Fungal Community Complexity and Stability in Clay Loam and Sandy Soils in Mangrove Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Sampling
2.2. Biochemical Factor Analyses
2.3. DNA Extraction and PCR Amplification
2.4. Bioinformatics Analysis
2.5. Statistical Analyses
3. Results
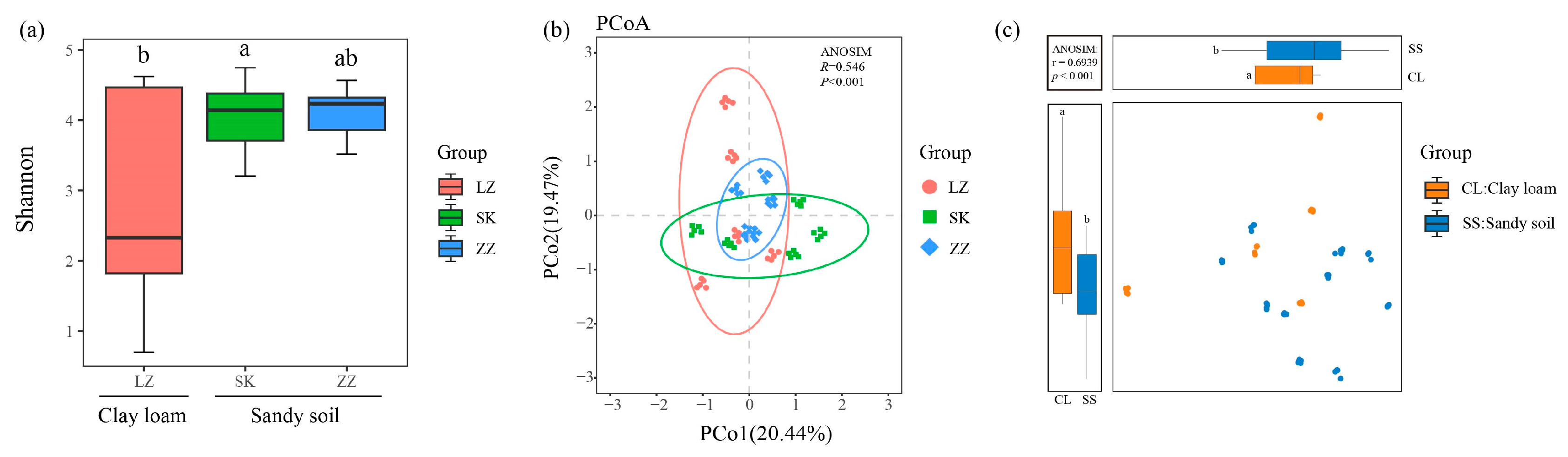
3.1. Composition and Diversity of Fungal Communities in Mangrove Clay Loam and Sandy Soil
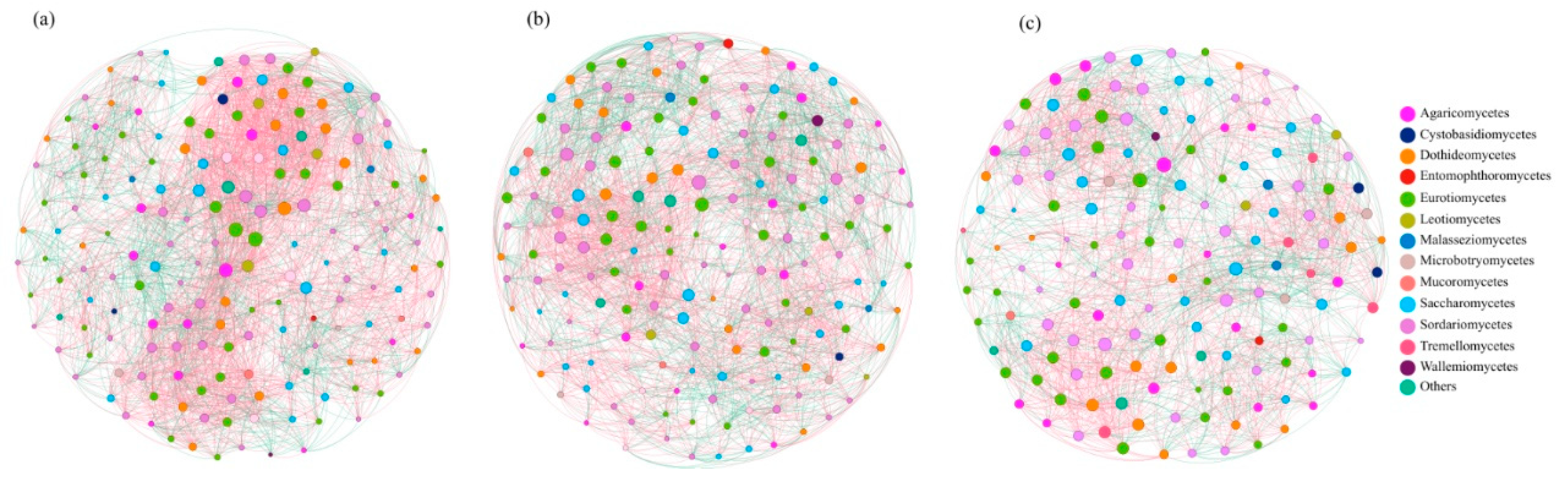
3.2. Topological Analysis of Fungal Community Structures in Mangrove Sediments
3.3. Environmental Drivers of Fungal Diversity, Complexity, and Stability in Mangrove Sediments
4. Discussion
4.1. Soil Types and Nutrient Availability Influencing Fungal Community Composition and Diversity in Mangrove Sediments
4.2. Higher Complexity and Stability of Fungal Communities in Clay Loam Compared to Sandy Soil in Mangrove Ecosystems
4.3. Main Driving Factors Influencing Complexity and Stability in Clay Loam and Sandy Soil in Mangrove Ecosystems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DO | Dissolved oxygen |
| TC | Total carbon |
| TOC | Total organic carbon |
| TP | Total phosphorus |
| TS | Total sulfur |
| TN | Total nitrogen |
| TIC | Total inorganic carbon |
| ORP | Oxidation-reduction potential |
| PCoA | Principal coordinate analysis |
| ANOSIM | Analysis of Similarity |
References
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [PubMed]
- Kuske, C.R.; Hesse, C.N.; Challacombe, J.F.; Cullen, D.; Herr, J.R.; Mueller, R.C.; Tsang, A.; Vilgalys, R. Prospects and challenges for fungal metatranscriptomics of complex communities. Fungal Ecol. 2015, 14, 133–137. [Google Scholar]
- Pettersson, S. Stability and Complexity of Ecosystems-Global Interaction Constraints, Landscape and Extinctions; Chalmers Tekniska Hogskola: Göteborg, Sweden, 2023. [Google Scholar]
- Luo, S.; Png, G.K.; Ostle, N.J.; Zhou, H.; Hou, X.; Luo, C.; Quinton, J.N.; Schaffner, U.; Sweeney, C.; Wang, D. Grassland degradation-induced declines in soil fungal complexity reduce fungal community stability and ecosystem multifunctionality. Soil Biol. Biochem. 2023, 176, 108865. [Google Scholar]
- Zhao, P.; Gao, G.; Ding, G.; Zhang, Y.; Ren, Y. Fungal complexity and stability across afforestation areas in changing desert environments. Sci. Total Environ. 2024, 912, 169398. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.; Sasekumar, A. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar]
- Jia, S.-L.; Chi, Z.; Liu, G.-L.; Hu, Z.; Chi, Z.-M. Fungi in mangrove ecosystems and their potential applications. Crit. Rev. Biotechnol. 2020, 40, 852–864. [Google Scholar]
- Barbi, F.; Prudent, E.; Vallon, L.; Buée, M.; Dubost, A.; Legout, A.; Marmeisse, R.; Fraissinet-Tachet, L.; Luis, P. Tree species select diverse soil fungal communities expressing different sets of lignocellulolytic enzyme-encoding genes. Soil Biol. Biochem. 2016, 100, 149–159. [Google Scholar] [CrossRef]
