Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants
2.2. Pathogen Inoculum and Inoculation
2.3. Disease Assessment
2.4. Geographic Information System for Longitudinal Assessment of CLS
2.5. Microscopic Investigations
2.6. Statistical Analysis
3. Results
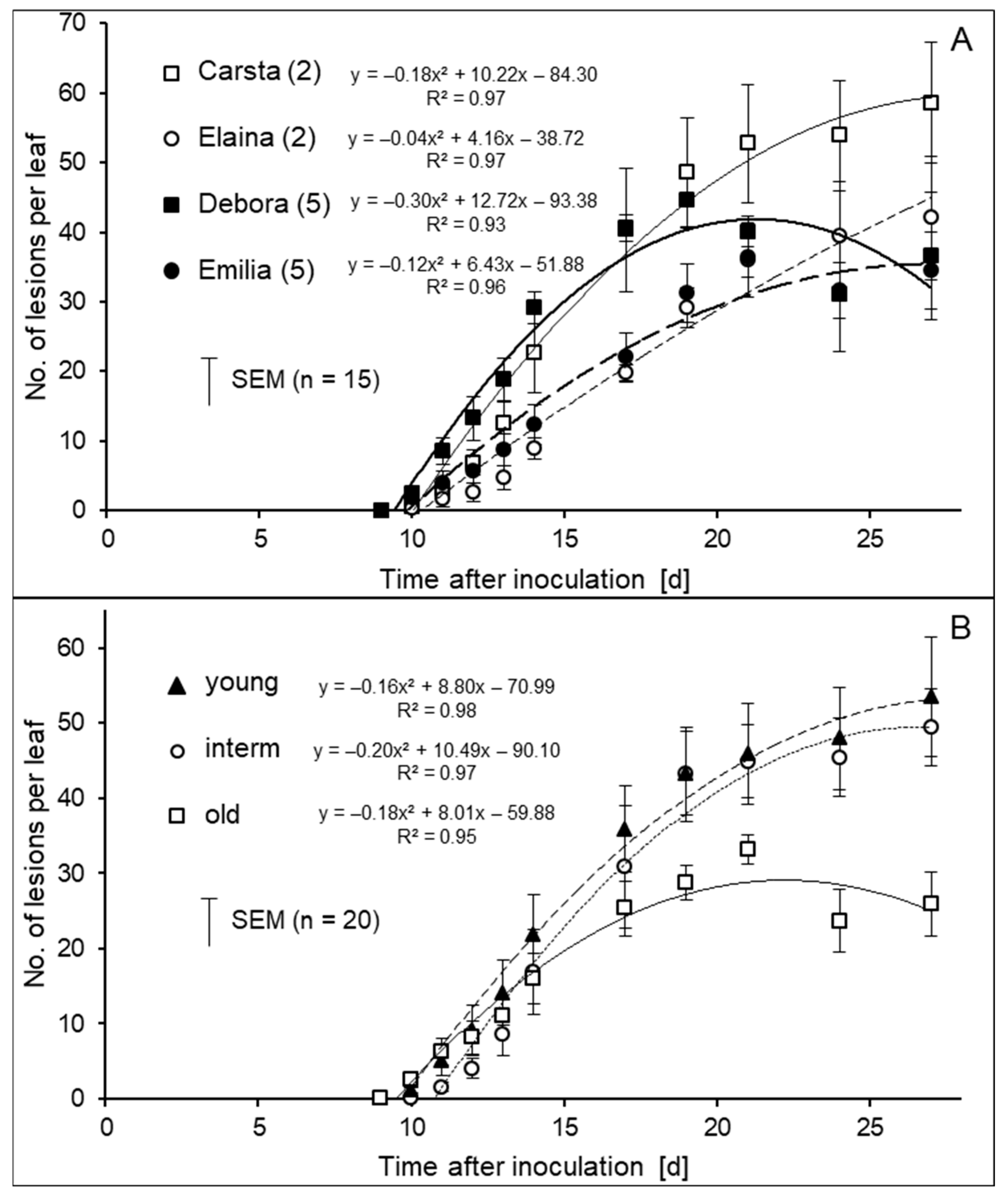
3.1. Variation in the Length of Incubation Period of CLS Lesions
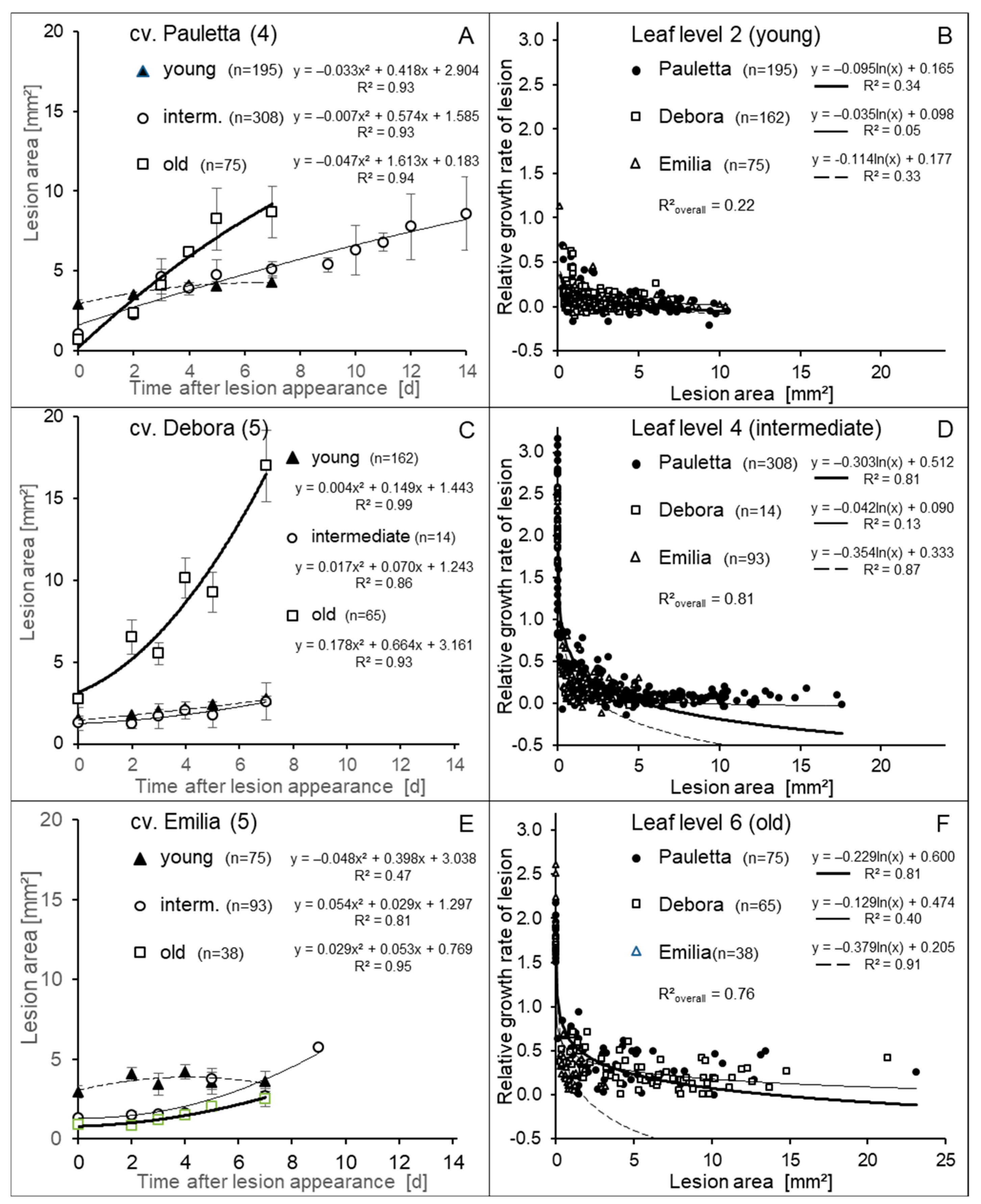
3.2. Development of Lesion Growth Depending on Age and Size
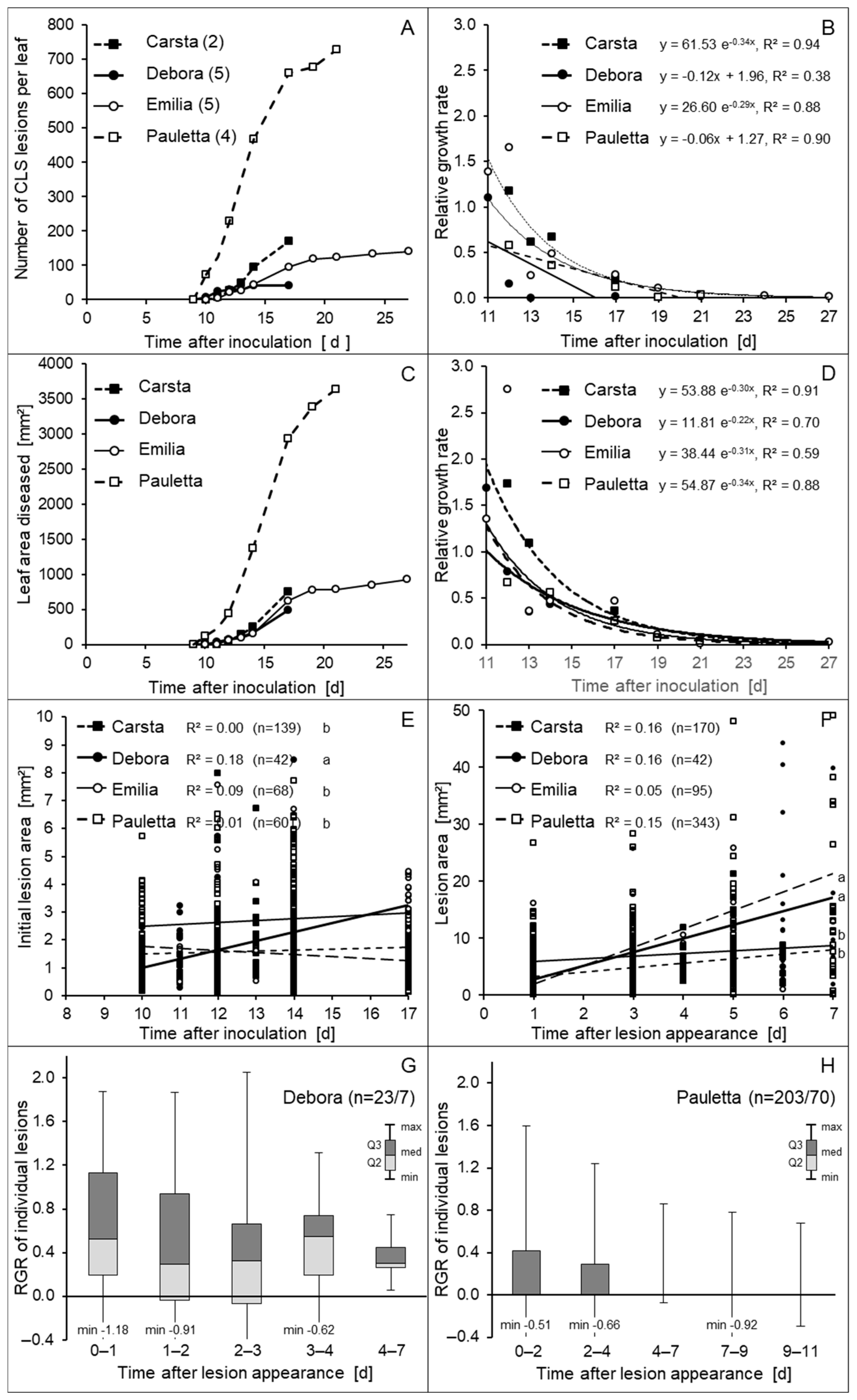
3.3. Effect of the Length of Optimal Infection Conditions on CLS Intensity and Epicuticular Development of C. beticola
3.4. Appearance and Growth of CLS Lesions on Individual Sugar Beet Leaves
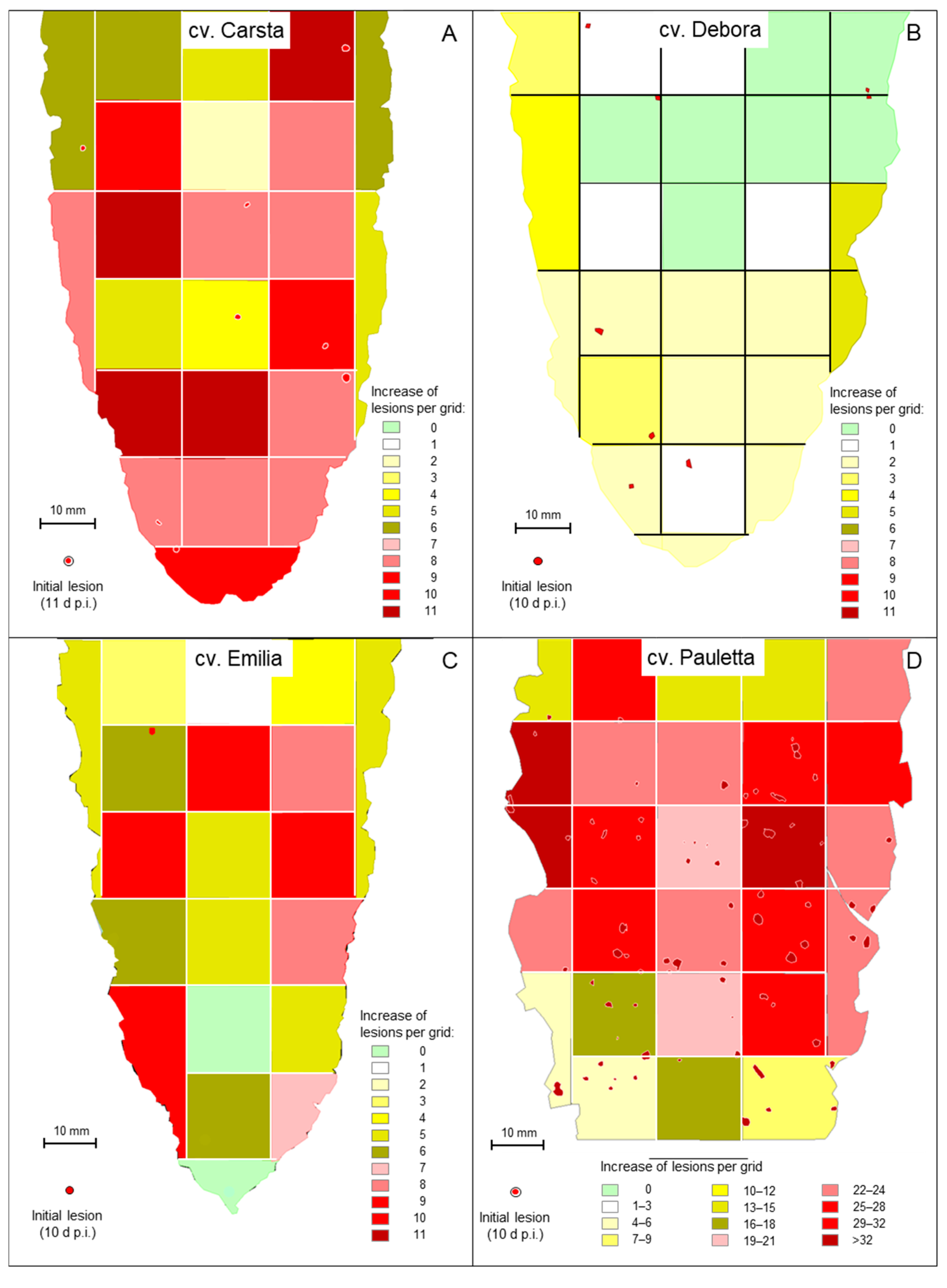
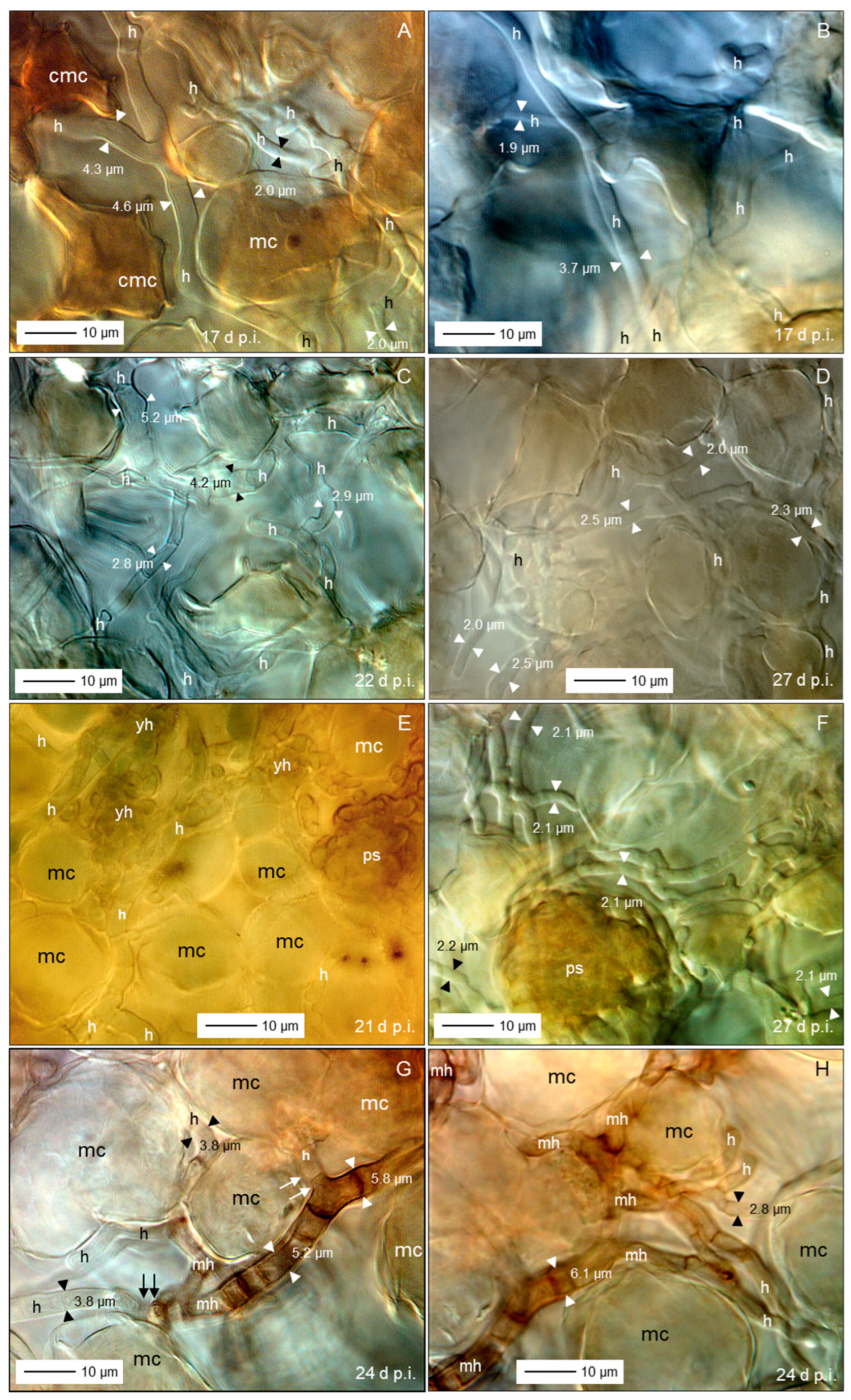
3.5. Spatial and Temporal Patterns of C. beticola Structures in Sugar Beet Leaves
4. Discussion
4.1. Length of Incubation Period
4.2. Initial Lesion Area and Lesion Growth
4.3. Tissue Colonization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shane, W.; Teng, P. Impact of Cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Dis. 1992, 76, 812–820. [Google Scholar] [CrossRef]
- Groenewald, M.; Groenewald, J.Z.; Harrington, T.; Abeln, E.C.A.; Crous, P.W. Mating type gene analysis in apparently asexual Cercospora species is suggestive of cryptic sex. Fungal Genet. Biol. 2006, 43, 813–825. [Google Scholar] [CrossRef]
- Groenewald, J.; Nakashima, C.; Nishikawa, J.; Shin, H.D.; Park, J.H.; Jama, A.; Groenewald, M.; Braun, U.; Crous, P.W. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013, 75, 115–170. [Google Scholar] [CrossRef]
- Rangel, L.I.; Spanner, R.E.; Ebert, M.K.; Pethybridge, S.J.; Stukenbrock, E.H.; de Jonge, R.; Secor, G.A.; Bolton, M.D. Cercospora beticola: The intoxicating lifestyle of the leaf spot pathogen of sugar beet. Mol. Plant Pathol. 2020, 21, 1020–1041. [Google Scholar] [CrossRef]
- Weiland, J.; Koch, G. Sugarbeet leaf spot disease (Cercospora beticola Sacc.). Mol. Plant Pathol. 2004, 5, 157–166. [Google Scholar] [CrossRef]
- Groenewald, M.; Groenewald, J.Z.; Braun, U.; Crous, P.W. Host range of Cercospora apii and C. beticola, and description of C. apiicola, a novel species from celery. Mycologia 2006, 98, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, E.G. Negative relationship of stomatal size and density with resistance in sugar beet to Cercospora beticola. Phytopathology 1972, 62, 1095–1096. [Google Scholar] [CrossRef]
