Abstract
Human endemic mycoses are potentially fatal diseases caused by a diverse group of fungi that can alter their morphology in response to an increase in temperature. These thermally dimorphic fungi affect both healthy and immunocompromised hosts, causing a substantial health and economic burden. Despite this, the diagnosis of endemic mycoses is still a formidable challenge for several reasons, including similar symptomatology, limited utility of classical diagnostic methods, inaccessibility to reliable molecular approaches in most endemic areas, and a lack of clinical suspicion out of these regions. This review summarizes essential knowledge on thermally dimorphic fungi and the life-threatening diseases they cause. The principle, advantages and limitations of the methods traditionally used for their diagnosis are also described, along with the application status and future directions for the development of alternative diagnostic strategies, which could help to reduce the disease burden in endemic areas.
1. Introduction
Since the emergence of fungi ca. 700 million years ago, pathogenic forms have emerged independently in multiple lineages during evolution. Among them, there is a taxonomically diverse group of fungi distributed across four of the nine fungal lineages currently accepted ([1]; Figure 1) that drastically alter their morphology and developmental programs in response to different environmental stimuli, such as the concentration of nutrients, ammonium, O2 and CO2, pheromones, pH, or temperature (Table S1). Because of this morphological transition, which entails changes in the cell wall composition to ensure fungal survival [2], they are known as dimorphic fungi.
Given their impacts on public health, this review focuses on thermally dimorphic fungi (hereafter TDF), i.e., species that switch between two morphologies depending on the temperature at which they grow, and presents a summary of the methods currently available for the diagnosis of their associated diseases. Specifically, the TDF included here are primary pathogens of the genera Blastomyces, Coccidioides, Histoplasma, Paracoccidioides, Sporothrix, Talaromyces, which need to be handled in biosafety level 3 laboratories (BSL-3), and opportunistic species of Emergomyces (BSL-2), a recently described genus [3]. These fungi are responsible for different human systemic mycoses that can present as localized or disseminated and are associated with a variety of symptoms, including severe lung damage and stigmatizing cutaneous lesions (Table 1). Note that some close relatives to the species included in Table 1, such as Blastomyces silverae or Paracoccidioides ceti, cause pulmonary infections in animals, but have been only occasionally reported as human pathogens, and therefore will not be treated here.
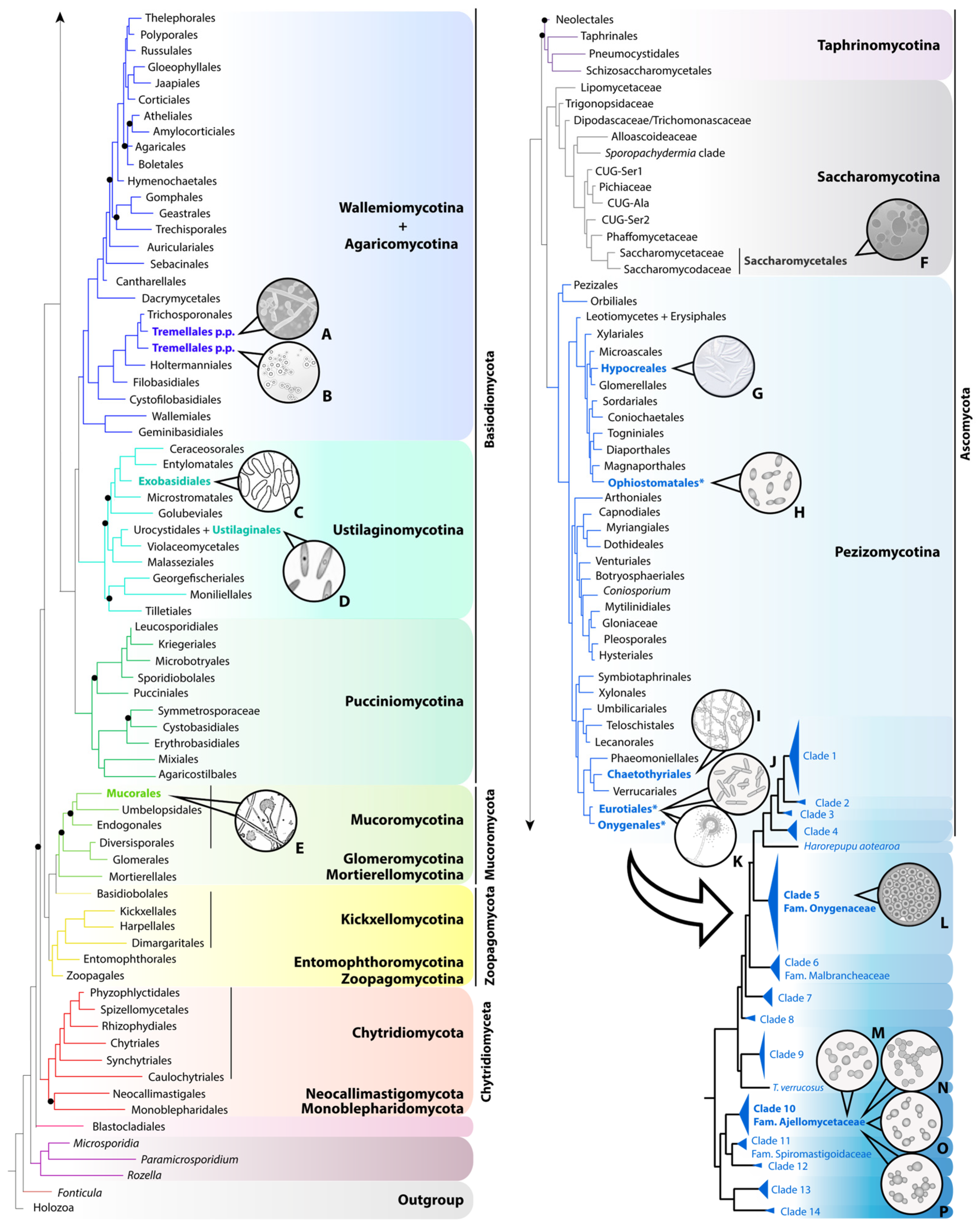

Figure 1.
Distribution of relevant human pathogenic species across fungi, based on the phylogenomic tree published by Li and colleagues [4] and the multigene phylogeny of the order Onygenales published by Kandemir and collaborators [5]. In the genome-scale tree of fungi, derived from a concatenated data matrix (290 genes), the nine lineages currently accepted are named and shaded in different colors. Terminals of the tree are labeled using order-level taxonomic names, except for Saccharomycotina, for which informal and family-level names of the major clades are used. Orders including pathogenic species appear in bold and are colored, with those three comprising thermally dimorphic fungi of clinical interest marked with an asterisk. The phylogeny of Onygenales (lower right corner of the figure) was obtained by combining eight loci. Only those clades comprising human pathogens are formally named. A. Trichosporon spp. B. Cryptococcus spp. C. Exobasidium spp. D. Ustilago spp. E. Mucor spp. F. Candida spp. G. Fusarium spp. H. Sporothrix spp. I. Fonsecaea spp. J. Talaromyces marneffei. K. Aspergillus spp. L. Coccidioides spp. M. Blastomyces spp. N. Emergomyces spp. O. Histoplasma spp. P. Paracoccidioides spp.
Figure 1.
Distribution of relevant human pathogenic species across fungi, based on the phylogenomic tree published by Li and colleagues [4] and the multigene phylogeny of the order Onygenales published by Kandemir and collaborators [5]. In the genome-scale tree of fungi, derived from a concatenated data matrix (290 genes), the nine lineages currently accepted are named and shaded in different colors. Terminals of the tree are labeled using order-level taxonomic names, except for Saccharomycotina, for which informal and family-level names of the major clades are used. Orders including pathogenic species appear in bold and are colored, with those three comprising thermally dimorphic fungi of clinical interest marked with an asterisk. The phylogeny of Onygenales (lower right corner of the figure) was obtained by combining eight loci. Only those clades comprising human pathogens are formally named. A. Trichosporon spp. B. Cryptococcus spp. C. Exobasidium spp. D. Ustilago spp. E. Mucor spp. F. Candida spp. G. Fusarium spp. H. Sporothrix spp. I. Fonsecaea spp. J. Talaromyces marneffei. K. Aspergillus spp. L. Coccidioides spp. M. Blastomyces spp. N. Emergomyces spp. O. Histoplasma spp. P. Paracoccidioides spp.


Table 1.
Symptoms of endemic fungal diseases produced by clinically relevant thermally dimorphic fungi of the orders Onygenales, Ophiostomatales and Eurotiales.
Most TDF belong to the family Ajellomycetaceae, a unique clade within the monophyletic order Onygenales, as it includes the highest number of species adapted to survival and replication within mammalian hosts [11,34]. The exceptions are Coccidioides (family Onygenaceae, Onygenales), Sporothrix (order Ophiostomatales) and Talaromyces marneffei (order Eurotiales). TDF generally develop as spore-producing mycelia (saprophytic phase) in nature (~25–30 °C) or when incubated at similar temperatures, and transform into yeast-like cells (parasitic phase) when infecting a host. In the case of Coccidioides, a special type of spore called arthroconidia, produced by segmentation of hyphae, convert into large parasitic structures (spherules) comprising numerous endospores that are cyclically released within the host [35]. Meanwhile, Emergomyces is characterized by a spectrum of infective forms from budding yeasts to adiaspores, i.e., large, thick-walled non-replicating structures resulting from the growth of inhaled spores that lead to the formation of granulomas [17].
Regardless the type of structure to which spores transform, morphological conversion is essential for the upregulation of genes involved in subverting host immune defenses and in the increased expression of virulence factors (Table S2). These gene products are not necessary for the growth of the parasitic phase in vitro, but are essential for fungal survival within the host [36], as they enable immune response evasion and host colonization.
The outcome of the infections caused by TDF not only depends on the immune status of the host but also on the dose of infectious particles. For this reason, unlike most fungal pathogens, TDF can affect both immunocompromised and healthy individuals if enough spores are inhaled. Moreover, TDF may persist as latent infections for years and reactivate when the immune system weakens (due to ageing, concomitant diseases, or immunosuppressive treatments).
Historically, TDF have been considered regionally endemic, that is, they have been thought to occur in limited geographic ranges (Figure 2), being responsible for substantial morbidity and mortality in their respective regions, i.e., tropical areas of Africa, Asia, and Central and South America [37]. As such, the infections caused by TDF are commonly referred to as “endemic mycoses”. However, due to climate change, the spatial range of different TDF is widening [38,39], which contributes to the already difficult task of diagnosing TDF-caused infections because physicians may have not been in contact with these etiological agents before.
The diagnosis of endemic mycoses is challenging per se due to considerable clinical overlap among them. Indeed, symptoms are not only nonspecific but also hardly distinguishable from those associated with unrelated diseases. For instance, some endemic mycoses, including blastomycosis, coccidioidomycosis and histoplasmosis, are frequently misdiagnosed as pulmonary or intestinal tuberculosis [17,40,41], bacterial or viral pneumonia [42,43], bowel disease [44,45], or diverse malignancies [6,46,47]. In turn, sporotrichosis often presents as lymphocutaneous lesions mimicking those caused by atypical mycobacterial infections, nocardiosis, and leishmaniasis [30].
Classical diagnostic methods are not as accurate as desired, which, coupled with the mentioned syndromic similarities, makes a high index of suspicion crucial to ensure pathogen identification and appropriate treatment [12], avoiding unnecessary antimicrobial use and development of antibiotic resistance. This is especially true in non-endemic areas, where cases associated with immigrants and travelers who returned from endemic countries [48,49] may be undetected or misdiagnosed due to a lack of awareness. On the other hand, reliable molecular diagnostic methods are not available in most endemic areas.
Furthermore, given that person-to-person transmission has not been documented, except for rare cases of transmission by organ transplantation [50], endemic mycoses are not notifiable diseases.
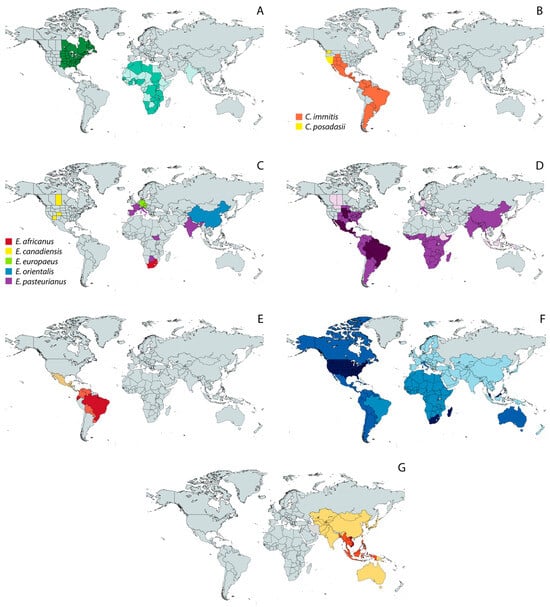
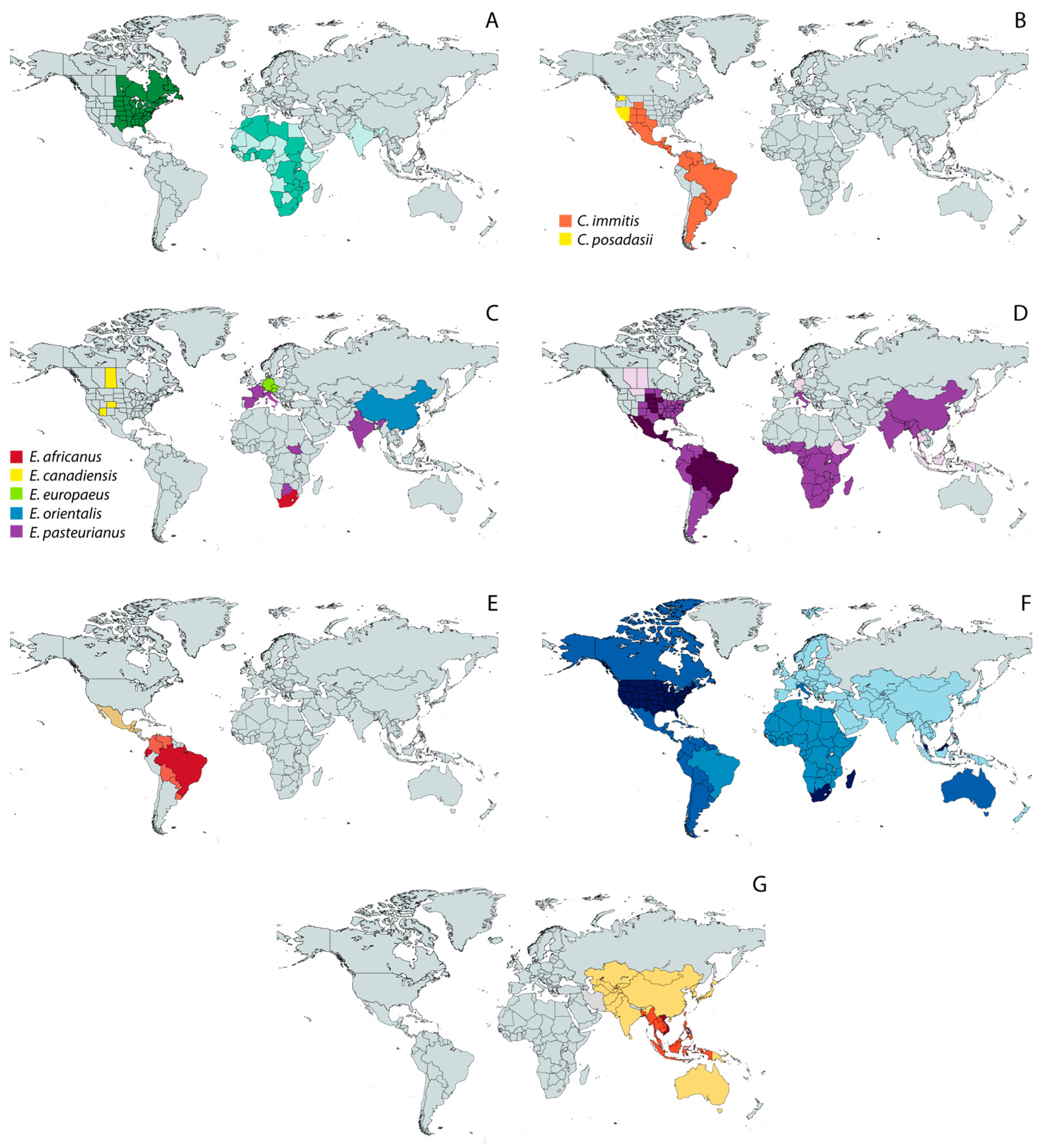

Figure 2.
Geographic distribution of endemic mycoses based on published data [18,39,51,52,53,54]. (A) Blastomycosis; (B) Coccidioidomycosis; (C) Emergomycosis; (D) Histoplasmosis; (E) Paracoccidioidomycosis; (F) Sporotrichosis; and (G) Talaromycosis. Except for the maps of coccidioidomycosis (B) and emergomycosis (C), in which different colors represent different species, color shades represent different levels of incidence, with darker tones corresponding to the highest incidence.
Figure 2.
Geographic distribution of endemic mycoses based on published data [18,39,51,52,53,54]. (A) Blastomycosis; (B) Coccidioidomycosis; (C) Emergomycosis; (D) Histoplasmosis; (E) Paracoccidioidomycosis; (F) Sporotrichosis; and (G) Talaromycosis. Except for the maps of coccidioidomycosis (B) and emergomycosis (C), in which different colors represent different species, color shades represent different levels of incidence, with darker tones corresponding to the highest incidence.

Notably, zoonotic transmission of sporotrichosis through bites or scratches from infected cats is now considered a public health problem in hyperendemic areas [50,55], which makes a prompt and accurate diagnosis necessary to break the chain of transmission and to improve epidemiological knowledge. In this regard, the disease burden and distribution of Sporothrix and other TDF, specially the recently described genus Emergomyces, are probably much larger than currently estimated [19]. In the USA alone, TDF of the order Onygenales collectively cause more than 650,000 new infections each year [8], and the global disease burden continues to rise yearly [56], even if numerous cases remain underdiagnosed and neglected in some endemic regions [57,58].
As for the treatment of TDF-related mycoses, four main classes of antifungal drugs are currently available: (1) polyenes, such as amphotericin B (AmB), available in different intravenous formulations like liposomal (L-AmB) or deoxycholate (AmB-d), which inhibit fungal growth by forming pores in the membranes that lead to cytoplasm leakage; (2) azoles, such as fluconazole (FLZ), itraconazole (ITZ), voriconazole (VRC), or posaconazole (PSZ), which interfere with the biosynthesis of ergosterol, a key cell membrane component; (3) echinocandins, including caspofungin, micafungin, and anidulafungin, are less toxic and inhibit cell wall synthesis, forcing cell rupture and/or aberrant hyphal growth; and (4) synthetic antimetabolites, like flucytosine, which inhibit DNA and RNA synthesis, thus causing cell death [59]. Additionally, several new drugs are in clinical trials [17,60].
