Associations Between Indoor Fungal Community Structures and Environmental Factors: Insights from the Evidence-Driven Indoor Air-Quality Improvement Study
Abstract
1. Introduction
2. Materials and Methods
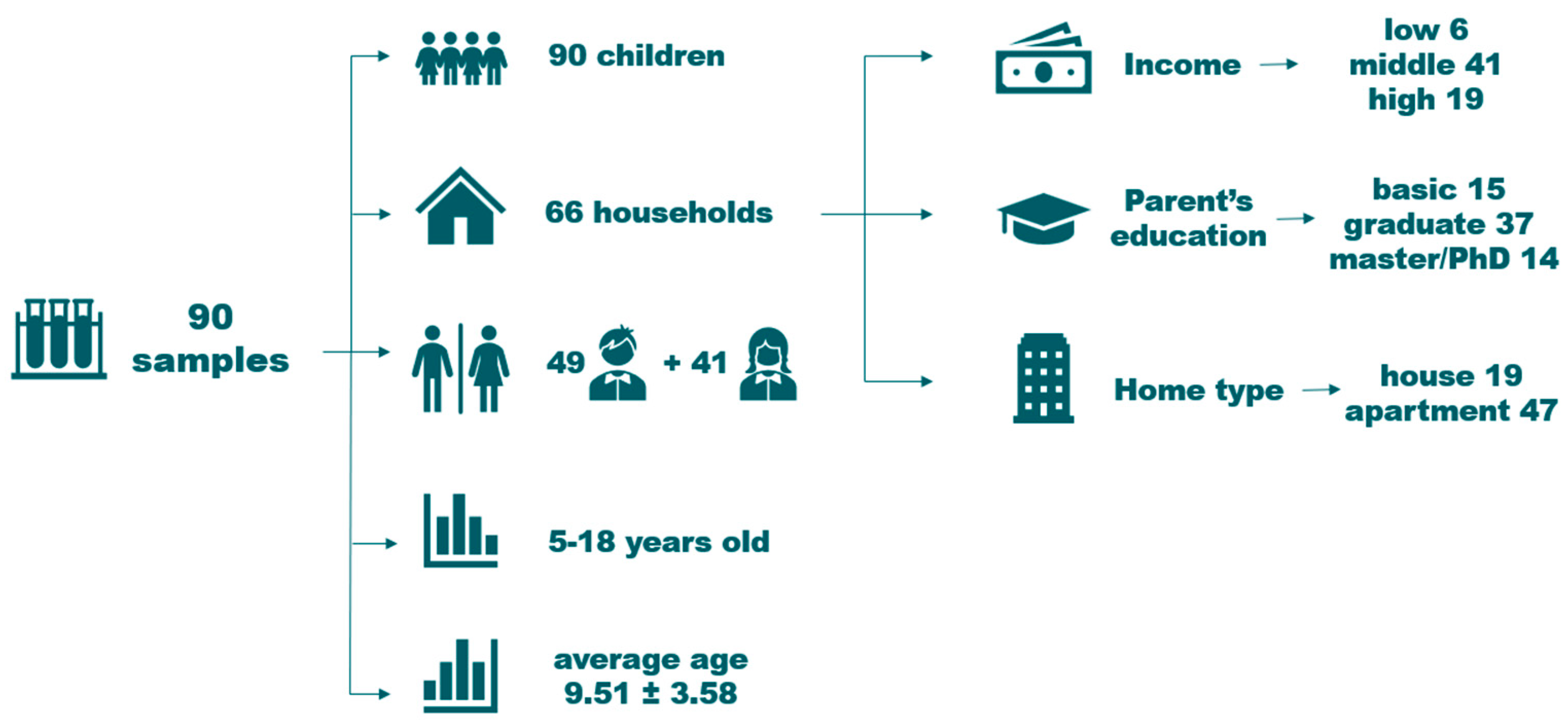
2.1. Study Group and Sample Collection
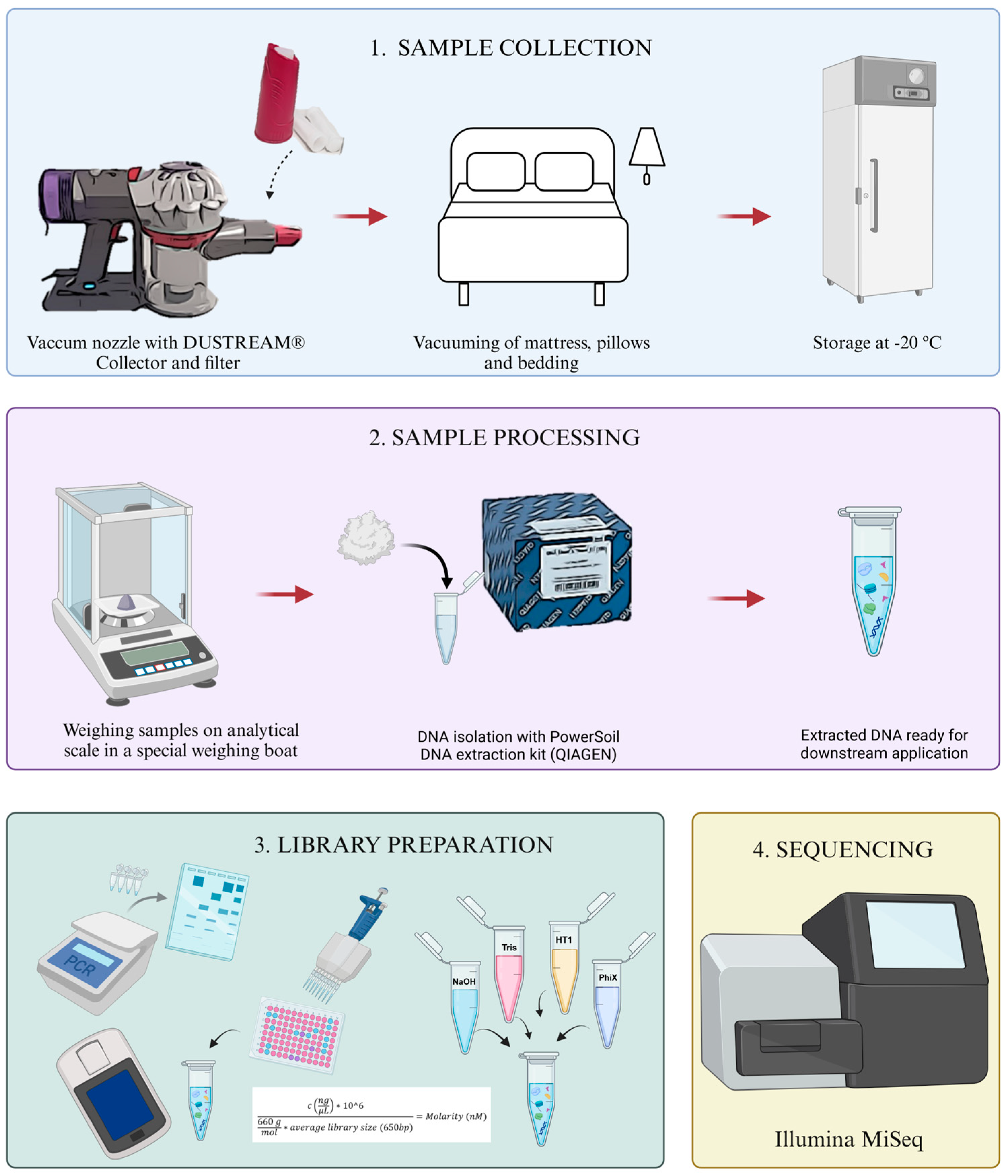
2.2. Isolation of Fungal DNA
2.3. Library Preparation and Sequencing
2.4. Statistical Analysis
2.4.1. Data Preprocessing and Taxonomic Classification
2.4.2. Statistical Analysis
2.4.3. Covariates
3. Results
3.1. Taxonomic Composition
3.2. Alpha Diversity of Fungal Communities in Relation to Environmental and Socioeconomic Factors
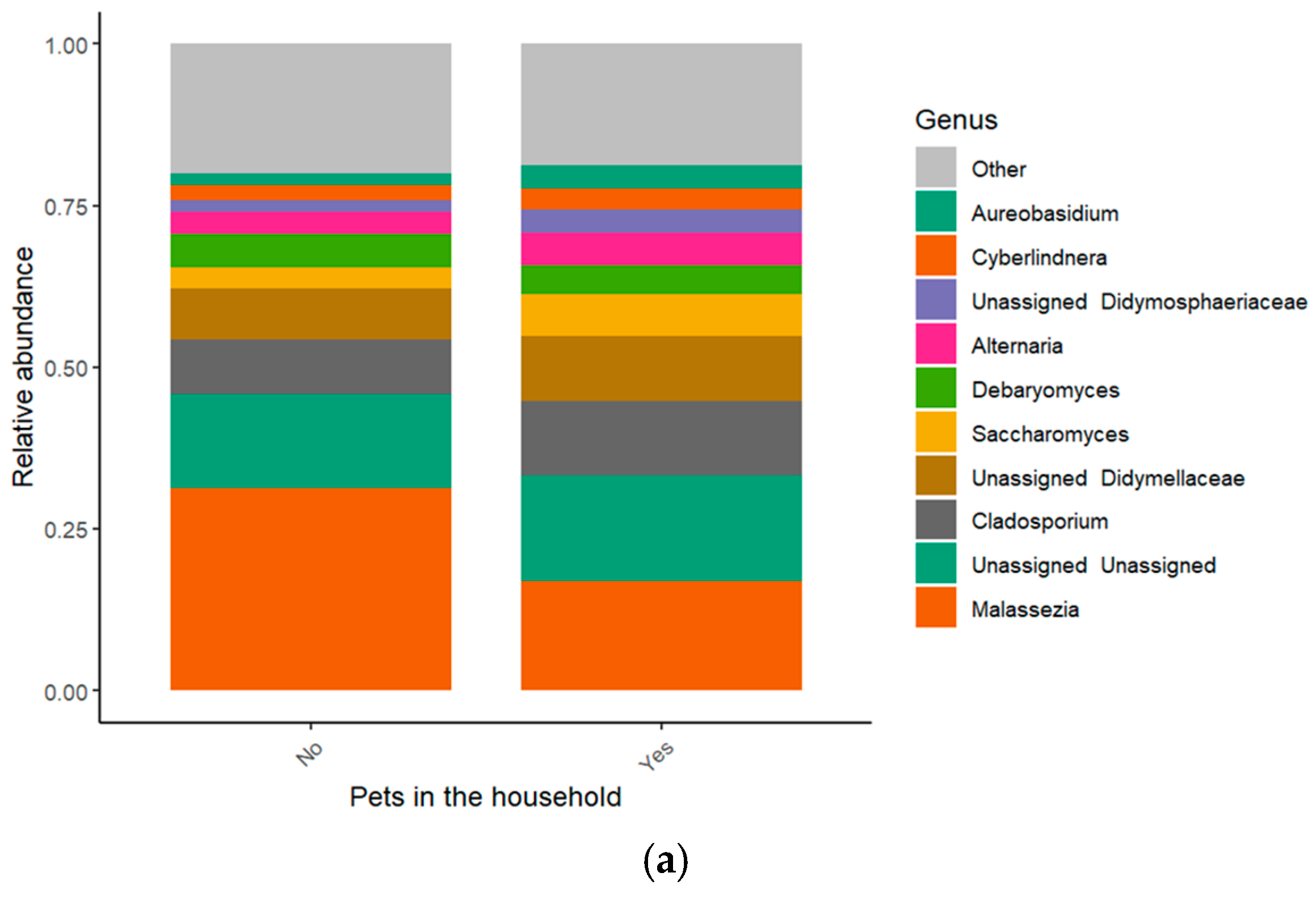
3.3. Influence of Pet Ownership and Sibling Count
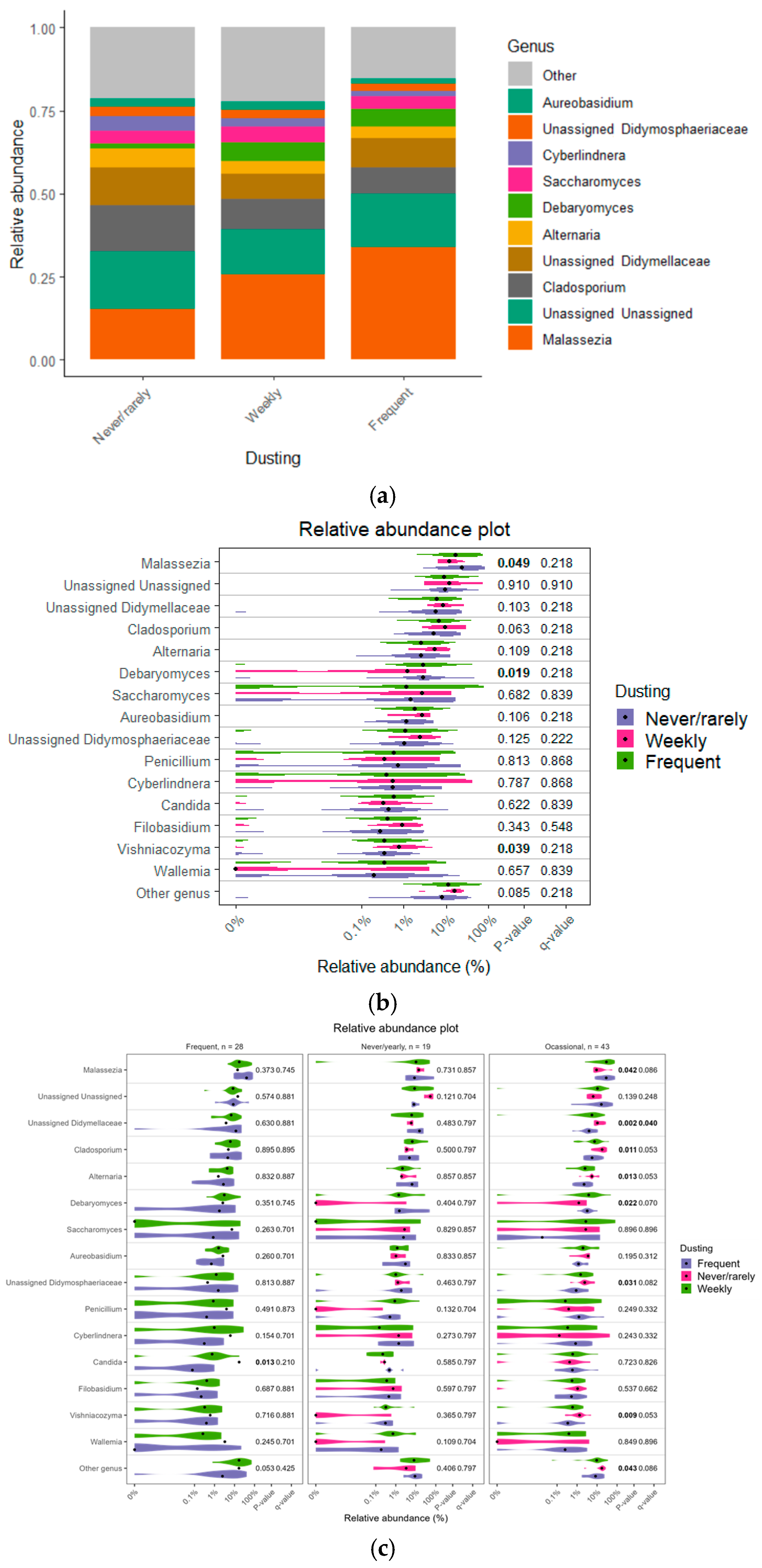
3.4. Impact of Cleaning Practices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amend, A.S.; Seifert, K.A.; Samson, R.; Bruns, T.D. Indoor Fungal Composition Is Geographically Patterned and More Diverse in Temperate Zones than in the Tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 13748–13753. [Google Scholar] [CrossRef]
- Coombs, K.; Taft, D.; Ward, D.V.; Green, B.J.; Chew, G.L.; Shamsaei, B.; Meller, J.; Indugula, R.; Reponen, T. Variability of Indoor Fungal Microbiome of Green and Non-Green Low-Income Homes in Cincinnati, Ohio. Sci. Total Environ. 2018, 610–611, 212–218. [Google Scholar] [CrossRef]
- Cox, J.; Stone, T.; Ryan, P.; Burkle, J.; Jandarov, R.; Mendell, M.J.; Adams, R.I.; Niemeier-Walsh, C.; Reponen, T. Associations of Observed Home Dampness and Mold with the Fungal and Bacterial Dust Microbiomes. Environ. Sci. Process. Impacts 2021, 23, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Norbäck, D.; Yuan, Q.; Li, Y.; Zhu, X.; Hashim, J.H.; Hashim, Z.; Ali, F.; Zheng, Y.-W.; Lai, X.-X.; et al. Indoor Microbiome, Environmental Characteristics and Asthma among Junior High School Students in Johor Bahru, Malaysia. Environ. Int. 2020, 138, 105664. [Google Scholar] [CrossRef]
