Trichoderma asperellum 22043: Inoculation Promotes Salt Tolerance of Tomato Seedlings Through Activating the Antioxidant System and Regulating Stress-Resistant Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Trichoderma Strain and Plant Material
2.2. Plate Culture Experiment
2.3. Pot Experiment
2.4. Physiological and Biochemical Characteristics in Tomato Seedlings
2.5. Expression of Genes Related to Salt-Tolerance
2.6. Statistical Analysis
3. Results
3.1. Effect of T. asperellum 22043 on Tomato Seed Germination and Seedling Growth In Vitro
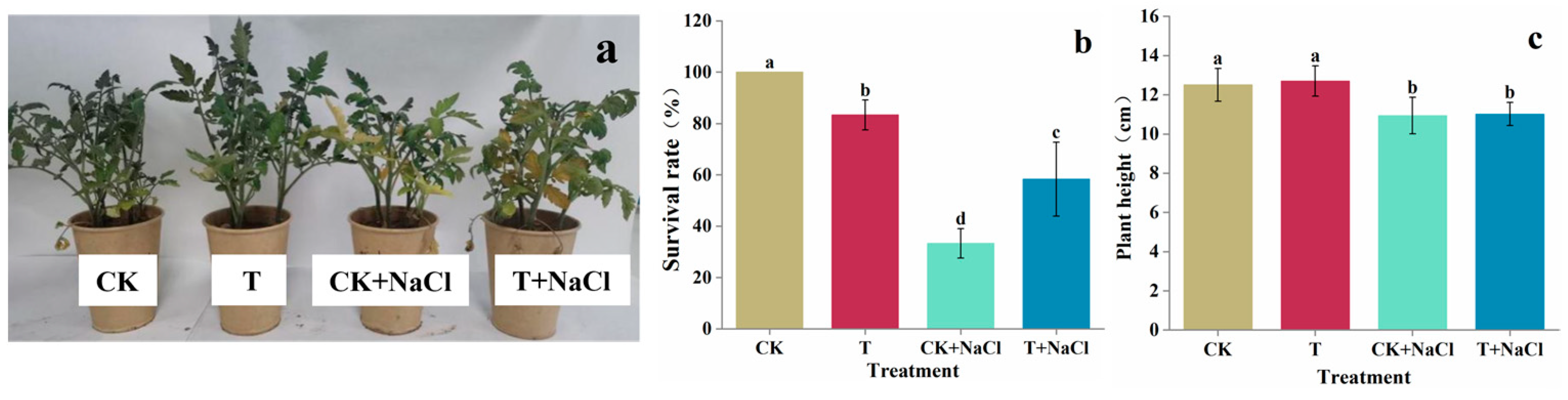
3.2. Effect of T. asperellum 22043 on Tomato Seedling Growth in Greenhouse
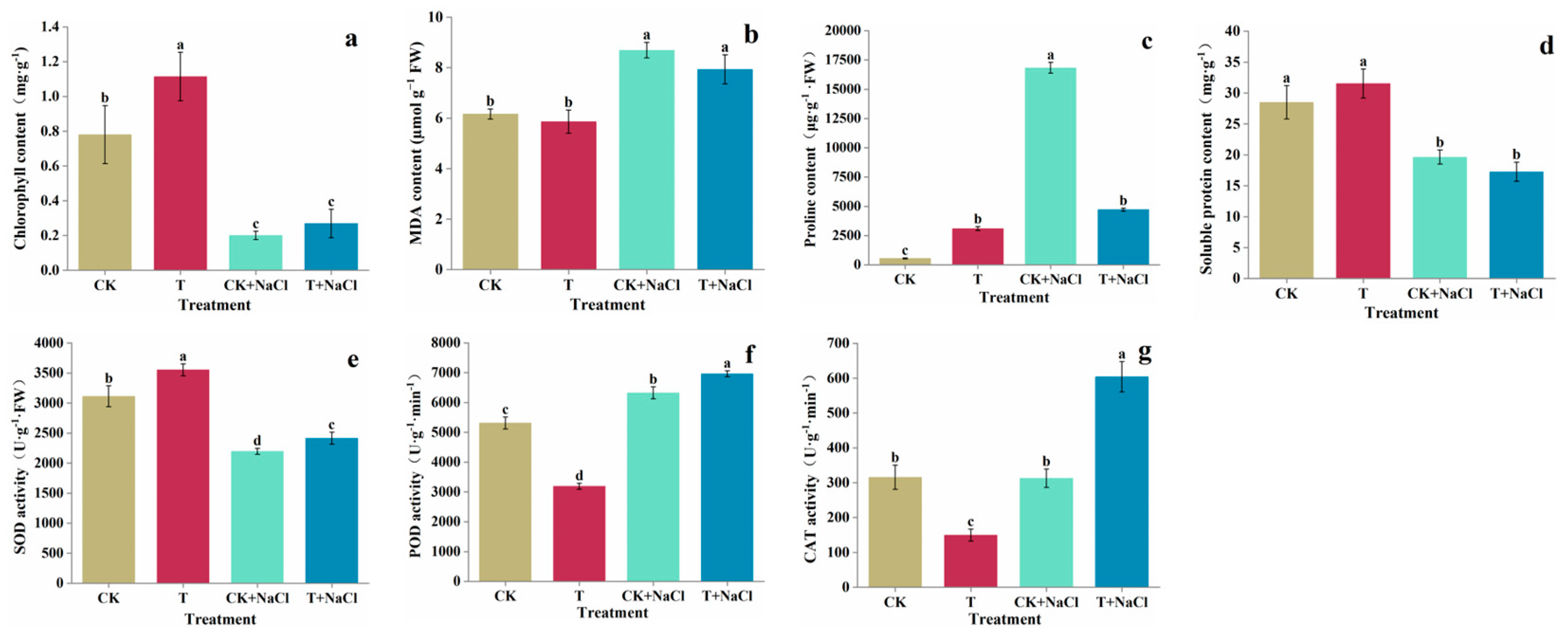
3.3. Chlorophyll, MDA, Proline and Soluble Protein Content in Tomato Seedling
3.4. SOD, POD and CAT Activity Assay
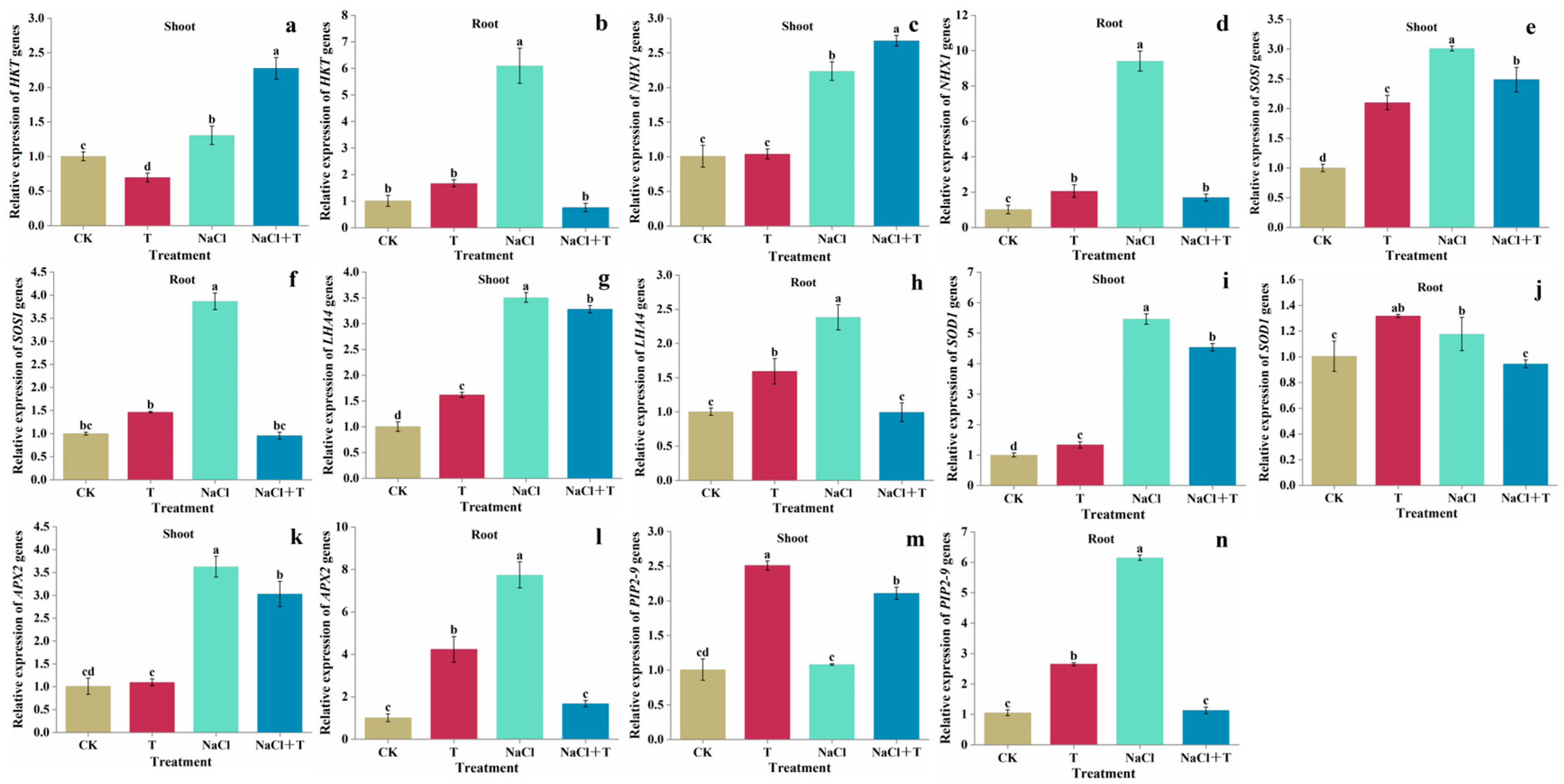
3.5. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarai, B.; Walter, C.; Michot, D.; Montoroi, J.P.; Hachicha, M. Integrating Multiple Electromagnetic Data to Map Spatiotemporal Variability of Soil Salinity in Kairouan Region, Central Tunisia. J. Arid Land 2022, 14, 186–202. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric Oxide-Induced Salt Stress Tolerance in Plants: ROS Metabolism, Signaling, and Molecular Interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Li, Y.; Chang, C.; Wang, Z.; Zhao, G. Upscaling Remote Sensing Inversion and Dynamic Monitoring of Soil Salinization in the Yellow River Delta, China. Ecol. Indic. 2023, 148, 110087. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Liu, W.; Zhang, D.; Dong, D.; Wu, H.; Zhang, T.; Liu, D. Transcriptomic Insights into Growth Promotion Effect of Trichoderma afroharzianum TM2-4 Microbial Agent on Tomato Plants. J. Integr. Agric. 2021, 20, 1266–1276. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of Salt Stress on Tomato Plant and the Role of Calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Khasa, P.D.; Hambling, B.; Kernaghan, G.; Fung, M.; Ngimbi, E. Genetic Variability in Salt Tolerance of Selected Boreal Woody Seedlings. For. Ecol. Manag. 2002, 165, 257–269. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed Priming to Alleviate Salinity Stress in Germinating Seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Ali, A.; Tucker, T.C.; Thompson, T.L.; Salim, M. Effects of Salinity and Mixed Ammonium and Nitrate Nutrition on the Growth and Nitrogen Utilization of Barley. J. Agron. Crop Sci. 2001, 186, 223–228. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The Integration of Activity in Saline Environments: Problems and Perspectives. Funct. Plant Biol. 2013, 40, 759. [Google Scholar] [CrossRef]
- Migicovsky, Z.; Myles, S. Exploiting Wild Relatives for Genomics-Assisted Breeding of Perennial Crops. Front. Plant Sci. 2017, 8, 460. [Google Scholar] [CrossRef]
- Dodd, I.C.; Perez-Alfocea, F. Microbial Amelioration of Crop Salinity Stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The Growth Promotion of Two Salt-Tolerant Plant Groups with PGPR Inoculation: A Meta-Analysis. Sustainability 2019, 11, 378. [Google Scholar] [CrossRef]
- Kumar, V. Optimal Physical Parameters for Growth of Trichoderma Species at Varying PH, Temperature and Agitation. Virol. Mycol 2013, 03, 1000127. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in Mitigating NaCl Stress in Indian Mustard (Brassica juncea L.) through Antioxidative Defense System. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef]
- Azarmi, R. Effect of Trichoderma Isolates on Tomato Seedling Growth Response and Nutrient Uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Pascual, J.A.; Tittarelli, F.; Hernández, J.A.; Diaz-Vivancos, P. Trichoderma Harzianum T-78 Supplementation of Compost Stimulates the Antioxidant Defence System in Melon Plants: Trichoderma Inoculated Compost Enhances Antioxidant Defences. J. Sci. Food Agric. 2015, 95, 2208–2214. [Google Scholar] [CrossRef]
- Ruppel, S.; Franken, P.; Witzel, K. Properties of the Halophyte Microbiome and Their Implications for Plant Salt Tolerance. Funct. Plant Biol. 2013, 40, 940. [Google Scholar] [CrossRef]
- Maurel, C.; Verdoucq, L.; Rodrigues, O. Aquaporins and Plant Transpiration. Plant Cell Environ. 2016, 39, 2580–2587. [Google Scholar] [CrossRef]
