
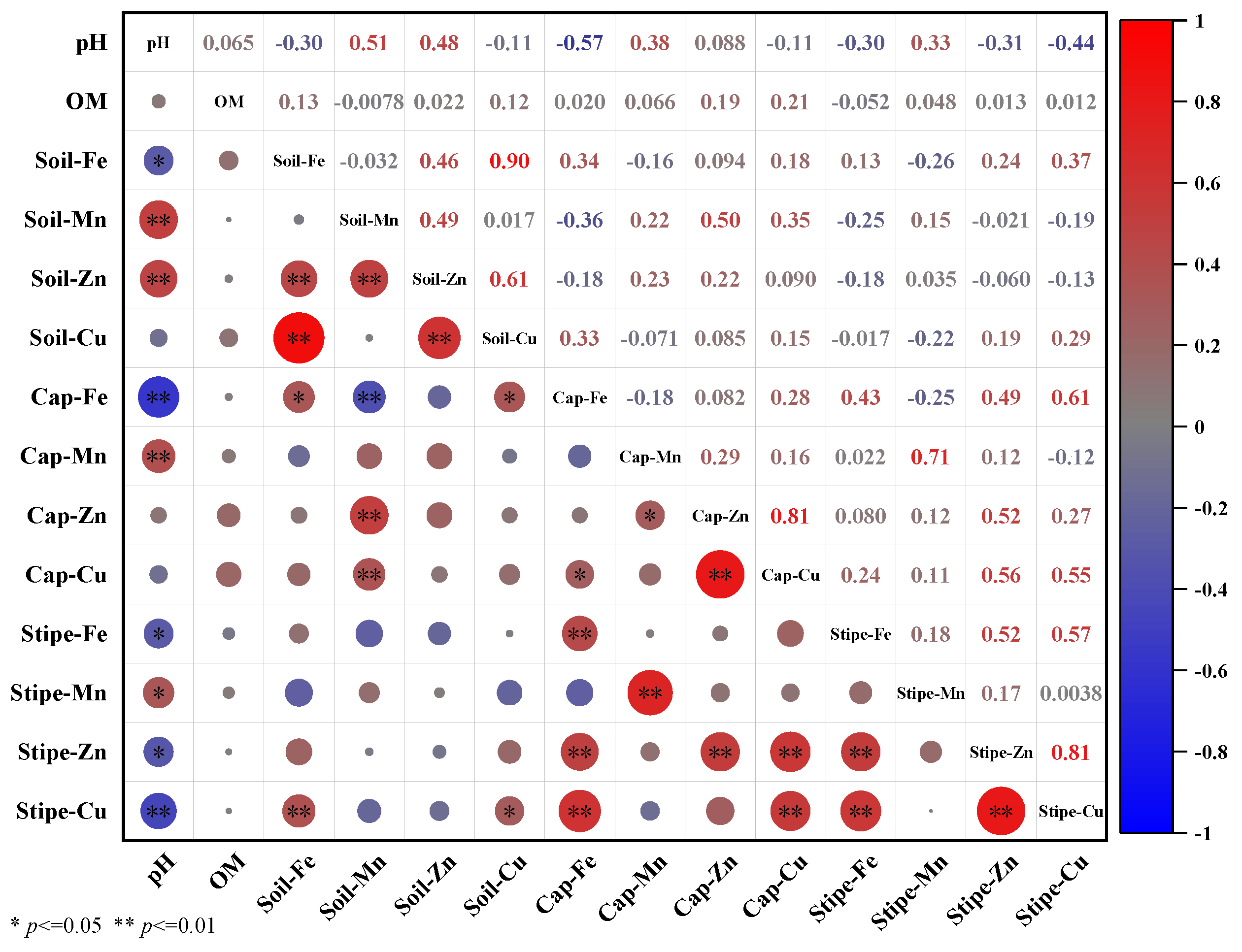
| Sample ID | Soil pH | Soil Organic Matter Content (%) | DI (µg kg−1 bw d−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | |||||||
| Cap | Stipe | Cap | Stipe | Cap | Stipe | Cap | Stipe | |||
| 1 | 4.57 | 6.96 | 1413 | 244 | 34.7 | 30.5 | 81.6 | 50.9 | 62.1 | 35.2 |
| 2 | 5.33 | 11.9 | 689 | 493 | 26.0 | 16.2 | 75.6 | 34.1 | 53.1 | 25.2 |
| 3 | 4.32 | 8.79 | 1708 | 2464 | 50.9 | 20.3 | 77.8 | 36.5 | 59.9 | 27.1 |
| 4 | 4.39 | 10.6 | 1437 | 3564 | 26.4 | 37.2 | 53.2 | 44.3 | 37.8 | 31.4 |
| 5 | 4.64 | 13.7 | 1013 | 2412 | 37.0 | 34.4 | 69.3 | 45.2 | 34.0 | 35.5 |
| 6 | 4.58 | 9.63 | 596 | 416 | 26.5 | 11.0 | 55.8 | 23.3 | 31.8 | 18.8 |
| 7 | 4.32 | 9.58 | 1115 | 1613 | 43.0 | 25.3 | 88.0 | 49.0 | 60.3 | 29.1 |
| 8 | 4.99 | 18 | 1303 | 2301 | 27.0 | 19.2 | 63.7 | 37.3 | 47.4 | 28.5 |
| 9 | 5.02 | 27.8 | 2033 | 3077 | 39.9 | 26.6 | 74.8 | 32.5 | 47.6 | 22.4 |
| 10 | 4.19 | 3.86 | 2097 | 6799 | 33.0 | 39.8 | 63.5 | 53.4 | 39.8 | 37.4 |
| 11 | 5.06 | 11.4 | 1329 | 6857 | 45.8 | 54.0 | 98.6 | 55.6 | 68.5 | 39.5 |
| 12 | 4.06 | 7.71 | 948 | 7216 | 50.8 | 82.2 | 98.7 | 44.6 | 82.2 | 33.0 |
| 13 | 4.32 | 7.87 | 1416 | 8058 | 34.3 | 49.2 | 73.0 | 47.1 | 44.8 | 28.7 |
| 14 | 4.74 | 10.6 | 1033 | 1073 | 22.8 | 12.9 | 56.2 | 30.0 | 39.1 | 26.0 |
| 15 | 4.23 | 1.29 | 1119 | 2132 | 40.4 | 22.9 | 65.9 | 38.2 | 48.1 | 24.6 |
| 16 | 4.84 | 5.07 | 727 | 3145 | 26.8 | 29.0 | 55.2 | 32.7 | 36.3 | 25.3 |
| 17 | 4.68 | 6.84 | 697 | 3495 | 38.6 | 33.1 | 52.9 | 28.0 | 30.8 | 18.4 |
| 18 | 4.83 | 15.1 | 1330 | 4760 | 25.5 | 24.4 | 73.7 | 38.7 | 71.6 | 32.7 |
| 19 | 4.8 | 6.23 | 1964 | 3912 | 34.3 | 26.9 | 89.4 | 52.6 | 65.4 | 33.8 |
| 20 | 4.31 | 17.3 | 935 | 2419 | 29.2 | 26.4 | 65.5 | 44.1 | 43.0 | 27.2 |
| 21 | 4.81 | 3.77 | 1434 | 1784 | 67.4 | 27.7 | 79.0 | 51.5 | 51.2 | 28.7 |
