Exploring the Diversity and Ecological Dynamics of Palm Leaf Spotting Fungi—A Case Study on Ornamental Palms in Portugal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Examination
2.2. Fungal Isolation
2.3. Morphological Observation and Characterisation and Fungal Genera Identification
2.4. DNA Extraction and Genomic Fingerprinting
2.5. Genomic Discrimination and Molecular Identification of Fungal Genera
2.6. Biodiversity and Ecological Data Analysis and Statistical Inference
2.6.1. Population Status and Structural Diversity Measures
2.6.2. Accumulation Curves and Abundance Distributions
2.6.3. Exploratory Analysis of the Assembly of Fungal Communities
Statistical Analyses of Composition and Diversity of Fungal Assemblages
Principal Component Analyses and Hierarchical Cluster Analyses of Fungal Assemblages
3. Results
3.1. Disease Symptoms and Foliar Lesion Types
3.2. Overall Structural Diversity
3.2.1. Composition of Mitosporic Fungi and Taxa Abundance Distributions
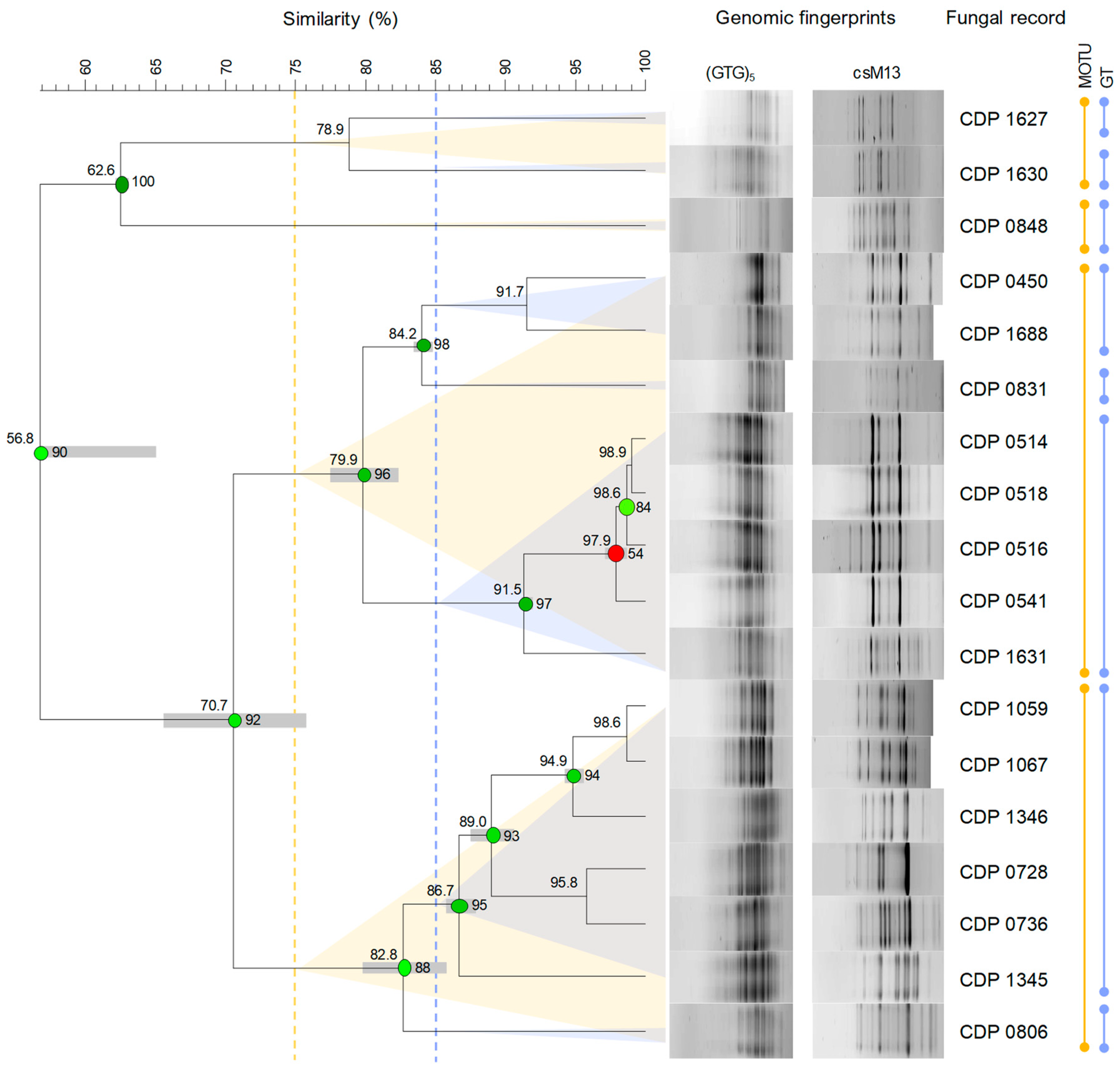
3.2.2. Taxonomic Structure and Genetic Diversity
3.3. Fungal Assemblages and Biodiversity Analyses
3.3.1. Fungal Assemblages and Host Species
Quantitative and Qualitative Diversity
Assessment of the Sampling Process
3.3.2. Fungal Assemblages and Foliar Lesion Types
Number of Taxa in a Single Foliar Lesion
Taxa Co-Occurrence
4. Discussion
4.1. Biodiversity and Ecological Dynamics of Palm Leaf Spotting Fungal Communities
4.1.1. Ecological Significance of Dominant Taxa and Functional Implications in Disease Development
4.1.2. Ecological Significance of Uncommon Taxa and Impact of Knowledge Gaps in Palm Health
4.2. Taxonomic Structure of Palm Leaf Spotting Fungal Communities
4.2.1. Differences in the Typical Mycota of Tropical Palm Fungi
4.2.2. Coelomycetous Taxa as Components of Temperate Palm Fungal Assemblages
4.3. Host-Related Patterns in Communities of Palm Leaf Spotting Fungi
4.4. Foliar Lesions as Hyperdiverse Microhabitats with Co-Occurrence Patterns
Co-Occurrence in Palm Leaf Spotting Fungal Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Branco, S. Fungal diversity—An overview. In The Dynamical Processes of Biodiversity: Case Studies of Evolution and Spatial Distribution; Grillo, O., Venora, G., Eds.; InTech: London, UK, 2011; pp. 211–226. [Google Scholar] [CrossRef]
- Branco, S. Fungal diversity from communities to genes. Fungal Biol. Rev. 2019, 33, 225–237. [Google Scholar] [CrossRef]
- Devkota, S.; Fang, W.; Arunachalam, K.; Phyo, K.M.M.; Shakya, B. Systematic review of fungi, their diversity and role in ecosystem services from the Far Eastern Himalayan Landscape (FHL). Heliyon 2023, 9, e12756. [Google Scholar] [CrossRef]
- Hickman, K.J.E.; Atherton, K.F. Fungi are the future: Realizing the potential of fungi in policy. MIT Sci. Policy Rev. 2024, 5, 5–19. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, FUNK-0052-2016. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Hawksworth, D.L. Formal description of sequence-based voucherless Fungi: Promises and pitfalls, and how to resolve them. IMA Fungus 2018, 9, 143–165. [Google Scholar] [CrossRef]
- Cheek, M.; Lughadha, E.N.; Kirk, P.; Lindon, H.; Carretero, J.; Looney, B.; Douglas, B.; Haelewaters, D.; Gaya, E.; Llewellyn, T. New scientific discoveries: Plants and fungi. Plants People Planet 2020, 2, 371–388. [Google Scholar] [CrossRef]
- Tang, A.M.C.; Shenoy, B.D.; Hyde, K.D. Fungal diversity. In Reconstructing the Tree of Life: Taxonomy and Systematics of Species Rich Taxa; Hodkinson, T.R., Parnell, J.A.N., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 221–249. [Google Scholar]
- Hyde, K.D.; Bussaban, B.; Paulus, B.; Crous, P.W.; Lee, S.; McKenzie, E.H.C.; Photita, W.; Lumyong, S. Diversity of saprobic microfungi. Biodivers. Conserv. 2007, 16, 7–35. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2014, 29, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, T.; Lücking, R.; Dahlberg, A.; Gaya, E.; Suz, L.M.; Mikryukov, V.; Liimatainen, K.; Druzhinina, I.; Westrip, J.R.S.; Mueller, G.M.; et al. Pushing the frontiers of biodiversity research: Unveiling the global diversity, distribution, and conservation of fungi. Annu. Rev. Environ. Resour. 2023, 48, 149–176. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2015, 40, 182–207. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Netherway, T. Fungi as mediators linking organisms and ecosystems. FEMS Microbiol. Rev. 2022, 46, fuab058. [Google Scholar] [CrossRef] [PubMed]
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.Ș.; Vrâncianu, C.O.; Șesan, T.E. Current insights in fungal importance—A comprehensive review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Priyashantha, A.K.H.; Dai, D.-Q.; Bhat, D.J.; Stephenson, S.L.; Promputtha, I.; Kaushik, P.; Tibpromma, S.; Karunarathna, S.C. Plant-fungi interactions: Where it goes? Biology 2023, 12, 809. [Google Scholar] [CrossRef]
- Dransfield, J.; Uhl, N.W.; Asmussen, C.B.; Baker, W.J.; Harley, M.M.; Lewis, C.E. Genera Palmarum. The Evolution and Classification of the Palms; Royal Botanic Gardens: London, UK, 2008. [Google Scholar] [CrossRef]
- Johnson, D.V. Tropical Palms—2010 Revision. Non-Wood Forest Products No. 10/Rev.1; FAO: Rome, Italy, 2011; Available online: https://www.fao.org/4/i1971e/i1971e00.htm (accessed on 15 October 2024).
- Solanki, K.S.; Patel, G.D.; Sindha, M.; Chaudhari, V.M.; Shivaswamy, C. Palm: Versatile group of plant material for landscape gardening. Int. J. Adv. Biochem. Res. 2023, 7, 538–544. [Google Scholar] [CrossRef]
- Azam, M.; Qadri, R.; Akram, M.T.; Ejaz, S. Cultivation and growth constraints of ornamental palms. In Etiology and Integrated Management of Economically Important Fungal Diseases of Ornamental Palms; Ul Haq, I., Ijaz, S., Eds.; Sustainability in Plant and Crop Protection No. 16; Springer: Cham, Switzerland, 2020; pp. 73–81. [Google Scholar] [CrossRef]
- Ijaz, S.; Babar, M. Ornamental palms: Molecular taxonomy, ecology and distribution. In Etiology and Integrated Management of Economically Important Fungal Diseases of Ornamental Palms; Ul Haq, I., Ijaz, S., Eds.; Sustainability in Plant and Crop Protection No. 16; Springer: Cham, Switzerland, 2020; pp. 41–72. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Mustapic, L.; Ivic, D.; Delbianco, A. Characterisation of palms and ornamentals in the EU: A tool for crop-based surveys of Union quarantine pests. EFSA Support. Publ. 2024, 21, 8818E. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Wu, Q.; Lu, Y.; Shi, J. A review of plant leaf fungal diseases and its environment speciation. Bioengineered 2019, 10, 409–424. [Google Scholar] [CrossRef]
- Beaury, E.M.; Allen, J.M.; Evans, A.E.; Fertakos, M.E.; Pfadenhauer, W.G.; Bradley, B.A. Horticulture could facilitate invasive plant range infilling and range expansion with climate change. BioScience 2023, 73, 635–642. [Google Scholar] [CrossRef]
- Elliott, M.L.; Broschat, T.K.; Uchida, J.Y.; Simone, G.W. (Eds.) Compendium of Ornamental Palm Diseases and Disorders; American Phytopathological Society (APS) Press: Saint Paul, MN, USA, 2004. [Google Scholar]
- Broschat, T.K.; Elliott, M.L.; Hodel, D.R. Ornamental palms: Biology and horticulture. In Horticultural Reviews, Volume 42; Janick, J., Ed.; Wiley Blackwell: Hoboken, NJ, USA, 2014; pp. 1–120. [Google Scholar] [CrossRef]
- Ul Haq, I.; Ijaz, S.; Shakeel, Q.; Li, G.; Yang, L.; Rashid, I. Fungi: Cynosure of ornamental palms diseases. In Etiology and Integrated Management of Economically Important Fungal Diseases of Ornamental Palms; Ul Haq, I., Ijaz, S., Eds.; Sustainability in Plant and Crop Protection No. 16; Springer: Cham, Switzerland, 2020; pp. 85–113. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D. Biodiversity of palm fungi in the tropics: Are global fungal diversity estimates realistic? Biodivers. Conserv. 1999, 8, 977–1004. [Google Scholar] [CrossRef]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. The biogeographical distribution of microfungi associated with three palm species from tropical and temperate habitats. J. Biogeogr. 2000, 27, 297–310. [Google Scholar] [CrossRef]
- Elliott, M.L. Fusarium Wilt of Queen Palm and Mexican Fan Palm [Fact Sheet]. Publication #PP278. 2017. Available online: https://edis.ifas.ufl.edu/pp278 (accessed on 15 October 2024).
- Elliott, M.L. Ganoderma Butt Rot of Palms [Fact Sheet]. Publication #PP54. 2018. Available online: https://edis.ifas.ufl.edu/publication/pp100 (accessed on 15 October 2024).
- Elliott, M.L. Bud Rot of Palm [Fact Sheet]. Publication #PP-220. 2018. Available online: https://edis.ifas.ufl.edu/publication/PP144 (accessed on 15 October 2024).
- Elliott, M.L. Thielaviopsis Trunk Rot of Palm [Fact Sheet]. Publication #PP-219. 2018. Available online: https://edis.ifas.ufl.edu/publication/PP143 (accessed on 15 October 2024).
- Elliott, M.L. Fusarium Wilt of Canary Island Date Palm [Fact Sheet]. Publication #PP-215. 2019. Available online: https://edis.ifas.ufl.edu/publication/pp139 (accessed on 15 October 2024).
- Ayika, M.-G.; Rosano, A.; Valiente, J.; Chakrabarti, S.; Rollins, J.A.; Dhillon, B. Characterizing the palm pathogenic Thielaviopsis species from Florida. J. Fungi 2024, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Cannon, P.F. Fungi causing tar spots on palms. Mycol. Pap. 1999, 175, 1–114. [Google Scholar]
- Piepenbring, M.; Nold, F.; Trampe, T.; Kirschner, R. Revision of the genus Graphiola (Exobasidiales, Basidiomycota). Nova Hedwig. 2012, 94, 67–96. [Google Scholar] [CrossRef]
- Elliott, M.L. Petiole (Rachis) Blight of Palm [Fact Sheet]. Publication #PP-221. 2018. Available online: https://edis.ifas.ufl.edu/publication/PP145 (accessed on 15 October 2024).
- Elliott, M.L. Graphiola Leaf Spot (False Smut) of Palm [Fact Sheet]. Publication #PP-216. 2018. Available online: https://edis.ifas.ufl.edu/publication/PP140 (accessed on 15 October 2024).
- Elliott, M.L. Leaf Spots and Leaf Blights of Palm [Fact Sheet]. Publication #PP-218. 2018. Available online: https://edis.ifas.ufl.edu/publication/PP142 (accessed on 15 October 2024).
- Elliott, M.L. Pestalotiopsis (Pestalotia) Diseases of Palm [Fact Sheet]. Publication #PP-217. 2018. Available online: https://edis.ifas.ufl.edu/publication/PP141 (accessed on 15 October 2024).
- Yu, J.; Elliott, M.L. Calonectria (Cylindrocladium) Leaf Spot of Palm [Fact Sheet]. Publication #PP-302. 2019. Available online: https://edis.ifas.ufl.edu/publication/PP302 (accessed on 15 October 2024).
- Forsberg, L.I. Foliar diseases of nursery-grown ornamental palms in Queensland. Australas. Plant Pathol. 1985, 14, 67–71. [Google Scholar] [CrossRef]
- Pereira, D.S.; Phillips, A.J.L. Palm fungi and their key role in biodiversity surveys: A review. J. Fungi 2023, 9, 1121. [Google Scholar] [CrossRef]
- Chase, A.R.; Broschat, T.K. (Eds.) Diseases and Disorders of Ornamental Palms; American Phytopathological Society (APS) Press: Saint Paul, MN, USA, 1991. [Google Scholar]
- Hyde, K.D.; Philemon, E. Capitorostrum cocoes sp. nov., causing leaf spot of Cocos nucifera. Mycotaxon 1991, 42, 95–97. [Google Scholar]
- Hyde, K.D.; Philemon, E. Some disease-associated microorganisms on plants in the Western Province of Papua New Guinea. Australas. Plant Pathol. 1994, 23, 69–76. [Google Scholar] [CrossRef]
- Hyde, K.D.; Alcorn, J.L. Some disease-associated microorganisms on plants of Cape York Peninsula and Torres Strait islands. Australas. Plant Pathol. 1993, 22, 73–83. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D. New Oxydothis species associated with palm leaf spots in north Queensland, Australia. Mycol. Res. 1994, 98, 213–218. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D. Guignardia candeloflamma sp. nov. causing leaf spots of Pinanga spp. Mycol. Res. 1995, 99, 110–112. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D. Astrosphaeriella fronsicola sp. nov. associated with leaf spots of Oraniopsis and other palms. Mycol. Res. 1995, 99, 453–456. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D. Maculatipalma fronsicola gen. et sp. nov. causing leaf spots on palm species in north Queensland with descriptions of related genera: Apioplagiostoma and Plagiostoma. Mycol. Res. 1995, 99, 727–734. [Google Scholar] [CrossRef]
- Hyde, K.D.; Fröhlich, J. Mycosphaerella palmicola associated with leaf spots of Cocos nucifera in Australia, Irian Jaya and Papua New Guinea. Mycol. Res. 1995, 99, 704–706. [Google Scholar] [CrossRef]
- Hyde, K.D.; Stanley, S.J.; Steinke, T.D. Fungi associated with leaf spots of palms: Maculatifrondis aequatoriensis gen. et sp. nov., with a Cyclodomus anamorph, and Myelosperma parasitica sp. nov. Mycol. Res. 1996, 100, 1509–1514. [Google Scholar] [CrossRef]
- Shivas, R.G.; Alcorn, J.L. A checklist of plant pathogenic and other microfungi in the rainforests of the wet tropics of northern Queensland. Australas. Plant Pathol. 1996, 25, 158–173. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D.; Guest, D.I. Fungi associated with leaf spots of palms in north Queensland, Australia. Mycol. Res. 1997, 101, 721–732. [Google Scholar] [CrossRef]
- Yanna; Ho, W.H.; Goh, T.K.; Hyde, K.D. A new species of Everhartia associated with leaf spots of Phoenix hanceana from Hong Kong. Bot. J. Linn. Soc. 2000, 134, 465–470. [Google Scholar] [CrossRef]
- To-anun, C.; Nguenhom, J.; Meeboon, J.; Hidayat, I. Two fungi associated with necrotic leaflets of areca palms (Areca catechu). Mycol. Prog. 2009, 8, 115–121. [Google Scholar] [CrossRef]
- Wulandari, N.F.; To-Anun, C.; McKenzie, E.H.C.; Hyde, K.D. Guignardia bispora and G. ellipsoidea spp. nov. and other Guignardia species from palms (Arecaceae). Mycosphere 2011, 2, 115–128. [Google Scholar]
- Forsberg, L.I. Diseases of ornamental palms. Queensl. Agric. J. 1987, 113, 279–286. [Google Scholar]
- Polizzi, G.; Vitale, A. First report of leaf spot and blight caused by Botrytis cinerea on pygmy date palm in Italy. Plant Dis. 2003, 87, 1398. [Google Scholar] [CrossRef]
- Suwannarach, N.; Sujarit, K.; Kumla, J.; Bussaban, B.; Lumyong, S. First report of leaf spot disease on oil palm caused by Pestalotiopsis theae in Thailand. J. Gen. Plant Pathol. 2013, 79, 277–279. [Google Scholar] [CrossRef]
- Oliveira, R.R.; Aguiar, R.L.; Tessmann, D.J.; Nunes, W.M.C.; Santos, A.F.; Vida, J.B. First report of leaf spot caused by Cladosporium perangustum on Syagrus oleracea in Brazil. Plant Dis. 2014, 98, 280. [Google Scholar] [CrossRef] [PubMed]
- Kumla, J.; Suwannarach, N.; Lumyong, S. First report of Phoma leaf spot disease on cherry palm caused by Phoma herbarum in Thailand. Can. J. Plant Pathol. 2016, 38, 103–106. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J.; Gené, J.; Guarro, J.; Baseia, I.G.; García, D.; Gusmão, L.F.P.; Souza-Motta, C.M.; et al. Fungal Planet description sheets: 716–784. Persoonia 2018, 40, 240–393. [Google Scholar] [CrossRef]
- Mukhtar, I.; Quan, X.; Khokhar, I.; Chou, T.; Huang, Q.; Jiang, S.; Yan, J.; Chen, B.; Huang, R.; Ashraf, H.J.; et al. First report of leaf spot on Caryota mitis (fishtail palm) caused by Neodeightonia palmicola in China. Plant Dis. 2019, 103, 2675–2676. [Google Scholar] [CrossRef]
- Nasehi, A.; Sathyapriya, H.; Wong, M.Y. First report of leaf spot on oil palm caused by Phyllosticta capitalensis in Malaysia. Plant Dis. 2020, 104, 288. [Google Scholar] [CrossRef]
- Pereira, D.S.; Phillips, A.J.L. A new leaf spot disease of Chamaerops humilis caused by Palmeiromyces chamaeropicola gen. et sp. nov. Phytopathol. Mediterr. 2020, 59, 353–363. [Google Scholar] [CrossRef]
- Pereira, D.S.; Phillips, A.J.L. Two new Morinia species from palms (Arecaceae) in Portugal. Mycol. Prog. 2021, 20, 83–94. [Google Scholar] [CrossRef]
- Alam, M.W.; Rehman, A.; Ahmad, S.; Sarwar, M.; Nawaz, A.; Khan, S.M.; Ali, S.; Aslam, S.; Mannan, A. First report of Nigrospora sphaerica causing leaf spot of date palm in Pakistan. J. Plant Pathol. 2020, 102, 223. [Google Scholar] [CrossRef]
- Yang, C.; Xu, X.; Zeng, Q.; Lv, Y.; Liu, S.; Liu, Y.; Qiao, T.; Han, S. First report of Neovaginatispora fuckelii causing leaf spot on Phoenix canariensis. Plant Dis. 2021, 105, 223. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, X.; Zeng, Q.; Lv, Y.; Deng, Y.; Liu, Y. First report of leaf blight caused by Cladosporium perangustum on Livistona chinensis in China. Plant Dis. 2021, 105, 223. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, M.; Chen, G.; Sun, J.; Zhang, W.; Cui, J.; Wang, Y.; Gong, D.; Hu, M. First report of Colletotrichum siamense causing leaf spot on silver date palm in China. Crop Prot. 2024, 180, 106646. [Google Scholar] [CrossRef]
- Lekete, E.; Osekre, E.A.; Andoh-Mensah, E. First report of Curvularia pseudobrachyspora causing leaf spots disease on coconut (Cocos nucifera L.) seedlings in Ghana. Am. J. Plant Sci. 2022, 13, 972–983. [Google Scholar] [CrossRef]
- Douanla-Meli, C.; Scharnhorst, A. Palm Foliage as Pathways of Pathogenic Botryosphaeriaceae Fungi and Host of New Lasiodiplodia Species from Mexico. Pathogens 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.P.; Caetano, M.F.; Rocha, M.; Belchior, S.; Lima, A. Doenças e pragas que condicionam o uso de palmeiras em espaços verdes. Rev. Assoc. Port. Hortic. 2013, 112, 37–40. Available online: http://hdl.handle.net/10400.5/10216 (accessed on 15 October 2024).