- Rathaiah, Y. Stomatal tropism of Cercospora beticola in sugarbeet. Phytopathology 1977, 67, 358–362. [Google Scholar] [CrossRef]
- Steinkamp, M.; Martin, S.; Hoefert, L.; Ruppel, E. Ultrastructure of lesions produced by Cercospora beticola in leaves of Beta vulgaris. Physiol. Plant Pathol. 1979, 15, 13–26. [Google Scholar] [CrossRef]
- Windels, C.E.; Lamey, H.A.; Hilde, D.; Widner, J.; Knudsen, T. A Cerospora leaf spot model for sugar beet: In practice by an industry. Plant Dis. 1998, 82, 716–726. [Google Scholar] [CrossRef]
- Rataiah, Y. Infection of sugarbeet by Cercospora beticola in relation to stomatal condition. Phytopathology 1976, 66, 737–740. [Google Scholar] [CrossRef]
- Jacobsen, B.; Franc, G. Cercospora leaf spot. In Compendium of Beet Diseases and Pests, 2nd ed.; Harveson, R., Hanson, L., Hein, G., Eds.; APS Press: Saint Paul, MN, USA, 2009; pp. 7–10. [Google Scholar]
- Franc, G.D. Ecology and epidemiology of Cercospora beticola. In Cercospora Leaf Spot of Sugar Beet and Related Species; Lartey, R.T., Weiland, J.W., Panella, L., Crous, P., Windels, C., Eds.; APS Press: Saint Paul, MN, USA, 2010; pp. 7–19. [Google Scholar]
- Rossi, V.; Battilani, P.; Chiusa, G.; Giosuè, S.; Languasco, L.; Racca, P. Components of rate-reducing resistance to Cercospora leaf spot in sugar beet: Conidiation length, spore yield. J. Plant Pathol. 2000, 82, 125–131. [Google Scholar]
- Wolf, P.F.J.; Verreet, J.A. Factors affecting the onset of Cercospora leaf spot epidemics in sugar beet and establishment of disease-monitoring thresholds. Phytopathology 2005, 95, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tedford, S.L.; Burlakoti, R.R.; Schaafsma, A.W.; Trueman, C.L. Relationships among airborne Cercospora beticola conidia concentration, weather variables and Cercospora leaf spot severity in sugar beet (Beta vulgaris L.). Can. J. Plant Pathol. 2018, 40, 1–10. [Google Scholar] [CrossRef]
- Imbusch, F.; Liebe, S.; Erven, T.; Varrelmann, M. Dynamics of Cercospora leaf spot disease determined by aerial spore dispersal in artificially inoculated sugar beet fields. Plant Pathol. 2021, 70, 853–861. [Google Scholar] [CrossRef]
- Spanner, R.; Neubauer, J.; Heick, T.M.; Grusak, M.A.; Hamilton, O.; Rivera-Varas, V.; de Jonge, R.; Pethybridge, S.; Webb, K.M.; Leubner-Metzger, G.; et al. Seedborne Cercospora beticola can initiate Cercospora leaf spot from sugar beet (Beta vulgaris) fruit tissue. Phytopathology 2022, 112, 1016–1028. [Google Scholar] [CrossRef]