| 22 | 4.13 | 6.21 | 836 | 1695 | 34.8 | 22.3 | 52.7 | 31.4 | 25.6 | 19.9 |
| 23 | 4.52 | 11.1 | 1907 | 2185 | 30.6 | 19.9 | 80.4 | 40.1 | 47.7 | 21.7 |
| 24 | 4.26 | 2.78 | 1253 | 1427 | 33.3 | 16.1 | 70.7 | 35.5 | 41.7 | 25.6 |
| 25 | 4.12 | 7.55 | 1246 | 3285 | 19.4 | 16.4 | 71.1 | 39.9 | 39.3 | 19.4 |
| 26 | 4.1 | 3.85 | 667 | 840 | 39.7 | 21.6 | 67.0 | 34.1 | 34.9 | 19.1 |
| 27 | 4.7 | 6.2 | 860 | 2811 | 37.1 | 20.1 | 78.5 | 40.1 | 49.2 | 26.8 |
| 28 | 4.48 | 6.68 | 1008 | 2877 | 51.1 | 26.8 | 94.2 | 46.7 | 51.3 | 22.3 |
| 29 | 4.44 | 3.09 | 2099 | 3081 | 26.5 | 30.7 | 58.1 | 34.3 | 40.5 | 26.7 |
| 30 | 4.19 | 44.5 | 962 | 1764 | 60.7 | 40.0 | 99.1 | 43.2 | 64.4 | 24.6 |
| 31 | 4.57 | 8.4 | 1178 | 4609 | 29.9 | 28.7 | 63.3 | 42.2 | 46.6 | 28.2 |
| 32 | 4.2 | 8.61 | 669 | 2996 | 14.5 | 18.1 | 46.9 | 32.0 | 31.4 | 23.9 |
| 33 | 4.55 | 10.3 | 810 | 5716 | 26.4 | 36.5 | 73.3 | 46.1 | 50.5 | 31.0 |
| 34 | 4.74 | 8.76 | 584 | 1733 | 15.6 | 14.3 | 46.2 | 23.4 | 23.8 | 21.8 |
| 35 | 3.95 | 12.2 | 1250 | 2414 | 26.7 | 22.8 | 72.8 | 36.7 | 52.0 | 25.4 |
| 36 | 4.89 | 10.6 | 566 | 2739 | 15.2 | 20.0 | 62.5 | 33.4 | 36.1 | 19.3 |
| 37 | 4.66 | 8.77 | 1005 | 3172 | 22.5 | 28.7 | 52.9 | 41.0 | 31.2 | 25.1 |
| 38 | 4.68 | 5.59 | 539 | 1485 | 36.0 | 23.3 | 76.5 | 40.8 | 43.8 | 24.8 |
| 39 | 4.55 | 7.71 | 1175 | 1479 | 30.5 | 16.2 | 83.5 | 29.4 | 54.1 | 21.1 |
| 40 | 4.68 | 10.6 | 800 | 3722 | 30.4 | 39.0 | 86.6 | 49.3 | 58.3 | 30.3 |
| 41 | 6.37 | 5.53 | 417 | 627 | 61.8 | 34.5 | 93.6 | 27.1 | 26.6 | 13.1 |
| 42 | 5.52 | 10.3 | 261 | 2027 | 103 | 637 | 74.7 | 38.9 | 44.0 | 19.8 |
| 43 | 5.94 | 13.4 | 408 | 2473 | 60.1 | 128 | 82.7 | 39.2 | 59.6 | 24.8 |
| 44 | 5.08 | 7.12 | 490 | 744 | 49.9 | 56.5 | 44.5 | 23.9 | 27.1 | 15.0 |
| 45 | 6.29 | 4.48 | 335 | 2092 | 26.0 | 29.4 | 48.0 | 28.0 | 17.4 | 16.7 |