In general, antifungal therapy is effective, with AmB and ITZ being the most frequently prescribed drugs (Table 1), but both are associated with potentially severe pharmacokinetic interactions and toxicity. Not to mention that in Africa, some of these drugs are either prohibitively expensive (e.g., ITZ or VRC) or totally out of reach (e.g., PSZ) [61].
Some TDF, like Coccidioides immitis [62], Paracoccidioides brasiliensis [63], or Histoplasma capsulatum [64], have the ability to colonize surfaces and form biofilms, a phenotype that may induce high levels of resistance and enhanced virulence. Indeed, multiple strains are increasingly reported to be resistant to different antifungals [65,66,67]. Although less-publicized than antibiotic resistance, this real threat is accelerating over time [68], since most fungi have highly plastic genomes and reproduce rapidly, quickly generating variants selected for resistance. Understandably, antifungal resistance has undesirable consequences not only for human and animal health but also for forest protection and agriculture, because the already limited therapeutic options are becoming significantly reduced [69].
In the last two decades, some progress towards vaccine development against some TDF, like Blastomyces dermatitidis, Coccidioides spp., H. capsulatum [70,71], Paracoccidioides spp. [72], Sporothrix spp. [73], and T. marneffei [74], has been made using mice as a model. However, the design of safe and effective vaccines is still in its infancy due to the lack of knowledge about immunity against these fungal infections but also because of the scarcity of epidemiological data regarding the incidence and prevalence, which would allow pharmaceutical companies to decide whether human vaccines are cost-effective. For all these reasons, having reliable, affordable, and widely accessible diagnostic methods for TDF-related mycoses should be a priority for researchers in the subject and policymakers in order to improve epidemiological knowledge and surveillance. This would probably promote vaccine research but also could be useful to prevent disease, avoid incapacitating sequelae, tackle drug resistance, and, eventually, minimize mortality due to these fungal pathogens.
It is also important to note that the generalized lack of awareness about TDF-related mycoses leads to the non-use of protective means for people exposed and to the underutilization of testing, even in well-equipped centers, where the diagnosis is still mainly based on the inadequate traditional methods described next. For this and other reasons mentioned above, faster and more reliable diagnostic methods are needed in order to (1) quickly stablish the best available therapy to improve patient outcomes and increase survival by preventing pathogen dissemination, and to (2) avoid the administration of inappropriate treatments leading to antibiotic and antifungal resistance.
2. Diagnostic Methods
2.1. Culture-Based Diagnosis
Culture of clinical specimens (e.g., bone marrow, blood, urine, sputum, cerebrospinal and bronchoalveolar fluids, tissues, etc.) followed by a microscopic examination of colony morphology and sporulation pattern is the most frequently used method to diagnose endemic mycoses, such as histoplasmosis [75], blastomycosis [76], or sporotrichosis [77]. The main reason for this may be the widespread availability of affordable culture media, which makes this approach ideal for routine diagnostic laboratories. Table 2 summarizes the culture media commonly used to grow different TDF and the main genus-level morphological characters used for a preliminary diagnosis.

Table 2.
Different culture media used to grow TDF and main genus-level morphological characters useful for a diagnosis.
Following best practices, clinical specimens should be cultured in plates containing the most appropriate medium and incubated at 25–30 °C until mycelial growth is observed. For instance, samples from patients with suspected blastomycosis are often plated on Sabouraud dextrose agar (SDA) and incubated at 25–27 °C for 4 weeks, although other media, incubation periods, and temperatures can also be used. Similarly, the time required for growth of Coccidioides spp. ranges from 2 to 16 days [80], but the final report of negative results is usually completed at 28 days [103]. Then, to confirm thermal dimorphism, isolated colonies should be transferred to new plates containing enriched media (e.g., blood–chocolate agar, blood agar or brain heart infusion agar) that will be incubated at higher temperatures (35–37 °C). This triggers a phase conversion, allowing the observation of yeast morphology; for example, in samples from patients with paracoccidioidomycosis, one would typically observe yeasts surrounded by multiple budding daughter cells [104], while in those from patients with sporotrichosis, yeast are elongated and cigar-shaped [96]. Notably, despite careful culture maintenance, the dimorphic transition may not always occur since it depends not only on temperature but also on nutrient requirements and the physiology of each strain [105].
Culture remains the gold standard for the diagnosis of most endemic mycoses, but this method is not ideal for several reasons. First, the fastidious and slow-growing nature of TDF (see Table 2) renders it a time-consuming and labor-intensive process that delays the correct diagnosis and initiation of treatment. Second, some morphological features considerably overlap among different genera of TDF, and so culture-based diagnosis involves the subjective observation of subtle differences in colony morphology and sporulation characteristics, which is one reason why it requires well-trained staff. For example, the conidia of Emergomyces crescens are arranged in complex “florets” on slightly swollen stalks reminiscent of those of Blastomyces parvus [18], while the mycelial form of Emergomyces africanus closely resembles Sporothrix schenckii [16]. Additionally, culturing may be confusing due to contaminations, and phenotypic features can vary depending on the medium [106], which leads to misidentifications and, ultimately, to the prescription of unnecessary drugs, causing toxicity and resistance in patients [107]. Third, the overall sensitivity of culturing is relatively low. For instance, in patients with mild to moderate pulmonary histoplasmosis, cultures of respiratory specimens are often negative, and positive results may be masked due to the overgrowth of commensal organisms [75]. Moreover, some TDF, like Paracoccidioides lobogeorgii (formerly, P. loboi, and also known as Lacazia loboi) are unculturable [27,108]. Last but not least, culture maintenance involves a significant risk of laboratory-acquired infections, given the specially high spore load, orders of magnitude higher than in nature, which can be aerosolized accidentally [109]. Indeed, as previously mentioned, most TDF must be handled in BSL-3 facilities, which further discourages the use of culture techniques for identification of these pathogens.
2.2. Direct Microscopic Examination and Histopathology
A second low-cost classic approach to a presumptive diagnosis, with much less risk than cultures, is the direct microscopic visualization of the causative agent in freshly collected clinical specimens. This approach has several advantages: (i) different histochemical stains providing good staining properties with minimal background are generally available (Table 3); (ii) it enables a rapid diagnosis while waiting for cultures; and (iii) it allows complete characterization of the pathogenic phase, including the analysis of micromorphological traits and mode of reproduction. Therefore, when combined with complete clinical information, detailed histopathological studies may provide sufficient information for the correct identification of a TDF, being particularly suited when cultures are negative or unobtainable, and when dealing with very slow-growing organisms [110].

Table 3.
Stains used for the diagnosis of endemic mycoses and main morphological traits at the genus level.
This seems to be the case for African histoplasmosis, a disease caused by Histoplasma capsulatum var. duboisii, also referred to as H. duboisii, with predominant involvement of skin and subcutaneous tissues (nodules, umbilicate papules, abscesses, ulcers, etc.) but also lymph nodes and bones.
This form of histoplasmosis is frequently diagnosed based on histopathological findings [20], in the same way that the diagnosis of sporotrichosis, a deep cutaneous mycosis, relies on histopathology combined with identification by culture [114].
However, similar to culture-based diagnosis, histopathology is also expertise-dependent, again, due to morphological similarities shared by different TDF [17]. For example, the yeast-like cells of H. duboisii can be confused with those of Blastomyces spp. because of their similar size and the presence of thick refractile walls [115]. Similarly, the etiologic agents of emergomycosis, Emergomyces spp., develop as small budding yeasts resembling those of H. capsulatum in affected tissues (although, as previously mentioned, in culture, the conidia of some Emergomyces species are arranged in stalked “florets” similar to those of B. parvus). Consequently, every effort should be made for an adequate differential diagnosis to exclude histoplasmosis and blastomycosis, but also tuberculosis, talaromycosis and listeriosis [76]. Indeed, only coccidioidomycosis and paracoccidioidomycosis can be relatively safely diagnosed by histopathologic identification, since both infections are characterized by the presence of easily distinguishable structures in secretions and tissues, i.e., spherules and “pilot’s wheels”, respectively (Table 2 and Table 3). In both cases, a simple, inexpensive, and reliable potassium hydroxide (KOH) preparation is particularly useful for a prompt and accurate diagnosis since it provides excellent visualization of the mentioned pathognomonic signs [79].
It is also important to take into account that histopathology sensitivity varies depending on the organism. For example, it ranges from 50% to 90% when trying to detect Blastomyces spp. [6], and it is low for sporotrichosis, given the scarcity of fungal elements typically found in infected tissues [96]. Moreover, appropriate tissue samples may not be easily obtained from all patients [106], which makes this approach difficult to implement.
As mentioned, identifying a specific TDF based solely on histopathology can be difficult, even impossible, given the almost complete absence of morphological singularities among TDF and the impossibility of using this method in some instances. These shortcomings paved the way for sero- and immunological tests, detailed in the next paragraph, for the diagnosis of endemic mycoses and treatment response assessments.
2.3. Serological and Immunological Tests
Cultures may become positive only late on the course of infection, and it may be difficult to obtain proper specimens for histopathology; so, alternative methods based on the detection of circulating antibodies (Ab) or antigens (Ag) in different body fluids, such as blood, urine, saliva or respiratory secretions, have been proposed (Figure 3).
Antigen tests provide direct proof of a fungal infection by reveling the presence of biological molecules produced by the pathogen (mainly proteins, but also polysaccharides or carbohydrates), such as Blastomyces adhesin 1 (BAD1), the surface protein adhesion WI-1 or galactomannan (Blastomyces spp.), coccidioidin (Coccidioides spp.), or gp43 (Paracoccidioides spp.).
As shown in Figure 3, there are several strategies for Ag testing, including immunodiffusion (ID) and tests using enzyme-labeled antibodies, i.e., enzyme immunoassays (EIA) and enzyme-linked immunosorbent assays (ELISA); the latter are also useful to detect and quantify Abs. In the case of EIAs, Ags are immobilized on a solid surface and then complexed with the Ab linked to a reporter enzyme. Antigen detection is accomplished by measuring the activity of the reporter, after an incubation with the appropriate substrate to produce a measurable product.
Urine and serum Ag EIAs designed for H. capsulatum are not able to distinguish it from its close relative H. duboisii. In contrast, ID assays effectively differentiate these species, although this type of Ag test is not widely available [116]. An in-depth explanation of these and other immunological tests is out of the scope of the present review. For this purpose, see, for example, Cáceres, DH, T Chiller and MD Lindsley [117].
What is clear is that, in immunocompetent patients, Ag tests are more likely to be positive during the early acute stage of the infection because Ag levels tend to diminish over time. In contrast, in immunocompromised patients, Ag testing is often used in early and in late disease stages, since antigens might be presents for long periods [117]. It is important to note though the wide variation in Ag production among species and isolates (for instance, Paracoccidiodes lutzii often produces either very low levels or no gp43, in contrast to its sister species P. brasiliensis [118]).
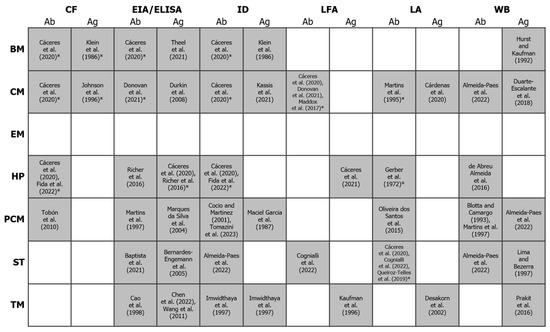
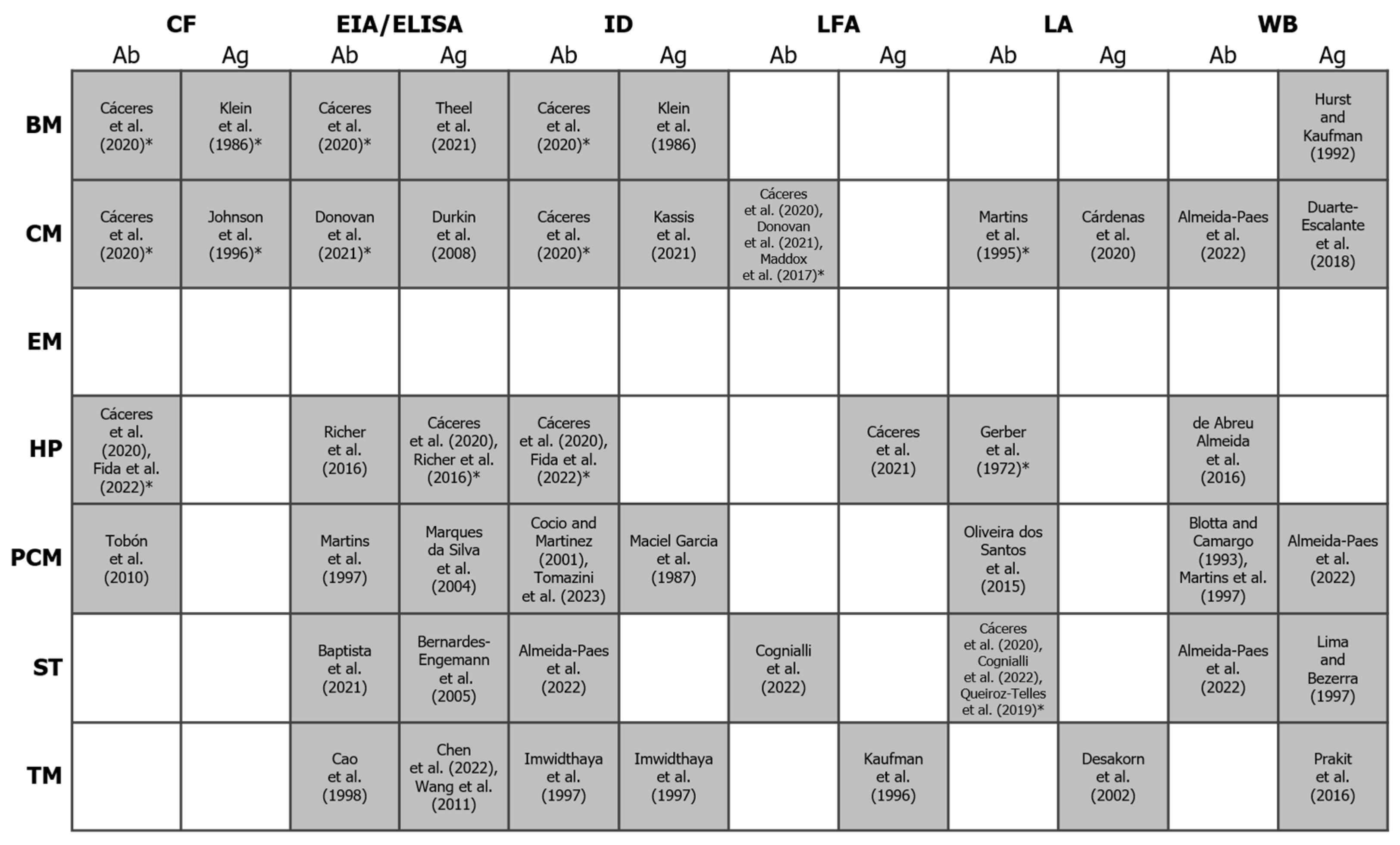

Figure 3.
Binary heatmap showing the existence/absence of antigen and antibody detection methods (x-axis) for the diagnosis of different endemic mycoses (y-axis). Shaded cells represent the existence of a test for a given mycosis. References to studies using these methods (or citing their use) are indicated between square brackets. An asterisk indicates that at least one commercial test is available (others may exist). For more details, see the review by Cáceres and colleagues [117] and https://www.immy.com (accessed on 31 July 2024). Abbreviations: Ab = antibody; Ag = antigen; BM = blastomycosis; CF = complement fixation; CM = coccidioidomycosis; EIA = enzyme immunoassay; ELISA = enzyme-linked immunosorbent assays; EM = emergomycosis; HP = histoplasmosis; ID = immunodiffusion; LFA = lateral flow assay; LA = latex agglutination; PCM = paracoccidioidomycosis; ST = sporotrichosis; TM = talaromycosis; WB = Western blot. References in this figure: [76,117,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154].
Figure 3.
Binary heatmap showing the existence/absence of antigen and antibody detection methods (x-axis) for the diagnosis of different endemic mycoses (y-axis). Shaded cells represent the existence of a test for a given mycosis. References to studies using these methods (or citing their use) are indicated between square brackets. An asterisk indicates that at least one commercial test is available (others may exist). For more details, see the review by Cáceres and colleagues [117] and https://www.immy.com (accessed on 31 July 2024). Abbreviations: Ab = antibody; Ag = antigen; BM = blastomycosis; CF = complement fixation; CM = coccidioidomycosis; EIA = enzyme immunoassay; ELISA = enzyme-linked immunosorbent assays; EM = emergomycosis; HP = histoplasmosis; ID = immunodiffusion; LFA = lateral flow assay; LA = latex agglutination; PCM = paracoccidioidomycosis; ST = sporotrichosis; TM = talaromycosis; WB = Western blot. References in this figure: [76,117,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154].

As mentioned, Ag testing can be performed on different body fluids, with urine Ag tests being particularly interesting for several reasons: (i) They are based on the use of readily available non-invasive samples. (ii) The turnaround time is relatively short (for example, in patients with blastomycosis, results are frequently available within 48 h [155]). (iii) Their sensitivity seems to be higher than that of Ag tests using serum or bronchoalveolar lavage to diagnose blastomycosis [13] or histoplasmosis [156], among other endemic mycoses, and are considered particularly advantageous for disseminated forms of histoplasmosis [157]. Indeed, urinary Ag tests represent an improvement in the management of histoplasmosis, especially in highly endemic areas, where it frequently coexists with tuberculosis [158]. (iv) They are very easy to perform. For these reasons, urine Ag tests often guide physicians on the therapy to be administered until cultures are available [33], and they seem to be a suitable option for point-of-care testing (POCT) close to the site of patient care.
Assays for the detection of Abs are also frequently used for the diagnosis of endemic mycoses. Several common testing strategies are available for Ab detection (Figure 3), including latex agglutination (LA), complement fixation (CF), and immunodiffusion (ID). LA tests are based on the ability of a specific Ab to bind to a suspension of antigen-coated polystyrene latex beads. When Abs are present in the sample, beads agglutinate and large clumps are then observed on a clear background. In contrast, when samples are negative, the suspension of beads has a milky aspect with no background clearing. Regarding CF assays, they are based on the lytic properties of the complement system, a set of proteins naturally present in human serum that react with Ag-Ab complexes. These proteins first need to be destroyed by heat and replaced by synthetic complement proteins with a known concentration. Then, the Ag of interest is added to the serum, along with an indicator system, i.e., sheep red blood cells (sRBCs) coated with anti-sRBC antibodies. When the serum sample contains an Ab against the Ag of interest, both bind together, forming Ag-Ab complexes. Complement proteins get fixed to these Ag-Ab complexes, avoiding sRBC lysis. When serum samples do not contain the Ab, the complement remains free in the mixture, and so they can fixed on the sRBC-Ab complex, which results in hemolysis of the sRBCs (the solution turns pink and the test is considered negative).