- Ramos, A.P.; Rocha, M.; Belchior, S.; Peixoto, R.; Caetano, M.F.; Lima, A. Micobiota associada a adultos do escaravelho das palmeiras (Rhynchophorus ferrugineus) provenientes de Cascais, Portugal. Rev. Ciências Agrár. 2015, 38, 220–229. [Google Scholar] [CrossRef]
- da Ponte, N.H.T.; dos Santos, A.V.F.; Pinho, R.C. Ocorrência das principais doenças e pragas que causam danos a palmeiras ornamentais. Rev. Soc. Cient. 2024, 7, 1647–1658. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project; 2024. Available online: http://qgis.osgeo.org (accessed on 15 July 2024).
- Choi, Y.W.; Hyde, K.D.; Ho, W.H. Single Spore Isolation of Fungi. Fungal Divers. 1999, 3, 29–38. [Google Scholar]
- Zhang, K.; Sun, Y.-Y.; Cai, L. An optimized protocol of single spore isolation for fungi. Cryptogam. Mycol. 2013, 34, 349–356. [Google Scholar] [CrossRef]
- Schulz, B.; Guske, S.; Dammann, U.; Boyle, C. Endophyte–Host Interactions. II. Defining Symbiosis of the Endophyte–Host Interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Chomnunti, P.; Hongsanan, S.; Aguirre-Hudson, B.; Tian, Q.; Peršoh, D.; Dhami, M.K.; Alias, S.A.; Xu, J.; Liu, X.; Stadler, M.; et al. The Sooty Moulds. Fungal Divers. 2014, 66, 1–36. [Google Scholar] [CrossRef]
- Sutton, B.C. The Coelomycetes: Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute (CMI): London, UK, 1980. [Google Scholar]
- Seifert, K.A.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS Biodiversity Series No. 9; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2011. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Wanasinghe, D.N.; Papizadeh, M.; Goonasekara, I.D.; Camporesi, E.; Bhat, D.J.; McKenzie, E.H.C.; Phillips, A.J.L.; Diederich, P.; et al. Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers. 2016, 77, 1–316. [Google Scholar] [CrossRef]
- Li, W.-J.; McKenzie, E.H.C.; Liu, J.-K.; Bhat, D.J.; Dai, D.-Q.; Camporesi, E.; Tian, Q.; Maharachchikumbura, S.S.N.; Luo, Z.-L.; Shang, Q.-J.; et al. Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Divers. 2020, 100, 279–801. [Google Scholar] [CrossRef]
- Pitcher, D.G.; Saunders, N.A.; Owen, R.J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 1989, 8, 151–156. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Meyer, W.; Koch, A.; Niemann, C.; Beyermann, B.; Epplen, J.T.; Börner, T. Differentiation of species and strains among filamentous fungi by DNA fingerprinting. Curr. Genet. 1991, 19, 239–242. [Google Scholar] [CrossRef]
- Meyer, W.; Lieckfeldt, E.; Kuhls, K.; Freedman, E.Z.; Börner, T.; Mitchell, T.G. DNA- and PCR-fingerprinting in fungi. In DNA Fingerprinting: State of the Science; Pena, S.D.J., Chakraborty, R., Epplen, J.T., Jeffreys, A.J., Eds.; Birkhäuser: Basel, Switzerland, 1993; pp. 311–320. [Google Scholar] [CrossRef]
- Soll, D.R. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 2000, 13, 332–370. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Cullen, D.; Kurtzman, C.P.; Khachatourians, G.G.; Hegedus, D.D. Molecular Methods for Discriminating Taxa, Monitoring Species, and Assessing Fungal Diversity. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 77–102. [Google Scholar] [CrossRef]
- Weising, K.; Atkinson, R.G.; Gardner, R.C. Genomic Fingerprinting by Microsatellite-Primed PCR: A Critical Evaluation. PCR Methods Appl. 1995, 4, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.M.; Scolnik, P.A. Direct Amplifications from Microsatellites: Detection of Simple Sequence Repeat-Based Polymorphisms without Cloning. In DNA Markers: Protocols, Applications and Overviews; Caetano-Anollés, G., Gresshoff, P.M., Eds.; Wiley: New York, NY, USA, 1997; pp. 133–150. [Google Scholar]
- Olive, D.M.; Bean, P. Principles and Applications of Methods for DNA-Based Typing of Microbial Organisms. J. Clin. Microbiol. 1999, 37, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Phillips, A.J.L.; Henriques, I.; Correia, A. Rapid differentiation of species of Botryosphaeriaceae by PCR fingerprinting. Res. Microbiol. 2007, 158, 112–121. [Google Scholar] [CrossRef]
- Mohali, S.; Slippers, B.; Wingfield, M.J. Identification of Botryosphaeriaceae from Eucalyptus, Acacia, and Pinus in Venezuela. Fungal Divers. 2007, 25, 103–125. [Google Scholar]
- Palencia, E.R.; Klich, M.A.; Glenn, A.E.; Bacon, C.W. Use of a rep-PCR system to predict species in the Aspergillus section Nigri. J. Microbiol. Methods 2009, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Redondo, C.; Cubero, J.; Melgarejo, P. Characterization of Penicillium species by ribosomal DNA sequencing and BOX, ERIC and REP-PCR analysis. Mycopathologia 2009, 168, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Piškur, B.; Pavlic, D.; Slippers, B.; Ogris, N.; Maresi, G.; Wingfield, M.J.; Jurc, D. Diversity and pathogenicity of Botryosphaeriaceae on declining Ostrya carpinifolia in Slovenia and Italy following extreme weather conditions. Eur. J. For. Res. 2011, 130, 235–249. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Zolfaghari, S. Efficiency of rep-PCR fingerprinting as a useful technique for molecular typing of plant pathogenic fungal species: Botryosphaeriaceae species as a case study. FEMS Microbiol. Lett. 2014, 361, 144–157. [Google Scholar] [CrossRef]
- Ramírez-Castrillón, M.; Mendes, S.D.C.; Inostroza-Ponta, M.; Valente, P. (GTG)5 MSP-PCR Fingerprinting as a Technique for Discrimination of Wine Associated Yeasts? PLoS ONE 2014, 9, e105870. [Google Scholar] [CrossRef]
- Vassart, G.; Georges, M.; Monsieur, R.; Brocas, H.; Lequarre, A.S.; Christophe, D. A Sequence in M13 Phage Detects Hypervariable Minisatellites in Human and Animal DNA. Science 1987, 235, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Ryskov, A.P.; Jincharadze, A.G.; Prosnyak, M.I.; Ivanov, P.L.; Limborska, S.A. M13 phage DNA as a universal marker for DNA fingerprinting of animals, plants and microorganisms. FEBS Lett. 1988, 233, 388–392. [Google Scholar] [CrossRef]
- Walmsley, R.M.; Wilkinson, B.M.; Kong, T.H. Genetic fingerprinting for yeasts. Nat. Biotechnol. 1989, 7, 1168–1170. [Google Scholar] [CrossRef]
- Lieckfeldt, E.; Meyer, W.; Börner, T. Rapid identification and differentiation of yeasts by DNA and PCR fingerprinting. J. Basic Microbiol. 1993, 33, 413–426. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Johnson, R. The Influence on Numerical Taxonomic Similarities of Errors in Microbiological Tests. J. Gen. Microbiol. 1972, 72, 377–392. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- O’Donnell, K. Fusarium and Its Near Relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Aime, M.C.; Robbertse, B.; Miller, A.N.; Ariyawansa, H.A.; Aoki, T.; Cardinali, G.; Crous, P.W.; Druzhinina, I.S.; Geiser, D.M.; et al. Unambiguous identification of fungi: Where do we stand and how accurate and precise is fungal DNA barcoding? IMA Fungus 2020, 11, 14. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 July 2024).
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R. Fungal Biodiversity Patterns. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 59–75. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R.E. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Ludwig, J.A.; Reynolds, J.F. Statistical Ecology: A Primer on Methods and Computing; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Taylor, L.R. Bates, Williams, Hutchinson—A Variety of Diversities. In Diversity of Insect Faunas; Mound, L.A., Waloff, N., Eds.; Symposia of the Royal Entomological Society of London No. 9; Blackwell Scientific Publications: Oxford, UK, 1978; pp. 1–18. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Menhinick, E.F. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 1964, 45, 859–861. [Google Scholar] [CrossRef]
- McGill, B.J.; Etienne, R.S.; Gray, J.S.; Alonso, D.; Anderson, M.J.; Benecha, H.K.; Dornelas, M.; Enquist, B.J.; Green, J.L.; He, F.; et al. Species abundance distributions: Moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007, 10, 995–1015. [Google Scholar] [CrossRef]
- Matthews, T.; Whittaker, R.J. Fitting and comparing competing models of the species abundance distribution: Assessment and prospect. Front. Biogeogr. 2014, 6, 67–82. [Google Scholar] [CrossRef]
- Baldridge, E.; Harris, D.J.; Xiao, X.; White, E.P. An extensive comparison of species-abundance distribution models. PeerJ 2016, 4, e2823. [Google Scholar] [CrossRef]
- MacArthur, R.H. On the relative abundance of bird species. Proc. Natl. Acad. Sci. USA 1957, 43, 293–295. [Google Scholar] [CrossRef]
- Motomura, I. A statistical treatment of associations. Jpn. J. Zool. 1932, 44, 379–383. [Google Scholar]
- Preston, F.W. The commonness, and rarity, of species. Ecology 1948, 29, 254–283. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Monographs in Population Biology No. 32; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Volkov, I.; Banavar, J.R.; Hubbell, S.P.; Maritan, A. Neutral theory and relative species abundance in ecology. Nature 2003, 424, 1035–1037. [Google Scholar] [CrossRef]
- McGill, B.J. Species abundance distributions. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 105–122. [Google Scholar]
- Prado, P.I.; Miranda, M.D.; Chalom, A. sads: Maximum Likelihood Models for Species Abundance Distributions, R Package Version 0.6.3. 2024. Available online: https://CRAN.R-project.org/package=sads (accessed on 15 July 2024).
- McGill, B.J. Strong and weak tests of macroecological theory. Oikos 2003, 102, 679–685. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Connolly, S.R.; Dornelas, M. Fitting and Empirical Evaluation of Models for Species Abundance Distributions. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 123–140. [Google Scholar]
- Schmit, J.P.; Lodge, D.J. Classical Methods and Modern Analysis for Studying Fungal Diversity. In The Fungal Community: Its Organization and Role in the Ecosystem, 3rd ed.; Dighton, J., White, J.F., Oudemans, P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 193–214. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar] [CrossRef]
- Burnham, K.P.; Overton, W.S. Estimation of the Size of a Closed Population When Capture Probabilities Vary Among Animals. Biometrika 1978, 65, 625–633. [Google Scholar] [CrossRef]
- Burnham, K.P.; Overton, W.S. Robust Estimation of Population Size When Capture Probabilities Vary Among Animals. Ecology 1979, 60, 927–936. [Google Scholar] [CrossRef]
- Heltshe, J.F.; Forrester, N.E. Estimating Species Richness Using the Jackknife Procedure. Biometrics 1983, 39, 1–11. [Google Scholar] [CrossRef]
- Smith, E.P.; van Belle, G. Nonparametric Estimation of Species Richness. Biometrics 1984, 40, 119–129. [Google Scholar] [CrossRef]
- Chao, A.; Lee, S.-M. Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Chao, A.; Ma, M.-C.; Yang, M.C.K. Stopping rules and estimation for recapture debugging and unequal failure rates. Biometrika 1993, 80, 193–201. [Google Scholar] [CrossRef]
- Lee, S.-M.; Chao, A. Estimating population size via sample coverage for closed capture-recapture models. Biometrics 1994, 50, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2011; pp. 39–54. [Google Scholar]
- Ratkowsky, D.A. Nonlinear Regression Modeling: A Unified Practical Approach; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Ratkowsky, D.A. Handbook of Nonlinear Regression Models; Statistics: Textbooks and Monographs; Marcel Dekker: New York, NY, USA, 1990; Volume 107. [Google Scholar]
- Lomolino, M.V. Ecology’s most general, yet protean pattern: The species–area relationship. J. Biogeogr. 2000, 27, 17–26. [Google Scholar] [CrossRef]
- Michaelis, L.; Menten, M.L. Die Kinetik der Invertinwirkung. Biochem. Z. 1913, 49, 333–369. [Google Scholar]
- Weibull, W. A statistical distribution function of wide applicability. J. Appl. Mech. 1951, 18, 293–296. [Google Scholar] [CrossRef]
- Tjørve, E. Shapes and functions of species–area curves: A review of possible models. J. Biogeogr. 2003, 30, 827–835. [Google Scholar] [CrossRef]
- Tjørve, E. Shapes and functions of species–area curves (II): A review of new models and parameterizations. J. Biogeogr. 2009, 36, 1435–1445. [Google Scholar] [CrossRef]
- Dengler, J. Which function describes the species–area relationship best? A review and empirical evaluation. J. Biogeogr. 2009, 36, 728–744. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H. Species Richness: Estimation and Comparison. In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package, R Package Version 2.6–6.1. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 July 2024).
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C. SpadeR: Species-Richness Prediction and Diversity Estimation with R, R Package Version 0.1.1. 2016. Available online: https://CRAN.R-project.org/package=SpadeR (accessed on 15 July 2024).
- Wang, J. SPECIES: Statistical Package for Species Richness Estimation, R Package Version 1.2.0. 2024. Available online: https://CRAN.R-project.org/package=SPECIES (accessed on 15 July 2024).
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Gao, C.-H.; Chen, C.; Akyol, T.; Dușa, A.; Yu, G.; Cao, B.; Cai, P. ggVennDiagram: Intuitive Venn diagram software extended. iMeta 2024, 3, e177. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 15 July 2024).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions, R Package Version 2.1.4. 2022. Available online: https://CRAN.R-project.org/package=cluster (accessed on 15 July 2024).