- Van der Plank, J.E. Plant Diseases: Epidemics and Control; Academic Press Inc.: New York, NY, USA, 1963. [Google Scholar]
- Parlevliet, J.E. Components of resistance that reduce the rate of epidemic development. Annu. Rev. Phytopathol. 1979, 17, 203–222. [Google Scholar] [CrossRef]
- Précigout, P.A.; Claessen, D.; Makowski, D.; Robert, C. Does the latent period of fungal pathogens reflect their trophic type? A meta-analysis of biotrophs, hemibiotrophs and necrotrophs. Phytopathology 2020, 110, 345–361. [Google Scholar] [CrossRef]
- Jacobsen, B.J.; Johnson, M.; Zidack, N.K.; Eckhoff, J.; Bergman, J. Effects of high temperatures on Cercospora leaf spot infection and sporulation and effects of variety and number of fungicide sprays on yield. Sugarbeet Res. Ext. Rep. 2004, 35, 205–208. [Google Scholar]
- Pool, V.W.; McKay, M.B. Climatic conditions as related to Cercospora beticola. J. Agric. Res. 1916, 6, 21–60. [Google Scholar]
- Rossi, V.; Battilani, P.; Chiusa, G.; Giosuè, S.; Languasco, L.; Racca, P. Components of rate-reducing resistance to Cercospora leaf spot in sugar beet: Incubation length, infection efficiency, lesion size. J. Plant Pathol. 1999, 81, 25–35. [Google Scholar]
- Ebert, M.K. Effector Biology of the Sugar Beet Pathogen Cercospora beticola. Ch 3: Homologs of Verticillium dahliae Effector Ave1 Contribute to Virulence of Fungal Pathogens of Diverse Plant Hosts. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Ebert, M.K.; Rangel, L.I.; Spanner, R.E.; Taliadoros, D.; Wang, X.; Friesen, T.L.; de Jonge, R.; Neubauer, J.D.; Secor, G.A.; Thomma, B.P.; et al. Identification and characterization of Cercospora beticola necrosis-inducing effector CbNip1. Mol. Plant Pathol. 2021, 22, 301–316. [Google Scholar] [CrossRef]
- Daub, M.E.; Ehrenshaft, M. The photoactivated Cercospora toxin cercosporin: Contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 2000, 38, 461–490. [Google Scholar] [CrossRef]
- Weiland, J.; Chung, K.; Suttle, J. The role of cercosporin in the virulence of Cercospora spp. to plant hosts. In Cercospora Leaf Spot of Sugar Beet and Related Species; Lartey, R.T., Weiland, J.J., Panella, L., Crous, P.W., Windels, C.E., Eds.; APS Press: Saint Paul, MN, USA, 2010; pp. 109–117. [Google Scholar]
- Steinkamp, M.P.; Martin, S.S.; Hoefert, L.L.; Ruppel, E.G. Ultrastructure of lesions produced in leaves of Beta vulgaris by cercosporin, a toxin from Cercospora beticola. Phytopathology 1981, 71, 1272–1281. [Google Scholar]