As for ID tests, these solid-phase assays are performed in an agar matrix by placing the Ag and Ab in opposing wells (generally, a known Ag is placed in a center well within the matrix and serum samples and controls are placed in surrounding wells). Reagents are allow to diffuse toward each other so, if Abs are present in the sample, a thin white line will be observed due to Ag/Ab precipitation.
Notably, the sensitivity of different types of Ab tests greatly differs. For example, compared to EIA, the sensitivity of LFA can be as low as 30% in patients with coccidioidomycosis. This seems to be directly related to the duration of illness, with subjects with positive LFA tests tending to be sick longer than those with false-negative LFA tests [123]. Despite this, the detection of Abs with different tests is still the main method for the diagnosis of coccidioidomycosis [125]. First, Ab production can occur up to six weeks after exposure in immunocompetent patients, while those with a weakened immune system are generally unable to mount an immune response. Indeed, the rate of false-negatives can be high in immunocompromised patients, but also during the acute phase because disease development can be so fast that there is no time to produce detectable Ab titers [99,159]. This could be also the case of Ab ID and Ab CF tests for the detection of Blastomyces spp., which present a very low sensitivity (<45%) [160]. A second limitation of Ab testing is that distinguishing between past and current active infections is not possible because Abs may persist if patients are recurrently exposed to the fungus [131], as it might occur in endemic areas. In other words, Ab tests are mainly helpful in immunocompetent patients able to produce a quantifiable humoral response after the acute phase, when high levels of Ab are still present in the host. Only under these circumstances, Ab assays have shown acceptable sensitivity and specificity, thus representing a relatively accurate means of diagnosing endemic mycoses, especially when used in combination with Ag tests [125,131,161].
Sero- and immunological tests remain valuable assets to support endemic mycosis diagnoses, particularly when direct detection fails, as they provide faster results than culture and microscopy. Furthermore, they also offer reasonable analytical sensitivity, tending to be most useful for severely ill patients, with, presumably, a greater fungal burden [13]. In these patients, determining Ag and Ab titers before and after treatment can serve as indicator of its efficiency. Moreover, several seroimmunological tests for the diagnosis of TDF are commercially available (see, for example, [162,163] and references in Figure 3), which enables clinicians to select the best-fitting diagnostic option. However, sensitivity and specificity variations, as well as significant disparities in inter-laboratory results, have been observed among certain commercial tests [164], which evidences the need for standardization in order to minimize the effect of pre-analytical and analytical factors on their performance. Additionally, most countries where diseases caused by TDF are endemic still face production, distribution, and cost problems [93], not to mention that commercial assays are only available for the most prevalent endemic mycoses [165]. For instance, so far, no commercial or in-house Ag/Ab test has been designed for emergomycosis [61], an endemic mycosis with involvement of the skin, lungs, gastrointestinal tract, bone marrow, etc., common in immunocompromised patients. It is frequently misdiagnosed as blastomycosis or histoplasmosis [19], or even as varicella or scrofuloderma, which significantly alters treatment outcomes [166]. Commercial tests are also scarce for the diagnosis of talaromycosis, paracoccidioidomycosis, and sporotrichosis. For the latter, in particular, there used to be an Ab LA test on the market (IMMY©, Norman, OK, USA), whose sensitivity greatly varied from 100% in disseminated forms to 56% in cutaneous forms [145,167]. This Ab LA test seems to be no longer manufactured since it is not available on the company’s website (https://www.immy.com; last accessed on 31 July 2024).
Additionally, on the negative side, the specificity of both immunological and serological tests can be very low, and so diagnoses exclusively based only on Ag or Ab assays are often classified as “probable” or “possible cases” [117]. In this regard, it is well known that some Ags produced by different TDF (e.g., Emergomyces spp. and Histoplasma spp.) are extremely similar, which is a reason why a high degree of cross-reactivity has been observed. For instance, Ag detection via EIA in urine and serum (also in bronchoalveolar lavage and cerebrospinal fluid samples) is a fast method for a probable diagnosis of infections caused by Blastomyces spp., but cross-reactions with Histoplasma spp. [13] and Emergomyces spp. [166,168] are frequently reported. Blastomyces cross-reactivity with T. marneffei, the most important TDF causing systemic mycosis in immunocompromised patients in Southeast Asia, has also been observed in urine Ag tests [33]. Likewise, using Histoplasma antibody EIAs, cross-reactions have been noted in urine samples from patients with coccidioidomycosis [131].
Furthermore, some Ags, like galactomannan, are shared not only among different TDF but also with other not so closely related filamentous fungi. For instance, in patients with confirmed blastomycosis, false-positive results for Aspergillus galactomannan have been reported when using bronchoalveolar lavage fluid samples [169]. As can be imagined, cross-reactivity is an undesirable drawback that limits clinical use, in particular for evaluating patients suspected to have blastomycosis or histoplasmosis, since their endemicity areas greatly overlap. For this reason, these two endemic mycoses could be easily misdiagnosed if only based on sero-immunological results [75].
Ag and Ab tests still play an important role in clinical settings, but they may be of little help on their own for the diagnosis of endemic mycoses. In the case of histoplasmosis, it has been proved that the combination of Ab and Ag EIA tests improves the diagnostic accuracy [131], in a similar way that combining Ab and Ag ID assays increases the diagnostic sensitivity in patients with coccidioidomycosis [125]. Therefore, efforts should be directed to design new generations of Ab/Ag tests with enhanced sensitivity and specificity to be used in combination so they could be truly useful for the diagnosis of TDF-related mycoses and to monitor the treatment response.
The methods mentioned so far (i.e., cultures, histopathology, and Ag/Ab detection) can serve as basis for the presumptive diagnosis of different endemic mycoses. However, they all have some shortcomings (most TDF grow very slowly in culture, some species are non-culturable, others share considerable clinical and histopathological overlap, and, in some cases, clinical manifestations can even be easily confused with those of unrelated diseases). Therefore, researchers have tried to obtain diagnoses not affected by misleading positive results due to cross-reactions and false-negatives related to low sensitivity by introducing methods based on the detection of organic molecules by spectrophotometry, as explained next.
2.4. Matrix-Assisted Laser Desorption/Ionization–Time of Flight Mass Spectrometry (MALDI-ToF MS)
In recent years, matrix-assisted laser desorption ionization–time of flight mass spectrometry (“MALDI-ToF MS”) has become a popular alternative for pathogen detection [170], with two main systems being available, i.e., MALDI Bruker Biotyper (Bruker Daltonik GmbH, Bremen, Germany) and VITEK MS (bioMérieux, Craponne, France). Regardless of the platform, this culture-based method relies on the detection of highly abundant species-specific proteins in different clinical samples. In short, a protein spectrum is generated for each target specimen and then is used as a signature profile with peaks that are unique to the species represented in the specimen analyzed. The spectrum is compared to a reference database, and so the specimen can be successfully identified at species level if its spectrum matches with some of the reference spectra (for a further explanation, see Patel, R [171]).
To achieve reliable results, the appropriate matrix, which isolates individual proteins, protects them from breaking up, and allows desorption by laser energy [171], and an optimized sample preparation protocol need to be used, as both aspects may influence the quality and accuracy of the spectra [172].
MALDI-ToF MS has been extensively used to identify countless pathogenic bacteria, becoming an almost indispensable tool in microbiology laboratories [173]. It has been also introduced into the identification of clinically relevant yeasts (mainly Candida and Cryptococcus species), for which a cutoff value of 1.7 has been defined for optimal identification [174]. Indeed, MALDI-ToF MS has changed the diagnostic workflow at medical centers working with fungal pathogens worldwide [170], but little progress has been made with respect to TDF. The reason is that its application to TDF diagnosis is limited and subjected to a major caveat: a comprehensive curated database does not exist, since spectral data have been generated for only a few strains of some species. Specifically, the Vitek MS system includes the spectra of B. dermatitidis, C. immitis, C. posadasii, H. capsulatum, and S. schenckii [175]. It seems to be especially good at identifying different genera (100% accuracy), but its utility greatly varies depending on the species. For example, it is unclear whether it is able to distinguish between H. capsulatum and H. duboisii [116]. The same seems to occur with the other MALDI-ToF MS system (Bruker Biotype). Due to database incompleteness, this system is not able to identify the species T. marneffei [176,177]. Indeed, it fails to identify up to four members of the genera Talaromyces and Penicillium at the species level.
On the other hand, MALDI-ToF MS seems to be a robust method for Paracoccidioides species differentiation, with all isolates being correctly identified as P. brasiliensis or P. lutzii, with log score values of >2.0, although this result was obtained using a small in-house database [178]. There is another in-house library that includes spectra for both yeast and mycelial phases of H. capsulatum [179]. Interestingly, these spectra are completely different, probably due to differences in gene expression, which allows the identification of both morphological stages. Still, the most reliable results were obtained for mycelial phases.
Some authors claim that MALDI-ToF MS shows promise for the fast and accurate identification of pathogens causing human mycoses, including TDF [170]. However, it should be noted that, currently, this methodology is only successful using clinical isolates, and that it is not widespread among researchers working on TDF. Moreover, results are somewhat contradictory. What seems clear is that MALDI-ToF MS-based identification of the species level requires reference database expansion and refinement by obtaining spectral data from as many species, strains, and phases as possible. Equally important is that curated reference libraries become publicly available and interoperable to allow practical use.
2.5. PCR-Based Molecular Diagnosis
Given the already mentioned drawbacks associated with cultures, histopathology, Ag/Ab detection, and MALDI-ToF MS, in well-equipped laboratories, these approaches have lately been replaced by nucleic acid-based detection methods. Indeed, their incorporation into what has been traditionally a morphology-based discipline has revolutionized the diagnosis of countless diseases, including those caused by TDF. However, in most endemic areas, the application of molecular techniques, which are crucial for taxonomic studies, to disentangle species complexes is far from routine [165].
Two main molecular strategies are commonly used for the detection of pathogenic fungal species or varieties, i.e., polymerase chain reaction (PCR) and quantitative real-time PCR (qPCR). Standard PCR, developed by the Nobel Prize winner Kary Mullis and collaborators, allows the exponential amplification of a particular DNA fragment (generally a multi-copy gene) in vitro using a thermostable DNA polymerase and one specific primer pair [180]. If the targeted gene is present in the sample(s) analyzed, thousands to millions of gene copies are obtained after several rounds of DNA denaturation, annealing, and extension conducted in a thermocycler. Thus, using intercalating dyes, these PCR products can be detected by gel electrophoresis as fluorescent bands. In general, a negative result (absence of the expected band in agarose gels) may be sufficient to exclude a diagnosis of proven or probable endemic mycosis, while two positive PCRs would be required to confirm the diagnosis, as in other fungal diseases like aspergillosis [181].
The popularization and optimization of the original technique has led to a broad range of variants. These include, among others, (i) nested PCR (it involves two different primer pairs and the use of the PCR products of a first PCR as template for a second amplification round [180]); (ii) multiplex PCR for the simultaneous detection of several gene targets [182]; (iii) reverse transcription PCR (RT-PCR), which allows the detection of RNA, instead of DNA, starting with reverse transcription of RNA and following with DNA amplification by two different enzymes [183]; and (iv) quantitative real-time PCR (qPCR), another frequently used molecular technique that allows the detection of amplification in the exponential growth phase of the reaction [184]. This latter PCR variant is performed in a closed system and gel electrophoresis is not required after amplification, which reduces the chances for contamination and shortens the detection time. Interestingly, qPCR allows a fungal burden quantification, which is very convenient for clinical diagnosis and treatment follow up [165].
PCR-based approaches offer particularly high specificity and sensitivity if the target choice and primer design are carefully performed. Indeed, they can detect very low amounts of DNA of an etiological agent in samples collected from patients with a low fungal burden [162]. Not only this, PCR and qPCR assays are useful for detecting point mutations and differentially expressed genes potentially associated with antifungal resistance, respectively [67]. Furthermore, conventional PCR and its variants are suitable for both fresh and archival paraffin-embedded clinical samples; so, they allow not only diagnosis and screening but also large-scale retrospective epidemiological studies [13,75]. Given that PCR-based assays involve the use of DNA samples, instead of highly infectious cultures, and are much faster than culturing, they are very convenient to use in well-equipped laboratories, particularly to confirm the diagnosis in cases where more than one fungal infection is possible [75]. Because of these advantages, numerous PCR-based tests have been developed in the last decades for the detection of the causative agents of most endemic mycoses (Figure 4; for an extensive review, see Valero, C, MT Martín-Gómez and MJ Buitrago [165]). These include, for example, a qPCR assay for the simultaneous detection and differentiation of B. dermatitidis and H. capsulatum [185], which are responsible for most cases of two overlapping endemic mycoses, i.e., blastomycosis and histoplasmosis, respectively.
As can be seen in Figure 4, regardless of the use of PCR or qPCR, the multi-copy nuclear ribosomal internal transcribed spacers (ITS1 and ITS2) are the regions most frequently targeted. The nuclear small ribosomal subunit gene (SSU or 18S) is also a common target, although others, such as BAD1 [78], gp43 [186], Pb27 [187], or the mitochondrial small ribosomal subunit gene (mtSSU) [188], have also been used for the PCR-based diagnosis of endemic mycoses. In this regard, and despite the increasing use of PCR-based diagnosis to detect low abundance nucleic acids, there is no consensus among laboratories on which molecular region or method is best to use, even if ITSs are the universal DNA barcodes for the identification and taxonomical classification of fungi [189,190].
Apart from this lack of agreement, PCR-based assays have other limitations. For example, they require complex pretreatment (DNA must be extracted using expensive commercial kits) but, even so, they can be inhibited by different substances, mostly in formalin-fixed and paraffin-treated samples [191]. Likewise, no amplification can occur due to template degradation, or if the concentration of fungal DNA in clinical samples is below the detection limit [192]. That is to say, PCR and its variants are highly sensitive and specific, but not infallible. For instance, according to the results presented by O’Dowd and collaborators [160], the sensitivity of PCR assays designed for the detection of Blastomyces in sputum and bronchoalveolar lavage fluid ranges from very low to moderate (40–75%), respectively.
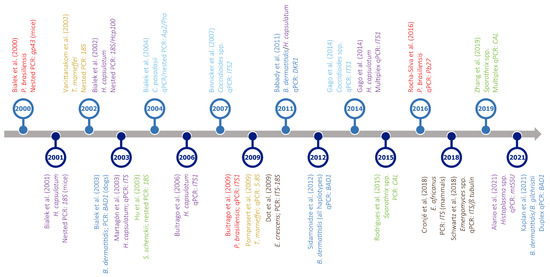
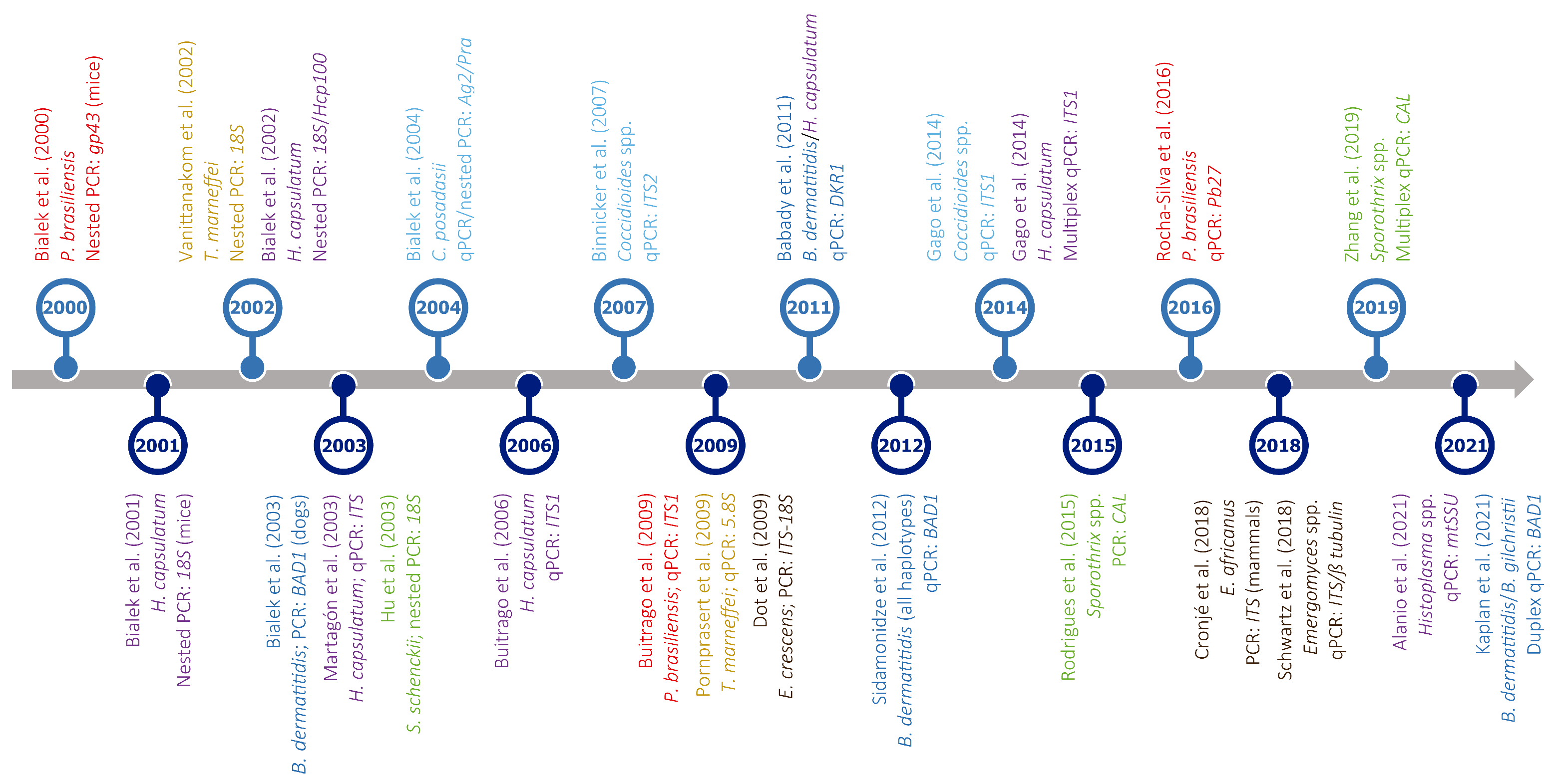

Figure 4.