- Agrios, G.N. Plant Pathology, 5th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Varanda, C.M.R.; Materatski, P.; Landum, M.; Campos, M.D.; Félix, M.d.R. Fungal Communities Associated with Peacock and Cercospora Leaf Spots in Olive. Plants 2019, 8, 169. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Meneses-Sánchez, M.d.l.C.; Martínez-Contreras, R.D.; Martínez-Montiel, N. Unraveling the Fungal Community Associated with Leaf Spot on Crataegus sp. Microorganisms 2020, 8, 459. [Google Scholar] [CrossRef]
- Tao, J.; Cao, P.; Xiao, Y.; Wang, Z.; Huang, Z.; Jin, J.; Liu, Y.; Yin, H.; Liu, T.; Zhou, Z. Distribution of the Potential Pathogenic Alternaria on Plant Leaves Determines Foliar Fungal Communities around the Disease Spot. Environ. Res. 2021, 200, 111715. [Google Scholar] [CrossRef]
- Marčiulynas, A.; Marčiulynienė, D.; Lynikienė, J.; Bakys, R.; Menkis, A. Fungal Communities in Leaves and Roots of Healthy-Looking and Diseased Ulmus glabra. Microorganisms 2022, 10, 2228. [Google Scholar] [CrossRef] [PubMed]
- Mekapogu, M.; Jung, J.A.; Kwon, O.K.; Ahn, M.S.; Song, H.Y.; Jang, S. Recent Progress in Enhancing Fungal Disease Resistance in Ornamental Plants. Int. J. Mol. Sci. 2021, 22, 7956. [Google Scholar] [CrossRef] [PubMed]
- Dewdney, A.K. A dynamical model of communities and a new species-abundance distribution. Biol. Bull. 2000, 198, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, E.; Crawford, J.W.; Bown, J.L.; Squire, G. Towards a General Theory of Biodiversity. Nature 2001, 410, 923–926. [Google Scholar] [CrossRef]
- Magurran, A.E.; Henderson, P.A. Explaining the Excess of Rare Species in Natural Species Abundance Distributions. Nature 2003, 422, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Neubert, K.; Mendgen, K.; Brinkmann, H.; Wirsel, S.G.R. Only a Few Fungal Species Dominate Highly Diverse Mycofloras Associated with the Common Reed. Appl. Environ. Microbiol. 2006, 72, 1118–1128. [Google Scholar] [CrossRef]
- van Schalkwyk, J.; Pryke, J.S.; Samways, M.J. Contribution of Common vs. Rare Species to Species Diversity Patterns in Conservation Corridors. Ecol. Indic. 2019, 104, 279–288. [Google Scholar] [CrossRef]
- Fröhlich, J.; Hyde, K.D.; Petrini, O. Endophytic Fungi Associated with Palms. Mycol. Res. 2000, 104, 1202–1212. [Google Scholar] [CrossRef]
- Pinnoi, A.; Lumyong, S.; Hyde, K.D.; Jones, E.B.G. Biodiversity of fungi on the palm Eleiodoxa conferta in Sirindhorn peat swamp forest, Narathiwat, Thailand. Fungal Divers. 2006, 22, 205–218. [Google Scholar]
- Pinnoi, A.; Phongpalchit, S.; Hyde, K.D.; Jones, E.B.G. Biodiversity of fungi on Calamus (Palmae) in Thailand. Cryptogam. Mycol. 2009, 30, 181–190. [Google Scholar]
- Pinruan, U.; Hyde, K.D.; Lumyong, S.; McKenzie, E.H.C.; Jones, E.B.G. Occurrence of fungi on tissues of the peat swamp palm Licuala longicalycata. Fungal Divers. 2007, 25, 157–173. [Google Scholar]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. Contribution of rarity and commonness to patterns of species richness. Ecol. Lett. 2004, 7, 81–87. [Google Scholar] [CrossRef]
- Heino, J.; Soininen, J. Are common species sufficient in describing turnover in aquatic metacommunities along environmental and spatial gradients? Limnol. Oceanogr. 2010, 55, 2397–2402. [Google Scholar] [CrossRef]
- Alahuhta, J.; Johnson, L.B.; Olker, J.; Heino, J. Species sorting determines variation in the community composition of common and rare macrophytes at various spatial extents. Ecol. Complex. 2014, 20, 61–68. [Google Scholar] [CrossRef]
- Avolio, M.L.; Forrestel, E.J.; Chang, C.C.; La Pierre, K.J.; Burghardt, K.T.; Smith, M.D. Demystifying dominant species. New Phytol. 2019, 223, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Lennon, J.J.; Beale, C.M.; Reid, C.L.; Kent, M.; Pakeman, R.J. Are richness patterns of common and rare species equally well explained by environmental variables? Ecography 2011, 34, 529–539. [Google Scholar] [CrossRef]
- Pearman, P.B.; Weber, D. Common species determine species richness patterns in biodiversity indicator taxa. Biol. Conserv. 2007, 138, 109–119. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Tzanopoulos, J.; Kallimanis, A.S.; Matsinos, Y.G.; Sgardelis, S.P.; Pantis, J.D. The contribution of common and rare species to plant species richness patterns: The effect of habitat type and size of sampling unit. Biodivers. Conserv. 2008, 17, 3567–3577. [Google Scholar] [CrossRef]
- Pérez-Quesada, A.; Brazeiro, A. Contribution of rarity and commonness to patterns of species richness in biogeographic transition regions: Woody plants of Uruguay. Austral Ecol. 2013, 38, 639–645. [Google Scholar] [CrossRef]
- Tsang, T.P.N.; Bonebrake, T.C. Contrasting roles of environmental and spatial processes for common and rare urban butterfly species compositions. Landsc. Ecol. 2017, 32, 47–57. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Graves, G.R. Null Models in Ecology; Smithsonian Institution Press: Washington, DC, USA, 1996. [Google Scholar]
- May, R.M. Patterns of Species Abundance and Diversity. In Ecology and Evolution of Communities; Cody, M.L., Diamond, J.M., Eds.; Harvard University Press: Cambridge, MA, USA, 1975; pp. 81–120. [Google Scholar]
- García-Laviña, C.X.; Bettucci, L.; Tiscornia, S. Fungal Communities Associated with Eugenia uruguayensis (Myrtaceae) Leaf Litter in Early Stages of Decomposition in Uruguay. Sydowia 2016, 68, 139–150. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Mir, Z.A. Plant Immunity: At the Crossroads of Pathogen Perception and Defense Response. Plants 2024, 13, 1434. [Google Scholar] [CrossRef]
- Persiani, A.M.; Maggi, O. Species-Abundance Distribution Patterns of Soil Fungi: Contribution to the Ecological Understanding of Their Response to Experimental Fire in Mediterranean Maquis (Southern Italy). Mycologia 2013, 105, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Horst, R.K. Westcott’s Plant Disease Handbook, 8th ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of Phytopathogenic Fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Iturrieta-González, I.; García, D.; Gené, J.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Quaedvlieg, W.; Schumacher, R.K.; et al. Genera of Phytopathogenic Fungi: GOPHY 3. Stud. Mycol. 2019, 94, 1–124. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.L. Alternaria Leaf Spot Caused by Alternaria Species: An Emerging Problem on Ornamental Plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef]
- Alasadi, R.M.S. Alternaria alternata: The Most Common Pathogen on Date Palm. Stud. Fungi 2024, 9, e012. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Lumyong, S. First Report of Alternaria Leaf Blight Disease on Oil Palm Caused by Alternaria longipes in Thailand. Phytoparasitica 2015, 43, 57–59. [Google Scholar] [CrossRef]
- Robles-Yerena, L.; Ayala-Escobar, V.; Leyva-Mir, S.G.; Lima, N.B.; Camacho-Tapia, M.; Tovar-Pedraza, J.M. First Report of Cladosporium cladosporioides Causing Leaf Spot on Tomato in Mexico. J. Plant Pathol. 2019, 101, 759. [Google Scholar] [CrossRef]
- Basavand, E.; Babaeizad, V.; Mirhosseini, H.A.; Niri, M.D. Occurrence of Leaf Spot Disease Caused by Phoma herbarum on Oregano in Iran. J. Plant Pathol. 2020, 102, 575–576. [Google Scholar] [CrossRef]
- Atik, O. Phoma Diseases: Identification, Epidemiology, and Strategies for Management. In Phoma: Diversity, Taxonomy, Bioactivities, and Nanotechnology; Rai, M., Zimowska, B., Kövics, G.J., Eds.; Springer: Cham, Switzerland, 2022; pp. 121–134. [Google Scholar] [CrossRef]
- Batson, A.M.; Spawton, K.A.; Katz, R.; du Toit, L.J. Cladosporium Leaf Spot Caused by Cladosporium variabile in Winter High Tunnel Production of Spinach (Spinacia oleracea) in Maine, United States. Plant Dis. 2022, 106, 2260. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, G.; Chai, J.; Zeng, L.; Gong, W.; Ran, F.; Nie, X.; Zhang, R.; Lin, D. Occurrence of Cladosporium herbarum Causing Leaf Spot on Avena sativa in China. Crop Prot. 2024, 177, 106555. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Dijksterhuis, J.; Starink-Willemse, M.; Andersen, B.; Summerell, B.A.; Shin, H.-D.; Dugan, F.M.; Schroers, H.-J.; Braun, U.; et al. Species and Ecological Diversity within the Cladosporium cladosporioides Complex (Davidiellaceae, Capnodiales). Stud. Mycol. 2010, 67, 1–94. [Google Scholar] [CrossRef] [PubMed]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The Genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Anjum, M.A.; Haider, M.S.; Ali, M.; Mubashar, U.; Bashir, U.; Aslam, H.M.U.; Sajjad, M. Association of Cladosporium cladosporioides Brown Leaf Spot of Lady Palm in Pakistan. J. Anim. Plant Sci. 2020, 30, 371–376. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, P.; Panja, B.; Nath, P.S. First Report of Cladosporium cladosporioides as Causal Agent of Leaf Blight on Borassus flabellifer in India. J. Plant Pathol. 2020, 102, 937–938. [Google Scholar] [CrossRef]
- Xie, P.; Zhong, F.T.; Liu, Y.L. First Report of Phoma herbarum Causing Leaf Spot on Rhapis humilis in China. Plant Dis. 2021, 106, 767. [Google Scholar] [CrossRef] [PubMed]
- de Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Systematic Reappraisal of Species in Phoma Section Paraphoma, Pyrenochaeta, and Pleurophoma. Mycologia 2010, 102, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Restrepo, M.; Schumacher, R.K.; Wingfield, M.J.; Ishtiaq, A.; Cai, L.; Duong, T.A.; Edwards, J.; Gene, J.; Groenewald, J.Z.; Sana, J.; et al. Fungal Systematics and Evolution: FUSE 2. Sydowia 2016, 68, 193–230. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Jones, E.B.G.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal Diversity Notes 709–839: Taxonomic and Phylogenetic Contributions to Fungal Taxa with an Emphasis on Fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.B.J.; Maharachchikumbura, S.S.N.; Raspé, O.; Karunarathna, S.C.; Wanasinghe, D.N.; Hongsanan, S.; et al. Fungal Diversity Notes 929–1036: Taxonomic and Phylogenetic Contributions on Genera and Species of Fungal Taxa. Fungal Divers. 2019, 95, 1–273. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Thambugala, K.M.; Wanasinghe, D.N.; Gentekaki, E.; Promputtha, I.; Kuo, C.H.; Hyde, K.D. Additions to Phaeosphaeriaceae (Pleosporales): Elongaticollum gen. nov., Ophiosphaerella taiwanensis sp. nov., Phaeosphaeriopsis beaucarneae sp. nov. and a New Host Record of Neosetophoma poaceicola from Musaceae. MycoKeys 2020, 70, 59–88. [Google Scholar] [CrossRef]