- Luis, P.; Saint-Genis, G.; Vallon, L.; Bourgeois, C.; Bruto, M.; Marchand, C.; Record, E.; Hugoni, M. Contrasted ecological niches shape fungal and prokaryotic community structure in mangroves sediments. Environ. Microbiol. 2019, 21, 1407–1424. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Pan, Y.-P.; Liu, Y.; Li, M. High-level diversity of basal fungal lineages and the control of fungal community assembly by stochastic processes in mangrove sediments. Appl. Environ. Microbiol. 2021, 87, e00928-21. [Google Scholar]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.; Yu, X.; Gu, H.; Zheng, X.; Wang, C.; Xiao, F.; Shu, L. Co-symbiosis of arbuscular mycorrhizal fungi (AMF) and diazotrophs promote biological nitrogen fixation in mangrove ecosystems. Soil Biol. Biochem. 2021, 161, 108382. [Google Scholar]
- Behera, B.; Singdevsachan, S.K.; Mishra, R.; Dutta, S.; Thatoi, H. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Wei, P.; Lei, A.; Zhou, H.; Hu, Z.; Wong, Y.; Tam, N.F.; Lu, Q. Comparison of microbial community structure and function in sediment between natural regenerated and original mangrove forests in a National Nature Mangrove Reserve, South China. Mar. Pollut. Bull. 2021, 163, 111955. [Google Scholar] [PubMed]
- Ghizelini, A.M.; Mendonça-Hagler, L.C.S.; Macrae, A. Microbial diversity in Brazilian mangrove sediments: A mini review. Braz. J. Microbiol. 2012, 43, 1242–1254. [Google Scholar] [CrossRef]
- Nagati, M.; Roy, M.; Manzi, S.; Richard, F.; Desrochers, A.; Gardes, M.; Bergeron, Y. Impact of local forest composition on soil fungal communities in a mixed boreal forest. Plant Soil 2018, 432, 345–357. [Google Scholar] [CrossRef]
- Kang, P.; Hu, J.; Pan, Y.; Qu, X.; Ran, Y.; Yang, C.; Liu, B. Response of soil fungal-community structure and function to land conversion to agriculture in desert grassland. Front. Microbiol. 2024, 15, 1413973. [Google Scholar]
- Wang, Q.; Wen, Y.; Zhao, B.; Hong, H.; Liao, R.; Li, J.; Liu, J.; Lu, H.; Yan, C. Coastal soil texture controls soil organic carbon distribution and storage of mangroves in China. Catena 2021, 207, 105709. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar]
- Wijayawardene, N.N.; Dai, D.-Q.; Jayasinghe, P.K.; Gunasekara, S.S.; Nagano, Y.; Tibpromma, S.; Suwannarach, N.; Boonyuen, N. Ecological and oceanographic perspectives in future marine fungal taxonomy. J. Fungi 2022, 8, 1141. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Cohen, R.; Hadar, Y. The roles of fungi in agricultural waste conversion. Fungi Bioremediat. 2001, 23, 305–334. [Google Scholar]