- Staerkel, C.; Boenisch, M.J.; Kröger, C.; Bormann, J.; Schäfer, W.; Stahl, D. CbCTB2, an O-methyltransferase is essential for biosynthesis of the phytotoxin cercosporin and infection of sugar beet by Cercospora beticola. BMC Plant Biol. 2013, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Goudet, C.; Milat, M.L.; Sentenac, H.; Thibaud, J.B. Beticolins, nonpeptidic, polycyclic molecules produced by the phytopathogenic fungus Cercospora beticola, as a new family of ion channel-forming toxins. Mol. Plant-Microbe Interact. 2000, 13, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.K.; Spanner, R.E.; de Jonge, R.; Smith, D.J.; Holthusen, J.; Secor, G.A.; Thomma, B.; Bolton, M.D. Gene cluster conservation identifies melanin and perylenequinone biosynthesis pathways in multiple plant pathogenic fungi. Environ. Microbiol. 2019, 21, 913–927. [Google Scholar] [CrossRef]
- Bhuiyan, M.Z.R.; Solanki, S.; del Rio Mendoza, L.E.; Borowicz, P.; Lakshman, D.K.; Qi, A.; Ameen, G.; Khan, M.F.R. Histopathological investigation of varietal responses to Cercospora beticola infection process on sugar beet leaves. Plant Dis. 2023, 107, 3906–3912. [Google Scholar] [CrossRef]
- Chen, C.; Keunecke, H.; Neu, E.; Kopisch-Obuch, F.J.; McDonald, B.A.; Stapley, J. Molecular epidemiology of Cercospora leaf spot on resistant and susceptible sugar beet hybrids. Plant Pathol. 2025, 74, 69–83. [Google Scholar] [CrossRef]
- Bundessortenamt. Beschreibende Sortenliste: Getreide, Mais, Öl- und Faserpflanzen, Leguminosen, Rüben, Zwischenfrüchte 2013; Bundessortenamt: Hannover, Germany, 2013. [Google Scholar]
- Meier, U.; Bachmann, L.; Buhtz, H.; Hack, H.; Klose, R.; Märländer, B.; Weber, E. Phänologische Entwicklungsstadien der Beta-Rüben (Beta vulgaris L. ssp.). Codierung und Beschreibung nach der erweiterten BBCH-Skala (mit Abbildungen). Nachrichtenblatt Dtsch. Pflanzenschutzd. 1993, 45, 37–41. [Google Scholar]
- De Coninck, B.M.A.; Amand, O.; Delauré, S.L.; Lucas, S.; Hias, N.; Weyens, G.; Mathys, J.; De Bruyne, E.; Cammue, B.P.A. The use of digital image analysis and real-time PCR fine-tunes bioassays for quantification of Cercospora leaf spot disease in sugar beet breeding. Plant Pathol. 2012, 61, 76–84. [Google Scholar] [CrossRef]
- Hood, M.E.; Shew, H.D. Applications of KOH–aniline blue fluorescence in the study of plant–fungal interactions. Phytopathology 1996, 86, 704–708. [Google Scholar] [CrossRef]
