Fusarium Species Associated with Diseases of Citrus: A Comprehensive Review
Abstract
1. Introduction
| Fusarium Species | Mycotoxin(s) | Impact on Citrus | Other Affected Crops | Reference |
|---|---|---|---|---|
| Neocosmospora solani | Fusaric acid | Contributes to dry root rot and decline and impairs plant root health, reducing nutrient uptake and chlorosis, and causing overall decline in tree health. | Various fruit and vegetable crops | [39] |
| Fusarium oxysporum | Beauvericin, Enniatins | Contributes to wilt and root diseases and compromises vascular health, leading to blockage, leaf yellowing, and tree death. | Bananas, cotton, tomatoes, melons | [40] |
| Fusarium proliferatum | Fumonisins, Moniliformin | Rare in citrus but can cause fruit decay. Contributes to plant stress and reduced quality. | Asparagus, garlic, maize, onions rice | [41] |
| Fusarium verticillioides | Fumonisins | No direct reports of citrus infection. Contributes to seedling diseases, root rot, and reduced growth. | Maize (primary host), wheat, rice | [42] |
| Fusarium graminearum | Zearalenone, Trichothecenes | Not typically associated with citrus; contributes to ear rot, grain contamination, and reduced crop yield in cereals. | Barley, maize, wheat | [43] |
| Citrus Host | Fusarium Species | Disease | Symptoms | Country | References |
|---|---|---|---|---|---|
| Citrus reticulata | F. oxysporum F. equiseti | Fusarium rot | Stem end discolouration and fruit rot | Pakistan | [39,43] |
| Poncirus trifoliata | F. oxysporum F. solani | Fusarium root rot | Leaf yellowing, wilting, and decline | China | [44] |
| Citrus spp. | N. citricola N. ferruginea N. solani | Dry root rot | yellowing, wilting leaves, and dieback cracked trunks above the crowns | South Africa | [45] |
| Citrus spp. | F. solani F. oxysporum F. equiseti F. brachygibbosum | Dry root rot | Root rot Necrotic roots Purple wood discoloration Plant yellowing | Morocco | [25] |