- Isa, K.N.M.; Jalaludin, J.; Hashim, Z.; Than, L.T.L.; Hashim, J.H.; Norbäck, D. Fungi Composition in Settled Dust Associated with Fractional Exhaled Nitric Oxide in School Children with Asthma. Sci. Total Environ. 2022, 853, 158639. [Google Scholar] [CrossRef]
- Vandenborght, L.-E.; Enaud, R.; Urien, C.; Coron, N.; Girodet, P.-O.; Ferreira, S.; Berger, P.; Delhaes, L. Type 2-High Asthma Is Associated with a Specific Indoor Mycobiome and Microbiome. J. Allergy Clin. Immunol. 2021, 147, 1296–1305.e6. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Dunn, R.R.; Reich, B.J.; Pacifici, K.; Laber, E.B.; Menninger, H.L.; Morton, J.M.; Henley, J.B.; Leff, J.W.; Miller, S.L.; et al. The Ecology of Microscopic Life in Household Dust. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151139. [Google Scholar] [CrossRef] [PubMed]
- Estensmo, E.L.F.; Smebye Botnen, S.; Maurice, S.; Martin-Sanchez, P.M.; Morgado, L.; Bjorvand Engh, I.; Høiland, K.; Skrede, I.; Kauserud, H. The Indoor Mycobiomes of Daycare Centers Are Affected by Occupancy and Climate. Appl. Environ. Microbiol. 2022, 88, e0211321. [Google Scholar] [CrossRef]
- Park, J.-H.; Lemons, A.R.; Croston, T.L.; Park, Y.; Roseman, J.; Green, B.J.; Cox-Ganser, J.M. Mycobiota and the Contribution of Yeasts in Floor Dust of 50 Elementary Schools Characterized with Sequencing Internal Transcribed Spacer Region of Ribosomal DNA. Environ. Sci. Technol. 2022, 56, 11493–11503. [Google Scholar] [CrossRef]
- Pitkäranta, M.; Meklin, T.; Hyvärinen, A.; Paulin, L.; Auvinen, P.; Nevalainen, A.; Rintala, H. Analysis of Fungal Flora in Indoor Dust by Ribosomal DNA Sequence Analysis, Quantitative PCR, and Culture. Appl. Environ. Microbiol. 2008, 74, 233–244. [Google Scholar] [CrossRef]
- Gupta, S.; Hjelmsø, M.H.; Lehtimäki, J.; Li, X.; Mortensen, M.S.; Russel, J.; Trivedi, U.; Rasmussen, M.A.; Stokholm, J.; Bisgaard, H.; et al. Environmental Shaping of the Bacterial and Fungal Community in Infant Bed Dust and Correlations with the Airway Microbiota. Microbiome 2020, 8, 115. [Google Scholar] [CrossRef]