- Zhang, S.N.; Hyde, K.D.; Jones, E.B.G.; Yu, X.-D.; Cheewangkoon, R.; Liu, J.-K. Current Insights into Palm Fungi with Emphasis on Taxonomy and Phylogeny. Fungal Divers. 2024, 127, 55–301. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J. Alternaria spp.: From General Saprophyte to Specific Parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Deb, D.; Khan, A.; Dey, N. Phoma Diseases: Epidemiology and Control. Plant Pathol. 2020, 69, 1203–1217. [Google Scholar] [CrossRef]
- Adigun, B.A.; Nahar, S.B.M.; Oyebamiji, Y.O.; Daba, T.M.; Sinumvayo, J.P.; Adegboyega, T.T.; Akinola, S.A.; Adebayo, I.A. Opportunistic Fungi, Plant, and Nematode Interactions in Agricultural Crops. In Opportunistic Fungi, Nematode and Plant Interactions; Akhtar, M.S., Ed.; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Affichard, M.; Jacquelin, M.; Khalil, T.; Andrivon, D.; Le May, C. Consideration of the Disease Complexes, the Missing Link to Correctly Analyze the Impact of Intercropping on Disease Development. Agronomy 2024, 14, 1210. [Google Scholar] [CrossRef]
- Madriz-Ordeñana, K.; Jørgensen, H.J.L.; Balan, A.; Sørensen, D.M.; Nielsen, K.L.; Thordal-Christensen, H. Prevalence of Soil-Borne Diseases in Kalanchoe blossfeldiana Reveals a Complex of Pathogenic and Opportunistic Fungi. Plant Dis. 2019, 103, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Escalante, M.; Damas, D.; Márquez, D.; Gelvez, W.; Chacón, H.; Díaz, A.; Moreno, B. Diagnóstico y Evaluación de Pestalotiopsis e Insectos Inductores, en Plantaciones de Palma Aceitera al Sur del Lago de Maracaibo, Venezuela. Bioagro 2010, 22, 211–216. Available online: https://ve.scielo.org/scielo.php?script=sci_arttext&pid=S1316-33612010000300006 (accessed on 15 October 2024).
- Martínez, L.C.; Plata-Rueda, A. Lepidoptera Vectors of Pestalotiopsis Fungal Disease: First Record in Oil Palm Plantations from Colombia. Int. J. Trop. Insect Sci. 2013, 33, 239–246. [Google Scholar] [CrossRef]
- Anuar, M.A.S.S.; Ali, N.S. Significant Oil Palm Diseases Impeding Global Industry: A Review. Sains Malays. 2022, 51, 707–721. [Google Scholar] [CrossRef]
- Mohamed-Azni, I.N.A.; Sritharan, K.; Ho, S.-H.; Roslan, N.D.; Arulandoo, X.; Sundram, S. Isolation, Identification and Pathogenicity of Fungi Associated with Leaf Blotches in Tenera × Tenera (TxT) Variety of Oil Palm in Malaysia. J. Plant Pathol. 2022, 104, 167–177. [Google Scholar] [CrossRef]
- Betancourt-Ortiz, W.F.; Medina-Cárdenas, H.C.; Padilla-Agudelo, J.L.; Varón, F.H.; Mestizo-Garzón, Y.A.; Morales-Rodríguez, A.; Sarria-Villa, G.A. Foliar Lesions Induced by Pestalotiopsis arengae in Oil Palm (O × G) in the Colombian Southwest Palm Zone. J. Fungi 2024, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host-Multi-Pathogen Warfare: Pathogen Interactions in Co-Infected Plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.; Kohn, L.M. Host-Synthesized Secondary Compounds Influence the In Vitro Interactions between Fungal Endophytes of Maize. Appl. Environ. Microbiol. 2008, 74, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, S.I.I.; Abo-Elyousr, K.A.M.; Abdel-Rahim, I.R. Leaf Surface and Endophytic Fungi Associated with Onion Leaves and Their Antagonistic Activity against Alternaria porri. Czech Mycol. 2015, 67, 1–22. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, S.; Manawasinghe, I.S.; Sun, D.; Song, J.; Xu, B. Taxonomic Revision of Neodidymelliopsis with N. cynanchi sp. nov., Associated with Cynanchum sibiricum Leaf Spot in Xinjiang, China. N. Z. J. Bot. 2023, 62, 151–164. [Google Scholar] [CrossRef]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and Nomenclature of the Genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Brahamanage, R.S.; Hyde, K.D.; Li, X.H.; Jayawardena, R.S.; McKenzie, E.H.C.; Yan, J.Y. Are Pathogenic Isolates of Stemphylium Host Specific and Cosmopolitan? Plant Pathol. Quar. 2018, 8, 153–164. [Google Scholar] [CrossRef]
- Shade, A.; Jones, S.E.; Caporaso, J.G.; Handelsman, J.; Knight, R.; Fierer, N.; Gilbert, J.A. Conditionally Rare Taxa Disproportionately Contribute to Temporal Changes in Microbial Diversity. mBio 2014, 5, e01371-14. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where Less May Be More: How the Rare Biosphere Pulls Ecosystems Strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Pascoal, F.; Costa, R.; Magalhães, C. The Microbial Rare Biosphere: Current Concepts, Methods and Ecological Principles. FEMS Microbiol. Ecol. 2021, 97, fiaa227. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Wingfield, M.J.; Akulov, A.; Carnegie, A.J.; Cheewangkoon, R.; Gramaje, D.; Groenewald, J.Z.; Guarnaccia, V.; Halleen, F.; et al. Genera of Phytopathogenic Fungi: GOPHY 2. Stud. Mycol. 2019, 92, 47–133. [Google Scholar] [CrossRef]
- Chen, Q.; Bakhshi, M.; Balci, Y.; Broders, K.D.; Cheewangkoon, R.; Chen, S.F.; Fan, X.L.; Gramaje, D.; Halleen, F.; Jung, M.H.; et al. Genera of Phytopathogenic Fungi: GOPHY 4. Stud. Mycol. 2022, 101, 417–564. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Rao, E.S.; Sandeepkumar, G.M.; Sriram, S. Stagonosporopsis cucurbitacearum, the Causal Agent of Gummy Stem Blight of Watermelon in India. Australas. Plant Dis. Notes 2020, 15, 7. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Huang, Y.H.; Manawasinghe, I.S.; Wanasinghe, D.N.; Liu, J.W.; Shu, Y.X.; Zhao, M.P.; Xiang, M.M.; Luo, M. Stagonosporopsis pogostemonis: A Novel Ascomycete Fungus Causing Leaf Spot and Stem Blight on Pogostemon cablin (Lamiaceae) in South China. Pathogens 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Bar, I.; Sambasivam, P.T.; Davidson, J.; Farfan-Caceres, L.M.; Lee, R.C.; Hobson, K.; Moore, K.; Ford, R. Current Population Structure and Pathogenicity Patterns of Ascochyta rabiei in Australia. Microb. Genom. 2021, 7, 000627. [Google Scholar] [CrossRef]
- Zou, J.; Dong, Y.; Wang, H.; Liang, W.; Li, D. Identification and Characterization of Nothophoma quercina Causing Bud Blight on Photinia × fraseri in China. Plant Dis. 2021, 105, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, G.K.; Nelson, A.C.; Williams, S.J.; Solomon, P.S.; Faris, J.D.; Friesen, T.L. The Necrotrophic Pathogen Parastagonospora nodorum Is a Master Manipulator of Wheat Defense. Mol. Plant-Microbe Interact. 2023, 36, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.; Crane, C.; Barrett, S.; Cano-Lira, J.F.; Le Roux, J.J.; Thangavel, R.; Guarro, J.; et al. Fungal Planet Description Sheets: 469–557. Persoonia 2016, 37, 218–403. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Begoude, B.A.D.; Boers, J.; Braun, U.; Declercq, B.; Dijksterhuis, J.; Elliott, T.F.; Garay-Rodriguez, G.A.; Jurjević, Ž.; Kruse, J.; et al. New and Interesting Fungi. 5. Fungal Syst. Evol. 2022, 10, 19–90. [Google Scholar] [CrossRef]
- Hyde, K.D.; Cai, L.; McKenzie, E.H.C.; Yang, Y.L.; Zhang, J.Z.; Prihastuti, H. Colletotrichum: A Catalogue of Confusion. Fungal Divers. 2009, 39, 1–17. [Google Scholar]
- Mehl, J.W.; Slippers, B.; Roux, J.; Wingfield, M.J. Overlap of Latent Pathogens in the Botryosphaeriaceae on a Native and Agricultural Host. Fungal Biol. 2017, 121, 405–419. [Google Scholar] [CrossRef]
- Cao, X.; Xu, X.; Che, H.; West, J.S.; Luo, D. Eight Colletotrichum Species, Including a Novel Species, Are Associated with Areca Palm Anthracnose in Hainan, China. Plant Dis. 2020, 104, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.D.; Patel, P.R.; Prajapati, V.P. First Report of Colletotrichum gloeosporioides Causing Leaf Spot Disease on Areca Palm (Chrysalidocarpus lutescens H. Wendl.) in India. Int. J. Econ. Plants 2019, 6, 147–149. [Google Scholar] [CrossRef]
- Pereira, D.S.; Phillips, A.J.L. Botryosphaeriaceae on Palms—A New Species of Neodeightonia, N. chamaeropicola, and New Records from Diseased Foliage of Ornamental Palms in Portugal. Phytotaxa 2023, 627, 1–64. [Google Scholar] [CrossRef]
- Lin, L.; Jing, X.; Lucas-Borja, M.E.; Shen, C.; Wang, Y.; Feng, W. Rare Taxa Drive the Response of Soil Fungal Guilds to Soil Salinization in the Taklamakan Desert. Front. Microbiol. 2022, 13, 862245. [Google Scholar] [CrossRef]
- Tang, L.; Xue, K.; Pang, Z.; Jiang, L.; Zhang, B.; Wang, W.; Wang, S.; Xu, Z.; Rui, Y.; Zhong, L.; et al. Plant Community Associates with Rare Rather than Abundant Fungal Taxa in Alpine Grassland Soils. Appl. Environ. Microbiol. 2023, 89, e01862-22. [Google Scholar] [CrossRef]
- Wang, H.; Tian, D.; Liu, H.; Wang, Z.; Yijun, H.; Lu, J.; Zhu, Y.; Wei, S.; Wang, H.; Wu, L.; et al. Rare Rather Than Abundant Taxa of Soil Bacteria and Fungi Regulate Soil Multifunctionality in Eucalyptus Plantations. Catena 2024, 245, 108303. [Google Scholar] [CrossRef]
- Nnadi, N.E.; Carter, D.A. Climate Change and the Emergence of Fungal Pathogens. PLoS Pathog. 2021, 17, e1009503. [Google Scholar] [CrossRef] [PubMed]
- Ristaino, J.B.; Anderson, P.K.; Bebber, D.P.; Brauman, K.A.; Cunniffe, N.J.; Fedoroff, N.V.; Finegold, C.; Garrett, K.A.; Gilligan, C.A.; Jones, C.M.; et al. The Persistent Threat of Emerging Plant Disease Pandemics to Global Food Security. Proc. Natl. Acad. Sci. USA 2021, 118, e2022239118, Erratum in Proc. Natl. Acad. Sci. USA 2021, 118, e2115792118. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Liang, J.J.; Li, K.Y.; Ling, X.F.; Yi, R.H. First Report of Brown Blotch Disease Caused by Diaporthe ueckeri on Hyophorbe lagenicaulis in China. Plant Dis. 2024, 108, 224. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.S.; Hilário, S.; Gonçalves, M.F.M.; Phillips, A.J.L. Diaporthe Species on Palms: Molecular Re-Assessment and Species Boundaries Delimitation in the D. arecae Species Complex. Microorganisms 2023, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.S.; Phillips, A.J.L. Diaporthe Species on Palms—Integrative Taxonomic Approach for Species Boundaries Delimitation in the Genus Diaporthe, with the Description of D. pygmaeae sp. nov. Stud. Mycol. 2024, 109, 487–594. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.; Hyde, K.D. Palm Microfungi; Fungal Diversity Research Series No. 3; Fungal Diversity Press: Hong Kong, China, 2000. [Google Scholar]