- Rossi, V.; Giosuè, S.; Racca, P. A model integrating components of rate-reducing resistance to Cercospora leaf spot in sugar beet. J. Phytopathol. 1999, 147, 339–346. [Google Scholar] [CrossRef]
- Bergamin Filho, A. Comparative epidemiology: Soybean rust and other diseases. In Facing the Challenge of Soybean Rust in South America; Kudo, H., Suenaga, K., Soares, R.M., Toledo, A., Eds.; JIRCAS: Tsukuba, Japan; Embrapa Soybean: Londrina, Brazil, 2008; pp. 31–38. [Google Scholar]
- Vaghefi, N.; Kikkert, J.R.; Bolton, M.D.; Hanson, L.E.; Secor, G.A.; Pethybridge, S.J. De novo genome assembly of Cercospora beticola for microsatellite marker development and validation. Fungal Ecol. 2017, 26, 125–134. [Google Scholar] [CrossRef]
- Moretti, M.; Karaoglanidis, G.; Saracchi, M.; Fontana, A.; Farina, G. Analysis of genotypic diversity in Cercospora beticola Sacc. field isolates. Ann. Microbiol. 2006, 56, 215–221. [Google Scholar] [CrossRef]
- Vaghefi, N.; Nelson, S.C.; Kikkert, J.R.; Pethybridge, S.J. Genetic structure of Cercospora beticola populations on Beta vulgaris in New York and Hawaii. Sci. Rep. 2017, 7, 1726. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Battilani, P.; Cavanna, M. Relationship between Cercospora leaf spot disease and photosynthetic activity in sugar beet. In Proceedings of 8th Congress Mediterranean Phytopathological Union, Agadir, Morocco; Mediterranean Phytopathological Union: Florence, Italy, 1990; pp. 209–210. [Google Scholar]
- Weltmeier, F.; Mäser, A.; Menze, A.; Hennig, S.; Schad, M.; Breuer, F.; Schulz, B.; Holtschulte, B.; Nehls, R.; Stahl, D.J. Transcript profiles in sugar beet genotypes uncover timing and strength of defense reactions to Cercospora beticola infection. Mol. Plant-Microbe Interact. 2011, 24, 758–772. [Google Scholar] [CrossRef]
- De Jonge, R.; Ebert, M.K.; Huitt-Roehl, C.R.; Pal, P.; Suttle, J.C.; Spanner, R.E.; Neubauer, J.D.; Jurick, W.M.; Stott, K.A.; Secor, G.A.; et al. Gene cluster conservation provides insight into cercosporin biosynthesis and extends production to the genus Colletotrichum. Proc. Natl. Acad. Sci. USA 2018, 115, E5459–E5466. [Google Scholar] [CrossRef] [PubMed]
- Callahan, T.M.; Rose, M.S.; Meade, M.J.; Ehrenshaft, M.; Upchurch, R.G. CFP, the putative cercosporin transporter of Cercospora kikuchii, is required for wild type cercosporin production, resistance, and virulence on soybean. Mol. Plant-Microbe Interact. 1999, 12, 901–910. [Google Scholar] [CrossRef]