Timeline of PCR-based assays developed for the detection of different thermally dimorphic fungi. Target organisms (represented in different colors: dark blue—Blastomyces spp.; light blue—Coccidioides spp.; brown—Emergomyces spp.; purple—Histoplasma spp.; red—Paracoccidioides spp.; yellow—Talaromyces spp.; green—Sporothrix spp.), type of assay (PCR, nested PCR, qPCR, or multiplex qPCR), and amplified gene(s) are indicated below each reference. For easy presentation, this graph shows only a selection of studies published in the last two decades. Most studies were based on clinical samples; otherwise, it is indicated between brackets after the gene name. References in this figure: [78,185,186,187,188,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211].
Figure 4.
Timeline of PCR-based assays developed for the detection of different thermally dimorphic fungi. Target organisms (represented in different colors: dark blue—Blastomyces spp.; light blue—Coccidioides spp.; brown—Emergomyces spp.; purple—Histoplasma spp.; red—Paracoccidioides spp.; yellow—Talaromyces spp.; green—Sporothrix spp.), type of assay (PCR, nested PCR, qPCR, or multiplex qPCR), and amplified gene(s) are indicated below each reference. For easy presentation, this graph shows only a selection of studies published in the last two decades. Most studies were based on clinical samples; otherwise, it is indicated between brackets after the gene name. References in this figure: [78,185,186,187,188,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211].

Furthermore, when using either nested PCR, which is prone to contamination, or qPCR assays on samples comprising high concentrations of DNA from closely related TDF, false-positive results can be obtained, although these are generally uncommon [103,212,213]. Additionally, it is important to note that PCR-based studies focused on the diagnosis of endemic mycoses other than histoplasmosis are relatively scarce, especially in the case of talaromycosis, emergomycosis, and blastomycosis. For example, PCR assays have been only infrequently used as an aid in the diagnosis of blastomycosis by a few laboratories [13,75], while no PCR assays have been validated for emergomycosis [61]. Moreover, there is limited availability of reagents on the market, and only a single specific PCR for the diagnosis of coccidioidomycosis is currently commercialized [201], which compromises the use of this method [165].
Still, the main disadvantage of DNA-based diagnosis, mainly if based on qPCR, is that sample processing and gene amplification have to be performed by trained personnel using sophisticated equipment, and so its use is essentially restricted to reference laboratories located in North America and Europe. Lastly, amplicons obtained by PCR should be sequenced, and newly generated sequences should be compared to credible molecular data deposited in public databases in order to confirm that no contamination occurred, which may be impractical when many clinical samples need to be screened.
Some authors envision that PCR-based diagnosis will eventually become standardized and widely available in strong health systems [13,214], but it will not easily be a routine assay in resource-limited endemic areas. Thus, molecular diagnostic approaches not relying on a generally unaffordable thermocycler may be the best solution to diagnose TDF-related infections [215].
2.6. Isothermal Amplification Methods
Although PCR-based methods are highly reliable for the diagnosis of endemic mycoses, they are not technically accessible for routine clinical practice, which makes it impossible to use them in POC settings. In this context, isothermal amplification methods (IAM), based on the replication of DNA at a constant temperature, could provide the most suitable and versatile alternative.
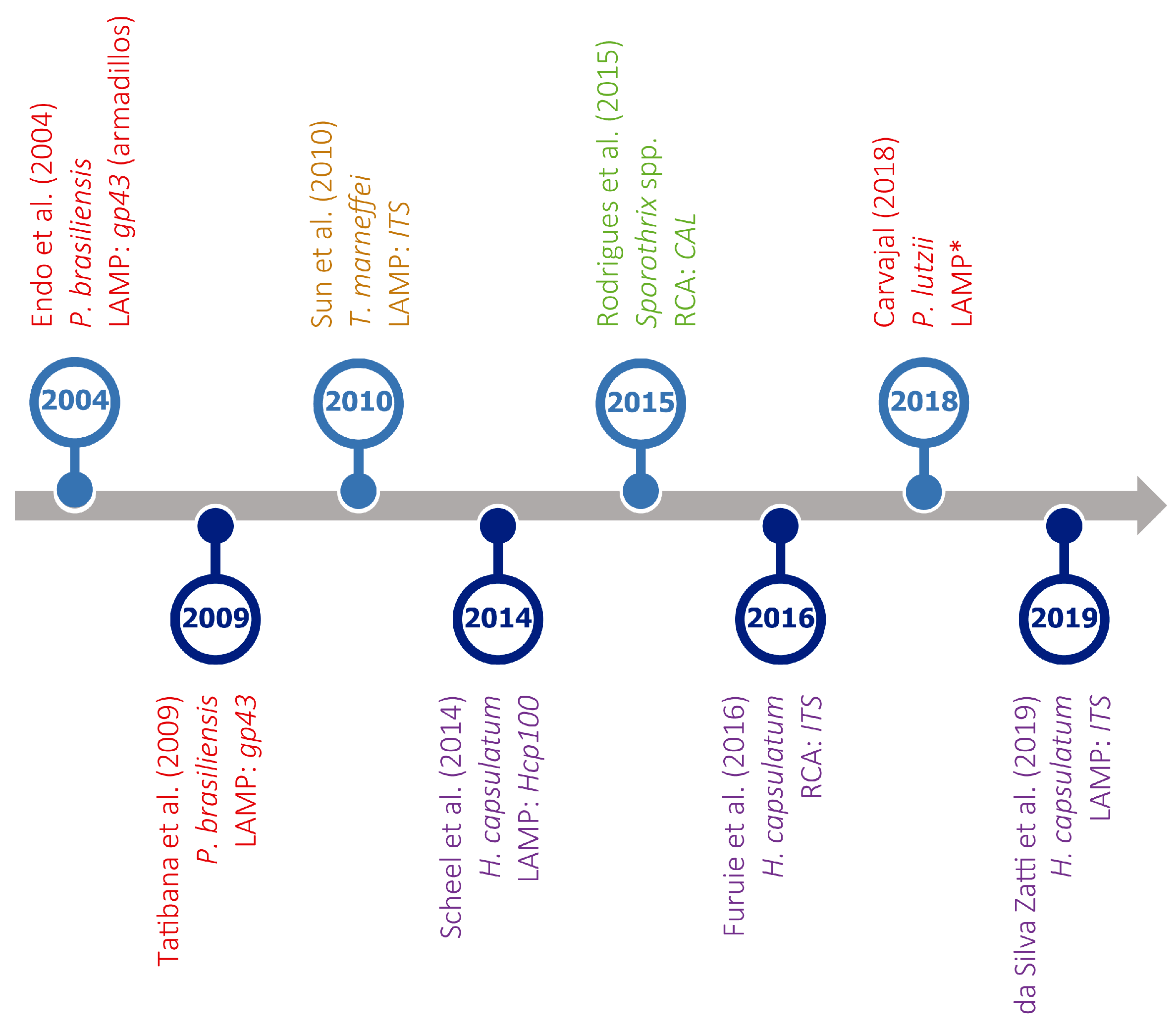
A number of methods for overcoming PCR-temperature dependence are currently available, including, among others, nucleic acid sequence-based amplification (NASBA), self-sustained sequence replication (SSR), strand displacement amplification (SDA), rolling circle amplification (RCA), and loop-mediated amplification (LAMP). For a review of IAM for the detection of pathogenic fungi see da Silva Zatti and colleagues [216]. However, only LAMP and RCA have been used to diagnose endemic mycoses (Figure 5), and the first is the most popular isothermal method.
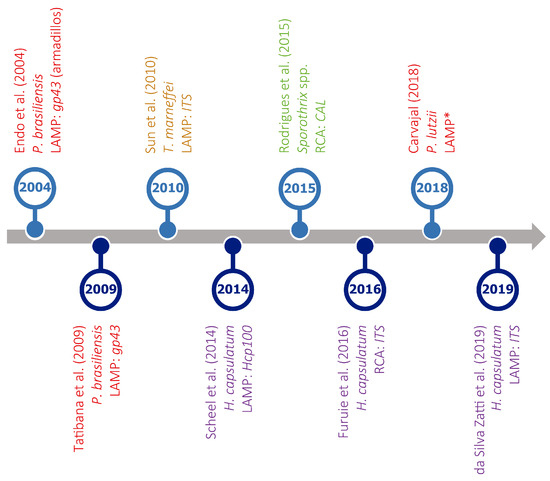
Figure 5.
Timeline of isothermal amplification assays developed for the detection of different thermally dimorphic fungi. Target organisms (represented in different colors), type of assay (LAMP or RCA), and amplified genes are indicated for each reference. * No information available. References in this figure: [217,218,219,220,221,222,223,224].
LAMP assays are based on the use of a thermophilic polymerase obtained from Bacillus stearothermophilus (Bst polymerase) and two specific primer sets [225]. These four primers bind to six different regions of the target (ideally a multi-copy gene to increase sensitivity without compromising specificity), which then is amplified at a constant temperature (60–65 °C). Additionally, a third primer set, the so-called loop primers, was introduced to further accelerate the amplification reaction [226]. The molecular mechanism behind LAMP is very complex, but the method is very easy to use, fast, and cost-effective because the Bst polymerase is not only highly resistant to inhibitors [227] but also cheaper than the enzyme traditionally used for PCR, Taq polymerase [228]. Furthermore, the amplification and detection of the gene(s) of interest can be completed in a single step, and the results can be directly read with the naked eye (color or turbidity changes are easily observable in the reaction tubes) or by real-time fluorescence, without gel electrophoresis. These are great advantages that could open new research avenues for fungal diagnosis. Indeed, LAMP could potentially be used in low-resource settings, such as endemic areas of mycoses caused by TDF, where the diagnosis is still primarily based on cultures.
LAMP has been widely applied to diagnose various tropical diseases caused by viruses [229,230,231], bacteria [232], protozoa [233,234,235], or helminths [236,237], offering good results. It has also been used for the diagnosis of mycoses caused by fungi not closely related to TDF (Table S3), and has been proposed as one of the best alternatives to classical PCR-based approaches, due to its simplicity, the low costs associated, and its relatively high sensitivity and specificity [216,238].
However, LAMP has not been sufficiently explored for the diagnosis of endemic mycoses, as indicated by the low number of assays currently available (Figure 5), often including a limited number of clinical specimens.
Focusing on the diagnosis of histoplasmosis, the most prevalent endemic mycosis worldwide, two LAMP assays have been designed (Table S4). Scheel and colleagues [220] developed a LAMP assay based on a single-copy gene (Hcp100), proved specific under laboratory conditions (results were negative in all healthy controls). Notably, the specificity of this assay was determined using DNA extracted from other fungi, but only some of them were closely related to Histoplasma, and no DNA samples of Emergomyces, its sister genus, and T. marneffei, a species causing symptoms very similar to those of histoplasmosis, were included (Table S4). Moreover, the sensitivity of the Hcp100 LAMP assay was not high enough, as it only detected the target in 67% of antigen-positive urine specimens. The presence of inhibitors as a cause of the decreased sensitivity was ruled out using urine samples spiked with known concentrations of H. capsulatum DNA (all were found positive). This suggested that DNA degradation may be responsible for the low sensitivity reported, although the fact that Hcp100 is a single-copy gene could also have contributed to some detection errors. Despite being a pioneer study, the authors acknowledged that further evaluation using additional fresh-frozen urine, serum, and blood samples is required to validate their test.
The second Histoplasma LAMP assay currently available was designed to amplify the ITS region [224]. In this study, 26 bone marrow specimens (which are much more difficult to obtain than urine, sputum, or blood samples) from HIV/AIDS patients with symptoms of progressive disseminated histoplasmosis were used. Only 11 of these samples corresponded to patients with positive cultures for Histoplasma spp. Additionally, one blood sample from a patient suspected to have histoplasmosis and another five from healthy individuals were also included (Table S4). Of interest, the authors analyzed a heparinized blood sample spiked with H. capsulatum yeasts directly, with no previous DNA extraction, which represents a huge advantage for molecular diagnosis. However, when using positive cultures as reference (that is, considering only the mentioned 11 bone marrow samples), the ITS LAMP assay showed only 54% sensitivity and a specificity of 95%. Using Hcp100 as reference, the test reached 83% sensitivity and 92% specificity, which are still limited values.
The sensitivity of the Histoplasma LAMP assays just mentioned is far from 100%. It is also important to note that none of these studies included more than one sample of the African lineage (Table S4), which could have influenced their results.
Other than these, LAMP assays have been designed for Paracoccidioides spp. [217,218,223] and T. marneffei [219], but, to the best of our knowledge, none are currently available for the diagnosis of potentially fatal diseases caused by species of Blastomyces, Coccidioides, Emergomyces, and Sporothrix. Therefore, their design and optimization should be a first priority for researchers working on the topic.
There is no doubt that LAMP has great utility and practical value for the diagnosis of endemic mycoses because of its high sensitivity, short turnaround time, and its potential for implementation in POCT. Nevertheless, it also has obvious disadvantages, such as strict and complex primer design and high rate of false-positives due to primer dimers [239,240], especially with long incubation times that may be necessary to amplify fungal DNA, according to the conditions reported for both Histoplasma LAMP assays [220,224]. In fact, it is well-known that LAMP assays are prone to non-specific amplifications and contamination, and so extremely careful procedures are required, including separate handling of DNA samples and reagents [241]. Considering this, the emphasis should be placed on the analysis of further data and the optimization of LAMP assays before the use of this IAM becomes popular among clinicians.
Several alternatives have been proposed to enhance LAMP analytical specificity by decreasing the probability of primer dimer formation. These include the use of species-specific primers with a highly controlled design [240], fluorescent probes [242], touchdown protocols [243], or the addition of chemical compounds to the reaction mixture to stabilize the structure of the primers and prevent the formation of dimers [244,245]. Even so, a new type of IAM, called stem-loop-primer-assisted isothermal amplification (SPA), has been recently developed using viral DNA as target [246]. Compared to conventional LAMP, SPA seems to offer a reduced risk of false-positives because it relies on a highly simplified primer design that provides ultralow background amplification. If this holds true when applied to fungal DNA amplification, SPA could be highly advantageous to perform disease screening and diagnosis in POC settings.
RCA, an IAM described by Fire, A and SQ Xu [247], relies on specially designed oligonucleotides whose ends are complementary to the target, known as padlock probes. To mimic bacterial plasmid amplification, RCA also depends on two enzymes, i.e., one thermostable polymerase, such as phi29 or Bst, and one ligase, like the T4 DNA ligase, used during the amplification reaction for strand displacement and padlock probe circularization, respectively. In the presence of dNTPs and only two primers, which makes RCA primer design much more flexible and simpler than that of LAMP primers [248], padlock probes hybridize to the target, enabling probe ends to be joined by the ligase and thus forming circular DNA molecules. These are amplified by an incubation at a constant temperature, giving rise to a large amount of DNA that can be visualized by fluorescence or colorimetric approaches [249]. Given the simplicity, sensibility, and robustness of this gel-free technology, and its ability to detect species-specific polymorphisms, the authors have claimed that RCA should be a routine molecular test for fungal diagnostics in low-income regions [189,221]. Indeed, this IAM is increasingly used to diagnose pathogenic fungi, including some species of the genera Aspergillus, Cryptococcus, Exophiala, Fonsecaea, and Trichophyton [189,222,250].
Nevertheless, as shown in Figure 5 and Table S4, RCA has been only occasionally applied to the diagnosis of TDF-related diseases, with only two assays being available. For the first RCA assay ever presented to diagnose an endemic mycosis, sporotrichosis specifically, six species-specific padlock probes targeting single nucleotide polymorphisms in the gene encoding calmodulin were developed [221]. Using DNA extracted from pure cultures, but also from complex environmental samples, the authors found no cross-reactivity with closely related species. The second RCA assay was designed to detect different ITS fragments in clinical samples from patients with histoplasmosis using two padlock probes, i.e., HcPL1 and HcPL2 [222]. Although the data on sensitivity and specificity are not detailed enough, HcPL2 seems to be effective for the specific identification of Histoplasma spp., but HcPL1 cross-reacted with the other fungi, an issue previously reported [189]. Future improvements to current probe design could address this issue, but its occurrence along with the limited number of studies based on RCA warrants further investigation to optimize this method and to reach a deeper understanding of its potential for endemic mycosis diagnoses.
The advent of IAM has allowed equipment simplification and a visual interpretation of results, has improved the speed of diagnosis, and has decreased the amplification costs, although few companies produce the reagents required [249]. Nevertheless, these methods may be limited to some extent by false-positives and low sensitivity. Some of them, in particular LAMP, are associated with difficult primer design. Therefore, there is an urgent need to demonstrate the applicability of optimized LAMP and RCA assays as user-friendly, fast, affordable, reliable, sensitive, and deliverable methods for the molecular identification TDF in a variety of human samples.
3. Concluding Remarks and Future Directions
Among the more than five millions of fungal species that probably exist [251], only a few species of the order Onygenales, plus the genus Sporothrix and T. marneffei are capable of undergoing a temperature-dependent morphological transformation and causing the so-called endemic mycoses in humans. These thermally dimorphic fungi have attracted scientists’ attention since their discovery, not only because of their striking morphological change but also because of the increasing number of infections caused. They often affect the lungs but can also disseminate hematogenously, causing disease in virtually any organ or tissue, with symptoms being similar among different endemic mycoses.
Treatments include similar drug combinations, although there are differences in terms of the drug, dosage, and duration. For example, FLZ should be avoided to treat emergomycosis because Emergomyces spp. are resistant to this antifungal, as indicated by the high minimum inhibitory concentrations observed [18]. In addition, because of its high toxicity and since, among other things, azole is known to cause hepatitis, it should not be administered to pregnant women because of possible teratogenicity [6]. Despite this, when WHO-listed essential systemic antifungal drugs (AmB, ITZ, VRC, and flucytosine) are not available, FLZ is frequently used to treat African histoplasmosis cases [20]. On the other hand, AmB, which is also highly toxic, is still considered a suitable treatment option for most TDF-related mycoses. However, its use is not recommended to treat non-life-threatening lymphocutaneous cases of sporotrichosis, also due to its side effects, such as nephro- and hepatotoxicity, hypokalemia, phlebitis, or anemia [252], and inconvenient administration [18]. Most importantly, the clinical manifestations of these mycoses can also mimic those of unrelated bacterial and viral diseases, and so an accurate diagnosis is vital to avoid increased morbidity, mortality, and antibiotic/antiviral resistance.
As mentioned in the first section, no human vaccines are available against TDF, so using optimal diagnostic methods is vital to improve epidemiological knowledge and surveillance systems, which would help to prevent and treat infections. Traditionally, the diagnosis of endemic mycoses has primarily relied on conventional methods, such as culture, serology, and histopathology, coupled with detailed knowledge of the patient’s history of exposure to the etiologic agents [75]. However, in the last decades, more advanced methodologies, including MALDI-ToF MS and molecular tests, have been developed. Given the wide range of methods, the choice of the most appropriate depends on the local epidemiology, treatment guidelines, and, overall, on its availability.
Currently, reliable PCR-based tests can only be implemented in high-income countries (even so, tests for some species are still under development or standardization), while conventional diagnostic techniques, which are not exempt from limitations, are used in routine clinical practice in most laboratories, including those located in endemic areas. Therefore, the development of an affordable, accessible, and reliable diagnostic tool for different endemic mycoses should be prioritized to allow a precise diagnosis in endemic areas, which would also improve epidemiological surveillance.