- Hyde, K.D.; Taylor, J.E.; Fröhlich, J. Genera of Ascomycetes from Palms; Fungal Diversity Research Series No. 2; Fungal Diversity Press: Hong Kong, China, 2000. [Google Scholar]
- Taylor, J.E.; Hyde, K.D. Microfungi of Tropical and Temperate Palms; Fungal Diversity Research Series No. 12; Fungal Diversity Press: Hong Kong, China, 2003. [Google Scholar]
- Khan, F.; Ismayil, M.S.; Ramesh, G.V.; Nayak, A.M.; Poonacha, T.T.; Tanuja, S.; Arti; Poojashree, M.S.; Palanna, K.B. Novel Report of Bipolaris heliconiae Causing Frog-Eye-Like Leaf Spot on Dypsis lutescens in Indian Sub-Continent. Microb. Pathog. 2024, 196, 106938. [Google Scholar] [CrossRef]
- Barbosa, T.J.A.; Feijó, M.G.L.; Silva, G.C.; Infante, N.B.; Feijó, F.M.; Assunção, I.P.; Lima, G.S.A. First Report of Neopestalotiopsis foedans Causing Pestalotia Spot in Leaf on Coconut in Brazil. Plant Dis. 2023, 10, 2552. [Google Scholar] [CrossRef] [PubMed]
- Guterres, D.C.; Silva, M.A.; Martins, M.D.; Azevedo, D.M.Q.; Lisboa, D.O.; Pinho, D.B.; Furtado, G.Q. Leaf Spot Caused by Neopestalotiopsis Species on Arecaceae in Brazil. Australas. Plant Pathol. 2023, 52, 47–62. [Google Scholar] [CrossRef]
- Xiong, Y.-R.; Manawasinghe, I.S.; Maharachchikumbura, S.S.N.; Lu, L.; Dong, Z.-Y.; Xiang, M.-M.; Xu, B. Pestalotioid Species Associated with Palm Species from Southern China. Curr. Res. Environ. Appl. Mycol. 2022, 12, 285–321. [Google Scholar] [CrossRef]
- Sarmiento-Chacón, M.; Hernández-García, V.; Rodríguez-Larramendi, L.A.; Salas-Marina, M.Á.; Ríos-Velasco, C. Neopestalotiopsis sp. and Colletotrichum karstii, Causal Agents of Leaf Spots on Camedor Palm (Chamaedorea quezalteca) in Mexico. Mex. J. Phytopathol. 2023, 41, 165–181. [Google Scholar] [CrossRef]
- Wikee, S.; Udayanga, D.; Crous, P.W.; Chukeatirote, E.; McKenzie, E.H.C.; Bahkali, A.H.; Dai, D.-Q.; Hyde, K.D. Phyllosticta—An Overview of Current Status of Species Recognition. Fungal Divers. 2011, 51, 43–61. [Google Scholar] [CrossRef]
- Lumyong, S.; Techa, W.; Lumyong, P.; McKenzie, E.H.C.; Hyde, K.D. Endophytic Fungi from Calamus kerrianus and Wallichia caryotoides (Arecaceae) at Doi Suthep-Pui National Park, Thailand. Chiang Mai J. Sci. 2009, 36, 158–167. [Google Scholar]
- Braun, U.; Freire, F.C.O. Some Cercosporoid Hyphomycetes from Brazil—II. Cryptogam. Mycol. 2002, 23, 295–328. [Google Scholar]
- Braun, U.; Hill, C.F.; Schubert, K. New Species and New Records of Biotrophic Micromycetes from Australia, Fiji, New Zealand and Thailand. Fungal Divers. 2006, 22, 13–35. [Google Scholar]
- Guatimosim, E.; Pinto, H.J.; Barreto, R.W. Passalora acrocomiae sp. nov. and Exosporium acrocomiae from the Palm Acrocomia aculeata in Puerto Rico. Mycotaxon 2013, 122, 61–67. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Jayawardena, R.S.; Bhunjun, C.S.; Harishchandra, D.L.; Hyde, K.D. Pseudocercospora dypsidis sp. nov. (Mycosphaerellaceae) on Dypsis lutescens Leaves in Thailand. Phytotaxa 2020, 474, 218–234. [Google Scholar] [CrossRef]
- Braun, U.; Crous, P.W.; Kamal. New Species of Pseudocercospora, Pseudocercosporella, Ramularia and Stenella (Cercosporoid Hyphomycetes). Mycol. Prog. 2003, 2, 197–208. [Google Scholar] [CrossRef]
- Braun, U.; Crous, P.W.; Nakashima, C. Cercosporoid Fungi (Mycosphaerellaceae) 2. Species on Monocots (Acoraceae to Xyridaceae, Excluding Poaceae). IMA Fungus 2014, 5, 203–390. [Google Scholar] [CrossRef]
- Crous, P.W. Taxonomy and Pathology of Cylindrocladium (Calonectria) and Allied Genera; American Phytopathological Society (APS) Press: Saint Paul, MN, USA, 2002. [Google Scholar]
- Yang, W.; Zheng, L.; Wang, C.; Xie, C.-P. First Report of Calonectria pteridis Causing a Leaf Spot Disease on Serenoa repens in China. Plant Dis. 2014, 98, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, G.; Grasso, F.M.; Vitale, A.; Aiello, D. First Occurrence of Calonectria Leaf Spot on Mexican Blue Palm in Italy. Plant Dis. 2007, 91, 1052. [Google Scholar] [CrossRef]
- Abass, M.H.; Hameed, M.A.; Ahmed, A.N. First Report of Nigrospora sphaerica (Sacc.) Mason as a Potential Pathogen on Date Palm (Phoenix dactylifera L.). Can. J. Plant Pathol. 2013, 35, 75–80. [Google Scholar] [CrossRef]
- Manzelat, S.F. Mycoflora Associated with Date Palm (Phoenix dactylifera) from Ad Darb, Jizan, Saudi Arabia. Microbiol. Res. J. Int. 2019, 27, 1–11. [Google Scholar] [CrossRef]
- Al-Nadabi, H.; Maharachchikumbura, S.S.N.; Al-Gahaffi, Z.S.; Al-Hasani, A.S.; Velazhahan, R.; Al-Sadi, A.M. Molecular Identification of Fungal Pathogens Associated with Leaf Spot Disease of Date Palms (Phoenix dactylifera). All Life 2020, 13, 587–597. [Google Scholar] [CrossRef]
- Tao, Y.; Quan, X.; Khokhar, I.; Anjum, T.; Song, H.; Mukhtar, I. First Report of Pseudopestalotiopsis theae Causing Leaf Spot of Date Palm (Phoenix dactylifera) in China. Plant Dis. 2021, 105, 508. [Google Scholar] [CrossRef] [PubMed]
- Rabaaoui, A.; Masiello, M.; Somma, S.; Crudo, F.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; et al. Phylogeny and Mycotoxin Profiles of Pathogenic Alternaria and Curvularia Species Isolated from Date Palm in Southern Tunisia. Front. Microbiol. 2022, 13, 1034658. [Google Scholar] [CrossRef] [PubMed]
- Arafat, K.H.; Hassan, M.; Hussein, E.A. Detection, Disease Severity, and Chlorophyll Prediction of Date Palm Leaf Spot Fungal Diseases. New Valley J. Agric. Sci. 2021, 1, 98–110. [Google Scholar] [CrossRef]
- Arafat, K.H.; Hassan, M.H.A.-R.; Hussein, E.A. Novel Fungal Pathogens Associated with Date Palm Leaf Spot in Egypt. New Valley J. Agric. Sci. 2024, 4, 84–106. [Google Scholar] [CrossRef]
- Jassim, N.S.; Ahmed, A.N. The Isolation and Molecular Identification of the Main Fungus Causing Leaf Spots on Date Palms (Phoenix dactylifera L.). Arch. Phytopathol. Plant Prot. 2024, 57, 542–554. [Google Scholar] [CrossRef]
- Kittimorakul, J.; Pornsuriya, C.; Sunpapao, A. Survey and Incidence of Leaf Blight and Leaf Spot Diseases of Oil Palm Seedlings in Southern Thailand. Plant Pathol. J. 2013, 12, 149–153. [Google Scholar] [CrossRef]
- Sunpapao, A.; Kittimorakul, J.; Pornsuriya, C. Disease Note: Identification of Curvularia oryzae as Cause of Leaf Spot Disease on Oil Palm Seedlings in Nurseries of Thailand. Phytoparasitica 2014, 42, 529–533. [Google Scholar] [CrossRef]
- Azlan, A.M.; Ahmad, Z.A.; Idris, A.S. Assessment of Leaf Spot and Anthracnose Diseases in Nurseries and Its Relationship with Oil Palm Seedling Ages. Int. J. Adv. Multidiscip. Res. 2018, 5, 529–533. [Google Scholar] [CrossRef]
- de Assis Costa, O.Y.; Tupinambá, D.D.; Bergmann, J.C.; Barreto, C.C.; Quirino, B.F. Fungal Diversity in Oil Palm Leaves Showing Symptoms of Fatal Yellowing Disease. PLoS ONE 2018, 13, e0191884, Erratum in PLoS ONE 2021, 16, e0254042. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, C.S.; Apriyanto, A.; Ernawan, R.; Neing, D.; Susilo, R.; Cordell, H.J.; Gatehouse, A.M.R.; Edwards, M.G. Genetic Variants Associated with Leaf Spot Disease Resistance in Oil Palm (Elaeis guineensis): A Genome-Wide Association Study. Plant Pathol. 2023, 72, 1626–1636. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Sunpapao, A.; Srihanant, N.; Worapattamasri, K.; Kittimorakul, J.; Phithakkit, S.; Petcharat, V. A Survey of Diseases and Disorders in Oil Palms of Southern Thailand. Plant Pathol. J. 2013, 12, 169–175. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Villarreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Fungal Biogeography: Global Diversity and Geography of Soil Fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A Meta-Analysis of Global Fungal Distribution Reveals Climate-Driven Patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.L.; Des Jardin, E.A. First Report of Cocoicola californica on Washingtonia robusta in Florida. Plant Health Prog. 2006, 7. [Google Scholar] [CrossRef]
- Elliott, M.L.; Des Jardin, E.A. First Report of a Serenomyces sp. from Copernicia × burretiana, Latania loddigesii, and Phoenix canariensis in Florida and the United States. Plant Health Prog. 2006, 7. [Google Scholar] [CrossRef]
- Elliott, M.L.; Des Jardin, E.A. Serenomyces Associated with Palms in Southeastern USA: Isolation, Culture Storage and Genetic Variation. Mycologia 2014, 106, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Downer, A.J.; Uchida, J.Y.; Hodel, D.R.; Elliott, M.L. Lethal Palm Diseases Common in the United States. HortTechnology 2009, 19, 710–716. [Google Scholar] [CrossRef]
- Cúndom, M.A.; Gutiérrez, S.A.; Cejas, P.; Cabrera, M.G. Exserohilum rostratum Patógeno de Caryota mitis en Argentina. Summa Phytopathol. 2006, 32, 277–279. [Google Scholar] [CrossRef]
- Colmán, A.; da Silva, R.A.; Alves, R.; Silva, M.; Barreto, R.W. First Report of Stigmina palmivora Causing Leaf Spots on Phoenix roebelenii in Brazil. Plant Dis. 2014, 98, 849. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, R.; Cáceres, O.; Piepenbring, M. Three New Records of Plant Parasitic Phyllosphere Fungi from Panama: Annellophora phoenicis, Cercospora corniculatae, and Sclerotium coffeicola. Check List 2018, 14, 93–100. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Pinruan, U.; Choeyklin, R.; Hattori, T.; Jones, E.B.G. Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis, and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers. 2008, 33, 139–161. [Google Scholar]