- Choquer, M.; Dekkers, K.L.; Chen, H.Q.; Cao, L.; Ueng, P.P.; Daub, M.E.; Chung, K.R. The CTB1 gene encoding a fungal polyketide synthase is required for cercosporin biosynthesis and fungal virulence of Cercospora nicotianae. Mol. Plant-Microbe Interact. 2005, 18, 468–476. [Google Scholar] [CrossRef]
- Daub, M.E. Cercosporin, a photosensitizing toxin from Cercospora species. Phytopathology 1982, 72, 370–374. [Google Scholar] [CrossRef]
- Harveson, R.M.; Hanson, L.E.; Hein, G.L. Compendium of Beet Diseases and Pests; APS Press: Saint Paul, MN, USA, 2009. [Google Scholar]
- Leucker, M.; Mahlein, A.K.; Steiner, U.; Oerke, E.C. Improvement of lesion phenotyping in Cercospora beticola–sugar beet interaction by hyperspectral imaging. Phytopathology 2016, 106, 177–184. [Google Scholar] [CrossRef]
- Leucker, M.; Wahabzada, M.; Kersting, K.; Peter, M.; Beyer, W.; Steiner, U.; Mahlein, A.K.; Oerke, E.C. Hyperspectral imaging reveals the effect of sugar beet quantitative trait loci on Cercospora leaf spot resistance. Funct. Plant Biol. 2017, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buanafina, M.M.d.O.; Morris, P. The impact of cell wall feruloylation on plant growth, responses to environmental stress, plant pathogens and cell wall degradability. Agronomy 2022, 12, 1847. [Google Scholar] [CrossRef]
- Jones, K.; Zhu, J.; Jenkinson, C.B.; Kim, D.W.; Pfeifer, M.A.; Khang, C.H. Disruption of the interfacial membrane leads to Magnaporthe oryzae effector re-location and lifestyle switch during rice blast disease. Front. Cell Dev. Biol. 2021, 9, 681734. [Google Scholar] [CrossRef]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef]
- Gow, N.A.R. Yeast-hyphal dimorphism. In The Growing Fungus; Gow, N.A.R., Gadd, G.M., Eds.; Chapman & Hall: London, UK, 1995; pp. 403–422. [Google Scholar]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [PubMed]
- Perfect, S.E.; Green, J.R.; O’Connell, R.J. Surface characteristics of necrotrophic secondary hyphae produced by the bean anthracnose fungus, Colletotrichum lindemuthianum. Eur. J. Plant Pathol. 2001, 107, 813–819. [Google Scholar] [CrossRef]
- Dean, R.A.; Talbot, N.J.; Ebbole, D.J.; Farman, M.L.; Mitchell, T.K.; Orbach, M.J.; Thon, M.; Kulkarni, R.; Xu, J.-R.; Pan, H.; et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [Google Scholar] [CrossRef]