There are many unresolved research questions about the disease burden and geographic ranges, both remaining highly speculative and underestimated, given the sporadic reports of cases from poor endemic areas (particularly Africa) with no or a very limited diagnostic capacity. In this context, novel isothermal assays, currently under development in our laboratory, hold promise for improved sensitivity and specificity. These include the analysis of multiple samples from geographically distant origins, DNA extraction simplification, and the selection of a target offering better results in terms of reliability, reaction time, sensibility, specificity, etc. Nevertheless, given the possibility of detecting false-positives derived from non-specific amplifications, including signals due to primer dimers, different enhancement strategies are currently being tested for performance. The reduction of false-positives in optimized LAMP assays will increase their specificity, eventually helping to minimize mortality due to endemic mycoses.
Until these much-needed molecular tests are validated in large clinical studies and are widely available in centers with restricted resources and/or no technical expertise, the best diagnostic option seems to be a multifaceted approach combining the use of several of the methods included in this review.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jof10090637/s1: Table S1. Data on selected dimorphic fungal pathogens affecting plants and animals. Abbreviations: Temp. = temperature; E = entomopathogen; H = human pathogen; M = mammal pathogen; P= phytopathogen; Z = zoopathogen; (?) = doubtful trigger. Table S2. Genes involved in virulence and or immune-evasion strategies in different TDF. Only genes with known function have been included. Note that some genes are present in different TDF, which gives an idea of their importance for virulence. Table S3. LAMP assays for pathogenic and food/drink spoilage fungi (not necessarily dimorphic fungi). Species are ordered first by taxonomic order and then alphabetically. Abbreviations: Acl1 = ATP citrate lyase subunit 1; amy1 = alpha-amylase 1; anxC4 = annexin C4; β-D glucan = (1→3)-β-D-glucan; βTUB = β-tubulin; CAP = capsule-associated gene; CHS-1 = chitin synthase 1; COX = mitochondrial cytochrome c oxidase; cyp51A = cyp51A gene promoter region; EF-1α = elongation factor 1 alpha; gaoA = galactose oxidase; Hyd = hydrophobin; IGS1 = intergenic spacer 1; ITS = internal transcribed spacer; LSU = nuclear large ribosomal subunit; MAT = mating type gene; mtSSU = mitochondrial small ribosomal subunit; PKS = calmodulin or polyketide synthase; rDNA = ribosomal operon (SSU-ITS1-5.8S-ITS2-LSU); SSU = nuclear small ribosomal subunit. * Former species name: 0 Exophiala jeanselmei; 1 Phomopsis phaseoli; 2 Pseudallescheria boydii; 3 Pseudallescheria boydii and Petriellidium boydii; and 4 Pneumocystis carinii. ** Sample origin: A = animal; H = human (clinical); P = plant; O = other origin (food or drink). If applicable, either the name of the disease caused by the particular fungus and/or the specific animal or plant affected appears between brackets. Table S4. Summary of LAMP and RCA assays designed so far for the diagnosis of endemic mycoses. Taxa are ordered alphabetically. Those closely related to the genera Histoplasma and Sporothrix are in bold. Abbreviations: HP = histoplasmosis; NS = not specified; SP = sporotrichosis; SPC = specificity; ST = sensitivity. *Twenty-six bone marrow samples were included, but only eleven corresponded to patients with positive cultures.
Author Contributions
Conceptualization, J.M.G.-M. and P.F.-S.; methodology, J.M.G.-M.; formal analysis, J.M.G.-M.; investigation, J.M.G.-M.; resources, P.F.-S. and A.M.; data curation, J.M.G.-M.; writing—original draft preparation, J.M.G.-M.; writing—review and editing, J.M.G.-M. and P.F.-S.; supervision, P.F.-S. and A.M.; project administration, P.F.-S. and A.M.; funding acquisition, P.F.-S. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Universities of the Spanish Government and financed by The European Next Generation Funds through a Margarita Salas postdoctoral contract awarded to J.M.G.-M. (host academic center: Faculty of Pharmacy, University of Salamanca; host researcher in charge: Prof. Dr. Pedro Fernández-Soto).
Data Availability Statement
Data supporting this review are available within the manuscript itself and in the studies referenced in the main manuscript and the Supplementary Materials.
Acknowledgments
We are thankful to the anonymous reviewers for their constructive comments on this review.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Srivastava, S.; Bhargava, A. Biological synthesis of nanoparticles: Fungi. In Green Nanoparticles: The Future of Nanobiotechnology; Srivastava, S., Bhargava, A., Eds.; Springer: Singapore, 2022; pp. 101–137. [Google Scholar]
- Valente-Navarro, M.; Nascimento de Barros, Y.; Dias Segura, W.; Alencar Chaves, A.F.; Pereira Jannuzzi, G.; Spadari Ferreira, K.; Xander, P.; Luiz Batista, W. The role of dimorphism regulating histidine kinase (Drk1) in the pathogenic fungus Paracoccidioides brasiliensis cell wall. JoF 2021, 7, 1014. [Google Scholar] [CrossRef]
- Dukik, K.; Muñoz, J.F.; Jiang, Y.; Feng, P.; Sigler, L.; Stielow, J.B.; Freeke, J.; Jamalian, A.; Gerrits van den Ende, B.; McEwen, J.G.; et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycoses 2017, 60, 296–309. [Google Scholar] [CrossRef]
- Li, Y.; Steenwyk, J.L.; Chang, Y.; Wang, Y.; James, T.Y.; Stajich, J.E.; Spatafora, J.W.; Groenewald, M.; Dunn, C.W.; Hittinger, C.T.; et al. A genome-scale phylogeny of the kingdom Fungi. Curr. Biol. 2021, 31, 1653–1665.e5. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, H.; Dukik, K.; de Melo Teixeira, M.; Stielow, J.B.; Delma, F.Z.; Al-Hatmi, A.; Ahmed, S.A.; Ilkit, M.; de Hoog, G.S. Phylogenetic and ecological reevaluation of the order Onygenales. Fungal Divers. 2022, 115, 1–72. [Google Scholar] [CrossRef]
- Chapman, S.W.; Dismukes, W.E.; Proia, L.A.; Bradsher, R.W.; Pappas, P.G.; Threlkeld, M.G.; Kauffman, C.A. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 1801–1812. [Google Scholar] [CrossRef]
- Jiang, Y.; Dukik, K.; Muñoz, J.F.; Sigler, L.; Schwartz, I.S.; Govender, N.P.; Kenyon, C.; Feng, P.; van den Ende, B.G.; Stielow, J.B.; et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018, 90, 245–291. [Google Scholar] [CrossRef]
- Muñoz, J.F.; McEwen, J.G.; Clay, O.K.; Cuomo, C.A. Genome analysis reveals evolutionary mechanisms of adaptation in systemic dimorphic fungi. Sci. Rep. 2018, 8, 4473. [Google Scholar] [CrossRef] [PubMed]
- Maphanga, T.G.; Birkhead, M.; Muñoz, J.F.; Allam, M.; Zulu, T.G.; Cuomo, C.A.; Schwartz, I.S.; Ismail, A.; Naicker, S.D.; Mpembe, R.S. Human blastomycosis in South Africa caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967 to 2014. J. Clin. Microbiol. 2020, 58, e01661-19. [Google Scholar] [CrossRef]
- Carod, J.-F.; Lortholary, O. Tropical fungal diseases in pediatrics. Int. J. Child Health Hum. Dev. 2021, 14, 281–294. [Google Scholar]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A global view on fungal infections in humans and animals: Infections caused by dimorphic fungi and dermatophytoses. J. Appl. Microbiol. 2021, 131, 2688–2704. [Google Scholar] [CrossRef]
- Pullen, M.F.; Alpern, J.D.; Bahr, N.C. Blastomycosis: Some progress but still much to learn. JoF 2022, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.A.; Kauffman, C.A.; Miceli, M.H. Blastomycosis: A review of mycological and clinical aspects. JoF 2023, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Wellinghausen, N.; Kern, W.V.; Haase, G.; Rozdzinski, E.; Kern, P.; Marre, R.; Essig, A.; Hetzel, J.; Hetzel, M. Chronic granulomatous lung infection caused by the dimorphic fungus Emmonsia sp. Int. J. Med. Microbiol. 2003, 293, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kenyon, C.; de Hoog, G.S.; Guo, L.; Fan, H.; Liu, H.; Li, Z.; Sheng, R.; Yang, Y.; Jiang, Y.; et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses 2017, 60, 310–319. [Google Scholar] [CrossRef]
- Govender, N.P.; Grayson, W. Emergomycosis (Emergomyces africanus) in advanced HIV disease. Dermatopathology 2019, 6, 63–69. [Google Scholar] [CrossRef]
- Samaddar, A.; Sharma, A. Emergomycosis, an emerging systemic mycosis in immunocompromised patients: Current trends and future prospects. Front. Med. 2021, 8, 670731. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Govender, N.P.; Sigler, L.; Jiang, Y.; Maphanga, T.G.; Toplis, B.; Botha, A.; Dukik, K.; Hoving, J.C.; Muñoz, J.F.; et al. Emergomyces: The global rise of new dimorphic fungal pathogens. PLoS Pathog. 2019, 15, e1007977. [Google Scholar] [CrossRef]
- Reddy, D.L.; Nel, J.; Govender, N.P. Review: Emergomycosis. J. Mycol. Med. 2023, 33, 101313. [Google Scholar] [CrossRef]
- Amona, F.M.; Denning, D.W.; Moukassa, D.; Develoux, M.; Hennequin, C. Histoplasmosis in the Republic of Congo dominated by African histoplasmosis, Histoplasma capsulatum var. duboisii. PLoS Negl. Trop. Dis. 2021, 15, e0009318. [Google Scholar] [CrossRef]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef]
- Sil, A.; Andrianopoulos, A. Thermally dimorphic human fungal pathogens—Polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harb. Perspect. Med. 2015, 5, a019794. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; De Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.; Nathan, A.; do Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Cattana, M.E.; Matute, D.R.; Muñoz, J.F.; Arechavala, A.; Isbell, K.; Schipper, R.; Santiso, G.; Tracogna, F.; Sosa, M.D.L.Á.; et al. Genomic diversity of the human pathogen Paracoccidioides across the South American continent. Fungal Genet. Biol. 2020, 140, 103395. [Google Scholar] [CrossRef]
- Felipe, C.R.A.; Silva, A.D.; Penido, M.G.M.G.; Felipe, C.R.; Silva, A. Disseminated paracoccidioidomycosis in a kidney transplant recipient. Cureus 2021, 13, e19007. [Google Scholar] [CrossRef]
- Cárcano, C.B.M.; Vanessa, D.; Alessi, C.; Tadin Reis, M.; Soares Ferreira, M. Paracoccidioidomycosis with sarcoid-like cutaneous lesion: A clinicopathological challenge. IDCases 2022, 29, e01574. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; de Hoog, S.; Bensch, K.; Bagagli, E.; Mendoza, L. A taxonomic review of the genus Paracoccidioides, with focus on the uncultivable species. PLoS Negl. Trop. Dis. 2023, 17, e0011220. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Koenig, G.; White, T.; Taylor, J. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002, 94, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Barker, B.M.; Wiederhold, N.P. Large-scale evaluation of in vitro amphotericin B, triazole, and echinocandin activity against Coccidioides species from U.S. institutions. Antimicrob. Agents Chemother. 2017, 61, e02634-16. [Google Scholar] [CrossRef]
- Kauffman, C.A.; Bustamante, B.; Chapman, S.W.; Pappas, P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 1255–1265. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Tse, H.; Chan, J.S.; Zhou, A.C.; Curreem, S.O.; Lau, C.C.; Yuen, K.Y.; Woo, P.C. Proteome profiling of the dimorphic fungus Penicillium marneffei extracellular proteins and identification of glyceraldehyde-3-phosphate dehydrogenase as an important adhesion factor for conidial attachment. FEBS J. 2013, 280, 6613–6626. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Chow, W.N.; Wong, A.Y.; Yeung, J.M.; Bao, J.; Zhang, N.; Lok, S.; Woo, P.C.; Yuen, K.Y. Identification of microRNA-like RNAs in mycelial and yeast phases of the thermal dimorphic fungus Penicillium marneffei. PLoS Negl. Trop. Dis. 2013, 7, e2398. [Google Scholar] [CrossRef] [PubMed]
- Vega, P.T.; Erramilli, S.; Lee, E. Talaromyces marneffei laboratory cross reactivity with Histoplasma and Blastomyces urinary antigen. Int. J. Infect. Dis. 2019, 86, 15–17. [Google Scholar] [CrossRef]
- Untereiner, W.A.; Scott, J.A.; Naveau, F.A.; Sigler, L.; Bachewich, J.; Angus, A. The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia 2004, 96, 812–821. [Google Scholar] [CrossRef]
- Mead, H.; Roe, C.; Higgins Keppler, E.; Van Dyke, M.C.; Laux, K.; Funke, A.; Miller, K.; Bean, H.; Sahl, J.; Barker, B.M. Defining critical genes during spherule remodeling and endospore development in the fungal pathogen, Coccidioides posadasii. Front. Genet. 2020, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Giannini, M.J.S.; Monteiro da Silva, J.L.; de Fátima da Silva, J.; Donofrio, F.C.; Miranda, E.T.; Andreotti, P.F.; Soares, C.P. Interactions of Paracoccidioides brasiliensis with host cells: Recent advances. Mycopathologia 2008, 165, 237–248. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Toda, M.; Benedict, K.; Cáceres, D.H.; Litvintseva, A.P. Endemic and other dimorphic mycoses in The Americas. JoF 2021, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Gadre, A.; Enbiale, W.; Andersen, L.K.; Coates, S.J. The effects of climate change on fungal diseases with cutaneous manifestations: A report from the International Society of Dermatology Climate Change Committee. J. Clim. Change Health 2022, 6, 100156. [Google Scholar] [CrossRef]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the maps for endemic mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Linder, K.A.; Kauffman, C.A. Histoplasmosis: Epidemiology, diagnosis, and clinical manifestations. Curr. Fungal Infect. Rep. 2019, 13, 120–128. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, X.; Huang, S. Disseminated Talaromyces marneffei infection mimicking intestinal tuberculosis. Lancet Infect. Dis. 2021, 21, 1469. [Google Scholar] [CrossRef]
- Azar, M.M.; Malo, J.; Hage, C.A. Endemic fungi presenting as community-acquired pneumonia: A review. Semin. Respir. Crit. Care Medicine. 2020, 41, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Benedict, K.; Kobayashi, M.; Garg, S.; Chiller, T.; Jackson, B.R. Symptoms in blastomycosis, coccidioidomycosis, and histoplasmosis versus other respiratory illnesses in commercially insured adult outpatients—United States, 2016–2017. Clin. Infect. Dis. 2021, 73, e4336–e4344. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tong, J.L.; Ran, Z.H.; Cao, Z.J. Talaromyces (Penicillium) infection in a patient presenting with intestinal ulcers mimicking inflammatory bowel disease. J. Dig. Dis. 2020, 21, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Nakshabendi, R.; Berry, A.C.; Torres-Miranda, D.; LaBarbera, F.D.; Kanar, O.; Nakshabandi, A.; Nakshabendi, I. Primary Histoplasma capsulatum enterocolitis mimicking peptic and inflammatory bowel disease. Case Rep. Gastrointest. Med. 2016, 2016, 7139573. [Google Scholar]
- Khalaf, S.A.; Patel, P.; Caruso, C.R.; Parrett, T.; Bran, A. CNS histoplasmosis as a gliosarcoma mimicker: The diagnostic dilemma of solitary brain lesions. IDCases 2022, 27, e01364. [Google Scholar] [CrossRef]
- Valera, E.T.; Mori, B.M.; Engel, E.E.; Costa, I.S.; Brandão, D.F.; Nogueira-Barbosa, M.H.; Queiroz, R.G.d.P.; Silveira, V.d.S.; Scrideli, C.A.; Tone, L.G. Fungal infection by Paracoccidioides brasiliensis mimicking bone tumor. Pediatr. Blood Cancer 2008, 50, 1284–1286. [Google Scholar] [CrossRef]
- Molina-Morant, D.; Sánchez-Montalva, A.; Salvador, F.; Sao-Avilés, A.; Molina, I. Imported endemic mycoses in Spain: Evolution of hospitalized cases, clinical characteristics and correlation with migratory movements, 1997–2014. PLoS Negl. Trop. Dis. 2018, 12, e0006245. [Google Scholar] [CrossRef]
- Zerbato, V.; Di Bella, S.; Pol, R.; D’Aleo, F.; Angheben, A.; Farina, C.; Conte, M.; Luzzaro, F.; Luzzati, R.; Principe, L.; et al. Endemic systemic mycoses in Italy: A systematic review of literature and a practical update. Mycopathologia 2023, 188, 307–334. [Google Scholar] [CrossRef]
- Wilmes, D.; Rickerts, V. Clinical syndromes: Rare fungi. In Clinically Relevant Mycoses: A Practical Approach; Presterl, E., Ed.; Springer: Cham, Switzerland, 2019; pp. 113–135. [Google Scholar]
- de Carvalho, J.A.; Monteiro, R.C.; Hagen, F.; Pires de Camargo, Z.; Rodrigues, A.M. Trends in molecular diagnostics and genotyping tools applied for emerging Sporothrix species. JoF 2022, 8, 809. [Google Scholar] [CrossRef]
- Bosco, S.M.G.; Bagagli, E. Paracoccidioidomycosis in animals and humans. In Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi, S., de Hoog, G.S., Guillot, J., Verweij, P.E., Eds.; Springer: Cham, Switzerland, 2018; pp. 129–145. [Google Scholar]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Williams, S.L.; Chiller, T. Update on the epidemiology, diagnosis, and treatment of coccidioidomycosis. JoF 2022, 8, 666. [Google Scholar] [CrossRef]