| 46 | 6.11 | 8.74 | 418 | 5031 | 90.8 | 57.3 | 81.1 | 36.9 | 37.8 | 17.9 |
| 47 | 4.79 | 7.49 | 102 | 217 | 12.3 | 9.0 | 43.9 | 20.0 | 18.0 | 10.7 |
| 48 | 5.96 | 10.6 | 334 | 861 | 39.6 | 25.3 | 67.5 | 27.0 | 35.8 | 13.3 |
| 49 | 6.3 | 6.51 | 273 | 1293 | 44.1 | 61.0 | 75.3 | 44.5 | 51.1 | 26.4 |
| 50 | 5.96 | 12.9 | 472 | 1502 | 56.2 | 25.8 | 35.5 | 16.6 | 42.2 | 17.9 |
| 51 | 5.38 | 3.94 | 650 | 2156 | 66.1 | 77.7 | 66.0 | 38.1 | 41.6 | 22.6 |
| 52 | 6.25 | 21.3 | 192 | 385 | 20.5 | 24.5 | 93.7 | 36.2 | 51.3 | 19.5 |
| 53 | 5.79 | 7.12 | 306 | 177 | 39.3 | 20.6 | 141 | 37.1 | 93.2 | 20.6 |
| 54 | 6.56 | 15.3 | 465 | 1476 | 59.1 | 80.6 | 57.2 | 30.4 | 27.3 | 15.2 |
| RfD a (µg kg−1 bw d−1) | 300 (JECEFA) c | 140 (USEPA) d | 300 d | 40 d | ||||||
| PTMDI b (µg kg−1 bw d−1) | – | – | 300–1000 c | 5000 c | ||||||
| Percentage of samples exceeding RfD (%) | 92.6% | 94.4% | 0 | 1.85% | 0 | 0 | 61.1% | 0 | ||
| Percentage of samples exceeding PTMDI (%) | – | – | 0 | 0 | 0 | 0 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Bi, J.; Zhang, Y.; Zhang, Y.; Liu, X. Metals Transfer in Mushroom Tricholoma matsutake from Regional High Geochemical Background Areas: Environmental Influences and Human Health Risk. J. Fungi 2024, 10, 608. https://doi.org/10.3390/jof10090608
Wang C, Bi J, Zhang Y, Zhang Y, Liu X. Metals Transfer in Mushroom Tricholoma matsutake from Regional High Geochemical Background Areas: Environmental Influences and Human Health Risk. Journal of Fungi. 2024; 10(9):608. https://doi.org/10.3390/jof10090608
Chicago/Turabian StyleWang, Cuiting, Jue Bi, Yukang Zhang, Yixuan Zhang, and Xue Liu. 2024. "Metals Transfer in Mushroom Tricholoma matsutake from Regional High Geochemical Background Areas: Environmental Influences and Human Health Risk" Journal of Fungi 10, no. 9: 608. https://doi.org/10.3390/jof10090608