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and how to kill a plant cell: Infection strategies of plant pathogenic fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Steiner, U.; Oerke, E.C. The hemibiotrophic apple scab fungus Venturia inaequalis induces a biotrophic interface but lacks a necrotrophic stage. J. Fungi 2024, 10, 831. [Google Scholar] [CrossRef]
- Horbach, R.; Deising, H.B. The biotrophy-necrotrophy switch in fungal pathogenesis. In Agricultural Applications, 2nd ed.; The Mycota XI; Kempken, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 343–360. [Google Scholar]
- Hernández-Oñate, M.A.; Herrera-Estrella, A. Damage response involves mechanisms conserved across plants, animals and fungi. Curr. Genet. 2015, 61, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Karimi, H.Z.; Baldrich, P.; Rutter, B.D.; Borniego, L.; Zajt, K.K.; Meyers, B.C.; Innes, R.W. Arabidopsis apoplastic fluid contains sRNA-and circular RNA–protein complexes that are located outside extracellular vesicles. Plant Cell 2022, 34, 1863–1881. [Google Scholar] [CrossRef]
- Oliver, R.P.; Ipcho, S.V. Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol. Plant Pathol. 2004, 5, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Valent, B. Communication between filamentous pathogens and plants at the biotrophic interface. Annu. Rev. Phytopathol. 2013, 51, 587–611. [Google Scholar] [CrossRef]
- Blein, M.; Levrel, A.; Lemoine, J.; Gautier, V.; Chevalier, M.; Barloy, D. Oculimacula yallundae lifestyle revisited: Relationships between the timing of eyespot symptom appearance, the development of the pathogen and the responses of infected partially resistant wheat plants. Plant Pathol. 2009, 58, 1–11. [Google Scholar] [CrossRef]
- Meinhardt, L.W.; Lacerda Costa, G.G.; Thomazella, D.P.T.; Teixeira, P.J.P.L.; Carazzolle, M.F.; Schuster, S.C.; Carlson, J.E.; Guiltinan, M.J.; Mieczkowski, P.; Farmer, A.; et al. Genome and secretome analysis of the hemibiotrophic fungal pathogen, Moniliophthora roreri, which causes frosty pod rot disease of cacao: Mechanisms of the biotrophic and necrotrophic phases. BMC Genom. 2014, 15, 164. [Google Scholar] [CrossRef]
- Guilengue, N.; Silva, M.d.C.; Talhinhas, P.; Neves-Martins, J.; Loureiro, A. Subcuticular–intracellular hemibiotrophy of Colletotrichum lupini in Lupinus mutabilis. Plants 2022, 11, 3028. [Google Scholar] [CrossRef]
- Sánchez-Vallet, A.; McDonald, M.C.; Solomon, P.S.; McDonald, B.A. Is Zymoseptoria tritici a hemibiotroph? Fungal Genet. Biol. 2015, 79, 29–32. [Google Scholar] [CrossRef]






| Parameter | Sugar Beet Genotype | |||