- Poester, V.R.; Stevens, D.A.; Basso, R.P.; Silveira Munhoz, L.; Zanchi, M.; Benelli, J.L.; Baracy Klafke, G.; Cardone, S.; Orzechowski Xavier, M. CATastrophe: Response to the challenges of zoonotic sporotrichosis in southern Brazil. Mycoses 2022, 65, 30–34. [Google Scholar] [CrossRef]
- Thompson, G.R.; Le, T.; Chindamporn, A.; Kauffman, C.A.; Alastruey-Izquierdo, A.; Ampel, N.M.; Andes, D.R.; Armstrong-James, D.; Ayanlowo, O.; Baddley, J.W.; et al. Global guideline for the diagnosis and management of the endemic mycoses: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect. Dis. 2021, 21, e364–e374. [Google Scholar] [CrossRef]
- Ocansey, B.K.; Kosmidis, C.; Agyei, M.; Dorkenoo, A.M.; Ayanlowo, O.O.; Oladele, R.O.; Darre, T.; Denning, D.W. Histoplasmosis in Africa: Current perspectives, knowledge gaps, and research priorities. PLoS Negl. Trop. Dis. 2022, 16, e0010111. [Google Scholar] [CrossRef]
- Narayanasamy, S.; Dat, V.Q.; Thanh, N.T.; Ly, V.T.; Chan, J.F.-W.; Yuen, K.-Y.; Ning, C.; Liang, H.; Li, L.; Chowdhary, A.; et al. A global call for talaromycosis to be recognised as a neglected tropical disease. Lancet Glob. Health 2021, 9, e1618–e1622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J. Molecular mechanisms of fungal adaptive evolution. In Molecular Mechanisms of Microbial Evolution. Grand Challenges in Biology and Biotechnology; Rampelotto, P., Ed.; Springer: Cham, Switzerland, 2018; pp. 409–435. [Google Scholar]
- Wiederhold, N.P. Review of the novel investigational antifungal olorofim. JoF 2020, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Ibe, C.; Mnyambwa, N.P.; Mfinanga, S.G. Emergomycosis in Africa: Time to pay attention to this emerging deadly fungal infection. Int. J. Gen. Med. 2023, 16, 2313–2322. [Google Scholar] [CrossRef]
- Davis, L.E.; Cook, G.; Costerton, J.W. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg. Infect. Dis. 2002, 8, 376–379. [Google Scholar] [CrossRef]
- Orlandi Sardi, J.; de Souza Pitangui, N.; Voltan, A.R.; Braz, J.D.; Pelajo Machado, M.; Fusco Almeida, A.M.; Mendes Giannini, M.J. In vitro Paracoccidioides brasiliensis biofilm and gene expression of adhesins and hydrolytic enzymes. Virulence 2015, 6, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Brilhante, R.S.; Chaves de Lima, R.A.; de Farias Marques, F.J.; Fechine Silva, N.; Pacheco Caetano, É.; Castelo-Branco, D.S.C.M.; Pinheiro Gomes Bandeira, T.J.; Bezerra Moreira, J.L.; de Aguiar Cordeiro, R.; Monteiro, A.J.; et al. Histoplasma capsulatum in planktonic and biofilm forms: In vitro susceptibility to amphotericin B, itraconazole and farnesol. J. Med. Microbiol. 2015, 64, 394–399. [Google Scholar] [CrossRef]
- Eltayeb, M.; Asad, S.; Malik, A.; Cao, Y.; Yu, W.Y.; Hong, A. Fluconazole non susceptible disseminated coccidioidomycosis in a critically ill patient without known immunosuppression. Am. J. Respir. Crit. Care Med. 2019, 199, A6871. [Google Scholar]
- Kai-Su, P.; Hong, L.; Dong-Yan, Z.; Yan-Qing, Z.; Andrianopoulos, A.; Latgé, J.P.; Cun-Wei, C. Study on the mechanisms of action of berberine combined with fluconazole against fluconazole-resistant strains of Talaromyces marneffei. Front. Microbiol. 2022, 13, 1033211. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.F.; Oliveira, A.F.; Landgraf, T.N.; Cunha, C.; Carvalho, A.; Vendruscolo, P.E.; Gonçales, R.A.; Almeida, F.; da Silva, T.A.; Rodrigues, F.; et al. Impact of paracoccin gene silencing on Paracoccidioides brasiliensis virulence. mBio 2017, 8, e00537-17. [Google Scholar] [CrossRef]
- Witzany, C.; Bonhoeffer, S.; Rolff, J. Is antimicrobial resistance evolution accelerating? PLoS Pathog. 2020, 16, e1008905. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, M.; Gern, B.; Hung, C.Y.; Ersland, K.; Rocco, N.; Pick-Jacobs, J.; Galles, K.; Filutowicz, H.; Warner, T.; Evans, M.; et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Investig. 2011, 121, 554–568. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Lerksuthirat, T.; Klein, B.; Wüthrich, M. The C-type lectin receptor MCL mediates vaccine-induced immunity against infection with Blastomyces dermatitidis. Infect. Immun. 2016, 84, 635–642. [Google Scholar] [CrossRef]
- Amaral, A.C.; Marques, A.F.; Muñoz, J.E.; Bocca, A.L.; Simioni, A.R.; Tedesco, A.C.; Morais, P.C.; Travassos, L.R.; Taborda, C.P.; Felipe, M.S.S. Poly (lactic acid-glycolic acid) nanoparticles markedly improve immunological protection provided by peptide P10 against murine paracoccidioidomycosis. Br. J. Pharmacol. 2010, 159, 1126–1132. [Google Scholar] [CrossRef]
- Fogaça de Almeida, J.R.; Kaihami, G.H.; Pereira Jannuzzi, G.; de Almeida, S.R. Therapeutic vaccine using a monoclonal antibody against a 70-kDa glycoprotein in mice infected with highly virulent Sporothrix schenckii and Sporothrix brasiliensis. Sabouraudia 2015, 53, 42–50. [Google Scholar] [CrossRef]
- Wong, L.-P.; Woo, P.C.; Wu, A.Y.; Yuen, K.-Y. DNA immunization using a secreted cell wall antigen Mp1p is protective against Penicillium marneffei infection. Vaccine 2002, 20, 2878–2886. [Google Scholar] [CrossRef]
- Linder, K.A.; Kauffman, C.A. Current and new perspectives in the diagnosis of blastomycosis and histoplasmosis. JoF 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Paes, R.; Bernardes-Engemann, A.R.; da Silva Motta, B.; Pizzini, C.V.; de Abreu Almeida, M.; de Medeiros Muniz, M.; Alves Barcelos Dias, R.; Zancopé-Oliveira, R.M. Immunologic diagnosis of endemic mycoses. JoF 2022, 8, 993. [Google Scholar] [CrossRef]
- Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef]
- Sidamonidze, K.; Peck, M.K.; Perez, M.; Baumgardner, D.; Smith, G.; Chaturvedi, V.; Chaturvedi, S. Real-time PCR assay for identification of Blastomyces dermatitidis in culture and in tissue. J. Clin. Microbiol. 2012, 50, 1783–1786. [Google Scholar] [CrossRef]
- Peçanha-Pietrobom, P.M.; Tirado-Sánchez, A.; Gonçalves, S.S.; Bonifaz, A.; Colombo, A.L. Diagnosis and treatment of pulmonary coccidioidomycosis and paracoccidioidomycosis. JoF 2023, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Saubolle, M.A. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Wen, S.L.; Ying, K.J. A case study of imported pulmonary coccidioidomycosis. J. Zhejiang Univ. Sci. B 2011, 12, 298–302. [Google Scholar] [CrossRef]
- Nogueira Brilhante, R.S.; Vago Bittencourt, P.; Chaves de Lima, R.A.; Castelo-Branco, D.S.C.M.; Sales Oliveira, J.; Pinheiro, A.; Cordeiro, R.; Pires de Camargo, Z.; Costa Sidrim, J.J.; Rocha, M.F.G. Coccidioidomycosis and histoplasmosis in equines: An overview to support the accurate diagnosis. J. Equine Vet. Sci. 2016, 40, 62–73. [Google Scholar] [CrossRef]
- Castañón-Olivares, L.R.; Güereña-Elizalde, D.; González-Martínez, M.R.; Licea-Navarro, A.F.; González-González, G.M.; Aroch-Calderón, A. Molecular identification of Coccidioides isolates from Mexican patients. Ann. N. Y. Acad. Sci. 2007, 1111, 326–335. [Google Scholar] [CrossRef]
- Reyes-Montes, M.d.R.; Frías-De-León, M.G.; Victoriano-Pastelín, I.; Acosta-Altamirano, G.; Duarte-Escalante, E. Design and evaluation of an AFLP molecular marker for the detection of Coccidioides spp. in biological samples. Braz. J. Infect. Dis. 2019, 23, 322–330. [Google Scholar] [CrossRef]
- Malik, R.; Capoor, M.R.; Vanidassane, I.; Gogna, A.; Singh, A.; Sen, B.; Rudramurthy, S.M.; Honnavar, P.; Gupta, S.; Chakrabarti, A. Disseminated Emmonsia pasteuriana infection in India: A case report and a review. Mycoses 2016, 59, 127–132. [Google Scholar] [CrossRef]
- Kenyon, C.; Bonorchis, K.; Corcoran, C.; Meintjes, G.; Locketz, M.; Lehloenya, R.; Vismer, H.F.; Naicker, P.; Prozesky, H.; van Wyk, M.; et al. A dimorphic fungus causing disseminated infection in South Africa. N. Engl. J. Med. 2013, 369, 1416–1424. [Google Scholar] [CrossRef]
- Maphanga, T.G. Diagnostic Methods for Detection and Characterisation of Dimorphic Fungi Causing Invasive Disease in Africa: Development and Evaluation; University of the Free State: Bloemfontein, South Africa, 2020. [Google Scholar]
- Gugnani, H.C.; Muotoe-Okafor, F. African histoplasmosis: A review. Rev. Iberoam. Micol. 1997, 14, 155–159. [Google Scholar]
- Castellanos Reynosa, M.E.; Caal, M.E.; Mercado, D.; Medina, N.; Pérez, J.C.; Emeto, T.I.; Arathoon, E. Clinical characteristics, diagnosis, treatment and outcomes of patients living with HIV and co-infected with tuberculosis and histoplasmosis: A 5-y retrospective case series. Trans. R. Soc. Trop. Med. Hyg. 2024, 118, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sahaza, J.H.; Rodríguez-Arellanez, G.; Canteros, C.E.; Reyes-Montes, M.d.R.; Taylor, M.L. Thermotolerance of Histoplasma capsulatum at 40° C predominates among clinical isolates from different Latin American regions. Braz. J. Infect. Dis. 2020, 24, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Niño-Vega, G.A.; Camacho, E.; Moreno, Á.R.; Tobón, A.M.; Gómez, B.L.; Teixeira, M.M.; Barker, B.M. Paracoccidioides spp. and paracoccidioidomycosis. In Current Progress in Medical Mycology; Mora-Montes, H.M., Lopes-Bezerra, L.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 281–308. [Google Scholar]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Mendes Peçanha, P.; Massaroni Peçanha-Pietrobom, P.; Grão-Velloso, T.R.; Rosa Júnior, M.; Falqueto, A.; Santos Gonçalves, S. Paracoccidioidomycosis: What we know and what is new in epidemiology, diagnosis, and treatment. JoF 2022, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zancopé-Oliveira, R.M.; Pizzini, C.V.; de Medeiros Muniz, M.; Francesconi do Valle, A.C.; Almeida-Paes, R. Diagnostic aspects of paracoccidioidomycosis. Curr. Trop. Med. Rep. 2014, 1, 111–118. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Macedo, P.M.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Bonifaz, A. Sporothrix and sporotrichosis. In Current Progress in Medical Mycology; Mora-Montes, H.M., Lopes-Bezerra, L.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 309–331. [Google Scholar]
- Sharma, B.; Sharma, A.K.; Sharma, U. Sporotrichosis: A comprehensive review on recent drug-based therapeutics and management. Curr. Dermatol. Rep. 2022, 11, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Salzer, H.J.; Burchard, G.; Cornely, O.A.; Lange, C.; Rolling, T.; Schmiedel, S.; Libman, M.; Capone, D.; Le, T.; Dalcolmo, M.P.; et al. Diagnosis and management of systemic endemic mycoses causing pulmonary disease. Respiration 2018, 96, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Zancopé-Oliveira, R.M.; Almeida-Paes, R.; Oliveira, M.; Saraiva Freitas, D.F.; Gutierrez-Galhardo, M. New diagnostic applications in sporotrichosis. In Skin Biopsy-Perspectives; Khopkar, U., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 53–72. [Google Scholar]
- Balajee, S.A.; Brandt, M.E. Aspergillus and Penicillium. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; John Wiley & Sons: Hoboken, NY, USA, 2011; pp. 1836–1852. [Google Scholar]
- Supparatpinyo, K.; Chiewchanvit, S.; Hirunsri, P.; Uthammachai, C.; Nelson, K.E.; Sirisanthana, T. Penicillium marneffei infection in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 1992, 14, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Vucicevic, D.; Blair, J.E.; Binnicker, M.J.; McCullough, A.E.; Kusne, S.; Vikram, H.R.; Parish, J.M.; Wengenack, N.L. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010, 170, 345–351. [Google Scholar] [CrossRef]
- Griffiths, J.; Lopes Colombo, A.; Denning, D.W. The case for paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef]
- Eissenberg, L.G.; Goldman, W.E. Histoplasma variation and adaptive strategies for parasitism: New perspectives on histoplasmosis. Clin. Microbiol. Rev. 1991, 4, 411–421. [Google Scholar] [CrossRef]
- Willinger, B. Culture-based techniques. In Human Fungal Pathogen Identification: Methods and Protocols; Lion, T., Ed.; John, M. Walker: Hatfield, UK, 2017; pp. 195–207. [Google Scholar]
- Duarte-Escalante, E.; Frías-De-León, M.G.; Zúñiga, G.; Martínez-Herrera, E.; Acosta-Altamirano, G.; del Rocío Reyes-Montes, M. Molecular markers in the epidemiology and diagnosis of coccidioidomycosis. Rev. Iberoam. Micol. 2014, 31, 49–53. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Hagen, F.; Puccia, R.; Hahn, R.C.; Pires de Camargo, Z. Paracoccidioides and paracoccidioidomycosis in the 21st Century. Mycopathologia 2023, 188, 129–133. [Google Scholar] [CrossRef]
- Sutton, D.A. Diagnosis of coccidioidomycosis by culture. Ann. N. Y. Acad. Sci. 2007, 1111, 315–325. [Google Scholar] [CrossRef]
- Gupta, E.; Bhalla, P.; Khurana, N.; Singh, T. Histopathology for the diagnosis of infectious diseases. Indian J. Med. Microbiol. 2009, 27, 100–106. [Google Scholar] [CrossRef]
- Brandt, M.E.; Gomez, B.L.; Warnock, D.W. Histoplasma, Blastomyces, Coccidioides, and other dimorphic fungi causing systemic mycoses. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Carroll, K.C., Funke, G., Jorgensen, J.H., Landry, M.L., Warnock, D.W., Eds.; John Wiley & Sons: Hoboken, NY, USA, 2011; pp. 1902–1918. [Google Scholar]
- Borman, A.M.; Jiang, Y.; Dukik, K.; Sigler, L.; Schwartz, I.S.; de Hoog, G.S. Adiaspiromycosis and diseases caused by related fungi in Ajellomycetaceae. In Emerging and Epizootic Fungal Infections in Animals; Seyedmousavi, S., de Hoog, G.S., Guillot, J., Verweij, P.E., Eds.; Springer: Cham, Switzerland, 2018; pp. 147–158. [Google Scholar]
- Rodríguez-Brito, S.; Camacho, E.; Mendoza, M.; Niño-Vega, G.A. Differential identification of Sporothrix spp. and Leishmania spp. by conventional PCR and qPCR in multiplex format. Med. Mycol. 2015, 53, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Maubon, D.; Garnaud, C.; Ramarozatovo, L.S.; Fahafahantsoa, R.R.; Cornet, M.; Rasamoelina, T. Molecular diagnosis of two major implantation mycoses: Chromoblastomycosis and sporotrichosis. JoF 2022, 8, 382. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Muñoz, J.F.; Kenyon, C.R.; Govender, N.P.; McTaggart, L.; Maphanga, T.G.; Richardson, S.; Becker, P.; Cuomo, C.A.; McEwen, J.G.; et al. Blastomycosis in Africa and the Middle East: A comprehensive review of reported cases and reanalysis of historical isolates based on molecular data. Clin. Infect. Dis. 2020, 73, e1560–e1569. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.N.; Chandler, B.; Dallas, S.D.; Wiederhold, N.P.; Cañete-Gibas, C.; Mais, D.D. The brief case of Histoplasma duboisii: An infection with a rare organism presenting as an abdominal mass. J. Clin. Microbiol. 2022, 60, e01201-21. [Google Scholar] [CrossRef]
- Cáceres, D.H.; Chiller, T.; Lindsley, M.D. Immunodiagnostic assays for the investigation of fungal outbreaks. Mycopathologia 2020, 185, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Leitão, N.P., Jr.; Vallejo, M.C.; Conceição, P.M.; Pires de Camargo, Z.; Hahn, R.; Puccia, R. Paracoccidioides lutzii Plp43 ps an active glucanase with partial antigenic identity with P. brasiliensis gp43. PLoS Negl. Trop. Dis. 2014, 8, e3111. [Google Scholar] [CrossRef]
- Klein, B.S.; Kuritsky, J.N.; Chappell, W.A.; Kaufman, L.; Green, J.; Davies, S.F.; Williams, J.E.; Sarosi, G.A. Comparison of the enzyme immunoassay, immunodiffusion, and complement fixation tests in detecting antibody in human serum to the A antigen of Blastomyces dermatitidis. Am. Rev. Respir. Dis. 1986, 133, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Theel, E.S.; Rodino, K.G.; Granger, D. Detection of Blastomyces dermatitidis antigen in urine using a commercially available quantitative enzyme immunoassay. J. Clin. Microbiol. 2021, 59, e0144421. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.F.; Kaufman, L. Western immunoblot analysis and serologic characterization of Blastomyces dermatitidis yeast form extracellular antigens. J. Clin. Microbiol. 1992, 30, 3043–3049. [Google Scholar] [CrossRef]
- Johnson, S.M.; Zimmermann, C.R.; Pappagianis, D. Use of a recombinant Coccidioides immitis complement fixation antigen-chitinase in conventional serological assays. J. Clin. Microbiol. 1996, 34, 3160–3164. [Google Scholar] [CrossRef]
- Donovan, F.M.; Ramadan, F.A.; Khan, S.A.; Bhaskara, A.; Lainhart, W.D.; Narang, A.T.; Mosier, J.M.; Ellingson, K.D.; Bedrick, E.J.; Saubolle, M.A.; et al. Comparison of a novel rapid lateral flow assay to enzyme immunoassay results for early diagnosis of coccidioidomycosis. Clin. Infect. Dis. 2021, 73, e2746–e2753. [Google Scholar] [CrossRef]
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73. [Google Scholar] [CrossRef] [PubMed]