| Citrus sinensis | Fusarium spp. F. polyphialidicum | Vascular wilt | Chlorosis, defoliation, and wilting of branches | Mexico | [46] |
| Citrus unshiu Citrus aurantium | N. solani | Dry root rot | Light purple, vascular discolouration, and dry decay of fibrous roots | Turkey | [47] |
| Poncirus trifoliata | N. solani F. oxysporum | Dry root rot | Chlorosis, canopy reduction, wilting, root necrosis, defoliation, and plant death symptoms | Chile | [48] |
2. Fusarium Species Associated with Citrus
2.1. Taxonomy and Diversity of Fusarium in Citrus Trees
2.2. Modern Molecular Techniques for Accurate Identification of Pathogens
2.3. Fusarium and Neocosmospora Species and Their Impact
3. Etiology and Symptomatology of Fusarium spp. Associated with Citrus
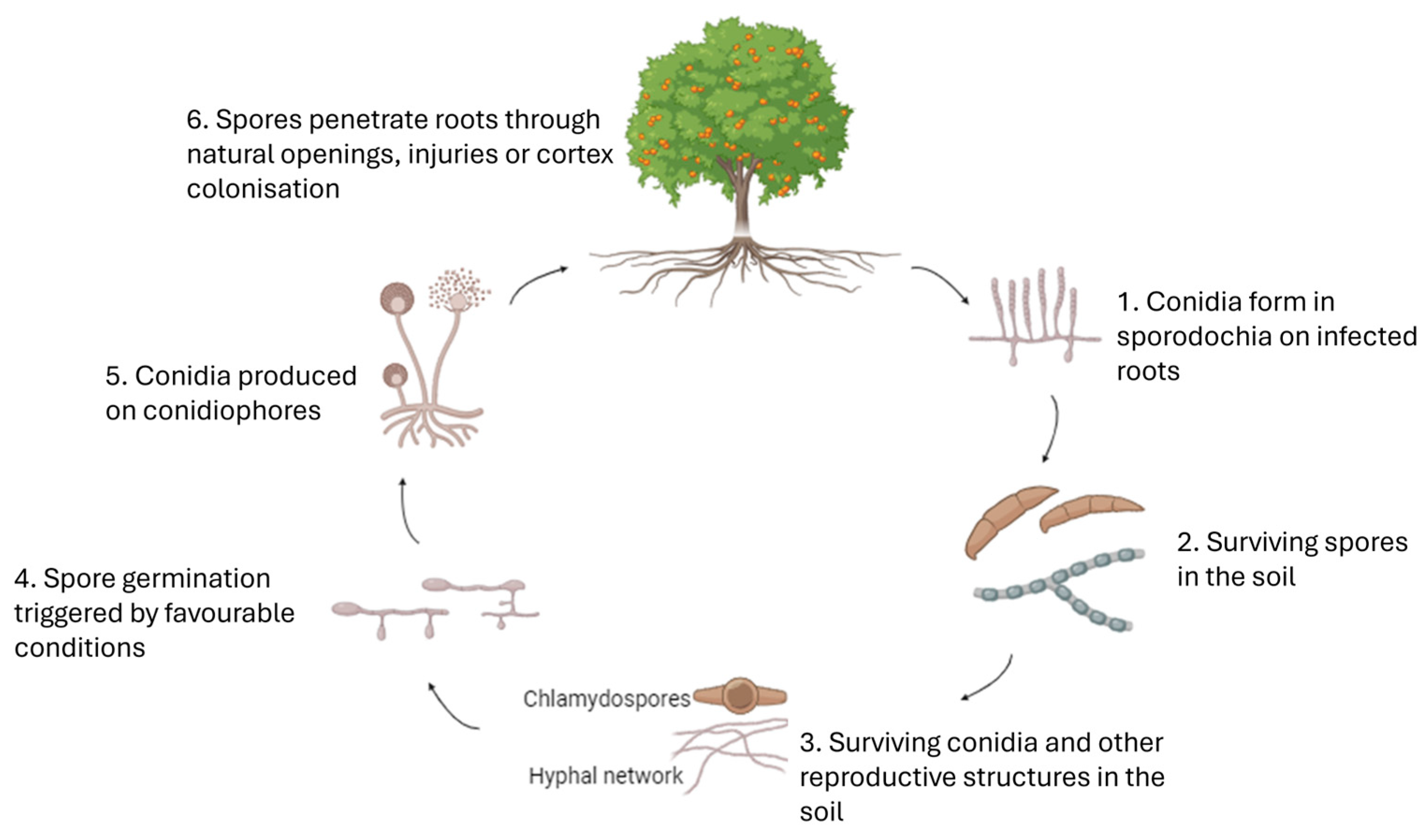
3.1. Etiology of the Associated Diseases
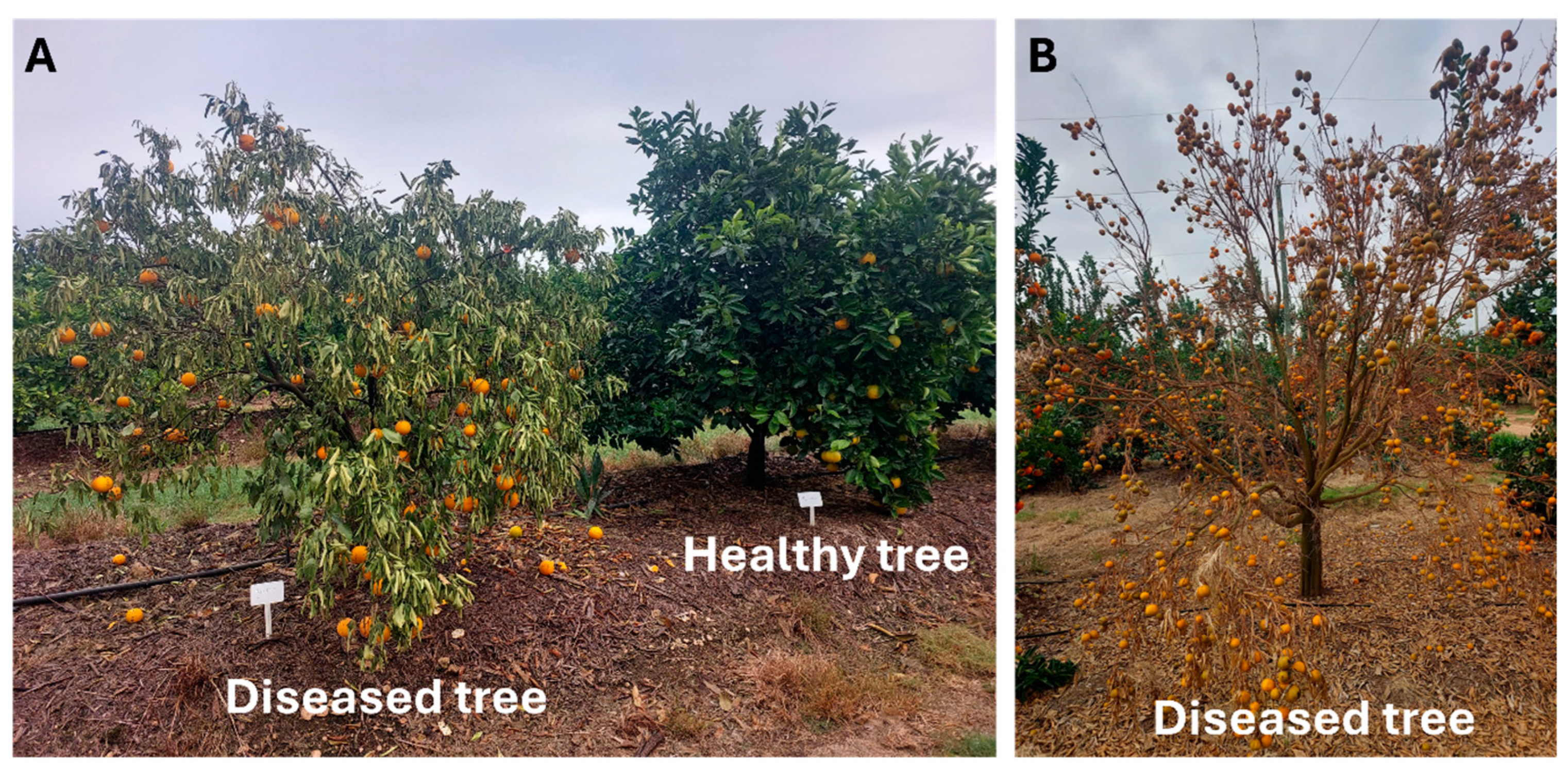
3.2. Symptomatology
4. Fusarium spp.—Host Plant Interaction and Molecular Mechanisms
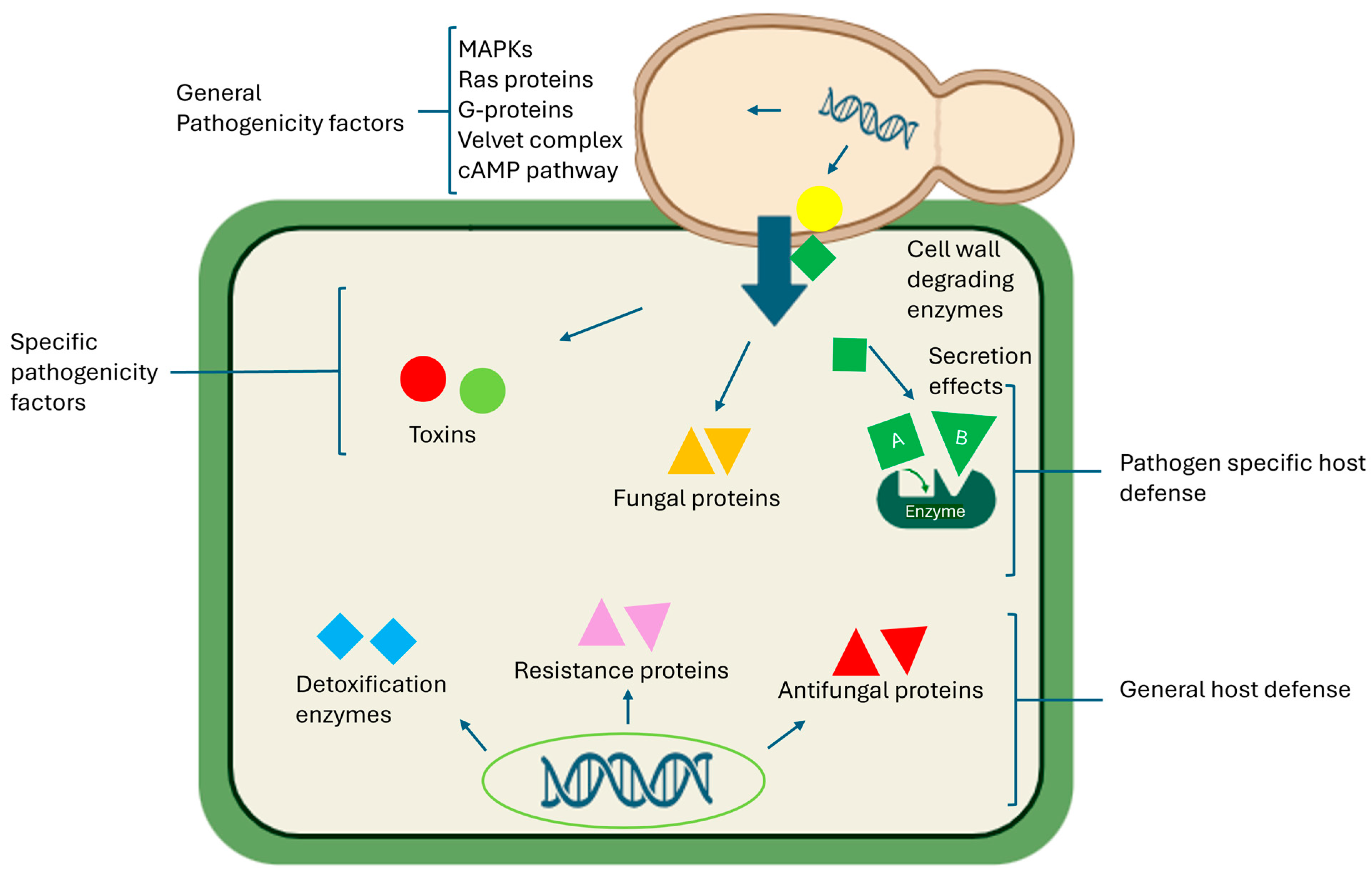
4.1. Host–Pathogen Interactions
4.2. Effector Proteins
4.3. Cell-Wall-Degrading Enzymes
4.4. Induced Systemic Resistance (ISR) in Citrus
4.5. Phytochemical Defence Mechanisms
4.6. Exploration of Novel Resistance Strategies
5. Management Strategies for Fusarium spp.
5.1. Cultural Practises
5.2. Chemical Control
5.3. Phytochemical Control
5.4. Biological Control
5.5. Mycoviruses Infecting Fusarium spp.
6. Conclusions and Future Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Hazarika, T.K. Citrus. In Fruit Nut Crops; Springer Nature: Singapore, 2023; pp. 1–44. [Google Scholar]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [PubMed]
- Zhong, G.; Nicolosi, E. Citrus origin, diffusion, and economic importance. In Citrus Genome; Springer: Berlin, Germany, 2020; pp. 5–21. [Google Scholar]
- Ahmed, W.; Azmat, R. Citrus: An ancient fruit of promise for health benefits. In Citrus: Health Benefits and Production Technology; IntechOpen: London, UK, 2019; pp. 19–30. Available online: https://www.intechopen.com/chapters/64826 (accessed on 31 October 2024).