|---|---|---|---|---|
| Carsta (2) 1 | Debora (5) | Emilia (5) | Pauletta (4) | |
| Total leaf area [mm2] | 6.486 | 6.867 | 5.944 | 9.455 |
| Initial CLS lesion(s): | ||||
| - First appearance [d p.i.] | 11 | 10 | 10 | 10 |
| - No. of lesions | 8 | 8 | 1 | 72 |
| - Lesion area [mm2] 2 | 1.18 | 1.13 | 1.11 | 1.27 |
| - Total lesion area [mm2] | 8.8 | 6.5 | 1.1 | 115 |
| CLS lesions 17 d p.i.: | ||||
| - No. of lesions | 170 | 43 | 95 | 661 |
| - Lesion area [mm2] 2 | 6.14 | 14.72 | 5.59 | 14.55 |
| - Total lesion area [mm2] | 759 | 494 | 628 | 2.922 |
| - Correlation time × area (r) | 0.40 (p ≤ 0.001) | 0.40 (p ≤ 0.01) | 0.21 (p ≤ 0.05) | 0.40 (p ≤ 0.001) |
| Mean growth duration [d] 3 | 5.8 ± 0.2 (n = 26) | 4.9 ± 0.5 (n = 27) | 2.5 ± 0.3 (n = 94) | 2.8 ± 0.2 (n = 231) |
| Parameter | Sugar Beet Genotype | |||
|---|---|---|---|---|
| Carsta | Debora | Emilia | Pauletta | |
| No. of grids | 23 | 25 | 20 | 27 |
| First CLS appearance [d p.i.] | 11 | 10 | 10 | 10 |
| - No. of lesions per leaf | 8 | 8 | 1 | 72 |
| - No. of lesions per grid | 0.35 ± 0.10 1 | 0.32 ± 0.09 | 0.05 ± 0.05 | 2.67 ± 0.40 |
| + Variance-to-mean ratio | 0.65 | 0.68 | 0.95 | 1.61 |
| CLS lesions, 17 d p.i.: | 170 | 43 | 95 | 661 |
| - No. of lesions per grid | 7.39 ± 0.491 | 1.72 ± 0.26 | 4.75 ± 0.53 | 24.48 ± 1.60 |
| + Variance-to-mean ratio | 0.76 | 1.00 | 1.20 | 2.83 |
| - Increase in no. of lesions | 162 | 35 | 94 | 589 |
| - Increase no. lesion per grid | 7.04 ± 0.48 1 | 1.40 ± 0.26 | 4.70 ± 0.52 | 21.81 ± 1.51 |
| + Variance-to-mean ratio | 0.75 | 1.20 | 1.17 | 2.84 |
| + Grids without lesion | 6.9 | 2.1 | 4.6 | 19.0 |
| + Grids with lesion(s) | 7.3 | 1.1 | 6.0 | 22.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oerke, E.-C.; Steiner, U. Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets. J. Fungi 2025, 11, 211. https://doi.org/10.3390/jof11030211
Oerke E-C, Steiner U. Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets. Journal of Fungi. 2025; 11(3):211. https://doi.org/10.3390/jof11030211
Chicago/Turabian StyleOerke, Erich-Christian, and Ulrike Steiner. 2025. "Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets" Journal of Fungi 11, no. 3: 211. https://doi.org/10.3390/jof11030211
APA StyleOerke, E.-C., & Steiner, U. (2025). Intra-Leaf Variability of Incubation Period Sheds New Light on the Lifestyle of Cercospora beticola in Sugar Beets. Journal of Fungi, 11(3), 211. https://doi.org/10.3390/jof11030211