- Kassis, C.; Durkin, M.; Holbrook, E.; Myers, R.; Wheat, L. Advances in diagnosis of progressive pulmonary and disseminated coccidioidomycosis. Clin. Infect. Dis. 2021, 72, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Maddox, S.; Doherty, B.; Pelfrey, J.; Thompson, G.; Bauman, S. Rapid detection of anti-coccidioides antibodies using the sona™ Coccidioides Ab lateral flow assay. In Proceedings of the 7th International Coccidioidomycosis Symposium, Stanford, CA, USA, 10–13 August 2017. [Google Scholar]
- Martins, T.B.; Jaskowski, T.D.; Mouritsen, C.L.; Hill, H.R. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J. Clin. Microbiol. 1995, 33, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, G.; Aristizábal, S.; Salinas, C.; Delgado-Hernández, R.; Angeles-Morales, V.; Soto-Hernández, J.; Castañón-Olivares, L.R.; Hernández, F. Coccidioidal meningitis in non-AIDS patients. A case series at a Mexican neurological referral center. Clin. Neurol. Neurosurg. 2020, 196, 106011. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Escalante, E.; Frías-De-León, M.G.; Martínez García, L.G.; Herrera, J.; Acosta Altamirano, G.; Cabello, C.; Palma, G.; Reyes Montes, M.D.R. Selection of specific peptides for Coccidioides spp. obtained from antigenic fractions through SDS-PAGE and Western blot methods by the recognition of sera from patients with coccidioidomycosis. Molecules 2018, 23, 3145. [Google Scholar] [CrossRef]
- Fida, M.; Misra, A.; Harring, J.A.; Kubbara, A.; Theel, E.S. Histoplasma capsulatum complement fixation and immunodiffusion assay sensitivity in culture-confirmed cases of histoplasmosis: A 10-year retrospective review (2011 to 2020). J. Clin. Microbiol. 2022, 60, e0105722. [Google Scholar] [CrossRef]
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Herman, K.M.; Hage, C.A.; Fuller, D.; Wheat, L.J. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin. Infect. Dis. 2016, 62, 896–902. [Google Scholar] [CrossRef]
- Cáceres, D.H.; Gómez, B.L.; Tobón, Á.M.; Minderman, M.; Bridges, N.; Chiller, T.; Lindsley, M.D. Validation and concordance analysis of a new lateral flow assay for detection of Histoplasma antigen in urine. JoF 2021, 7, 799. [Google Scholar] [CrossRef]
- Gerber, J.D.; Riley, R.E.; Jones, R. Evaluation of a microtiter latex agglutination test for histoplasmosis. Appl. Microbiol. 1972, 24, 191–197. [Google Scholar] [CrossRef]
- de Abreu Almeida, M.; Vera Pizzini, C.; Serra Damasceno, L.; de Medeiros Muniz, M.; Almeida-Paes, R.; Saramago Peralta, R.H.; Peralta, J.M.; de Vasconcelos Carvalhaes Oliveira, R.; Gomes Vizzoni, A.; de Andrade, C.L.T.; et al. Validation of western blot for Histoplasma capsulatum antibody detection assay. BMC Infect. Dis. 2016, 16, 87. [Google Scholar]
- Tobón, A.M.; Agudelo, C.A.; Restrepo, C.A.; Villa, C.A.; Quiceno, W.; Estrada, S.; Restrepo, A. Adrenal function status in patients with paracoccidioidomycosis after prolonged post-therapy follow-up. Am. J. Trop. Med. Hyg. 2010, 83, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Marques, S.; Alves, M.; Fecchio, D.; Franco, M.F.d. Serological follow-up of patients with paracoccidioidomycosis treated with itraconazole using dot-blot, ELISA and Western-blot. Rev. Inst. Med. Trop. São Paulo 1997, 39, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Marques da Silva, S.H.; de Mattos Grosso, M.; Lopes, J.D.; Lopes Colombo, A.; Souza Lima Blotta, M.H.; Queiroz-Telles, F.; Pires de Camargo, Z. Detection of Paracoccidioides brasiliensis gp70 circulating antigen and follow-up of patients undergoing antimycotic therapy. J. Clin. Microbiol. 2004, 42, 4480–4486. [Google Scholar] [CrossRef]
- Cocio, T.A.; Martinez, R. Serological diagnosis of paracoccidioidomycosis using a Paracoccidioides spp. comercial antigen and the counterimmunoelectrophoresis method. Braz. J. Infect. Dis. 2021, 25, 101607. [Google Scholar] [CrossRef]
- Tomazini, K.A.; Soares Pereira, B.A.; Sylvestre, T.F.; de Souza Cavalcante, R.; de Carvalho, L.R.; Poncio Mendes, R. Reproducibility of double agar gel immunodiffusion test using stored serum and plasma from paracoccidioidomycosis patients. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20220045. [Google Scholar] [CrossRef]
- Maciel Garcia, N.; Barbaro Del Negro, G.M.; Pinto Martins, H.; da Silva Lacaz, C. Detection of paracoccidioidomycosis circulating antigen by the immunoelectroosmophoresis-immunodiffusion technique. Preliminary report. Rev. Inst. Med. Trop. Sao Paulo 1987, 29, 327–328. [Google Scholar] [CrossRef]
- Oliveira dos Santos, P.; Rodrigues, A.M.; Fernandes, G.F.; da Silva, S.H.; Burger, E.; de Camargo, Z.P. Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: Detection of specific antibody anti-gp43 and specific antigen gp43. PLoS Negl. Trop. Dis. 2015, 9, e0003516. [Google Scholar]
- Blotta, M.H.; Camargo, Z.P. Immunological response to cell-free antigens of Paracoccidioides brasiliensis: Relationship with clinical forms of paracoccidioidomycosis. J. Clin. Microbiol. 1993, 31, 671–676. [Google Scholar] [CrossRef]
- Baptista, V.S.; Mothé, G.B.; Santos, G.M.P.; Melivilu, C.S.I.; Santos, T.O.; Virginio, E.D.; de Macêdo-Sales, P.A.; Pinto, M.R.; Machado, R.L.D.; Rocha, E.M. Promising application of the SsCBF ELISA test to monitor the therapeutic response of feline sporotrichosis caused by Sporothrix brasiliensis from Brazilian epidemics. Braz. J. Microbiol. 2021, 52, 145–153. [Google Scholar] [CrossRef]
- Bernardes-Engemann, A.R.; Costa, R.C.; Miguens, B.R.; Penha, C.V.; Neves, E.; Pereira, B.A.; Dias, C.M.; Mattos, M.; Gutierrez, M.C.; Schubach, A.; et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med. Mycol. 2005, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Cognialli, R.; Bloss, K.; Weiss, I.; Cáceres, D.H.; Davis, R.; Queiroz-Telles, F. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses 2022, 65, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Queiroz-Telles, F.; Buccheri, R.; Benard, G. Sporotrichosis in immunocompromised hosts. JoF 2019, 5, 8. [Google Scholar] [CrossRef]
- Lima, O.C.; Bezerra, L.M. Identification of a concanavalin A-binding antigen of the cell surface of Sporothrix schenckii. J. Med. Vet. Mycol. 1997, 35, 167–172. [Google Scholar] [CrossRef][Green Version]
- Cao, L.; Chen, D.L.; Lee, C.; Chan, C.M.; Chan, K.M.; Vanittanakom, N.; Tsang, D.N.; Yuen, K.Y. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 1998, 36, 3028–3031. [Google Scholar] [CrossRef]
- Chen, X.; Ou, X.; Wang, H.; Li, L.; Guo, P.; Chen, X.; Cai, W.; Tang, X.; Li, L. Talaromyces marneffei Mp1p antigen detection may play an important role in the early diagnosis of talaromycosis in patients with acquired immunodeficiency syndrome. Mycopathologia 2022, 187, 205–215. [Google Scholar] [CrossRef]
- Wang, Y.F.; Cai, J.P.; Wang, Y.D.; Dong, H.; Hao, W.; Jiang, L.X.; Long, J.; Chan, C.; Woo, P.C.; Yuen, K.-Y.; et al. Immunoassays based on Penicillium marneffei Mp1p derived from Pichia pastoris expression system for diagnosis of penicilliosis. PLoS ONE 2011, 6, e28796. [Google Scholar] [CrossRef]
- Imwidthaya, P.; Sekhon, A.S.; Mastro, T.D.; Garg, A.K.; Ambrosie, E. Usefulness of a microimmunodiffusion test for the detection of Penicillium marneffei antigenemia, antibodies, and exoantigens. Mycopathologia 1997, 138, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.; Standard, P.G.; Jalbert, M.; Kantipong, P.; Limpakarnjanarat, K.; Mastro, T.D. Diagnostic antigenemia tests for penicilliosis marneffei. J. Clin. Microbiol. 1996, 34, 2503–2505. [Google Scholar] [CrossRef]
- Desakorn, V.; Simpson, A.J.; Wuthiekanun, V.; Sahassananda, D.; Rajanuwong, A.; Pitisuttithum, P.; Howe, P.A.; Smith, M.D.; White, N.J. Development and evaluation of rapid urinary antigen detection tests for diagnosis of penicilliosis marneffei. J. Clin. Microbiol. 2002, 40, 3179–3183. [Google Scholar] [CrossRef]
- Prakit, K.; Nosanchuk, J.D.; Pruksaphon, K.; Vanittanakom, N.; Youngchim, S. A novel inhibition ELISA for the detection and monitoring of Penicillium marneffei antigen in human serum. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Baumgardner, D.J. Use of urine antigen testing for Blastomyces in an integrated health system. J. Patient Cent. Res. Rev. 2018, 5, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Kurowski, R.; Ostapchuk, M. Overview of histoplasmosis. Am. Fam. Physician 2002, 66, 2247. [Google Scholar]
- Azar, M.M.; Hage, C.A. Laboratory diagnostics for histoplasmosis. J. Clin. Microbiol. 2017, 55, 1612–1620. [Google Scholar] [CrossRef]
- Bansal, N.; Sethuraman, N.; Gopalakrishnan, R.; Ramasubramanian, V.; Kumar, D.S.; Nambi, P.S.; Chakrabarti, A. Can urinary Histoplasma antigen test improve the diagnosis of histoplasmosis in a tuberculosis endemic region? Mycoses 2019, 62, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, M.; Tarai, B.; Jena, P.P. Serodiagnosis in mycology. In Systemic Fungal Infections: Principles, Pathogenesis & Practice; Mahajan, M.E., Ed.; Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi, India, 2019; pp. 32–43. [Google Scholar]
- O’Dowd, T.; McHugh, J.; Wengenack, N.; Theel, E.S.; Vergidis, P. Performance characteristics of diagnostic assays in blastomycosis. Open Forum Infect. Dis. 2020, 7, S424. [Google Scholar] [CrossRef]
- Dizon, D.; Mitchell, M.; Dizon, B.; Libke, R.; Peterson, M.W. The utility of real-time polymerase chain reaction in detecting Coccidioides immitis among clinical specimens in the Central California San Joaquín Valley. Med. Mycol. 2019, 57, 688–693. [Google Scholar] [CrossRef]
- Inácio, M.M.; Milhomem Cruz-Leite, V.R.; Moreira, A.L.E.; Mattos, K.; Paccez, J.D.; Hernandez Ruiz, O.; Venturini, J.; de Souza Carvalho Melhem, M.; Paniago, A.M.M.; Soares, C.M.d.A.; et al. Challenges in serologic diagnostics of neglected human systemic mycoses: An overview on characterization of new targets. Pathogens 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J. Antigen detection, serology, and molecular diagnosis of invasive mycoses in the immunocompromised host. Transpl. Infect. Dis. 2006, 8, 128–139. [Google Scholar] [CrossRef]
- Khan, S.; Saubolle, M.A.; Oubsuntia, T.; Heidari, A.; Barbian, K.; Goodin, K.; Eguchi, M.; McCotter, O.Z.; Komatsu, K.; Park, B.J.; et al. Interlaboratory agreement of coccidioidomycosis enzyme immunoassay from two different manufacturers. Med. Mycol. 2019, 57, 441–446. [Google Scholar] [CrossRef]
- Valero, C.; Martín-Gómez, M.T.; Buitrago, M.J. Molecular diagnosis of endemic mycoses. JoF 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.S.; Govender, N.P.; Corcoran, C.; Dlamini, S.; Prozesky, H.; Burton, R.; Mendelson, M.; Taljaard, J.; Lehloenya, R.; Calligaro, G.; et al. Clinical characteristics, diagnosis, management, and outcomes of disseminated emmonsiosis: A retrospective case series. Clin. Infect. Dis. 2015, 61, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Rossow, J.A.; Queiroz-Telles, F.; Cáceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Ferreira Gremião, I.D.; Pereira, S.A. A One Health approach to combatting Sporothrix brasiliensis: Narrative review of an emerging zoonotic fungal pathogen in South America. JoF 2020, 6, 247. [Google Scholar] [CrossRef]
- Maphanga, T.G.; Naicker, S.D.; Gómez, B.L.; Mhlanga, M.; Mpembe, R.S.; Schwartz, I.S.; Bamford, C.; Nel, J.; Govender, N.P. Cross-reactivity of a Histoplasma capsulatum antigen enzyme immunoassay in urine specimens from persons with emergomycosis in South Africa. Med. Mycol. 2021, 59, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veer, J.; Lewis, R.J.; Emtiazjoo, A.M.; Allen, S.D.; Wheat, L.J.; Hage, C.A. Cross-reactivity in the Platelia™ Aspergillus enzyme immunoassay caused by blastomycosis. Med. Mycol. 2012, 50, 396–398. [Google Scholar] [CrossRef]
- Walsh, T.J.; McCarthy, M.W. The expanding use of matrix-assisted laser desorption/ionization-time of flight mass spectroscopy in the diagnosis of patients with mycotic diseases. Expert Rev. Mol. Diagn. 2019, 19, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. A moldy application of MALDI: MALDI-ToF mass spectrometry for fungal identification. JoF 2019, 5, 4. [Google Scholar] [CrossRef]
- Sanitá Lima, M.; Coutinho de Lucas, R.; Lima, N.; Teixeira de Moraes Polizeli, M.L.; Santos, C. Fungal community ecology using MALDI-TOF MS demands curated mass spectral databases. Front. Microbiol. 2019, 10, 315. [Google Scholar] [CrossRef]
- Gupta, A.; Agarwal, J.; Singh, V.; Das, A.; Sen, M.; Agarwal, J.; Singh, V. Matrix-Assisted Laser Desorption Ionization Time of Flight (MALDI-TOF) as an indispensable tool in diagnostic bacteriology: A comparative analysis with conventional technique. Cureus 2023, 15, e36984. [Google Scholar] [CrossRef]
- Vlek, A.; Kolecka, A.; Khayhan, K.; Theelen, B.; Groenewald, M.; Boel, E.; Group, M.S.; Boekhout, T. Interlaboratory comparison of sample preparation methods, database expansions, and cutoff values for identification of yeasts by matrix-assisted laser desorption ionization–time of flight mass spectrometry using a yeast test panel. J. Clin. Microbiol. 2014, 52, 3023–3029. [Google Scholar] [CrossRef]
- Rychert, J.; Slechta, E.S.; Barker, A.P.; Miranda, E.; Babady, N.E.; Tang, Y.-W.; Gibas, C.; Wiederhold, N.; Sutton, D.; Hanson, K.E. Multicenter evaluation of the Vitek MS v3. 0 system for the identification of filamentous fungi. J. Clin. Microbiol. 2018, 56, e01353-17. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Lam, C.S.K.; Ngan, A.H.Y.; Chow, W.-N.; Wu, A.K.L.; Tsang, D.N.C.; Tse, C.W.S.; Que, T.-L.; Tang, B.S.F.; Woo, P.C.Y. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for rapid identification of mold and yeast cultures of Penicillium marneffei. BMC Microbiol. 2016, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Liu, Y.-H.; Teng, S.-H.; Liao, C.-H.; Hung, C.-C.; Sheng, W.-H.; Teng, L.-J.; Hsueh, P.-R. Evaluation of the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry Bruker Biotyper for identification of Penicillium marneffei, Paecilomyces species, Fusarium solani, Rhizopus species, and Pseudallescheria boydii. Front. Microbiol. 2015, 6, 679. [Google Scholar] [CrossRef] [PubMed]
- Nobrega de Almeida, J., Jr.; Del Negro, G.M.; Grenfell, R.C.; Vidal, M.S.; Thomaz, D.Y.; De Figueiredo, D.S.; Bagagli, E.; Juliano, L.; Benard, G. Matrix-assisted laser desorption ionization–time of flight mass spectrometry for differentiation of the dimorphic fungal species Paracoccidioides brasiliensis and Paracoccidioides lutzii. J. Clin. Microbiol. 2015, 53, 1383–1386. [Google Scholar] [CrossRef]
- Valero, C.; Buitrago, M.J.; Gago, S.; Quiles-Melero, I.; García-Rodríguez, J. A matrix-assisted laser desorption/ionization time of flight mass spectrometry reference database for the identification of Histoplasma capsulatum. Med. Mycol. 2018, 56, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Mullis, K.; Faloona, F.; Scharf, S.; Saiki, R.; Horn, G.; Erlich, H. Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harbor Symp. Quant. Biol. 1986, 51, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. Current challenges in the diagnosis of fungal infections. Hum. Fungal Pathog. Identif. Methods Protoc. 2017, 1508, 3–15. [Google Scholar]
- Chamberlain, J.S.; Gibbs, R.A.; Rainer, J.E.; Nguyen, P.N.; Thomas, C. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988, 16, 11141–11156. [Google Scholar] [CrossRef]
- Rappolee, D.A.; Mark, D.; Banda, M.J.; Werb, Z. Wound macrophages express TGF-α and other growth factors in vivo: Analysis by mRNA phenotyping. Science 1988, 241, 708–712. [Google Scholar] [CrossRef]
- Holland, P.M.; Abramson, R.D.; Watson, R.; Gelfand, D.H. Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 1991, 88, 7276–7280. [Google Scholar] [CrossRef]
- Babady, N.E.; Buckwalter, S.P.; Hall, L.; Le Febre, K.M.; Binnicker, M.J.; Wengenack, N.L. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J. Clin. Microbiol. 2011, 49, 3204–3208. [Google Scholar] [CrossRef]
- Bialek, R.; Ibricevic, A.; Aepinus, C.; Najvar, L.K.; Fothergill, A.W.; Knobloch, J.; Graybill, J.R. Detection of Paracoccidioides brasiliensis in tissue samples by a nested PCR assay. J. Clin. Microbiol. 2000, 38, 2940–2942. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Silva, F.; Gomes, L.I.; Gracielle-Melo, C.; Goes, A.M.; Caligiorne, R.B. Real time polymerase chain reaction (rt-PCR): A new patent to diagnostic purposes for paracoccidioidomycosis. Recent Pat. Endocr. Metab. Immune Drug Discov. 2016, 10, 143–149. [Google Scholar] [CrossRef]