- Kettleson, E.M.; Adhikari, A.; Vesper, S.; Coombs, K.; Indugula, R.; Reponen, T. Key Determinants of the Fungal and Bacterial Microbiomes in Homes. Environ. Res. 2015, 138, 130–135. [Google Scholar] [CrossRef]
- Nygaard, A.B.; Charnock, C. Longitudinal Development of the Dust Microbiome in a Newly Opened Norwegian Kindergarten. Microbiome 2018, 6, 159. [Google Scholar] [CrossRef]
- Shan, Y.; Wu, W.; Fan, W.; Haahtela, T.; Zhang, G. House Dust Microbiome and Human Health Risks. Int. Microbiol. 2019, 22, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Weikl, F.; Tischer, C.; Probst, A.J.; Heinrich, J.; Markevych, I.; Jochner, S.; Pritsch, K. Fungal and Bacterial Communities in Indoor Dust Follow Different Environmental Determinants. PLoS ONE 2016, 11, e0154131. [Google Scholar] [CrossRef]
- Adams, R.I.; Bateman, A.C.; Bik, H.M.; Meadow, J.F. Microbiota of the Indoor Environment: A Meta-Analysis. Microbiome 2015, 3, 49. [Google Scholar] [CrossRef]
- Dannemiller, K.C.; Gent, J.F.; Leaderer, B.P.; Peccia, J. Indoor Microbial Communities: Influence on Asthma Severity in Atopic and Nonatopic Children. J. Allergy Clin. Immunol. 2016, 138, 76–83.e1. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Johnson, C.C.; Ownby, D.R.; Cox, M.J.; Brodie, E.L.; Havstad, S.L.; Zoratti, E.M.; Woodcroft, K.J.; Bobbitt, K.R.; Wegienka, G.; et al. Man’s Best Friend? The Effect of Pet Ownership on House Dust Microbial Communities. J. Allergy Clin. Immunol. 2010, 126, 410–412.e3. [Google Scholar] [CrossRef]
- Rintala, H.; Pitkäranta, M.; Täubel, M. Chapter 4—Microbial Communities Associated with House Dust. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 78, pp. 75–120. [Google Scholar]
- Karvonen, A.M.; Hyvärinen, A.; Rintala, H.; Korppi, M.; Täubel, M.; Doekes, G.; Gehring, U.; Renz, H.; Pfefferle, P.I.; Genuneit, J.; et al. Quantity and Diversity of Environmental Microbial Exposure and Development of Asthma: A Birth Cohort Study. Allergy 2014, 69, 1092–1101. [Google Scholar] [CrossRef]
- Beguin, H.; Nolard, N. Prevalence of Fungi in Carpeted Floor Environment: Analysis of Dust Samples from Living-Rooms, Bedrooms, Offices and School Classrooms. Aerobiologia 1996, 12, 113–120. [Google Scholar] [CrossRef]