- Pinruan, U.; Rungjindamai, N.; Choeyklin, R.; Lumyong, S.; Hyde, K.D.; Jones, E.B.G. Occurrence and Diversity of Basidiomycetous Endophytes from the Oil Palm, Elaeis guineensis in Thailand. Fungal Divers. 2010, 41, 71–88. [Google Scholar] [CrossRef]
- Kogel, K.-H.; Franken, P.; Hückelhoven, R. Endophyte or Parasite—What Decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Saikkonen, K. Forest structure and fungal endophytes. Fungal Biol. Rev. 2007, 21, 67–74. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology, and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Koivusaari, P.; Tejesvi, M.V.; Tolkkinen, M.; Markkola, A.; Mykrä, H.; Pirttilä, A.M. Fungi Originating from Tree Leaves Contribute to Fungal Diversity of Litter in Streams. Front. Microbiol. 2019, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Nwe, L.L.; Casonato, S.; Jones, E.E. Endophytic Fungal Isolates from Apple Tissue: Latent Pathogens Lurking Within? Fungal Biol. 2024, 128, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Azuddin, N.F.; Mohd, M.H.; Rosely, N.F.N.; Mansor, A.; Zakaria, L. Evaluation of the Pathogenicity of Endophytic Fungi Isolated from Spines of Rattan (Calamus castaneus) against Other Plant Hosts. J. Appl. Microbiol. 2022, 133, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.F. The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia 1994, 86, 376–385. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Samuels, G.J. Preliminary study of endophytic fungi in a tropical palm. Mycol. Res. 1990, 94, 827–830. [Google Scholar] [CrossRef]
- Taylor, J.E.; Hyde, K.D.; Jones, E.B.G. Endophytic Fungi Associated with the Temperate Palm, Trachycarpus fortunei, within and outside Its Natural Geographic Range. New Phytol. 1999, 142, 335–346. [Google Scholar] [CrossRef]
- Crous, P.W.; Summerell, B.A.; Mostert, L.; Groenewald, J.Z. Host Specificity and Speciation of Mycosphaerella and Teratosphaeria Species Associated with Leaf Spots of Proteaceae. Persoonia 2008, 20, 59–86. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, D.S.; Thambugala, K.M.; De Silva, N.I.; Kuo, C.H.; Hyde, K.D. Leaf Litter Saprobic Didymellaceae (Dothideomycetes): Leptosphaerulina longiflori sp. nov. and Didymella sinensis, a New Record from Roystonea regia. Asian J. Mycol. 2019, 2, 87–100. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Chen, Y.J.; Phukhamsakda, C.; Boekhout, T.; Groenewald, J.Z.; McKenzie, E.H.C.; Francisco, E.C.; Frisvad, J.C.; Groenewald, M.; Hurdeal, V.G.; et al. What Are the 100 Most Cited Fungal Genera? Stud. Mycol. 2024, 108, 1–411. [Google Scholar] [CrossRef]
- Thanos, M.; Schonian, G.; Meyer, W.; Schweynoch, C.; Graser, Y.; Mitchell, T.G.; Presber, W.; Tietz, H.J. Rapid Identification of Candida Species by DNA Fingerprinting with PCR. J. Clin. Microbiol. 1996, 34, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Latouche, G.N.; Daniel, H.M.; Thanos, M.; Mitchell, T.G.; Yarrow, D.; Schönian, G.; Sorrell, T.C. Identification of Pathogenic Yeasts of the Imperfect Genus Candida by Polymerase Chain Reaction Fingerprinting. Electrophoresis 1997, 18, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Czembor, P.C.; Arseniuk, E. Study of Genetic Variability among Monopycnidial and Monopycnidiospore Isolates Derived from Single Pycnidia of Stagonospora spp. and Septoria tritici with the Use of RAPD-PCR, MP-PCR and rep-PCR Techniques. J. Phytopathol. 1999, 147, 539–546. [Google Scholar] [CrossRef]
- Jedryczka, M.; Rouxel, T.; Balesdent, M.H. Rep-PCR Based Genomic Fingerprinting of Isolates of Leptosphaeria maculans from Poland. Eur. J. Plant Pathol. 1999, 105, 813–823. [Google Scholar] [CrossRef]
- Zhou, S.-G.; Smith, D.R.; Stanosz, G.R. Differentiation of Botryosphaeria Species and Related Anamorphic Fungi Using Inter Simple or Short Sequence Repeat (ISSR) Fingerprinting. Mycol. Res. 2001, 105, 919–926. [Google Scholar] [CrossRef]
- Mehta, Y.R.; Mehta, A.; Rosato, Y.B. ERIC and REP-PCR Banding Patterns and Sequence Analysis of the Internal Transcribed Spacer of rDNA of Stemphylium solani Isolates from Cotton. Curr. Microbiol. 2002, 44, 323–328. [Google Scholar] [CrossRef]
- de Arruda, M.C.C.; Miller, R.N.G.; Ferreira, M.A.S.V.; Felipe, M.S.S. Comparison of Crinipellis perniciosa Isolates from Brazil by ERIC Repetitive Element Sequence-Based PCR Genomic Fingerprinting. Plant Pathol. 2003, 52, 236–244. [Google Scholar] [CrossRef]
- Godoy, P.; Cano, J.; Gené, J.; Guarro, J.; Höfling-Lima, A.L.; Lopes Colombo, A. Genotyping of 44 Isolates of Fusarium solani, the Main Agent of Fungal Keratitis in Brazil. J. Clin. Microbiol. 2004, 42, 4494–4497. [Google Scholar] [CrossRef] [PubMed]
- Hierro, N.; González, A.; Mas, A.; Guillamón, J.M. New PCR-Based Methods for Yeast Identification. J. Appl. Microbiol. 2004, 97, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Pounder, J.I.; Williams, S.; Hansen, D.; Healy, M.; Reece, K.; Woods, G.L. Repetitive-Sequence-PCR-Based DNA Fingerprinting Using the DiversiLab System for Identification of Commonly Encountered Dermatophytes. J. Clin. Microbiol. 2005, 43, 2141–2147. [Google Scholar] [CrossRef]
- Surženko, M.; Kontram, K.; Sarand, I. PCR–based fingerprinting and identification of contaminative fungi isolated from rye breads. Agron. Res. 2017, 15, 288–297. [Google Scholar]
- Prakash, O.; Verma, M.; Sharma, P.; Kumar, M.; Kumari, K.; Singh, A.; Kumari, H.; Jit, S.; Gupta, S.K.; Khanna, M.; et al. Polyphasic Approach of Bacterial Classification – An Overview of Recent Advances. Indian J. Microbiol. 2007, 47, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dash, H.R.; Mangwani, N.; Chakraborty, J.; Kumari, S. Understanding Molecular Identification and Polyphasic Taxonomic Approaches for Genetic Relatedness and Phylogenetic Relationships of Microorganisms. J. Microbiol. Methods 2014, 103, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, J.L.W.; de Bruijn, F.J. Characterization and Classification of Microbes by Rep-PCR Genomic Fingerprinting and Computer Assisted Pattern Analyses. In DNA Markers: Protocols, Applications and Overviews; Caetano-Anollés, G., Gresshoff, P.M., Eds.; John Wiley & Sons: New York, NY, USA, 1997; pp. 151–171. [Google Scholar]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae Revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The Phoma-Like Dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef]
- de Gruyter, J.; Woudenberg, J.H.; Aveskamp, M.M.; Verkley, G.J.; Groenewald, J.Z.; Crous, P.W. Redisposition of Phoma-Like Anamorphs in Pleosporales. Stud. Mycol. 2012, 75, 1–36. [Google Scholar] [CrossRef]
- de Oliveira, R.J.V.; Bezerra, J.L.; Lima, T.E.F.; da Silva, G.A.; Cavalcanti, M.A.Q. Phaeosphaeria nodulispora, a New Endophytic Coelomycete Isolated from Tropical Palm (Cocos nucifera) in Brazil. Nova Hedwig. 2016, 103, 185–192. [Google Scholar] [CrossRef]
- Crous, P.W.; Schumacher, R.K.; Akulov, A.; Thangavel, R.; Hernández-Restrepo, M.; Carnegie, A.J.; Cheewangkoon, R.; Wingfield, M.J.; Summerell, B.A.; Quaedvlieg, W.; et al. New and Interesting Fungi. 2. Fungal Syst. Evol. 2019, 3, 57–134. [Google Scholar] [CrossRef]
- Xiong, Y.; Manawasinghe, I.S.; Wanasinghe, D.N.; Hongsanan, S.; Hyde, K.D.; Biao, X.; Dong, Z. Two New Species and a New Host Record of Pleosporales (Dothideomycetes) from Palm (Arecaceae) in Guangdong Province, China. N. Z. J. Bot. 2023, 62, 165–191. [Google Scholar] [CrossRef]
- Kularathnage, N.D.; Senanayake, I.C.; Wanasinghe, D.N.; Doilom, M.; Stephenson, S.L.; Song, J.; Dong, W.; Xu, B. Plant-Associated Novel Didymellaceous Taxa in the South China Botanical Garden (Guangzhou, China). J. Fungi 2023, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Tonellotto, M.; Fehr, V.; Conedera, M.; Hunziker, M.; Pezzatti, G.B. Iconic but Invasive: The Public Perception of the Chinese Windmill Palm (Trachycarpus fortunei) in Switzerland. Environ. Manag. 2022, 70, 618–632. [Google Scholar] [CrossRef]
- Obón, C.; Rivera, D.; Alcaraz, F.; Carreño, E.; Ríos, S.; Laguna, E.; Sánchez-Balibrea, J.; del Arco, M.; Bergmeier, E.; Johnson, D. What Are Palm Groves of Phoenix? Conservation of Phoenix Palm Groves in the European Union. Biodivers. Conserv. 2018, 27, 1905–1924. [Google Scholar] [CrossRef]
- Sosa, P.A.; Saro, I.; Johnson, D.; Obón, C.; Alcaraz, F.; Rivera, D. Biodiversity and conservation of Phoenix canariensis: A review. Biodivers. Conserv. 2021, 30, 275–293. [Google Scholar] [CrossRef]
- Fisher, P.J.; Petrini, O.; Petrini, L.E.; Sutton, B.C. Fungal Endophytes from the Leaves and Twigs of Quercus ilex L. from England, Majorca and Switzerland. New Phytol. 1994, 127, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.; Horton, T.R.; Pauchard, A.; Nuñnez, M.A. A Single Ectomycorrhizal Fungal Species Can Enable a Pinus Invasion. Ecology 2015, 96, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya’ei, M.N.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique Arbuscular Mycorrhizal Fungal Communities Uncovered in Date Palm Plantations and Surrounding Desert Habitats of Southern Arabia. Mycorrhiza 2011, 21, 195–209. [Google Scholar] [CrossRef]
- Gardiner, L.M.; Véla, E. Chamaerops humilis. The IUCN Red List of Threatened Species. Biodivers. Conserv. 2017, e.T13164373A95532812. [Google Scholar] [CrossRef]
- Alade, G.O.; Moody, J.O.; Bakare, A.G.; Awotona, O.R.; Adesanya, S.; Lai, D.; Debbab, A.; Proksch, P. Metabolites from Endophytic Fungus Pestalotiopsis clavispora Isolated from Phoenix reclinata Leaf. Fut. J. Pharm. Sci. 2018, 4, 273–275. [Google Scholar] [CrossRef]
- Crous, P.W.; Cowan, D.A.; Maggs-Kölling, G.; Yilmaz, N.; Thangavel, R.; Wingfield, M.J.; Noordeloos, M.E.; Dima, B.; Brandrud, T.E.; Jansen, G.M.; et al. Fungal Planet Description Sheets: 1182–1283. Persoonia 2021, 46, 313–528a. [Google Scholar] [CrossRef]