- Soliman, M.H.; Alnusaire, T.S.; Abdelbaky, N.F.; Alayafi, A.A.M.; Hasanuzzaman, M.; Rowezak, M.M.; El-Esawi, M.; Elkelish, A. Trichoderma-Induced Improvement in Growth, Photosynthetic Pigments, Proline, and Glutathione Levels in Cucurbita Pepo Seedlings under Salt Stress. Phyton 2020, 89, 473–486. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Salinity Tolerant Trichoderma harzianum Reinforces NaCl Tolerance and Reduces Population Dynamics of Fusarium oxysporum f.sp. Ciceri in Chickpea (Cicer arietinum L.) under Salt Stress Conditions. Arch. Phytopathol. Plant Prot. 2013, 46, 1442–1467. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-Plant Root Colonization: Escaping Early Plant Defense Responses and Activation of the Antioxidant Machinery for Saline Stress Tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Glick, B.R.; Santoyo, G. ACC Deaminase in Plant Growth-Promoting Bacteria (PGPB): An Efficient Mechanism to Counter Salt Stress in Crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Yang, T.; Ma, K.; Zhang, Y. Trichoderma harzianum Mitigates Salt Stress in Cucumber via Multiple Responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, K.; Li, J.; Wei, Y.; Wang, Y.; Wu, Y.; Yang, H.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Large-scale Trichoderma diversity was associated with ecosystem, climate and geographic location. Environ. Microbiol. 2020, 22, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B.; Xue, Y. The Parasitic and Lethal Effects of Trichoderma longibrachiatum Against Heterodera avenae. Biol. Control 2014, 72, 1–8. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Yang, A.; Zhang, K.; Wang, L.; Zhang, J. Increase of Glycinebetaine Synthesis Improves Drought Tolerance in Cotton. Mol. Breed. 2007, 20, 233–248. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shafi, A.; Pal, A.K.; Sharma, V.; Kalia, S.; Kumar, S.; Ahuja, P.S.; Singh, A.K. Transgenic Potato Plants Overexpressing SOD and APX Exhibit Enhanced Lignification and Starch Biosynthesis with Improved Salt Stress Tolerance. Plant Mol. Biol. Rep. 2017, 35, 504–518. [Google Scholar] [CrossRef]