- Onlamun, T.; Boonthavee, A.; Brooks, S. Diversity and Advantages of Culturable Endophytic Fungi from Tea (Camellia sinensis). J. Fungi 2023, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Diao, L.; Wang, Y.; Yang, X.; Zhang, H.; Wang, J.; Luo, Y.; An, S.; Cheng, X. Responses of soil fungal communities and functional guilds to ~160 years of natural revegetation in the Loess Plateau of China. Front. Microbiol. 2022, 13, 967565. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, H.; Cuevas, E.; Chacon, P. The role of soil organic matter in sustaining soil fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Deininger, A.; Frigstad, H. Reevaluating the role of organic matter sources for coastal eutrophication, oligotrophication, and ecosystem health. Front. Mar. Sci. 2019, 6, 210. [Google Scholar] [CrossRef]
- Musei, S.K.; Kuyah, S.; Nyawira, S.; Ng’ang’a, S.K.; Karugu, W.N.; Smucker, A.; Nkurunziza, L. Sandy soil reclamation technologies to improve crop productivity and soil health: A review. Front. Soil Sci. 2024, 4, 1345895. [Google Scholar] [CrossRef]
- Palit, K.; Rath, S.; Chatterjee, S.; Das, S. Microbial diversity and ecological interactions of microorganisms in the mangrove ecosystem: Threats, vulnerability, and adaptations. Environ. Sci. Pollut. Res. 2022, 29, 32467–32512. [Google Scholar] [CrossRef]
- Lai, J.; Cheah, W.; Palaniveloo, K.; Suwa, R.; Sharma, S. A systematic review of the physicochemical and microbial diversity of well-preserved, restored, and disturbed mangrove forests: What is known and what is the way forward? Forests 2022, 13, 2160. [Google Scholar] [CrossRef]
- Jiménez-Gómez, I.; Valdés-Muñoz, G.; Moreno-Perlin, T.; Mouriño-Pérez, R.R.; Sánchez-Carbente, M.d.R.; Folch-Mallol, J.L.; Pérez-Llano, Y.; Gunde-Cimerman, N.; Sánchez, N.d.C.; Batista-García, R.A. Haloadaptative responses of Aspergillus sydowii to extreme water deprivation: Morphology, compatible solutes, and oxidative stress at NaCl saturation. J. Fungi 2020, 6, 316. [Google Scholar] [CrossRef]
- Adame, M.F.; Neil, D.; Wright, S.F.; Lovelock, C.E. Sedimentation within and among mangrove forests along a gradient of geomorphological settings. Estuar. Coast. Shelf Sci. 2010, 86, 21–30. [Google Scholar] [CrossRef]
- Woodroffe, C.D.; Rogers, K.; McKee, K.L.; Lovelock, C.E.; Mendelssohn, I.; Saintilan, N. Mangrove sedimentation and response to relative sea-level rise. Annu. Rev. Mar. Sci. 2016, 8, 243–266. [Google Scholar]
- Vanegas, J.; Muñoz-García, A.; Pérez-Parra, K.A.; Figueroa-Galvis, I.; Mestanza, O.; Polanía, J. Effect of salinity on fungal diversity in the rhizosphere of the halophyte Avicennia germinans from a semi-arid mangrove. Fungal Ecol. 2019, 42, 100855. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, C.; Long, D.; Wang, S.; Li, J.; Lin, X. Unveiling sedimentary microbial communities: Restored mangrove wetlands show higher heterogeneity and network stability than natural ones. Ecol. Indic. 2024, 166, 112274. [Google Scholar]
- Bordoloi, R.; Das, B.; Yam, G.; Pandey, P.K.; Tripathi, O.P. Modeling of water holding capacity using readily available soil characteristics. Agric. Res. 2019, 8, 347–355. [Google Scholar] [CrossRef]
- Kheoruenromne, I.; Suddhiprakarn, A.; Kanghae, P. Properties, environment and fertility capability of sandy soils in Northeast Plateau, Thailand. Agric. Nat. Resour. 1998, 32, 355–373. [Google Scholar]