- Lovrić, M.; Gajski, G.; Fernández-Agüera, J.; Pöhlker, M.; Gursch, H.; EDIAQI Consortium; Borg, A.; Switters, J.; Mureddu, F. Evidence Driven Indoor Air Quality Improvement: An Innovative and Interdisciplinary Approach to Improving Indoor Air Quality. Biofactors 2024, 51, e2126. [Google Scholar] [CrossRef]
- Beck, J.M.; Young, V.B.; Huffnagle, G.B. The Microbiome of the Lung. Transl. Res. 2012, 160, 258–266. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.A.; Crown, M.; Bashton, M.; Pearce, D.; Entwistle, J.A.; Sangal, V. Environmental Microbiome in the Home and Daycare Settings during the COVID-19 Pandemic, and Potential Risk of Non-Communicable Disease in Children. Environ. Microbiol. Rep. 2024, 16, e13233. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- QIAGEN. DNeasy PowerSoil Pro Kit Handbook; QIAGEN: Germantown, MD, USA, 2023. [Google Scholar]
- Illumina. 16S Sample Preparation Guide; Illumina: San Diego, CA, USA, 2013. [Google Scholar]
- Wicaksono, W.A.; Reisenhofer-Graber, T.; Erschen, S.; Kusstatscher, P.; Berg, C.; Krause, R.; Cernava, T.; Berg, G. Phyllosphere-Associated Microbiota in Built Environment: Do They Have the Potential to Antagonize Human Pathogens? J. Adv. Res. 2023, 43, 109–121. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE Database for Molecular Identification and Taxonomic Communication of Fungi and Other Eukaryotes: Sequences, Taxa and Classifications Reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis (Use R); Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Stokholm, J. Rabuplot: Relative Abundance Plot. R Package Version 0.0.1.10 from GitHub 2024. Available online: https://github.com/jstokholm/rabuplot/tree/master (accessed on 12 September 2024).