- Crous, P.W.; Hernández-Restrepo, M.; Schumacher, R.K.; Cowan, D.A.; Maggs-Kölling, G.; Marais, E.; Wingfield, M.J.; Yilmaz, N.; Adan, O.C.G.; Akulov, A.; et al. New and Interesting Fungi. 4. Fungal Syst. Evol. 2021, 7, 255–343. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.; Xu, W.; Mukhtar, I.; Quan, X.; Jiang, S.; Huang, R.; Chen, B.; Xie, B. First Report of Leaf Spot Disease Caused by Colletotrichum siamense on Chrysalidocarpus lutescens in China. Plant Dis. 2019, 103, 1425. [Google Scholar] [CrossRef]
- Dhillon, B.; Chakrabarti, S. Ganoderma zonatum Causes Butt Rot of Areca (Dypsis lutescens) and Robellini (Phoenix roebelenii) Palms in Florida. Plant Dis. 2023, 107, 2844. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying Biodiversity: Procedures and Pitfalls in the Measurement and Comparison of Species Richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Chiarucci, A.; Enright, N.J.; Perry, G.L.W.; Miller, B.P.; Lamont, B.B. Performance of Nonparametric Species Richness Estimators in a High Diversity Plant Community. Divers. Distrib. 2003, 9, 283–295. [Google Scholar] [CrossRef]
- Hortal, J.; Borges, P.A.; Gaspar, C. Evaluating the Performance of Species Richness Estimators: Sensitivity to Sample Grain Size. J. Anim. Ecol. 2006, 75, 274–287. [Google Scholar] [CrossRef]
- Gwinn, D.C.; Allen, M.S.; Bonvechio, K.I.; Hoyer, M.V.; Beesley, L.S. Evaluating Estimators of Species Richness: The Importance of Considering Statistical Error Rates. Methods Ecol. Evol. 2016, 7, 294–302. [Google Scholar] [CrossRef]
- Hughes, J.B.; Hellmann, J.J.; Ricketts, T.H.; Bohannan, B.J.M. Counting the Uncountable: Statistical Approaches to Estimating Microbial Diversity. Appl. Environ. Microbiol. 2001, 67, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.A.; Moore, J.L. The Concepts of Bias, Precision and Accuracy, and Their Use in Testing the Performance of Species Richness Estimators, with a Literature Review of Estimator Performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Chao, A.; Hwang, W.-H.; Chen, Y.-C.; Kuo, C.-Y. Estimating the Number of Shared Species in Two Communities. Stat. Sin. 2000, 10, 227–246. [Google Scholar]
- Yanna; Ho, W.H.; Hyde, K.D. Fungal Communities on Decaying Palm Fronds in Australia, Brunei, and Hong Kong. Mycol. Res. 2001, 105, 1458–1471. [Google Scholar] [CrossRef]
- Yanna; Ho, W.H.; Hyde, K.D. Fungal Succession on Fronds of Phoenix hanceana in Hong Kong. Fungal Divers. 2002, 10, 185–211. [Google Scholar]
- Pinruan, U.; Pinnoi, A.; Hyde, K.D.; Jones, E.B.G. Tropical Peat Swamp Fungi with Special Reference to Palms. In Freshwater Fungi and Fungal-Like Organisms; Jones, E.B.G., Hyde, K.D., Pang, K.L., Eds.; de Gruyter: Berlin, Germany, 2014; pp. 371–388. [Google Scholar] [CrossRef]
- Pavicic, M.; Overmyer, K.; Rehman, A.U.; Jones, P.; Jacobson, D.; Himanen, K. Image-Based Methods to Score Fungal Pathogen Symptom Progression and Severity in Excised Arabidopsis Leaves. Plants 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Kaniyassery, A.; Sathish, S.B.; Thorat, S.A.; Murali, T.S.; Rao, M.R.; Muthusamy, A. Deciphering the Dynamics and Trophic Mode Distribution of the Leaf Spot-Associated Fungal Community of Eggplant (Solanum melongena L.). Phytopathol. Res. 2024, 6, 59. [Google Scholar] [CrossRef]
- Divon, H.H.; Fluhr, R. Nutrition Acquisition Strategies during Fungal Infection of Plants. FEMS Microbiol. Lett. 2007, 266, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Marroquin-Guzman, M.; Wilson, R.A. Mechanisms of Nutrient Acquisition and Utilization during Fungal Infections of Leaves. Annu. Rev. Phytopathol. 2014, 52, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Johns, L.E.; Goldman, G.H.; Ries, L.N.A.; Brown, N.A. Nutrient Sensing and Acquisition in Fungi: Mechanisms Promoting Pathogenesis in Plant and Human Hosts. Fungal Biol. Rev. 2021, 36, 1–14. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, Y.; Xu, G.; Guo, Q.; Tan, J.; Ding, G. Fungal diversity within the phyllosphere of Pinus massoniana and the possible involvement of phyllospheric fungi in litter decomposition. Fungal Biol. 2021, 125, 785–795. [Google Scholar] [CrossRef]
- Gulati, S.; Chitralekha, P.; Pandit, M.A.; Katyal, R.; Bhandari, N.; Mehta, P.; Rawat, C.D.; Kaur, S.; Kaur, J. Diversity, Succession and Seasonal Variation of Phylloplane Mycoflora of Leucaena leucocephala in Relation to Its Leaf Litter Decomposition. J. Fungi 2022, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Rippel, T.M.; Wimp, G.M. Succession of fungal communities and their functional profiles in a decaying foundation species. Microb. Ecol. 2023, 86, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Koide, K.; Osono, T.; Takeda, H. Colonization and Lignin Decomposition of Camellia japonica Leaf Litter by Endophytic Fungi. Mycoscience 2005, 46, 280–286. [Google Scholar] [CrossRef]
- Promputtha, I.; Lumyong, S.; Dhanasekaran, V.; McKenzie, E.; Hyde, K.; Jeewon, R. A Phylogenetic Evaluation of Whether Endophytes Become Saprotrophs at Host Senescence. Microb. Ecol. 2007, 53, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Voříšková, J.; Baldrian, P. Fungal Community on Decomposing Leaf Litter Undergoes Rapid Successional Changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Szink, I.; Davis, E.L.; Ricks, K.D.; Koide, R.T. New Evidence for Broad Trophic Status of Leaf Endophytic Fungi of Quercus gambelii. Fungal Ecol. 2016, 22, 2–9. [Google Scholar] [CrossRef]
- Wenndt, A.J.; Evans, S.E.; van Diepeningen, A.D.; Logan, J.R.; Jacobson, P.J.; Seely, M.K.; Jacobson, K.M. Why Plants Harbor Complex Endophytic Fungal Communities: Insights from Perennial Bunchgrass Stipagrostis sabulicola in the Namib Sand Sea. Front. Microbiol. 2021, 12, 691584. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria Host-Specific (HSTs) Toxins: An Overview of Chemical Characterization, Target Sites, Regulation and Their Toxic Effects. Toxicol. Rep. 2019, 6, 745–758, Erratum in Toxicol. Rep. 2020, 22, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Blagojević, J.; Vukojević, J.; Ivanović, B.; Ivanović, Ž. Characterization of Alternaria Species Associated with Leaf Spot Disease of Armoracia rusticana in Serbia. Plant Dis. 2020, 104, 1378–1389. [Google Scholar] [CrossRef]
- Jones, R.W.; Perez, F. Differential Plant Response to Toxins and Elicitor Proteins Released by the Potato and Tomato Pathogens Alternaria solani and Alternaria alternata. J. Plant Pathol. 2023, 105, 21–28. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phillips, A.J.L.; Jayawardena, R.S.; Promputtha, I.; Hyde, K.D. Importance of Molecular Data to Identify Fungal Plant Pathogens and Guidelines for Pathogenicity Testing Based on Koch’s Postulates. Pathogens 2021, 10, 1096. [Google Scholar] [CrossRef]
- Hawthorne, B.T.; Otto, C. Pathogenicity of Fungi Associated with Leaf Spots of Kiwifruit. N. Z. J. Agric. Res. 1986, 29, 533–538. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhang, X.; Yang, X.; Xu, X.; Lin, J.; Bing, H.; Wang, X.; Zhao, J.; Xiang, W. Identification and Pathogenicity of Fungi Associated with Leaf Spot of Muskmelon in Eastern Shandong Province, China. Plant Dis. 2022, 106, 872–890. [Google Scholar] [CrossRef] [PubMed]
- HilleRisLambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking Community Assembly through the Lens of Coexistence Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Williams, R.J.; Howe, A.; Hofmockel, K.S. Demonstrating Microbial Co-Occurrence Patterns within and between Ecosystems. Front. Microbiol. 2014, 5, 358. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J. Null Model Analysis of Species Co-Occurrence Patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]
- Veech, J.A. A Probability-Based Analysis of Temporal and Spatial Co-Occurrence in Grassland Birds. J. Biogeogr. 2006, 33, 2145–2153. [Google Scholar] [CrossRef]
- Veech, J.A. The Pairwise Approach to Analysing Species Co-Occurrence. J. Biogeogr. 2014, 41, 1029–1035. [Google Scholar] [CrossRef]
- Sarma, V.V.; Hyde, K.D. Fungal species consortia on Nypa fruticans at Brunei. Stud. Fungi 2018, 3, 19–26. [Google Scholar] [CrossRef]
- Unterseher, M.; Jumpponen, A.; Opik, M.; Tedersoo, L.; Moora, M.; Dormann, C.F.; Schnittler, M. Species Abundance Distributions and Richness Estimations in Fungal Metagenomics—Lessons Learned from Community Ecology. Mol. Ecol. 2011, 20, 275–285. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Yang, J.R.; Liu, M.; Huang, B.; Yang, J. Distinct Patterns and Processes of Abundant and Rare Eukaryotic Plankton Communities Following a Reservoir Cyanobacterial Bloom. ISME J. 2018, 12, 2263–2277. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Abundant Fungi Adapt to Broader Environmental Gradients Than Rare Fungi in Agricultural Fields. Glob. Chang. Biol. 2000, 26, 4506–4520. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, T.-S.; Park, H.-D. Transient-Rare Bacterial Taxa Are Assembled Neutrally Across Temporal Scales. Microbes Environ. 2021, 36, ME20110. [Google Scholar] [CrossRef] [PubMed]
- Pouska, V.; Svoboda, M.; Leps, J. Co-Occurrence Patterns of Wood-Decaying Fungi on Picea abies Logs: Does Fomitopsis pinicola Influence the Other Species? Pol. J. Ecol. 2013, 61, 119–134. [Google Scholar]
- Maria, G.L.; Sridhar, K.R. Pattern of Fungal Colonization and Co-Occurrence on Avicennia officinalis Woody Litter in a South-Western Mangrove of India. Kavaka 2017, 48, 17–21. [Google Scholar]
- Abrego, N.; Roslin, T.; Huotari, T.; Tack, A.J.M.; Lindahl, B.D.; Tikhonov, G.; Somervuo, P.; Schmidt, N.M.; Ovaskainen, O. Accounting for Environmental Variation in Co-Occurrence Modelling Reveals the Importance of Positive Interactions in Root-Associated Fungal Communities. Mol. Ecol. 2020, 29, 2736–2746. [Google Scholar] [CrossRef]
- Banik, M.T.; Lindner, D.L.; Jusino, M.A. Intraspecific Interactions among Wood-Decay Fungi Alter Decay Rates and Dynamics of Interspecific Interactions. Fungal Ecol. 2024, 68, 101314. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms Between Microbial Pathogens in Plant Disease Complexes: A Growing Trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef]