- Lurie, S.; Fallik, E.; Handros, A.; Shapira, R. The Possible Involvement of Peroxidase in Resistance ToBotrytis Cinereain Heat Treated Tomato Fruit. Physiol. Mol. Plant Pathol. 1997, 50, 141–149. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Tamoi, M.; Shigeoka, S. Evaluation of the Defense System in Chloroplasts to Photooxidative Stress Caused by Paraquat Using Transgenic Tobacco Plants Expressing Catalase from Escherichia Coli. Plant Cell Physiol. 2000, 41, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Daliakopoulos, I.N.; Apostolakis, A.; Wagner, K.; Deligianni, A.; Koutskoudis, D.; Stamatakis, A.; Tsanis, I.K. Effectiveness of Trichoderma harzianum in soil and yield conservation of tomato crops under saline irrigation. Catena 2019, 175, 144–153. [Google Scholar] [CrossRef]
- Adusumilli, N.; Kolli, S.C. Management of Salinity Stress by the Application of Trichoderma. In Advances in Trichoderma Biology for Agricultural Applications; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Fungal Biology; Springer: Cham, Switzerland, 2022; pp. 303–320. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Che Mohd Zain, C.R.; Wan Yusoff, W.M. Physiological and Growth Response of Rice Plants (Oryza sativa L.) to Trichoderma Spp. Inoculants. AMB Expr. 2014, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in Growth, Lipid Peroxidation and Some Key Antioxidant Enzymes in Chickpea Genotypes under Salt Stress. Acta Physiol. Plant 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.-J. Trichoderma reesei Improved the Nutrition Status of Wheat Crop under Salt Stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of Exogenous Melatonin on Root Physiology, Transcriptome and Metabolome of Cotton Seedlings Under Salt Stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Alleviation of the Adverse Effects of Salinity Stress in Wheat (Triticum aestivum L.) by Seed Biopriming with Salinity Tolerant Isolates of Trichoderma harzianum. Plant Soil. 2011, 347, 387–400. [Google Scholar] [CrossRef]
- Metwally, R.A.; Soliman, S.A. Alleviation of the adverse effects of NaCl stress on tomato seedlings (Solanum lycopersicum L.) by Trichoderma viride through the antioxidative defense system. Bot. Stud. 2023, 64, 4. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Solanki, M.K.; Kushwaha, P.; Kumar, S.; Srivastava, A.K. Biocontrol Potential of Salt-Tolerant Trichoderma and Hypocrea Isolates for the Management of Tomato Root Rot Under Saline Environment. J. Soil. Sci. Plant Nutr. 2020, 20, 160–176. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.-S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil Bacteria Confer Plant Salt Tolerance by Tissue-Specific Regulation of the Sodium Transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, Y.; Liu, X.; An, J.; Jiang, L.; Yu, B. Recretohalophyte Tamarix TrSOS1 Confers Higher Salt Tolerance to Transgenic Plants and Yeast than Glycophyte Soybean GmSOS1. Environ. Exp. Bot. 2019, 165, 196–207. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, M.P.; Gálvez, F.J.; Huertas, R.; Aranda, M.N.; Baghour, M.; Cagnac, O.; Venema, K. Plant NHX Cation/Proton Antiporters. Plant Signal. Behav. 2009, 4, 265–276. [Google Scholar] [CrossRef]
- Palmgren, M.G. P LANT P LASMA M EMBRANE H + -ATPases: Powerhouses for Nutrient Uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 817–845. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, Y.; Chen, X.; Song, J.; Guo, Z.; Li, K.; Zhang, K. Co-Expression of AtNHX1 and TsVP Improves the Salt Tolerance of Transgenic Cotton and Increases Seed Cotton Yield in a Saline Field. Mol. Breed. 2018, 38, 19. [Google Scholar] [CrossRef]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.-K. The Arabidopsis thaliana Salt Tolerance Gene SOS1 Encodes a Putative Na+/H+ Antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef]
- Rubio, M.B.; Quijada, N.M.; Pérez, E.; Domínguez, S.; Monte, E.; Hermosa, R. Identifying Beneficial Qualities of Trichoderma parareesei for Plants. Appl. Environ. Microbiol. 2014, 80, 1864–1873. [Google Scholar] [CrossRef]
- Boursiac, Y.; Chen, S.; Luu, D.-T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early Effects of Salinity on Water Transport in Arabidopsis Roots. Molecular and Cellular Features of Aquaporin Expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Boudet, J.; Postaire, O.; Luu, D.-T.; Tournaire-Roux, C.; Maurel, C. Stimulus-Induced Downregulation of Root Water Transport Involves Reactive Oxygen Species-Activated Cell Signalling and Plasma Membrane Intrinsic Protein Internalization. Plant J. 2008, 56, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma harzianum Alleviates Waterlogging-Induced Growth Alterations in Tomato Seedlings by Modulating Physiological, Biochemical, and Molecular Mechanisms. Environ. Exp. Bot. 2020, 171, 103946. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcon, C.; Aroca, R. Regulation by Arbuscular Mycorrhizae of the Integrated Physiological Response to Salinity in Plants: New Challenges in Physiological and Molecular Studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]

| Gene Symbol | Forward Primer (5->3) | Reverse Primer (5->3) |
|---|---|---|
| HKT | TCTAGCCCAAGAAACTCAAAT | CTAATGTTACAACTCCAAGGAATT |
| SOS1 | TCGAGTGATGATTCTGGTGG | ATCACAGTGTGGAAAGGCT |
| NHX1 | CACGATATGGTGGGCTGGTT | GGGTGTGGCCAAATCTCGTA |
| LHA4 | AAAGCAGAGAGAGAGAGACG | AGCACCACCCATTGAAAGGG |
| SOD1 | AGCGGTGGTGTCTGTCTTAG | ACCCCAATTCAAAAGGCGTC |
| APX2 | ATGGTAGCTGGAGGAGACC | TTGAGGGAGCATGGACCAAC |
| PIP2-9 | CCTGGTTACAACAATGGAA | GGTCAGTAGCAGAGAAGA |
| actin | AAAAGTGCGAGTGTCCTGTCT | TCAAAAAAACAAATTGACTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Zhao, Z.; Wei, Y.; Hu, J.; Zhou, Y.; Li, J.; Yang, H. Trichoderma asperellum 22043: Inoculation Promotes Salt Tolerance of Tomato Seedlings Through Activating the Antioxidant System and Regulating Stress-Resistant Genes. J. Fungi 2025, 11, 253. https://doi.org/10.3390/jof11040253
Hu G, Zhao Z, Wei Y, Hu J, Zhou Y, Li J, Yang H. Trichoderma asperellum 22043: Inoculation Promotes Salt Tolerance of Tomato Seedlings Through Activating the Antioxidant System and Regulating Stress-Resistant Genes. Journal of Fungi. 2025; 11(4):253. https://doi.org/10.3390/jof11040253
Chicago/Turabian StyleHu, Guangyan, Zhongjuan Zhao, Yanli Wei, Jindong Hu, Yi Zhou, Jishun Li, and Hetong Yang. 2025. "Trichoderma asperellum 22043: Inoculation Promotes Salt Tolerance of Tomato Seedlings Through Activating the Antioxidant System and Regulating Stress-Resistant Genes" Journal of Fungi 11, no. 4: 253. https://doi.org/10.3390/jof11040253
APA StyleHu, G., Zhao, Z., Wei, Y., Hu, J., Zhou, Y., Li, J., & Yang, H. (2025). Trichoderma asperellum 22043: Inoculation Promotes Salt Tolerance of Tomato Seedlings Through Activating the Antioxidant System and Regulating Stress-Resistant Genes. Journal of Fungi, 11(4), 253. https://doi.org/10.3390/jof11040253