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R Package for Data Mining in Microbial Community Ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.G.; Yang, J.I.; Kim, E.; Geum, S.W.; Park, J.-H.; Yeo, M.-K. Investigation of Bacterial and Fungal Communities in Indoor and Outdoor Air of Elementary School Classrooms by 16S rRNA Gene and ITS Region Sequencing. Indoor Air 2021, 31, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, P.M.; Estensmo, E.-L.F.; Morgado, L.N.; Maurice, S.; Engh, I.B.; Skrede, I.; Kauserud, H. Analysing Indoor Mycobiomes through a Large-Scale Citizen Science Study in Norway. Mol. Ecol. 2021, 30, 2689–2705. [Google Scholar] [CrossRef] [PubMed]
- Mäki, J.M.; Kirjavainen, P.V.; Täubel, M.; Piippo-Savolainen, E.; Backman, K.; Hyvärinen, A.; Tuoresmäki, P.; Jayaprakash, B.; Heinrich, J.; Herberth, G.; et al. Associations between Dog Keeping and Indoor Dust Microbiota. Sci. Rep. 2021, 11, 5341. [Google Scholar] [CrossRef]
- Hickman, B.; Kirjavainen, P.V.; Täubel, M.; de Vos, W.M.; Salonen, A.; Korpela, K. Determinants of Bacterial and Fungal Microbiota in Finnish Home Dust: Impact of Environmental Biodiversity, Pets, and Occupants. Front. Microbiol. 2022, 13, 1011521. [Google Scholar] [CrossRef]
- Alsammar, H.; Delneri, D. An Update on the Diversity, Ecology and Biogeography of the Saccharomyces Genus. FEMS Yeast Res. 2020, 20, foaa013. [Google Scholar] [CrossRef]
- Chen, T.; Wang, S.; Jiang, X.; Huang, Y.; Mo, M.; Yu, Z. New Species of Didymellaceae within Aquatic Plants from Southwestern China. J. Fungi 2023, 9, 761. [Google Scholar] [CrossRef]
- Estensmo, E.L.F.; Morgado, L.; Maurice, S.; Martin-Sanchez, P.M.; Engh, I.B.; Mattsson, J.; Kauserud, H.; Skrede, I. Spatiotemporal Variation of the Indoor Mycobiome in Daycare Centers. Microbiome 2021, 9, 220. [Google Scholar] [CrossRef]
- Fairs, A.; Wardlaw, A.J.; Thompson, J.R.; Pashley, C.H. Guidelines on Ambient Intramural Airborne Fungal Spores. J. Investig. Allergol. Clin. Immunol. 2010, 20, 490–498. [Google Scholar]
- Richardson, M.; Gottel, N.; Gilbert, J.A.; Gordon, J.; Gandhi, P.; Reboulet, R.; Hampton-Marcell, J.T. Concurrent Measurement of Microbiome and Allergens in the Air of Bedrooms of Allergy Disease Patients in the Chicago Area. Microbiome 2019, 7, 82. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The Genus Cladosporium: A Rich Source of Diverse and Bioactive Natural Compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Silva, M.; Vieira, D.; Soares, P.; Casal, M.; Soares-Silva, I. Expanding the Knowledge on the Skillful Yeast Cyberlindnera Jadinii. J. Fungi 2021, 7, 36. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Thambugala, K.M.; de Silva, N.I.; Suwannarach, N.; Lumyong, S. A Taxonomic Assessment of Novel and Remarkable Fungal Species in Didymosphaeriaceae (Pleosporales, Dothideomycetes) from Plant Litter. Front. Microbiol. 2022, 13, 1016285. [Google Scholar] [CrossRef]
- Wrent, P.; Rivas, E.M.; Gil de Prado, E.; Peinado, J.M.; de Silóniz, M.I. Debaryomyces. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 563–570. ISBN 978-0-12-384733-1. [Google Scholar]
- Raimondi, S.; Amaretti, A.; Gozzoli, C.; Simone, M.; Righini, L.; Candeliere, F.; Brun, P.; Ardizzoni, A.; Colombari, B.; Paulone, S.; et al. Longitudinal Survey of Fungi in the Human Gut: ITS Profiling, Phenotyping, and Colonization. Front. Microbiol. 2019, 10, 01575. [Google Scholar] [CrossRef]
- Zhu, T.; Duan, Y.-Y.; Kong, F.-Q.; Galzote, C.; Quan, Z.-X. Dynamics of Skin Mycobiome in Infants. Front. Microbiol. 2020, 11, 01790. [Google Scholar] [CrossRef]
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef]
- Hanson, B.; Zhou, Y.; Bautista, E.J.; Urch, B.; Speck, M.; Silverman, F.; Muilenberg, M.; Phipatanakul, W.; Weinstock, G.; Sodergren, E.; et al. Characterization of the Bacterial and Fungal Microbiome in Indoor Dust and Outdoor Air Samples: A Pilot Study. Environ. Sci. Process Impacts 2016, 18, 713–724. [Google Scholar] [CrossRef]
- Beigelman, A.; Weinstock, G.M.; Bacharier, L.B. The Relationships between Environmental Bacterial Exposure, Airway Bacterial Colonization, and Asthma. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 137–142. [Google Scholar] [CrossRef]
- Hallas, H.W.; Chawes, B.L.; Rasmussen, M.A.; Arianto, L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Airway Obstruction and Bronchial Reactivity from Age 1 Month until 13 Years in Children with Asthma: A Prospective Birth Cohort Study. PLoS Med. 2019, 16, e1002722. [Google Scholar] [CrossRef]