- Boixel, A.-L.; Rouxel, T.; Andrivon, D.; Affichard, M.; Le May, C. Slipping through the Cracks: Challenges and Prospects for Investigating Fungal Plant Disease Complexes. Crop Prot. 2024, 184, 106826. [Google Scholar] [CrossRef]
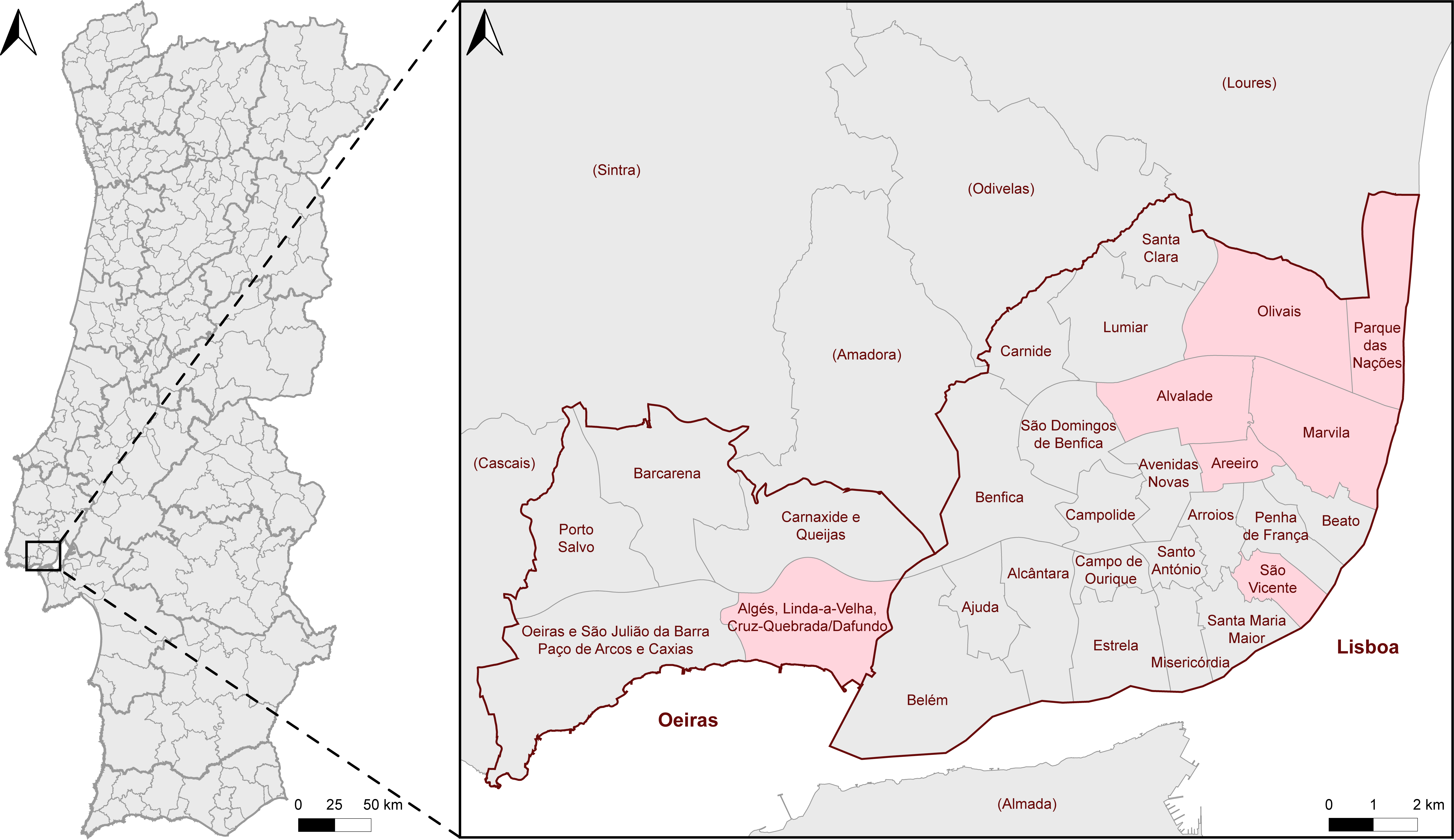

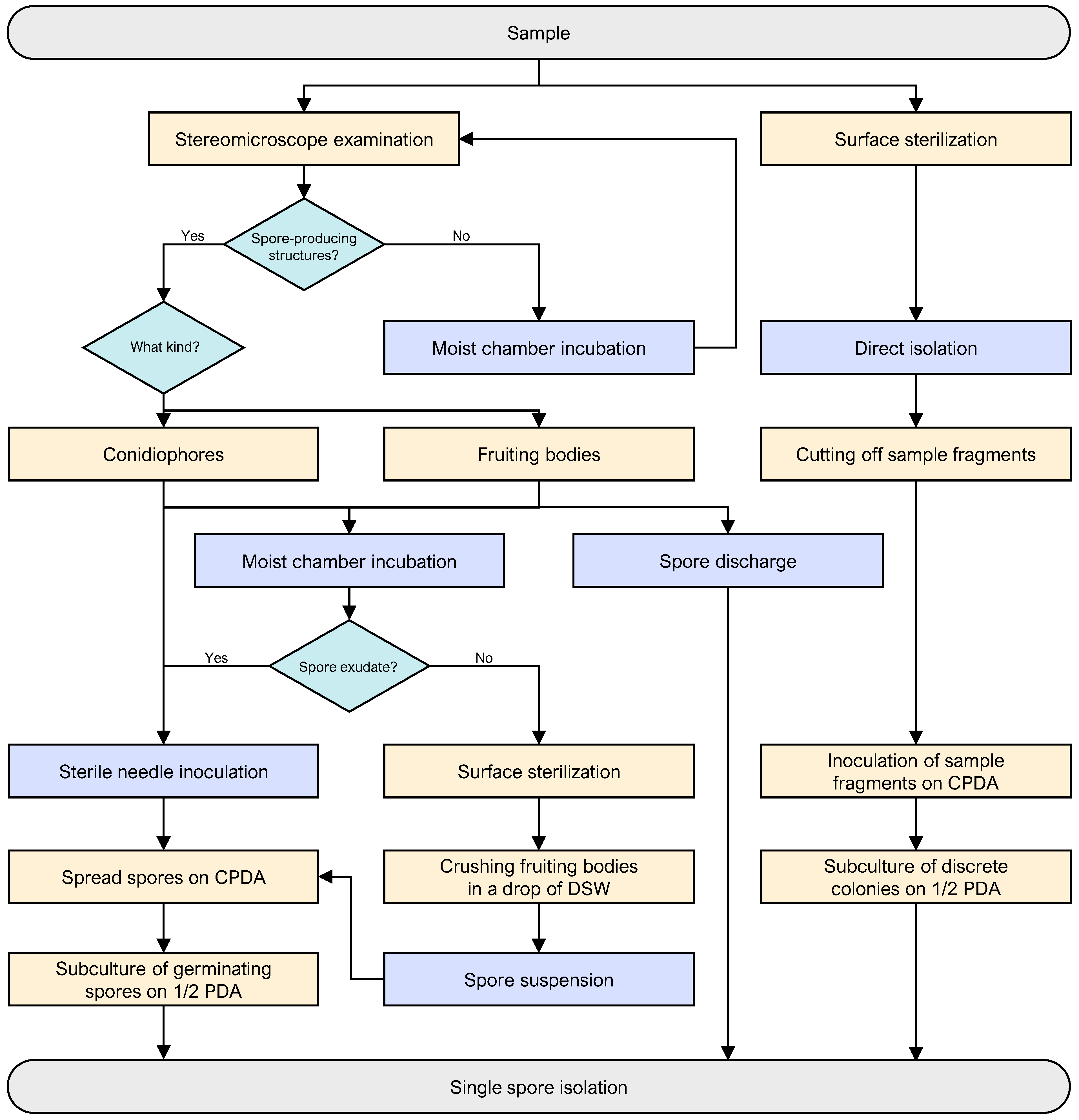
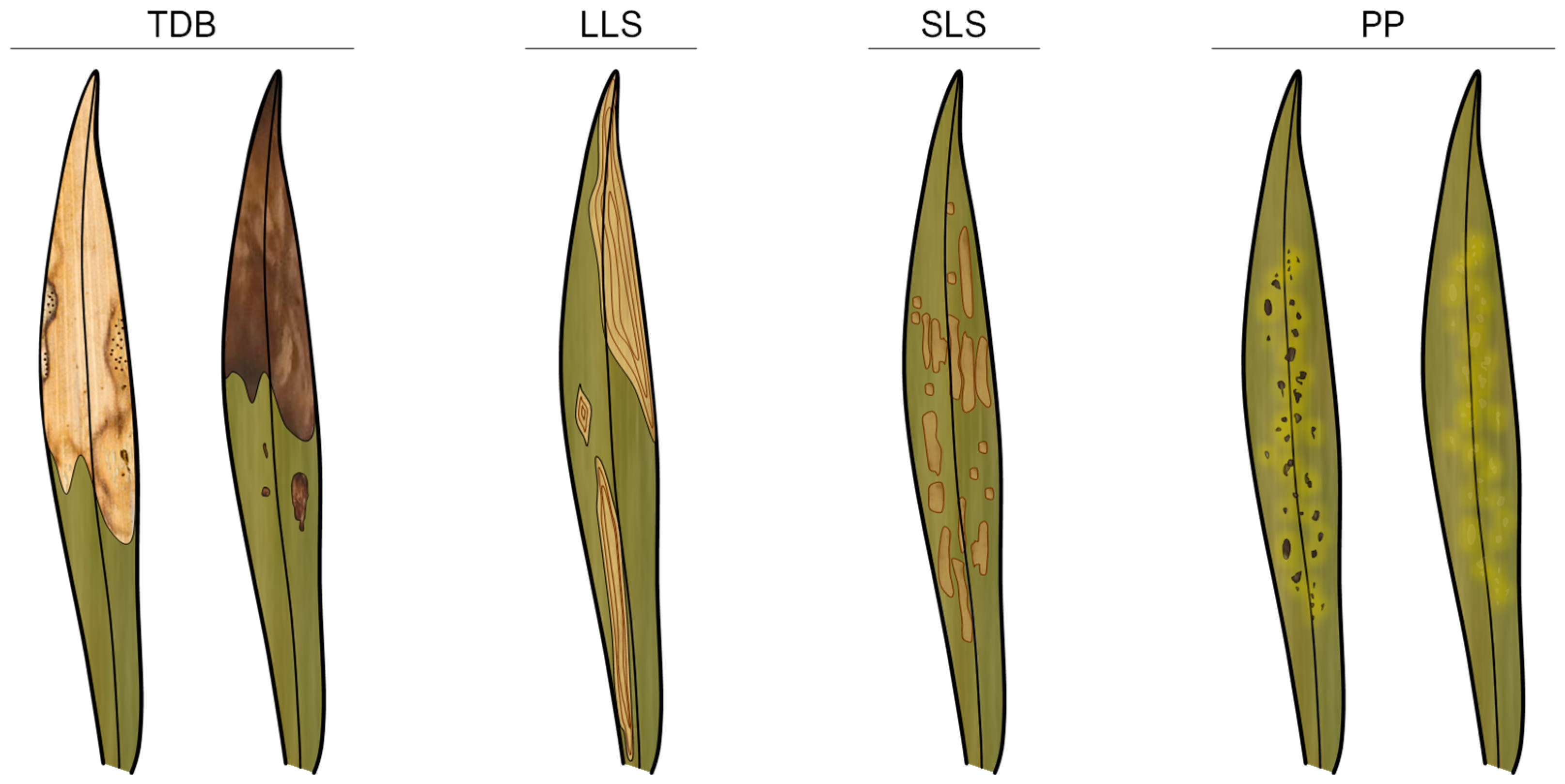

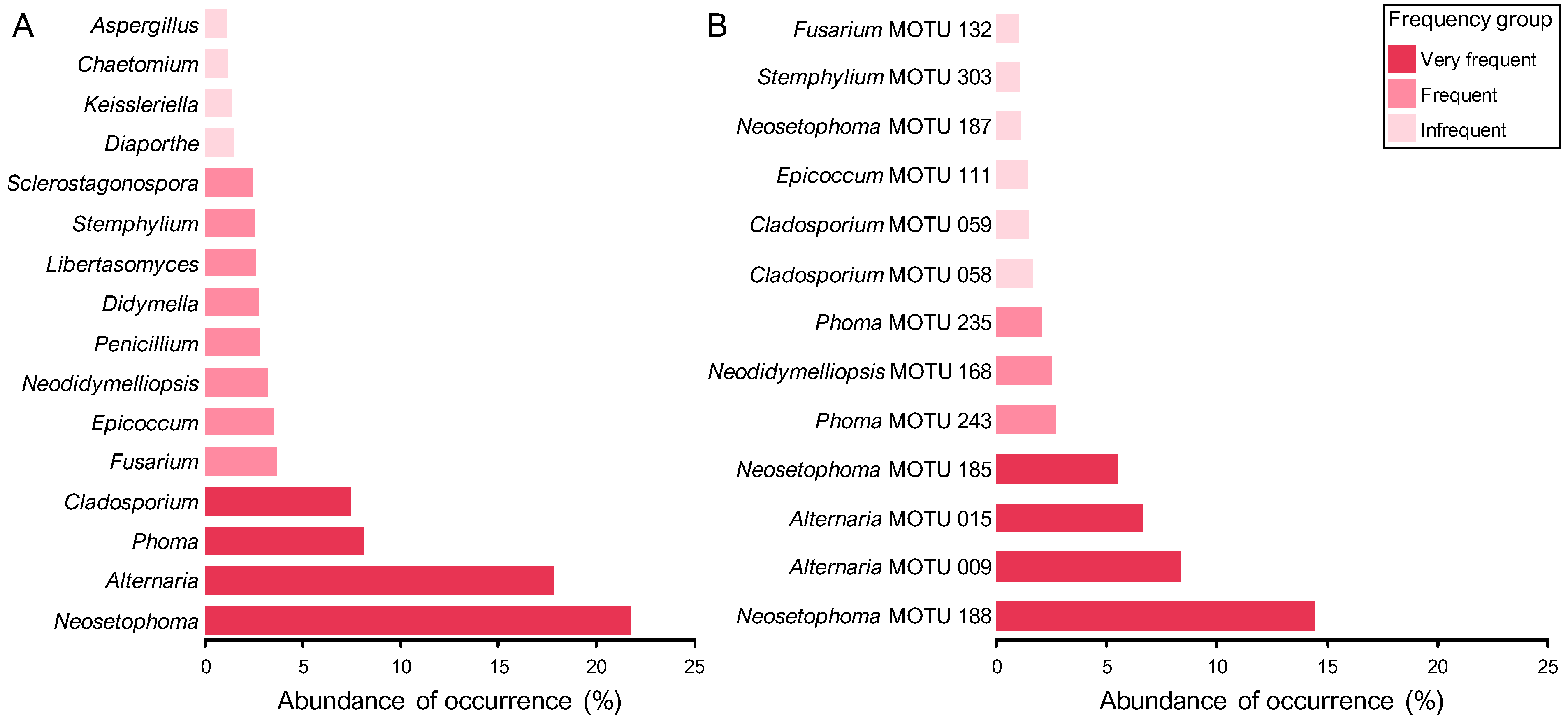
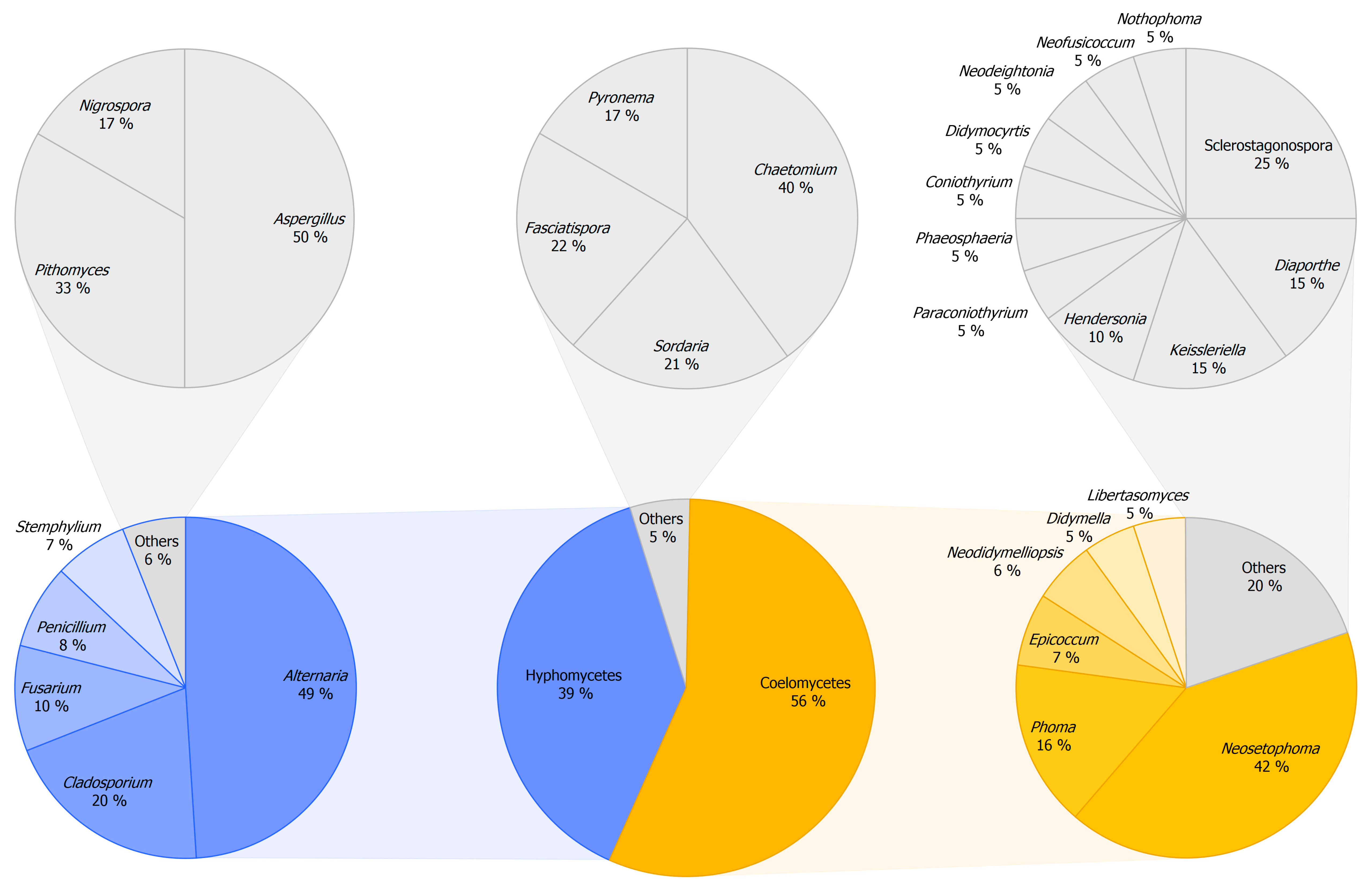

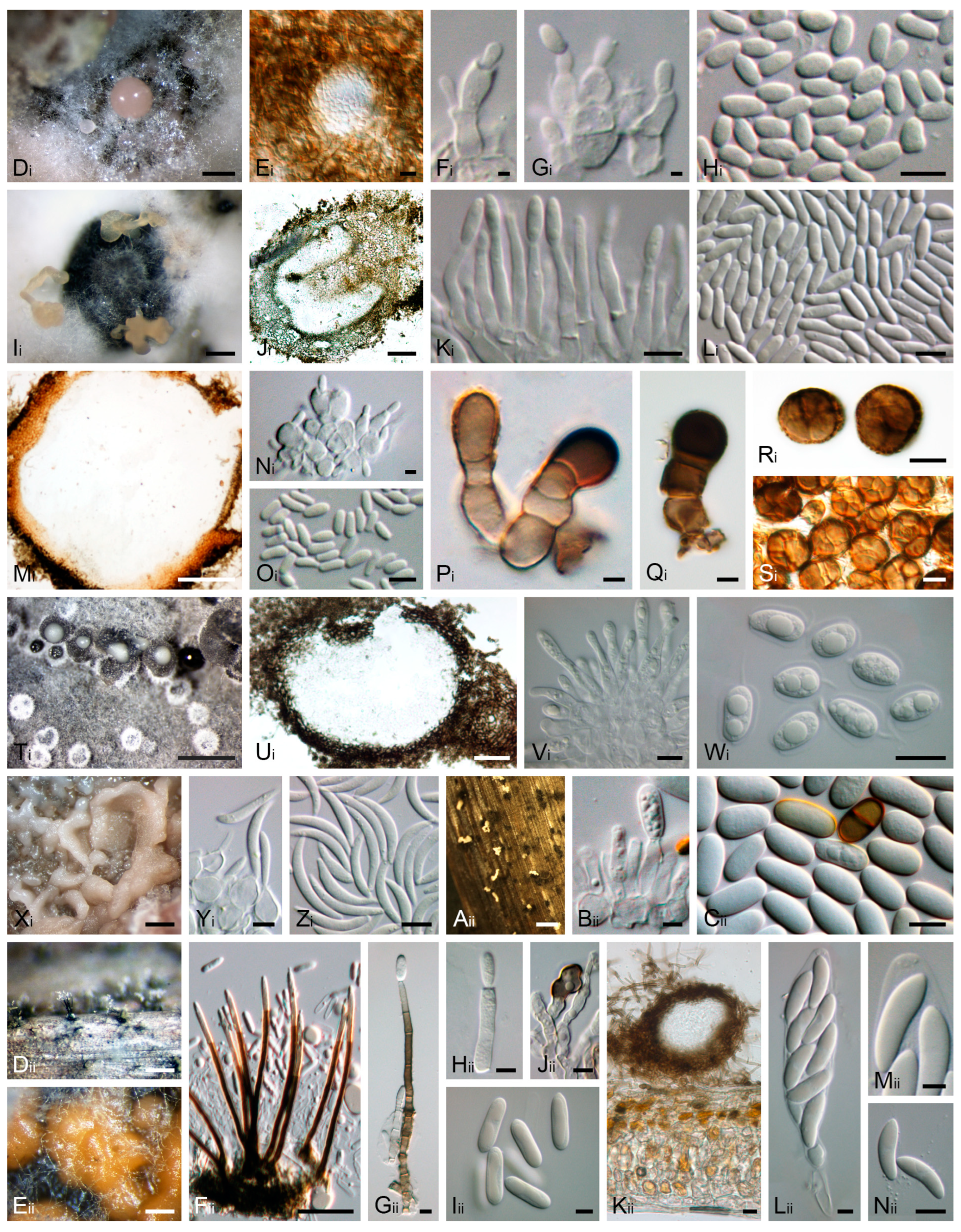
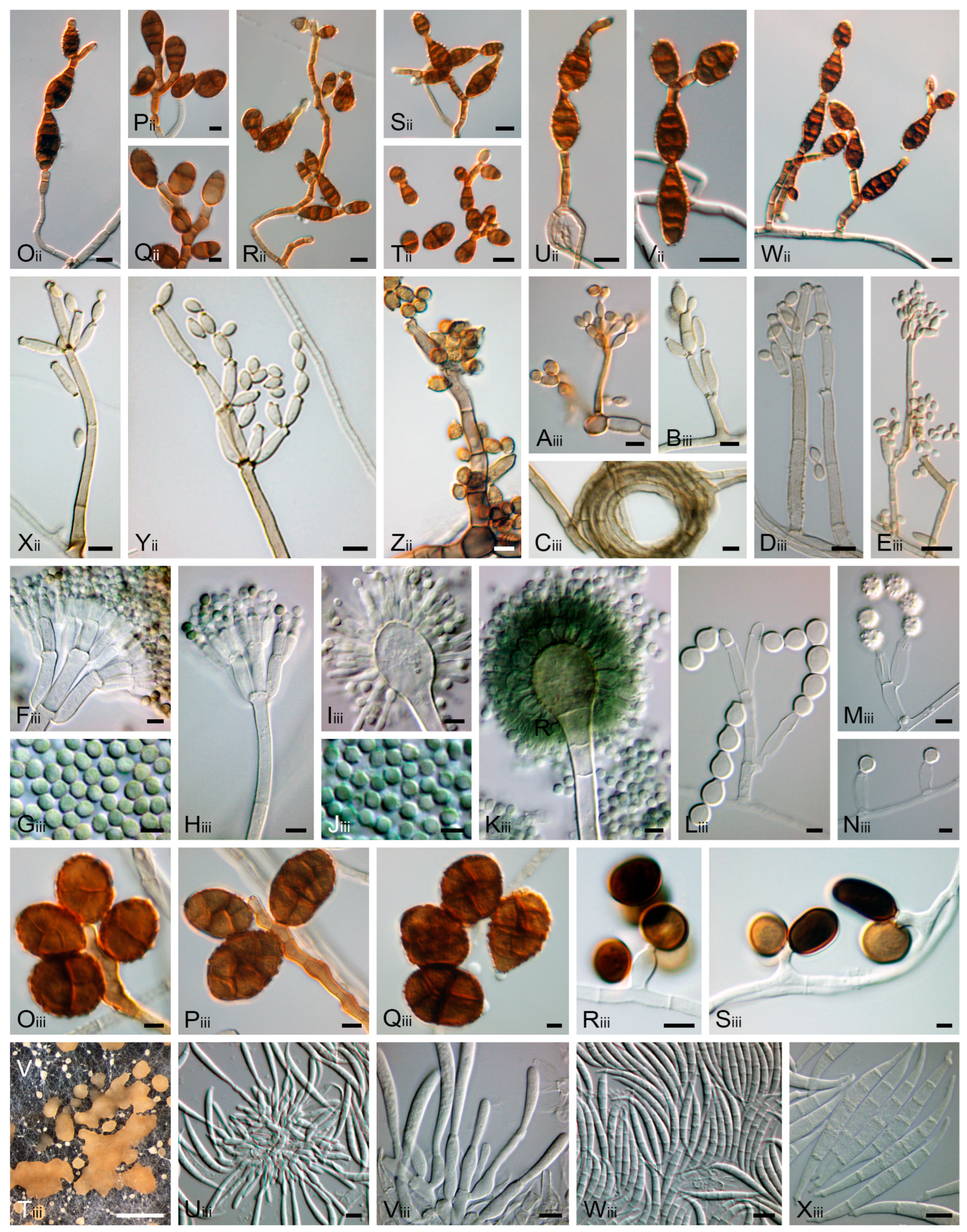

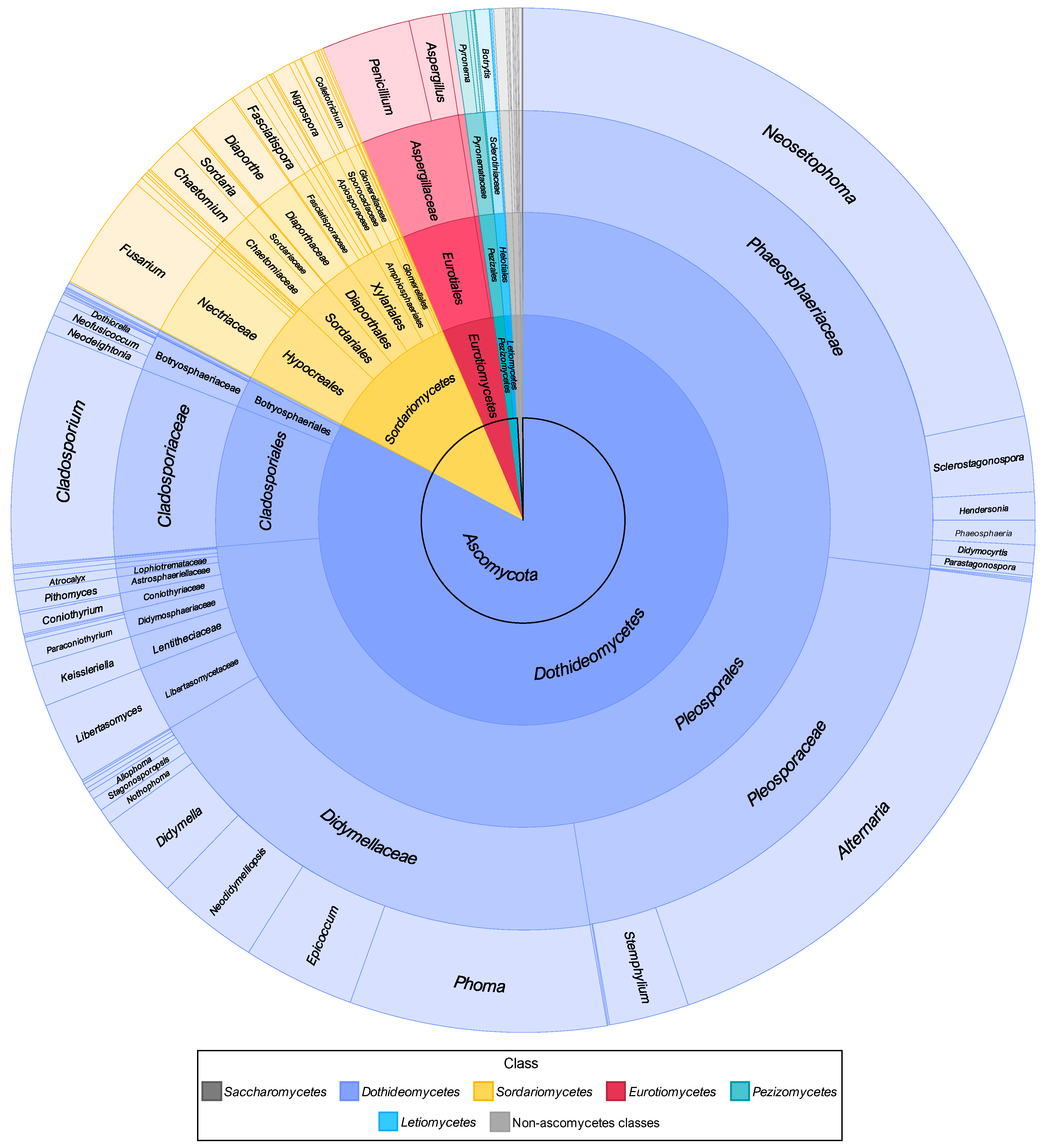
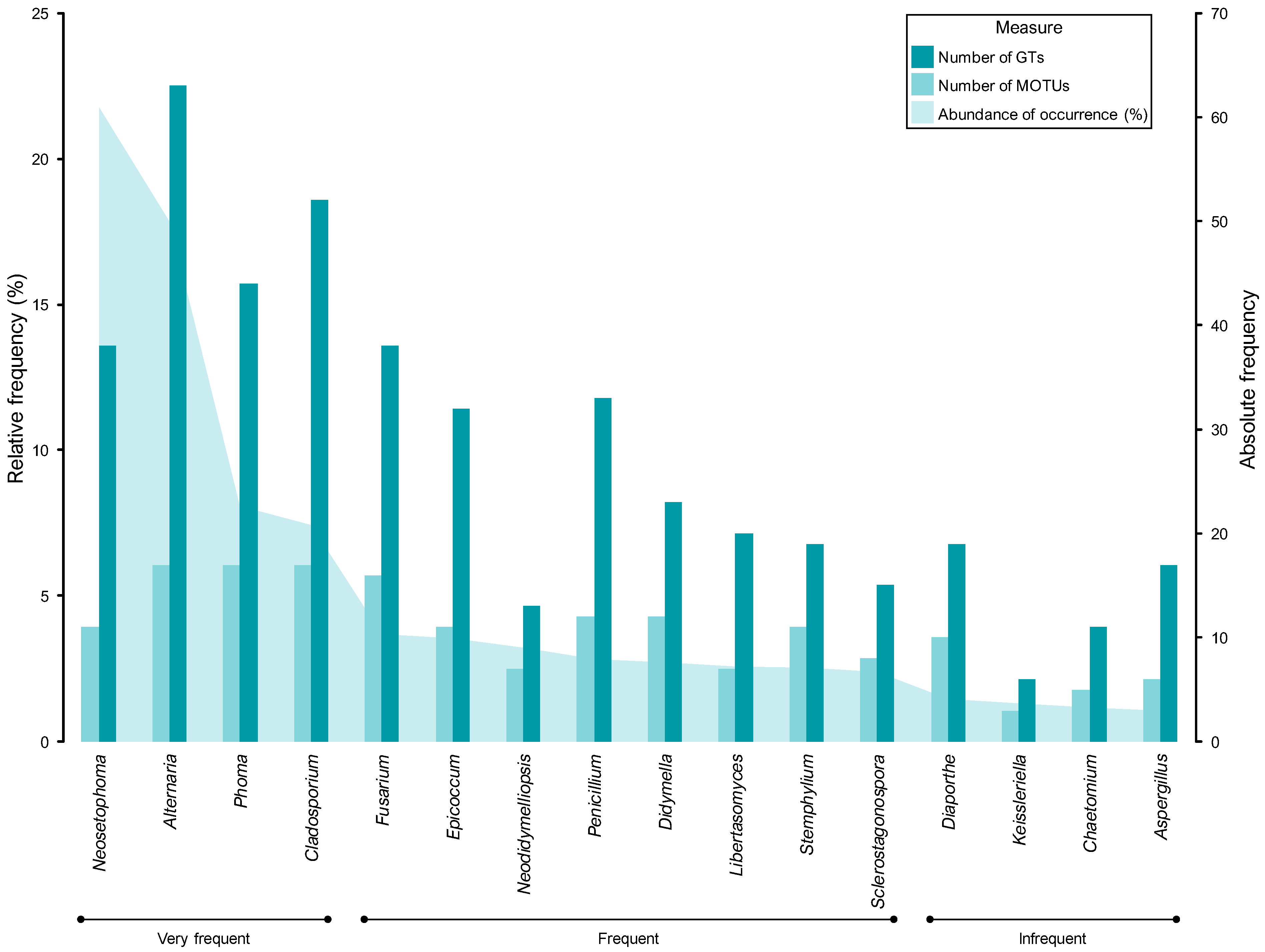


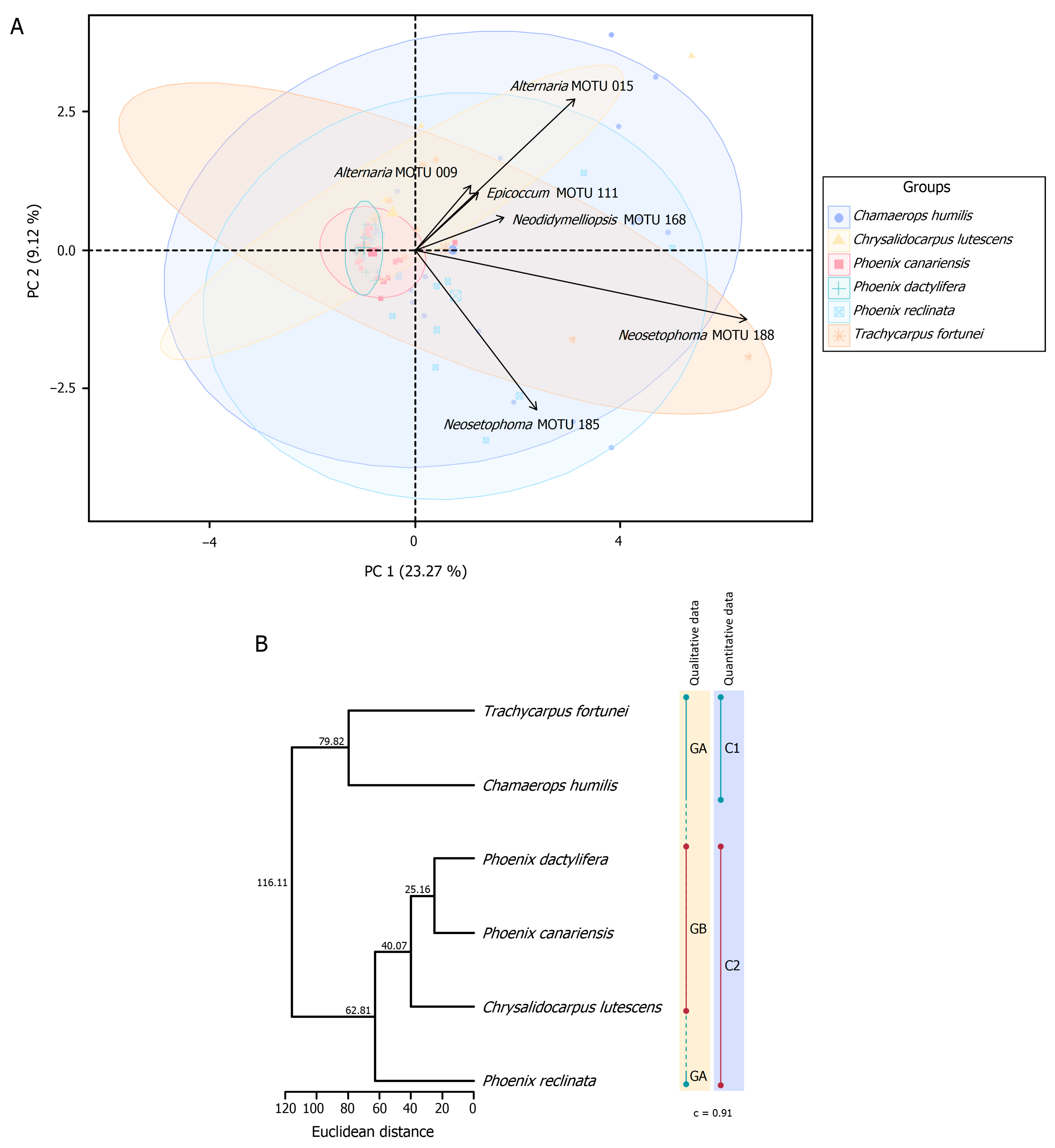
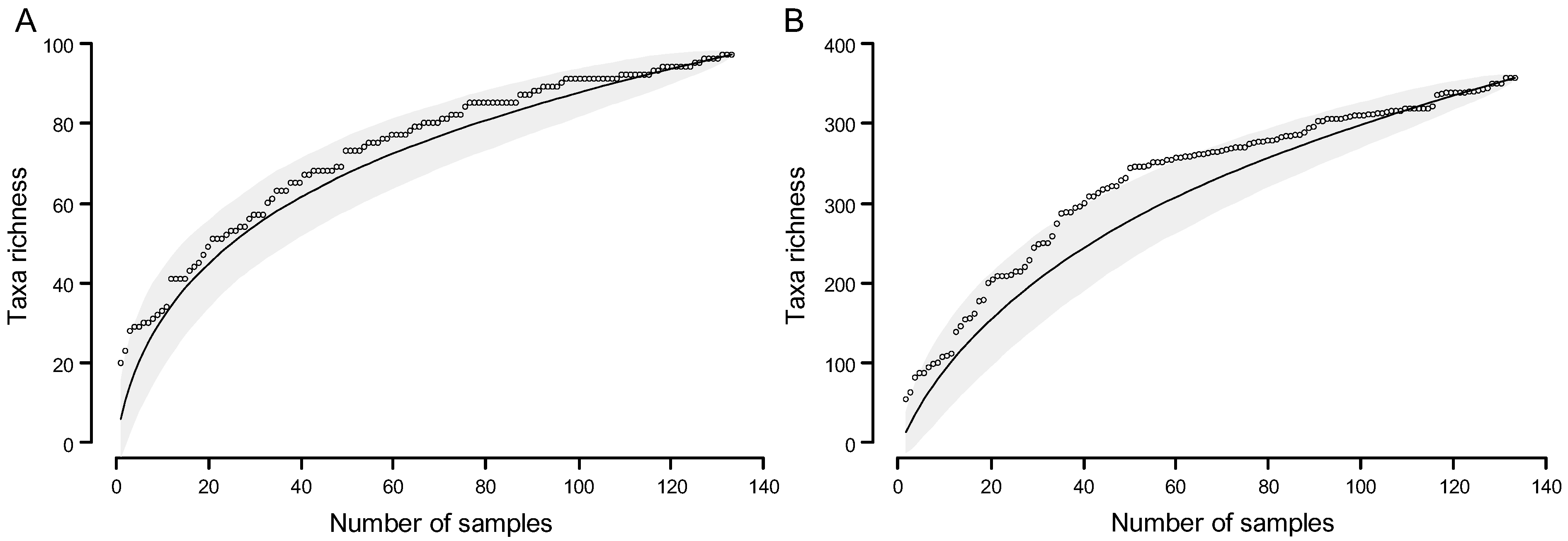
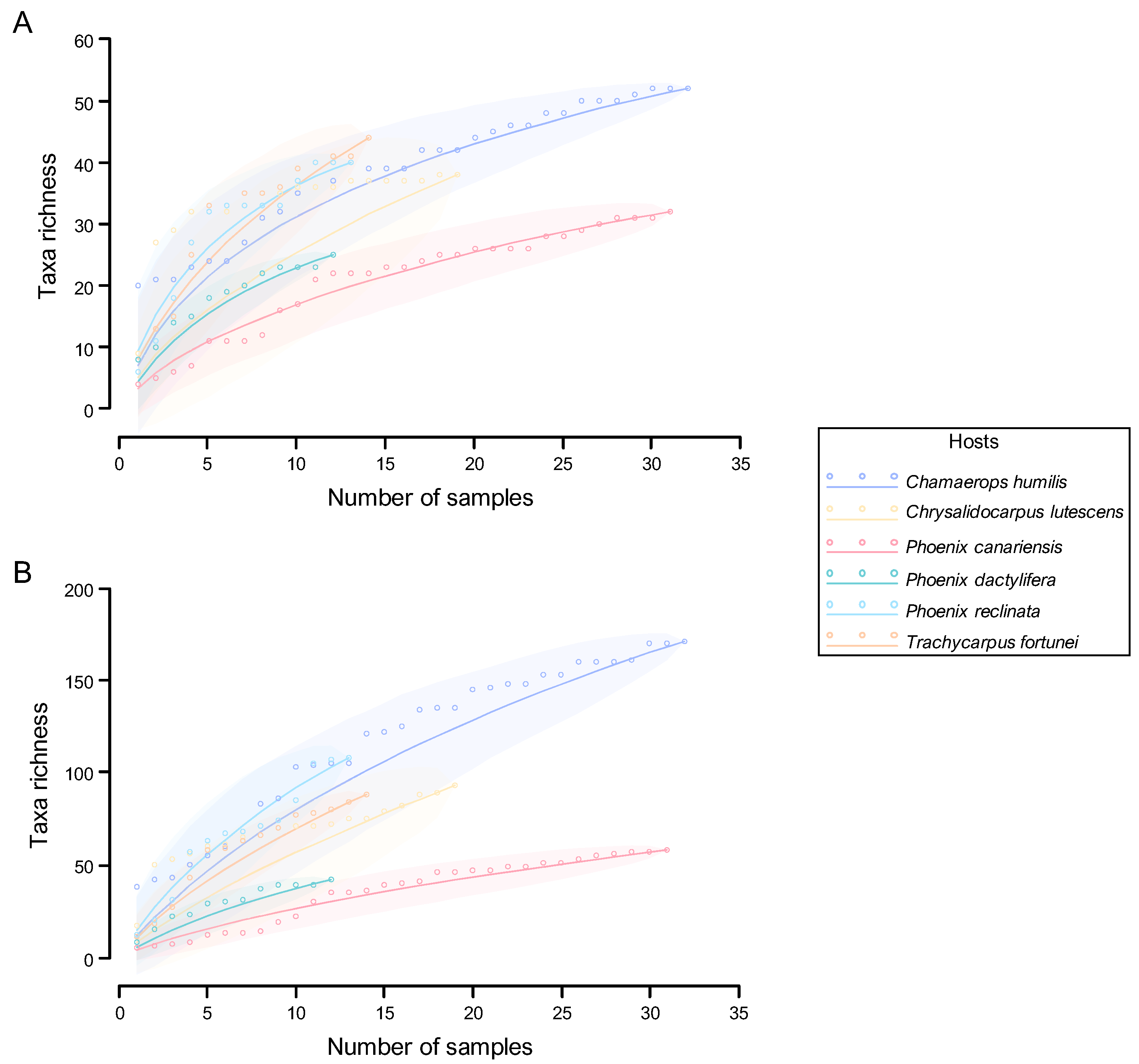
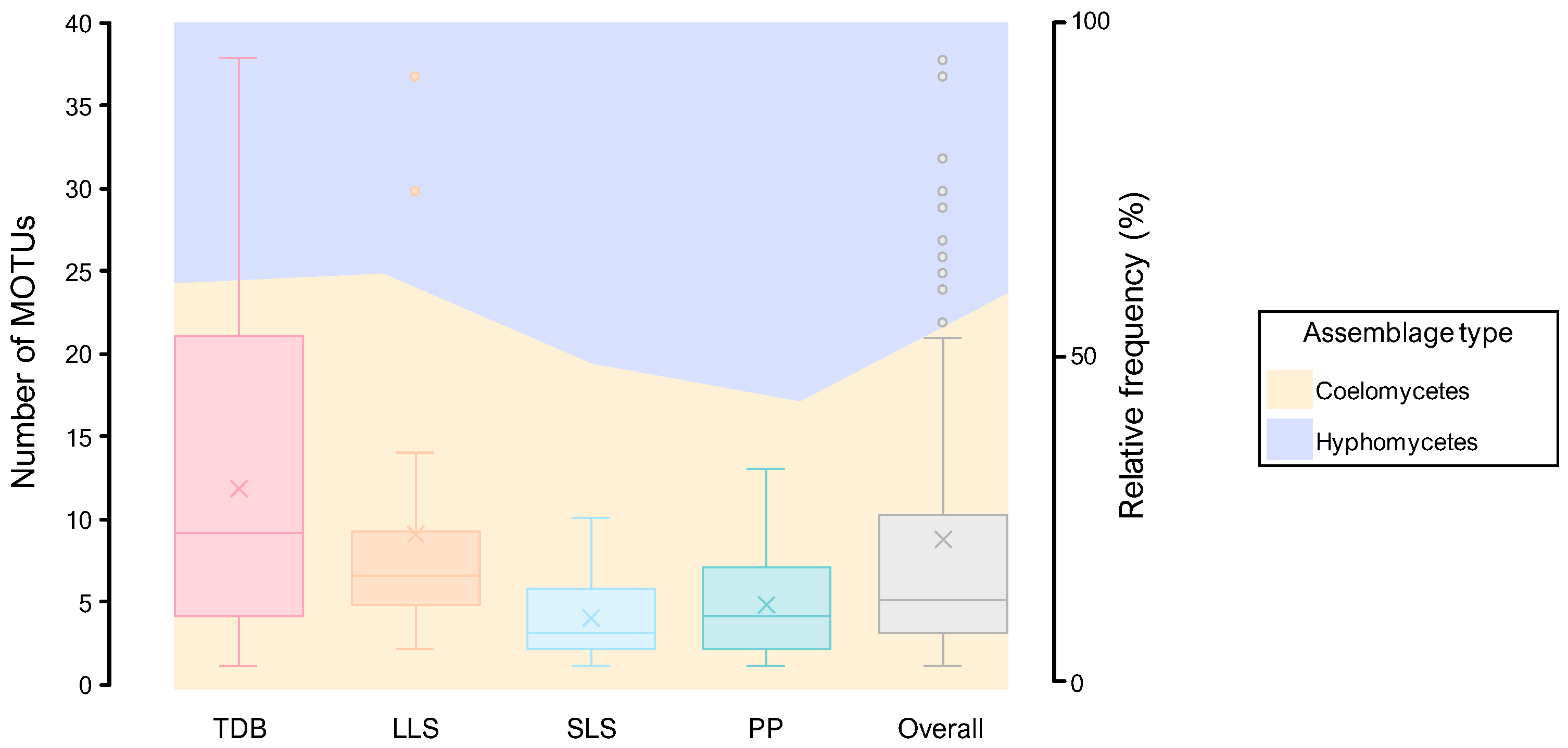
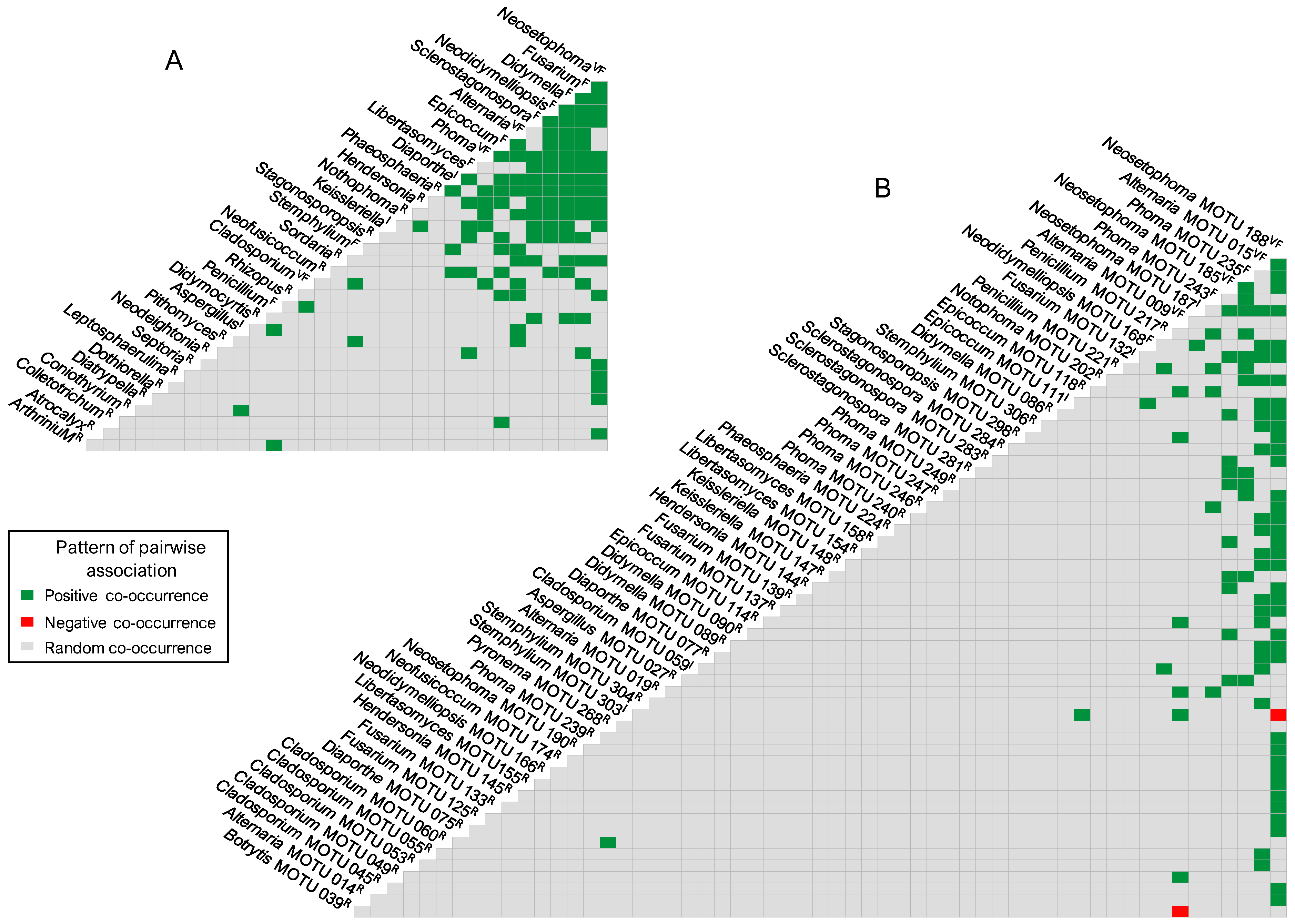
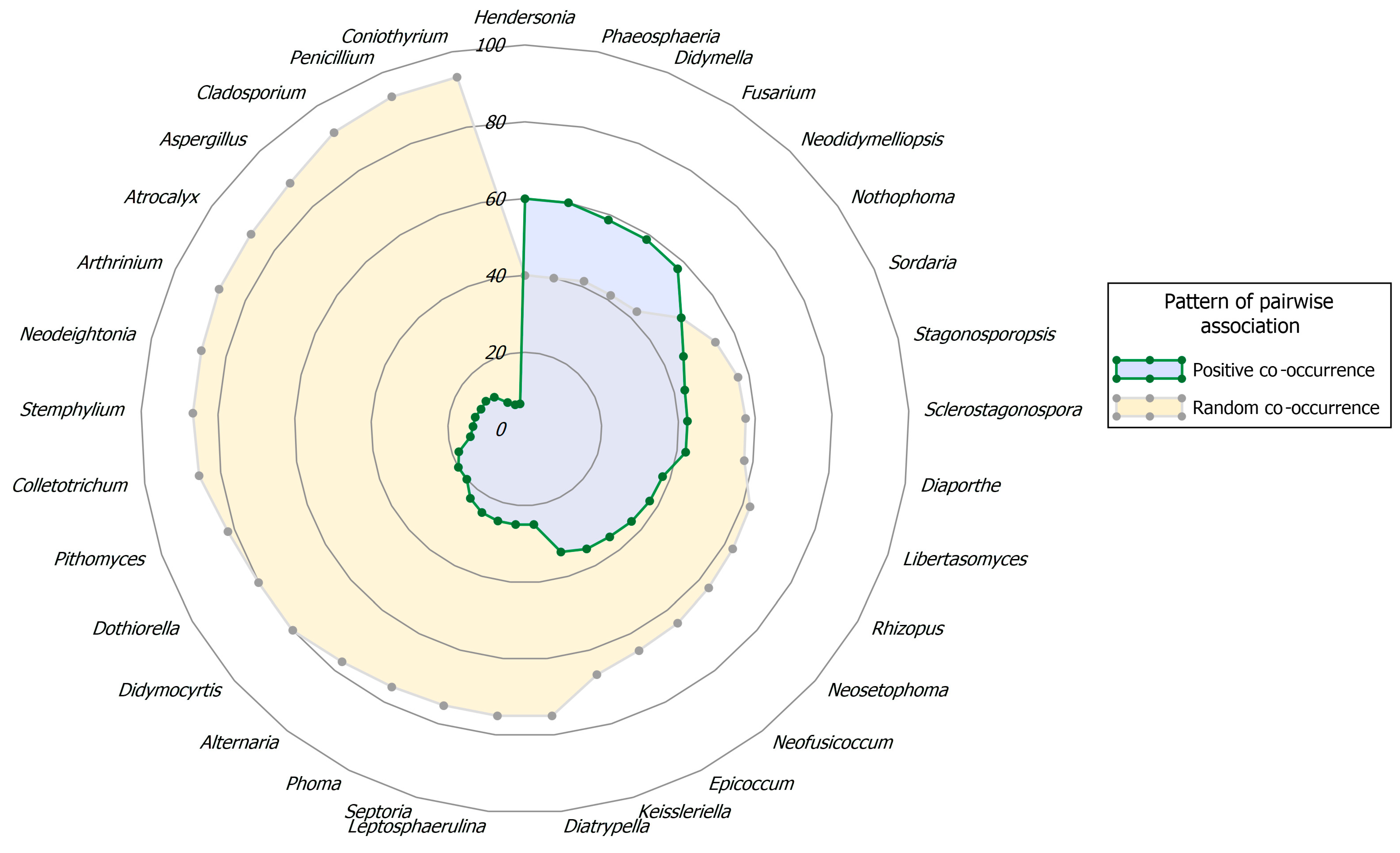
| Host | Number of Trees | Number of Samples 1 | ||||
|---|---|---|---|---|---|---|
| TDB | LLS | SLS | PP | Total | ||
| Chamaedorea elegans | 2 | 2 | 0 | 0 | 0 | 2 |
| Chamaerops humilis | 23 | 17 | 2 | 3 | 10 | 32 |
| Chrysalidocarpus lutescens | 18 | 11 | 5 | 2 | 1 | 19 |
| Phoenix canariensis | 17 | 10 | 3 | 11 | 7 | 31 |
| Phoenix dactylifera | 6 | 2 | 3 | 3 | 4 | 12 |
| Phoenix reclinata | 10 | 6 | 4 | 2 | 1 | 13 |
| Phoenix roebelenii | 3 | 2 | 0 | 1 | 0 | 3 |
| Trachycarpus fortunei | 13 | 10 | 3 | 1 | 0 | 14 |
| Washingtonia filifera | 8 | 5 | 2 | 1 | 0 | 8 |
| Total | 100 | 65 | 22 | 24 | 23 | 134 |
| Assemblage Type 1 | Number of Records | Number of Genera | Number of MOTUs 2 |
|---|---|---|---|
| Coelomycetes | 1163 | 47 | 160 |
| Hyphomycetes | 795 | 23 | 114 |
| Ascomycetes | 90 | 19 | 35 |
| Basidiomycetes | 8 | 7 | 8 |
| Zygomycetes | 8 | 1 | 3 |
| Total | 2064 | 97 | 320 |
| Frequency Group/Genus 1 | Measures of Population Status 2 | ||
|---|---|---|---|
| AO (%) | FO (%) | SMOTU | |
| Very frequent | |||
| Neosetophoma | 21.75 | 32.83 | 11 |
| Alternaria | 17.83 | 61.19 | 17 |
| Phoma | 8.09 | 49.25 | 17 |
| Cladosporium | 7.41 | 58.20 | 17 |
| Frequent | |||
| Fusarium | 3.68 | 16.42 | 16 |
| Epicoccum | 3.54 | 23.88 | 11 |
| Neodidymelliopsis | 3.20 | 14.18 | 7 |
| Penicillium | 2.81 | 25.37 | 12 |
| Didymella | 2.71 | 14.93 | 12 |
| Libertasomyces | 2.57 | 16.42 | 7 |
| Stemphylium | 2.52 | 21.64 | 7 |
| Sclerostagonospora | 2.37 | 17.16 | 8 |
| Infrequent | |||
| Diaporthe | 1.45 | 13.43 | 10 |
| Keissleriella | 1.31 | 11.94 | 3 |
| Chaetomium | 1.16 | 8.96 | 5 |
| Aspergillus | 1.07 | 12.69 | 6 |
| Rare | |||
| Hendersonia | 0.87 | 6.72 | 4 |
| Phaeosphaeria | 0.78 | 6.72 | 7 |
| Paraconiothyrium | 0.78 | 4.48 | 5 |
| Sordaria | 0.63 | 5.22 | 6 |
| Pithomyces | 0.63 | 5.22 | 5 |
| Coniothyrium | 0.63 | 6.72 | 2 |
| Fasciatispora | 0.63 | 5.22 | 1 |
| Didymocyrtis | 0.58 | 6.72 | 6 |
| Nigrospora | 0.58 | 5.97 | 5 |
| Neodeightonia | 0.53 | 6.72 | 2 |
| Neofusicoccum | 0.48 | 5.97 | 4 |
| Pyronema | 0.48 | 7.46 | 2 |
| Nothophoma | 0.48 | 5.97 | 1 |
| Colletotrichum | 0.44 | 3.73 | 4 |
| Stagonosporopsis | 0.44 | 5.97 | 2 |
| Botrytis | 0.44 | 5.97 | 1 |
| Rhizopus | 0.39 | 4.48 | 3 |
| Parastagonospora | 0.39 | 2.99 | 1 |
| Atrocalyx | 0.34 | 4.48 | 2 |
| Arthrinium | 0.29 | 4.48 | 3 |
| Nothodactylaria | 0.29 | 3.73 | 2 |
| Sarocladium | 0.24 | 3.73 | 4 |
| Dothiorella | 0.24 | 2.99 | 3 |
| Xenocylindrosporium | 0.24 | 3.73 | 2 |
| Allophoma | 0.24 | 2.99 | 1 |
| Preussia | 0.19 | 2.99 | 3 |
| Neopestalotiopsis | 0.19 | 2.99 | 3 |
| Morinia | 0.19 | 2.99 | 2 |
| Plenodomus | 0.19 | 2.99 | 2 |
| Leptosphaerulina | 0.15 | 2.24 | 2 |
| Plicaria | 0.15 | 2.24 | 2 |
| Acremonium | 0.15 | 1.49 | 2 |
| Pseudopithomyces | 0.15 | 1.49 | 2 |
| Diatrypella | 0.15 | 2.24 | 1 |
| Dimorpha | 0.15 | 2.24 | 1 |
| Septoria | 0.15 | 2.24 | 1 |
| Rare (doubletons) | 0.10 | 1.49 | 1–2 |
| Coniochaeta, Ectophoma, Foliophoma *, Lecanicillium *, Neosulcatispora, Phanerochaete *, Scopulariopsis *, Tricharina *, Trichoderma, Wallemia * | |||
| Rare (singletons) | 0.05 | 0.75 | 1 |
| Blastobotrys, Daldinia, Lanzia, Neoeutypella, Neurospora, Palmeiromyces, Peziza, Schizothecium, Thielavia, Antrodia, Coprinopsis, Coriolopsis, Cylindrobasidium, Phlebia, Trametes, Aplosporella, Ascochyta, Bartalinia, Botryosphaeria, Cryptovalsa, Cytospora, Didymosphaeria, Diplodia, Neostagonospora, Paraphaeosphaeria, Phyllosticta, Pseudoconiothyrium, Sardiniella, Tamaracicola, Wojnowiciella, Bipolaris, Harzia, Monilia, Pseudogymnoascus, Stachybotrys | |||
| Assemblage Type | Taxonomic Rank 1 | Diversity Measures 2 | |||||
|---|---|---|---|---|---|---|---|
| D | H′ | J′ | α | DMg | DMn | ||
| Coelomycetes | Genera | 0.82 | 1.05 | 0.63 | 9.83 | 6.52 | 1.38 |
| MOTU | 0.92 | 1.60 | 0.73 | 50.25 | 22.53 | 4.69 | |
| Hyphomycetes | Genera | 0.72 | 0.77 | 0.57 | 4.43 | 3.29 | 0.82 |
| MOTU | 0.92 | 1.51 | 0.74 | 36.46 | 16.92 | 4.04 | |
| Testing Method | Logseries | Neutral Model | Lognormal | Geometric Series | Broken Stick | ||
|---|---|---|---|---|---|---|---|
| GOF test 1 | χ2 (p-value) | 99.00 (0.24) * | 99.00 (0.24) * | 99.00 (0.24) * | 90.75 (0.22) * | 90.75 (0.22) * | |
| D (p-value) | 0.18 (0.99) * | 0.18 (0.99) * | 0.27 (0.81) * | 0.27 (0.81) * | 0.27 (0.81) * | ||
| SOF test 2 | log (L) | −755 | −755.1 | −771.8 | −890.3 | −922.3 | |
| AIC | AICc (ΔAICc) | 1512.0 (0.0) | 1514.2 (2.2) | 1547.6 (35.7) | 1782.6 (270.7) | 1844.6 (332.7) | |
| Weight | 0.75 | 0.25 | <0.001 | <0.001 | <0.001 | ||
| BIC | BIC (ΔBIC) | 1515.7 (0.0) | 1521.6 (5.9) | 1555.1 (39.4) | 1786.4 (270.7) | 1844.6 (328.9) | |
| Weight | 0.95 | 0.05 | <0.001 | <0.001 | <0.001 | ||
| Host | Richness Measures 1 | Diversity Measures 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| IR | MOTUs (Per Sample) | D | H′ | J′ | α | DMg | DMn | |
| Chamaerops humilis | 22.00 | 172 (5.38) | 0.95 | 1.66 | 0.74 | 72.56 | 26.08 | 6.48 |
| Chrysalidocarpus lutescens | 12.11 | 94 (4.95) | 0.97 | 1.72 | 0.87 | 59.32 | 17.10 | 6.20 |
| Phoenix canariensis | 4.68 | 58 (1.87) | 0.94 | 1.51 | 0.85 | 35.83 | 11.45 | 4.82 |
| Phoenix dactylifera | 7.67 | 42 (3.50) | 0.96 | 1.46 | 0.90 | 29.87 | 9.07 | 4.38 |
| Phoenix reclinata | 25.38 | 108 (8.31) | 0.96 | 1.71 | 0.84 | 55.90 | 18.45 | 5.95 |
| Trachycarpus fortunei | 21.50 | 86 (6.14) | 0.86 | 1.37 | 0.71 | 40.22 | 14.89 | 4.95 |
| Richness Estimator 1 | Extrapolated Taxon Richness 2 | |
|---|---|---|
| Genera ± SE [95% CI] | MOTUs ± SE (95% CI) | |
| ŜChao1 | 158.22 ± 29.40 [122.07, 246.50] | 505.88 ± 43.76 [437.91, 613.03] |
| ŜACE | 148.46 ± 17.68 [123.74, 196.03] | 535.95 ± 38.12 [473.20, 624.41] |
| Ŝjk1 | 131.98 ± 8.36 [119.04, 152.53] | 454.94 ± 16.43 [426.38, 491.14] |
| Ŝjk2 | 156.96 ± 14.48 [134.60, 192.62] | 540.88 ± 28.44 [491.79, 603.99] |
| Ŝboot | 111.83 ± 3.72 [104.53, 119.125] | 381.69 ± 13.51 [355.21, 408.17] |
| Ŝasymptotic * | 103.77 | 324.86 |
| Host Species | ||
|---|---|---|
| Genera | MOTUs | |
| Chamaerops humilis | 52/60–81 (71) | 172/212–326 (276) |
| Chrysalidocarpus lutescens | 38/47–79 (65) | 94//119–310 (201) |
| Phoenix canariensis | 32/38–56 (50) | 58/73–127 (104) |
| Phoenix dactylifera | 25/29–42 (36) | 42/53–106 (79) |
| Phoenix reclinata | 40/46–56 (50) | 108/135–213 (181) |
| Trachycarpus fortunei | 44/53–92 (74) | 86/111–241 (172) |
| Number of MOTUs (n) Per Sample | Break-Up of Number of Samples Examined | Foliar Lesion Type 1 | |||
|---|---|---|---|---|---|
| TDB | LLS | SLS | PP | ||
| n = 1 | 11 | 4 | 0 | 4 | 3 |
| n = 2 | 17 | 4 | 3 | 4 | 5 |
| n = 3 | 15 | 7 | 1 | 5 | 2 |
| n = 4 | 15 | 8 | 1 | 3 | 3 |
| n = 5 | 12 | 4 | 5 | 2 | 1 |
| n = 6 | 7 | 2 | 1 | 2 | 2 |
| n = 7 | 5 | 2 | 1 | 1 | 1 |
| n = 8 | 4 | 0 | 2 | 1 | 1 |
| n = 9 | 9 | 4 | 3 | 1 | 1 |
| n = 10 | 6 | 3 | 1 | 1 | 1 |
| 10 < n ≤ 20 | 14 | 10 | 2 | 0 | 2 |
| 20 < n ≤ 30 | 15 | 14 | 1 | 0 | 0 |
| 30 < n ≤ 40 | 4 | 3 | 1 | 0 | 0 |
| Taxonomic Rank | Palm Leaf Spotting Fungi (PLSF) | Tropical Palm Fungi (TrPF) | ||
|---|---|---|---|---|
| Genus | Alternaria Cladosporium Didymella * Epicoccum Fusarium Libertasomyces * Neodidymelliopsis * Neosetophoma * Penicillium Phoma Sclerostagonospora * Stemphylium | Acremonium-like taxa Anthostomella Apioclypea Apiospora Appendicospora Arecomyces Astrocystis Brunneiapiospora Capsulospora Cocoicola Endocalyx Fasciatispora | Frondispora Hypoxylon Lachnum Leptosporella Linocarpon Myelosperma Nectria-like taxa Neolinocarpon Oxydothis Palmicola Pemphidium Phaeochora | Phaeochoropsis Phomatospora Serenomyces Sorokinella Trematosphaeria Xylaria |
| Family | Aspergillaceae Cladosporiaceae Didymellaceae * Nectriaceae Phaeosphaeriaceae * Pleosporaceae | Apiosporaceae Appendicosporaceae Astrosphaeriellaceae Cainiaceae Clypeosphaeriaceae Fasciatisporaceae | Leptosporellaceae Linocarpaceae Oxydothidaceae Phaeochoraceae Phomatosporaceae Xylariaceae | |
| Order | Cladosporiales Eurotiales Hypocreales Pleosporales * | Amphisphaeriales Chaetosphaeriales Phomatosporales Phyllachorales Xylariales | ||
| Class | Dothideomycetes | Sordariomycetes | ||
| Phylum | Ascomycota | Ascomycota | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.S.; Phillips, A.J.L. Exploring the Diversity and Ecological Dynamics of Palm Leaf Spotting Fungi—A Case Study on Ornamental Palms in Portugal. J. Fungi 2025, 11, 43. https://doi.org/10.3390/jof11010043
Pereira DS, Phillips AJL. Exploring the Diversity and Ecological Dynamics of Palm Leaf Spotting Fungi—A Case Study on Ornamental Palms in Portugal. Journal of Fungi. 2025; 11(1):43. https://doi.org/10.3390/jof11010043
Chicago/Turabian StylePereira, Diana S., and Alan J. L. Phillips. 2025. "Exploring the Diversity and Ecological Dynamics of Palm Leaf Spotting Fungi—A Case Study on Ornamental Palms in Portugal" Journal of Fungi 11, no. 1: 43. https://doi.org/10.3390/jof11010043
APA StylePereira, D. S., & Phillips, A. J. L. (2025). Exploring the Diversity and Ecological Dynamics of Palm Leaf Spotting Fungi—A Case Study on Ornamental Palms in Portugal. Journal of Fungi, 11(1), 43. https://doi.org/10.3390/jof11010043