- Yang, T.; Tedersoo, L.; Liu, X.; Gao, G.F.; Dong, K.; Adams, J.M.; Chu, H. Fungi stabilize multi-kingdom community in a high elevation timberline ecosystem. Imeta 2022, 1, e49. [Google Scholar]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar]
- Craig, H.; Antwis, R.E.; Cordero, I.; Ashworth, D.; Robinson, C.H.; Osborne, T.Z.; Bardgett, R.D.; Rowntree, J.K.; Simpson, L.T. Nitrogen addition alters composition, diversity, and functioning of microbial communities in mangrove soils: An incubation experiment. Soil Biol. Biochem. 2021, 153, 108076. [Google Scholar]
- Rousk, J.; Brookes, P.C.; Bååth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Maria, G.; Sridhar, K.; Raviraja, N. Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J. Agric. Technol. 2005, 1, 67–80. [Google Scholar]
- Thatoi, H.; Behera, B.C.; Mishra, R.R.; Dutta, S.K. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann. Microbiol. 2013, 63, 1–19. [Google Scholar] [CrossRef]
- Ferreira, T.; Otero, X.; Vidal-Torrado, P.; Macías, F. Effects of bioturbation by root and crab activity on iron and sulfur biogeochemistry in mangrove substrate. Geoderma 2007, 142, 36–46. [Google Scholar] [CrossRef]
- Yang, H.; Cheng, L.; Che, L.; Su, Y.; Li, Y. Nutrients addition decreases soil fungal diversity and alters fungal guilds and co-occurrence networks in a semi-arid grassland in northern China. Sci. Total Environ. 2024, 926, 172100. [Google Scholar] [CrossRef]
- Dou, X.; Wang, R.; Li, C.; Zheng, C.; Zhou, X. Spatial distribution of soil water, plant roots, and water use pattern under different drip fertigation regimes in an apple-soybean intercropping system on the Loess Plateau, China. Agric. Water Manag. 2022, 269, 107718. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Deng, X.; Nethmini, R.T.; Zhao, H.; He, Q.; Jiang, G.; Hou, Q.; Chen, Q.; Li, X.; Dong, K.; et al. Fungal Community Complexity and Stability in Clay Loam and Sandy Soils in Mangrove Ecosystems. J. Fungi 2025, 11, 262. https://doi.org/10.3390/jof11040262
Zhou S, Deng X, Nethmini RT, Zhao H, He Q, Jiang G, Hou Q, Chen Q, Li X, Dong K, et al. Fungal Community Complexity and Stability in Clay Loam and Sandy Soils in Mangrove Ecosystems. Journal of Fungi. 2025; 11(4):262. https://doi.org/10.3390/jof11040262
Chicago/Turabian StyleZhou, Shengyao, Xiaojie Deng, Rajapakshalage Thashikala Nethmini, Huaxian Zhao, Qing He, Gonglingxia Jiang, Qinghua Hou, Qingxiang Chen, Xiaolei Li, Ke Dong, and et al. 2025. "Fungal Community Complexity and Stability in Clay Loam and Sandy Soils in Mangrove Ecosystems" Journal of Fungi 11, no. 4: 262. https://doi.org/10.3390/jof11040262
APA StyleZhou, S., Deng, X., Nethmini, R. T., Zhao, H., He, Q., Jiang, G., Hou, Q., Chen, Q., Li, X., Dong, K., & Li, N. (2025). Fungal Community Complexity and Stability in Clay Loam and Sandy Soils in Mangrove Ecosystems. Journal of Fungi, 11(4), 262. https://doi.org/10.3390/jof11040262