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2021, 12, 29. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus waste as source of bioactive compounds: Extraction and utilization in health and food industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Hosseini, S.H.; Ghobadian, B.; Motevali, A. Biofuel production from citrus wastes: A feasibility study in Iran. Renew. Sustain. Energy Rev. 2017, 69, 1100–1112. [Google Scholar]
- Varmie, E.B.; Thakur, M. Utilization of citrus processing waste: A review. Pharm. Innov. J. 2021, 10, 682–697. [Google Scholar]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar]
- Gohel, N.M.; Prajapati, B.K.; Srivastava, J.N. Major diseases of citrus and their management. In Diseases of Horticultural Crops; Apple Academic Press: New York, NY, USA, 2022; pp. 155–167. [Google Scholar]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 40, 1–25. [Google Scholar]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. General. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- O’Donnell, K. Molecular phylogeny of Fusarium inferred from partial rna polymerase ii gene sequences. J. Plant Pathol. 2008, 90, 11–14. [Google Scholar]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- Chandra, N.S.; Wulff, E.G.; Udayashankar, A.C.; Nandini, B.P.; Niranjana, S.R.; Mortensen, C.N.; Prakash, H.S. Prospects of molecular markers in Fusarium species diversity. Appl. Microbiol. Biotechnol. 2011, 90, 1625–1639. [Google Scholar]
- Summerell, B.A. Resolving Fusarium: Current status of the genus. Annu. Rev. Phytopathol. 2019, 57, 323–339. [Google Scholar]
- O’Donnell, K.; McCormick, S.P.; Busman, M.; Proctor, R.H.; Ward, T.J.; Geiser, D.M. Whole-genome phylogenetics and chemotyping of the Fusarium genus reveal new insights into species complexes. Fungal Genet. Biol. 2013, 52, 20–31. [Google Scholar] [PubMed]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application–A review. Food Control 2017, 76, 127–138. [Google Scholar]
- Marrez, D.A.; Ayesh, A.M. Mycotoxins: The threat to food safety. Egypt. J. Chem. 2022, 65, 353–372. [Google Scholar] [CrossRef]
- Misihairabgwi, J.M.; Ishola, A.; Quaye, I.; Sulyok, M.; Krska, R. Diversity and fate of fungal metabolites during the preparation of oshikundu, a Namibian traditional fermented beverage. World Mycotoxin J. 2018, 11, 471–481. [Google Scholar] [CrossRef]
- Adeyeye, S.O.; Ashaolu, T.J.; Idowu-Adebayo, F. Mycotoxins: Food safety, consumer health and Africa’s food security. Polycycl. Aromat. Compd. 2022, 42, 5779–5795. [Google Scholar]
- Donkersley, P.; Silva, F.W.; Carvalho, C.M.; Al-Sadi, A.M.; Elliot, S.L. Biological, environmental and socioeconomic threats to citrus lime production. J. Plant Dis. Prot. 2018, 125, 339–356. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhou, J.; Liao, J.; Yi, J.P.; Ma, D.F.; Deng, J.X. Grafted twig rot on Citrus sinensis caused by a member of the Fusarium solani species complex. Can. J. Plant Pathol. 2020, 42, 133–139. [Google Scholar]
- Ezrari, S.; Lahlali, R.; Radouane, N.; Tahiri, A.; Asfers, A.; Boughalleb-M’Hamdi, N.; Amiri, S.; Lazraq, A. Characterization of Fusarium species causing dry root rot disease of citrus trees in Morocco. J. Plant Dis. Prot. 2021, 128, 431–447. [Google Scholar]
- Umer, M.; Mubeen, M.; Ateeq, M.; Shad, M.A.; Atiq, M.N.; Kaleem, M.M.; Iqbal, S.; Shaikh, A.A.; Ullah, I.; Khan, M.; et al. Etiology, epidemiology and management of citrus black rot caused by Alternaria citri: An outlook. Plant Prot. 2021, 5, 105–115. [Google Scholar]
- Ezrari, S.; Radouane, N.; Tahiri, A.; El Housni, Z.; Mokrini, F.; Özer, G.; Lazraq, A.; Belabess, Z.; Amiri, S.; Lahlali, R. Dry root rot disease, an emerging threat to citrus industry worldwide under climate change: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101753. [Google Scholar]