- Alanio, A.; Gits-Muselli, M.; Lanternier, F.; Sturny-Leclère, A.; Benazra, M.; Hamane, S.; Rodrigues, A.M.; Garcia-Hermoso, D.; Lortholary, O.; Dromer, F.; et al. Evaluation of a new Histoplasma spp. quantitative RT-PCR assay. J. Mol. Diagn. 2021, 23, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Najafzadeh, M.J.; Dolatabadi, S.; Saradeghi Keisari, M.; Naseri, A.; Feng, P.; de Hoog, G.S. Detection and identification of opportunistic Exophiala species using the rolling circle amplification of ribosomal internal transcribed spacers. J. Microbiol. Methods 2013, 94, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal-Barcoding-Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Faria Luiz, R.L.; Caldas Menezes, R.; Pereira, S.A.; de Vasconcellos Carvalhaes de Oliveira, R.; Marques-Evangelista de Oliveira, M. Nested PCR for the diagnosis of feline sporotrichosis from formalin-fixed and paraffin-embedded samples using different DNA extraction protocols. Front. Vet. Sci. 2022, 8, 755897. [Google Scholar]
- Chaturvedi, S.; Victor, T.R.; Marathe, A.; Sidamonidze, K.; Crucillo, K.L.; Chaturvedi, V. Real-time PCR assay for detection and differentiation of Coccidioides immitis and Coccidioides posadasii from culture and clinical specimens. PLoS Negl. Trop. Dis. 2021, 15, e0009765. [Google Scholar] [CrossRef]
- Bialek, R.; Fischer, J.; Feucht, A.; Najvar, L.K.; Dietz, K.; Knobloch, J.; Graybill, J.R. Diagnosis and monitoring of murine histoplasmosis by a nested PCR assay. J. Clin. Microbiol. 2001, 39, 1506–1509. [Google Scholar] [CrossRef]
- Vanittanakom, N.; Vanittanakom, P.; Hay, R.J. Rapid identification of Penicillium marneffei by PCR-based detection of specific sequences on the rRNA gene. J. Clin. Microbiol. 2002, 40, 1739–1742. [Google Scholar] [CrossRef]
- Bialek, R.; Feucht, A.; Aepinus, C.; Just-Nübling, G.; Robertson, V.J.; Knobloch, J.; Hohle, R. Evaluation of two nested PCR assays for detection of Histoplasma capsulatum DNA in human tissue. J. Clin. Microbiol. 2002, 40, 1644–1647. [Google Scholar] [CrossRef]
- Bialek, R.; Cascante Cirera, A.; Herrmann, T.; Aepinus, C.; Shearn-Bochsler, V.I.; Legendre, A.M. Nested PCR assays for detection of Blastomyces dermatitidis DNA in paraffin-embedded canine tissue. J. Clin. Microbiol. 2003, 41, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Martagon-Villamil, J.; Shrestha, N.; Sholtis, M.; Isada, C.M.; Hall, G.S.; Bryne, T.; Lodge, B.A.; Reller, L.B.; Procop, G.W. Identification of Histoplasma capsulatum from culture extracts by real-time PCR. J. Clin. Microbiol. 2003, 41, 1295–1298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, S.; Chung, W.-H.; Hung, S.-I.; Ho, H.-C.; Wang, Z.-W.; Chen, C.-H.; Lu, S.-C.; Kuo, T.-T.; Hong, H.-S. Detection of Sporothrix schenckii in clinical samples by a nested PCR assay. J. Clin. Microbiol. 2003, 41, 1414–1418. [Google Scholar] [CrossRef]
- Bialek, R.; Kern, J.; Herrmann, T.; Tijerina, R.; Ceceñas, L.; Reischl, U.; González, G.M. PCR assays for identification of Coccidioides posadasii based on the nucleotide sequence of the antigen 2/proline-rich antigen. J. Clin. Microbiol. 2004, 42, 778–783. [Google Scholar] [CrossRef]
- Buitrago, M.J.; Berenguer, J.; Mellado, E.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Detection of imported histoplasmosis in serum of HIV-infected patients using a real-time PCR-based assay. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J.; Buckwalter, S.P.; Eisberner, J.J.; Stewart, R.A.; McCullough, A.E.; Wohlfiel, S.L.; Wengenack, N.L. Detection of Coccidioides species in clinical specimens by real-time PCR. J. Clin. Microbiol. 2007, 45, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, M.J.; Merino, P.; Puente, S.; Gomez-Lopez, A.; Arribi, A.; Zancopé-Oliveira, R.M.; Gutierrez, M.C.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Utility of real-time PCR for the detection of Paracoccidioides brasiliensis DNA in the diagnosis of imported paracoccidioidomycosis. Med. Mycol. 2009, 47, 879–882. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pornprasert, S.; Praparattanapan, J.; Khamwan, C.; Pawichai, S.; Pimsarn, P.; Samleerat, T.; Leechanachai, P.; Supparatpinyo, K. Development of TaqMan real-time polymerase chain reaction for the detection and identification of Penicillium marneffei. Mycoses 2009, 52, 487–492. [Google Scholar] [CrossRef]
- Dot, J.-M.; Debourgogne, A.; Champigneulle, J.; Salles, Y.; Brizion, M.; Puyhardy, J.; Collomb, J.; Plénat, F.; Machouart, M. Molecular diagnosis of disseminated adiaspiromycosis due to Emmonsia crescens. J. Clin. Microbiol. 2009, 47, 1269–1273. [Google Scholar] [CrossRef]
- Gago, S.; Esteban, C.; Valero, C.; Zaragoza, Ó.; Puig de la Bellacasa, J.; Buitrago, M.J. A multiplex real-time PCR assay for identification of Pneumocystis jirovecii, Histoplasma capsulatum, and Cryptococcus neoformans/Cryptococcus gattii in samples from AIDS patients with opportunistic pneumonia. J. Clin. Microbiol. 2014, 52, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Gago, S.; Buitrago, M.J.; Clemons, K.V.; Cuenca-Estrella, M.; Mirels, L.F.; Stevens, D.A. Development and validation of a quantitative real-time PCR assay for the early diagnosis of coccidioidomycosis. Diagn. Microbiol. Infect. Dis. 2014, 79, 214–221. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; Pires de Camargo, Z. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef]
- Cronjé, N.; Schwartz, I.S.; Retief, L.; Bastos, A.D.S.; Matthee, S.; Preiser, W.; Bennett, N.C.; Maphanga, T.; Govender, N.P.; Colebunders, R.; et al. Attempted molecular detection of the thermally dimorphic human fungal pathogen Emergomyces africanus in terrestrial small mammals in South Africa. Med. Mycol. 2018, 56, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.S.; McLoud, J.D.; Berman, D.; Botha, A.; Lerm, B.; Colebunders, R.; Levetin, E.; Kenyon, C. Molecular detection of airborne Emergomyces africanus, a thermally dimorphic fungal pathogen, in Cape Town, South Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Li, R.; Gong, J.; Zhao, F. Fast diagnosis of sporotrichosis caused by Sporothrix globosa, Sporothrix schenckii, and Sporothrix brasiliensis based on multiplex real-time PCR. PLoS Negl. Trop. Dis. 2019, 13, e0007219. [Google Scholar] [CrossRef]
- Kaplan, M.; Zhu, Y.; Kus, J.V.; McTaggart, L.; Chaturvedi, V.; Chaturvedi, S. Development of a duplex real-time PCR assay for the differentiation of Blastomyces dermatitidis and Blastomyces gilchristii and a retrospective analysis of culture and primary specimens from blastomycosis cases from New York (2005 to 2019). J. Clin. Microbiol. 2021, 59, e02078-20. [Google Scholar] [CrossRef]
- Buitrago, M.J.; Canteros, C.E.; Frías-De-León, M.G.; González, Á.; Marques-Evangelista de Oliveira, M.; Muñoz, C.O.; Ramirez, J.A.; Toranzo, A.I.; Zancopé-Oliveira, R.; Cuenca-Estrella, M. Comparison of PCR protocols for detecting Histoplasma capsulatum DNA through a multicenter study. Rev. Iberoamer. Micol. 2013, 30, 256–260. [Google Scholar] [CrossRef]
- Wilmes, D.; Hagen, F.; Verissimo, C.; Alanio, A.; Rickerts, V.; Buitrago, M.J. A multicentre external quality assessment: A first step to standardise PCR protocols for the diagnosis of histoplasmosis and coccidioidomycosis. Mycoses 2023, 66, 774–786. [Google Scholar] [CrossRef]
- Rocha-Silva, F.; de Figueiredo, S.M.; La Santrer, E.F.R.; Machado, A.S.; Fernandes, B.; Assunção, C.B.; Góes, A.M.; Caligiorne, R.B. Paracoccidioidomycosis: Detection of Paracoccidioides brasiliensis’ genome in biological samples by quantitative chain reaction polymerase (qPCR). Microb. Pathog. 2018, 121, 359–362. [Google Scholar] [CrossRef]
- Ahmed, S.A.; van de Sande, W.W.; Desnos-Ollivier, M.; Fahal, A.H.; Mhmoud, N.A.; de Hoog, G.S. Application of isothermal amplification techniques for identification of Madurella mycetomatis, the prevalent agent of human mycetoma. J. Clin. Microbiol. 2015, 53, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- da Silva Zatti, M.; Domingos Arantes, T.; Cordeiro Theodoro, R. Isothermal nucleic acid amplification techniques for detection and identification of pathogenic fungi: A review. Mycoses 2020, 63, 1006–1020. [Google Scholar] [CrossRef]
- Endo, S.; Komori, T.; Ricci, G.; Sano, A.; Yokoyama, K.; Ohori, A.; Kamei, K.; Franco, M.; Miyaji, M.; Kazuko, N. Detection of gp43 of Paracoccidioides brasiliensis by the loop-mediated isothermal amplification (LAMP) method. FEMS Microbiol. Lett. 2004, 234, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Tatibana, B.T.; Sano, A.; Uno, J.; Kamei, K.; Igarashi, T.; Mikami, Y.; Miyaji, M.; Nishimura, K.; Itano, E. Detection of Paracoccidioides brasiliensis gp43 gene in sputa by loop-mediated isothermal amplification method. J. Clin. Lab. Anal. 2009, 23, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Zeng, H.; Xie, Z.; Lu, C.; Xi, L.; de Hoog, G.S. Development and evaluation of loop-mediated isothermal amplification (LAMP) for the rapid diagnosis of Penicillium marneffei in archived tissue samples. FEMS Immunol. Med. Microbiol. 2010, 58, 381–388. [Google Scholar] [CrossRef]
- Scheel, C.M.; Zhou, Y.; Theodoro, R.C.; Abrams, B.; Balajee, S.A.; Litvintseva, A.P. Development of a loop-mediated isothermal amplification method for detection of Histoplasma capsulatum DNA in clinical samples. J. Clin. Microbiol. 2014, 52, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Najafzadeh, M.J.; de Hoog, G.S.; Pires de Camargo, Z. Rapid identification of emerging human-pathogenic Sporothrix species with rolling circle amplification. Front. Microbiol. 2015, 6, 1385. [Google Scholar] [CrossRef]
- Furuie, J.L.; Sun, J.; do Nascimento, M.M.F.; Gomes, R.R.; Waculicz-Andrade, C.E.; Sessegolo, G.C.; Rodrigues, A.M.; Galvão-Dias, M.A.; Pires de Camargo, Z.; Queiroz-Telles, F.; et al. Molecular identification of Histoplasma capsulatum using rolling circle amplification. Mycoses 2016, 59, 12–19. [Google Scholar] [CrossRef]
- Carvajal, D.L.M. Desenvolvimento de Método Diagnóstico Através do Desenho de Sondas para Identificação de Paracoccidioides Lutzii, Utilizando a Técnica de Loop-Mediated Isothermal Amplification (LAMP) [Recurso Eletrônico]; Faculdade de Ciências Médicas, Universidade Estadual de Campinas: Campinas, Brazil, 2018; p. 61. [Google Scholar]
- da Silva Zatti, M.; Domingos Arantes, T.; Lourenço Fernandes, J.A.; Baumgardt Bay, M.; Pipolo Milan, E.; Silva Naliato, G.F.; Cordeiro Theodoro, R. Loop-mediated isothermal amplification and nested PCR of the internal transcribed spacer (ITS) for Histoplasma capsulatum detection. PLoS Negl. Trop. Dis. 2019, 13, e0007692. [Google Scholar]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.L.; Wong, B.W.; Ma, E.H.; Chan, K.H.; Chow, L.M.; Abeyewickreme, W.; Tangpukdee, N.; Yuen, K.Y.; Guan, Y.; Looareesuwan, S.; et al. Sensitive and inexpensive molecular test for falciparum malaria: Detecting Plasmodium falciparum DNA directly from heat-treated blood by loop-mediated isothermal amplification. Clin. Chem. 2006, 52, 303–306. [Google Scholar] [CrossRef]
- Prattes, J.; Heldt, S.; Eigl, S.; Hoenigl, M. Point of care testing for the diagnosis of fungal infections: Are we there yet? Curr. Fungal Infect. Rep. 2016, 10, 43–50. [Google Scholar] [CrossRef]
- Wang, D.; Yu, J.; Wang, Y.; Zhang, M.; Li, P.; Liu, M.; Liu, Y. Development of a real-time loop-mediated isothermal amplification (LAMP) assay and visual LAMP assay for detection of African swine fever virus (ASFV). J. Virol. Methods 2020, 276, 113775. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.E.; Bartolone, S.N.; Tree, M.O.; Conway, M.J.; Rossignol, J.; Smith, C.P.; Chancellor, M.B. Rapid detection of Zika virus in urine samples and infected mosquitos by reverse transcription-loop-mediated isothermal amplification. Sci. Rep. 2018, 8, 3803. [Google Scholar] [CrossRef] [PubMed]
- Oloniniyi, O.K.; Kurosaki, Y.; Miyamoto, H.; Takada, A.; Yasuda, J. Rapid detection of all known ebolavirus species by reverse transcription-loop-mediated isothermal amplification (RT-LAMP). J. Virol. Methods 2017, 246, 8–14. [Google Scholar] [CrossRef]
- Beissner, M.; Phillips, R.O.; Battke, F.; Bauer, M.; Badziklou, K.; Sarfo, F.S.; Maman, I.; Rhomberg, A.; Piten, E.; Frimpong, M.; et al. Loop-mediated isothermal amplification for laboratory confirmation of Buruli ulcer disease–towards a point-of-care test. PLoS Negl. Trop. Dis. 2015, 9, e0004219. [Google Scholar] [CrossRef]
- Rivero, R.; Bisio, M.; Velázquez, E.B.; Esteva, M.I.; Scollo, K.; González, N.L.; Altcheh, J.; Ruiz, A.M. Rapid detection of Trypanosoma cruzi by colorimetric loop-mediated isothermal amplification (LAMP): A potential novel tool for the detection of congenital Chagas infection. Diagn. Microbiol. Infect. Dis. 2017, 89, 26–28. [Google Scholar] [CrossRef]
- Handa, D.; Gupta, M.; Lehl, S.S.; Gupta, A.; Singh, R. Utility of loop-mediated isothermal amplification as a point-of-care test in diagnosis of amoebic liver abscess. Trop. Doct. 2021, 51, 488–491. [Google Scholar] [CrossRef]
- Mthethwa, N.P.; Amoah, I.D.; Reddy, P.; Bux, F.; Kumari, S. Fluorescence and colorimetric LAMP-based real-time detection of human pathogenic Cryptosporidium spp. from environmental samples. Acta Trop. 2022, 235, 106606. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Febrer-Sendra, B.; Crego-Vicente, B.; Muro, A. Loop-mediated isothermal amplification in schistosomiasis. J. Clin. Med. 2021, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Soto, P.; Celis-Giraldo, C.T.; Collar-Fernández, C.; Gorgojo, Ó.; Camargo, M.; Muñoz, J.; Salas-Coronas, J.; Patarroyo, M.A.; Muro, A. Strong-LAMP assay based on a Strongyloides spp.-derived partial sequence in the 18S rRNA as potential biomarker for Strongyloidiasis diagnosis in human urine samples. Dis. Markers 2020, 2020, 5265198. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M. LAMP method as one of the best candidates for replacing with PCR method. Malays. J. Med. Sci. 2018, 25, 121–123. [Google Scholar] [CrossRef]
- Rolando, J.C.; Jue, E.; Barlow, J.T.; Ismagilov, R.F. Real-time kinetics and high-resolution melt curves in single-molecule digital LAMP to differentiate and study specific and non-specific amplification. Nucleic Acids Res. 2020, 48, e42. [Google Scholar] [CrossRef]
- Meagher, R.J.; Priye, A.; Light, Y.K.; Huang, C.; Wang, E. Impact of primer dimers and self-amplifying hairpins on reverse transcription loop-mediated isothermal amplification detection of viral RNA. Analyst 2018, 143, 1924–1933. [Google Scholar] [CrossRef]
- Sano, A.; Itano, E.N. Applications of loop-mediated isothermal amplificaton methods (LAMP) for identification and diagnosis of mycotic diseases: Paracoccidioidomycosis and Ochroconis gallopava infection. In Molecular Identification of Fungi; Gherbawy, Y., Voigt, K., Eds.; Springer: Heidelberg, Germany, 2010; pp. 417–437. [Google Scholar]
- Varona, M.; Anderson, J.L. Visual detection of single-nucleotide polymorphisms using molecular beacon loop-mediated isothermal amplification with centrifuge-free DNA extraction. Anal. Chem. 2019, 91, 6991–6995. [Google Scholar] [CrossRef]
- Wang, D.-G.; Brewster, J.D.; Paul, M.; Tomasula, P.M. Two methods for increased specificity and sensitivity in loop-mediated isothermal amplification. Molecules 2015, 20, 6048–6059. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, B.; Guan, Y. Pullulan reduces the non-specific amplification of loop-mediated isothermal amplification (LAMP). Anal. Bioanal. Chem. 2019, 411, 1211–1218. [Google Scholar] [CrossRef]
- Ku, J.; Chauhan, K.; Hwang, S.-H.; Jeong, Y.-J.; Kim, D.-E. Enhanced specificity in loop-mediated isothermal amplification with poly(ethylene glycol)-engrafted graphene oxide for detection of viral genes. Biosensors 2022, 12, 661. [Google Scholar] [CrossRef]
- Luo, G.; Yi, T.; Wang, Q.; Guo, B.; Fang, L.; Zhang, G.; Guo, X. Stem-loop-primer assisted isothermal amplification enabling high-specific and ultrasensitive nucleic acid detection. Biosensors Bioelectron. 2021, 184, 113239. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.Q. Rolling replication of short DNA circles. Proc. Natl. Acad. Sci. USA 1995, 92, 4641–4645. [Google Scholar] [CrossRef] [PubMed]
- Demidov, V.V. Rolling-circle amplification in DNA diagnostics: The power of simplicity. Expert Rev. Mol. Diagn. 2002, 2, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, Y.; Lv, X.; Deng, Y.; Li, X. Recent advances in cascade isothermal amplification techniques for ultra-sensitive nucleic acid detection. Talanta 2023, 260, 124645. [Google Scholar] [CrossRef]
- Najafzadeh, M.J.; Sun, J.; Vicente, V.A.; de Hoog, G.S. Rapid identification of fungal pathogens by rolling circle amplification using Fonsecaea as a model. Mycoses 2011, 54, e577–e582. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M. The Fungi: 1, 2, 3… 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Van Dyke, M.C.C.; Thompson, G.R.; Galgiani, J.N.; Barker, B.M. The rise of Coccidioides: Forces against the dust devil unleashed. Front. Immunol. 2019, 10, 2188. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).