- Al Bataineh, M.T.; Hamoudi, R.A.; Dash, N.R.; Ramakrishnan, R.K.; Almasalmeh, M.A.; Sharif, H.A.; Al-Hajjaj, M.S.; Hamid, Q. Altered Respiratory Microbiota Composition and Functionality Associated with Asthma Early in Life. BMC Infect. Dis. 2020, 20, 697. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-J.; Zhou, X.-Y.; Zhu, Y.-G. Microbiome and Antibiotic Resistome in Household Dust from Beijing, China. Environ. Int. 2020, 139, 105702. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Meng, Y.; Ou, Z.; Li, Y.; Zhang, M.; Chen, Y.; Zhang, Z.; Chen, X.; Mu, P.; Norbäck, D.; et al. Indoor Microbiome, Air Pollutants and Asthma, Rhinitis and Eczema in Preschool Children—A Repeated Cross-Sectional Study. Environ. Int. 2022, 161, 107137. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Ray, N.; Sorge, S.; Webster, J.; Merry, K.; Caserio, A.; Vecellio, D.J.; Kruczek, C.; Dowd, S.; Ardon-Dryer, K.; et al. Investigating the Culturable Atmospheric Fungal and Bacterial Microbiome in West Texas: Implication of Dust Storms and Origins of the Air Parcels. FEMS Microbes 2020, 1, xtaa009. [Google Scholar] [CrossRef]
- Cabañes, F.J.; Abarca, M.L.; Bragulat, M.R.; Castellá, G. Seasonal Study of the Fungal Biota of the Fur of Dogs. Mycopathologia 1996, 133, 1–7. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.-C.; Genuneit, J.; Cookson, W.O.C.M.; Braun-Fahrländer, C.; Heederik, D.; Piarroux, R.; von Mutius, E. GABRIELA Transregio 22 Study Group Exposure to Environmental Microorganisms and Childhood Asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef]


| Category | Variable | Total (n, %) | Alpha Diversity (p-Value) |
|---|---|---|---|
| Education parents | Basic | 23 (25.6) | 0.262 |
| Graduate | 48 (53.3) | ||
| Master/PhD | 19 (21.1) | ||
| Household monthly income | <EUR 1500 | 7 (7.8) | |
| EUR 1500–2500 | 25 (27.8) | 0.018 | |
| >EUR 2500 | 58 (64.4) | ||
| Suburban | 19 (21.1) | 0.548 | |
| Living environment | Urban (built surroundings) | 35 (38.9) | |
| Urban (green spaces) | 36 (40.0) | ||
| Type of home | House | 30 (33.3) | 0.451 |
| Apartment | 60 (66.7) | ||
| None | 12 (13.3) | ||
| Number of siblings | 1 | 54 (60.0) | 0.239 |
| 2 or more | 24 (26.7) | ||
| Pet ownership | No | 61 (67.8) | 0.117 |
| Yes | 29 (32.2) | ||
| Occasionally | 15 (16.7) | 0.543 | |
| Dusting furniture | Once a week | 45 (50.0) | |
| Frequently | 30 (33.3) | ||
| Never or once a year | 19 (21.1) | 0.193 | |
| Mattress vacuuming | Occasional | 43 (47.8) | |
| Frequent | 28 (31.1) | ||
| Season of sample collection | Heating | 52 (57.8) | 0.218 |
| No heating | 38 (42.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šunić, I.; Havaš Auguštin, D.; Šarac, J.; Michl, K.; Cernava, T.; Jakobsen, R.R.; Mešić, A.; Novokmet, N.; Lovrić, M. Associations Between Indoor Fungal Community Structures and Environmental Factors: Insights from the Evidence-Driven Indoor Air-Quality Improvement Study. J. Fungi 2025, 11, 261. https://doi.org/10.3390/jof11040261
Šunić I, Havaš Auguštin D, Šarac J, Michl K, Cernava T, Jakobsen RR, Mešić A, Novokmet N, Lovrić M. Associations Between Indoor Fungal Community Structures and Environmental Factors: Insights from the Evidence-Driven Indoor Air-Quality Improvement Study. Journal of Fungi. 2025; 11(4):261. https://doi.org/10.3390/jof11040261
Chicago/Turabian StyleŠunić, Iva, Dubravka Havaš Auguštin, Jelena Šarac, Kristina Michl, Tomislav Cernava, Rasmus Riemer Jakobsen, Armin Mešić, Natalija Novokmet, and Mario Lovrić. 2025. "Associations Between Indoor Fungal Community Structures and Environmental Factors: Insights from the Evidence-Driven Indoor Air-Quality Improvement Study" Journal of Fungi 11, no. 4: 261. https://doi.org/10.3390/jof11040261
APA StyleŠunić, I., Havaš Auguštin, D., Šarac, J., Michl, K., Cernava, T., Jakobsen, R. R., Mešić, A., Novokmet, N., & Lovrić, M. (2025). Associations Between Indoor Fungal Community Structures and Environmental Factors: Insights from the Evidence-Driven Indoor Air-Quality Improvement Study. Journal of Fungi, 11(4), 261. https://doi.org/10.3390/jof11040261