- Nemec, S. Microorganisms associated with healthy and sand hill-declined citrus roots. Plant Dis. Rep. 1975, 59, 210. [Google Scholar]
- Paguio, O.R. Citrus declinio in the State of Brazil: Occurrence and responses to blight diagnostic tests. Int. Organ. Citrus Virol. Conf. Proc. 1984, 9, 1957–2010. [Google Scholar]
- Malikoutsaki-Mathioudi, M.; Bourbos, V.A.; Skoudridakis, M.T. Dry root rot-a very serious citrus disease in Greece. Bull. OEPP 1987, 17, 335–340. [Google Scholar]
- Labuschagne, N.; Kotzé, J.M. Factors affecting feeder root rot of citrus caused by Fusarium solani. In Proceedings of the Sixth International Citrus Congress, Tel Aviv, Israel, 6–11 March 1988; Volume 14, pp. 839–845. [Google Scholar]
- El-Mohamedy, R.S.R.; Hasabo, S.A. Response of some citrus rootstocks to infection with Fusarium solani and citrus nematode Tylenchulus semipenetrans under greenhouse conditions. Egypt. J. Phytopathol. 2005, 33, 11–25. [Google Scholar]
- Chandran, M.R.; Kumar, M.R. Studies on cultural, morphological variability in isolates of Fusarium solani (Mart.) Sacc., incitant of dry root-rot of Citrus. Curr. Biot. 2012, 6, 152–162. [Google Scholar]
- Rehman, A.; Sahi, S.T.; Khan, M.A.; Mehboob, S. Fungi associated with bark, twigs and roots of declined shisham (Dalbergia sissoo roxb.) trees in Punjab, Pakistan. Pak. J. Phytopathol. 2012, 24, 152–158. [Google Scholar]
- Al-Sadi, A.M.; Al-Alawi, Z.A.; Deadman, M.L.; Patzelt, A. Etiology of four foliar and root diseases of wild plants in Oman. Can. J. Plant Pathol. 2014, 36, 517–522. [Google Scholar]
- Al-Karboli, M.H.; Kuthair, W.M. Isolation and pathogenicity of the fungus, Fusarium solani a causal of dry root rot on sour orange in Baghdad province. J. Agric. Technol. 2016, 12, 927–938. [Google Scholar]
- Liu, X.; Cao, A.; Wang, Q.; Yan, D.; Ouyang, C.; Fang, W. First report of small yellow ginger root rot caused by Fusarium solani in Jiangxi, China. Plant Dis. 2019, 103, 1415. [Google Scholar]
- Moosa, A.; Farzand, A.; Sahi, S.T.; Khan, S.A.; Abbas, M.F.; Abbas, A.; Binyamin, R. First report of post-harvest Fusarium rot caused by Fusarium oxysporum on Citrus reticulata Blanco cv. ‘Kinnow’ in Pakistan. J. Plant Pathol. 2020, 102, 945–946. [Google Scholar]
- Baker, R.A.; Tatum, J.H.; Nemec, S. Toxin production by Fusarium solani from fibrous roots of blight-diseased citrus. Phytopathology 1981, 71, 951–954. [Google Scholar]
- de HC Maciel, M.; do Amaral, A.C.T.; da Silva, T.D.; Bezerra, J.D.; de Souza-Motta, C.M.; da Costa, A.F.; Tiago, V.; de Oliveira, N.T. Evaluation of Mycotoxin Production and Phytopathogenicity of the Entomopathogenic Fungi Fusarium caatingaense and F. pernambucanum from Brazil. Curr. Microbiol. 2021, 78, 1218–1226. [Google Scholar]
- Gálvez, L.; Palmero, D. Fusarium dry rot of garlic bulbs caused by Fusarium proliferatum: A review. Horticulturae 2022, 8, 628. [Google Scholar] [CrossRef]
- Achar, P.N.; Sreenivasa, M.Y. Current perspectives of biocontrol agents for management of Fusarium verticillioides and its fumonisin in cereals—A review. J. Fungi 2021, 7, 776. [Google Scholar] [CrossRef]
- Gupta, R.C.; Doss, R.B.; Lall, R.; Srivastava, A.; Sinha, A. Trichothecenes and zearalenone. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2022; pp. 1003–1016. [Google Scholar]
- Ma, X.; Zhai, L.; Jiang, Y.; Wang, Z.; He, L.; Song, F.; Wu, L. First report of Fusarium oxysporum and Fusarium solani causing root rot on trifoliate orange rootstock in China. Plant Dis. 2023, 107, 944. [Google Scholar]
- Guarnaccia, V.; Van Niekerk, J.; Crous, P.; Sandoval-Denis, M. Neocosmospora spassociated with dry root rot of citrus in South Africa. Phytopathol. Mediterr. 2021, 60, 79–100. [Google Scholar]
- Ortiz-Martínez, L.E.; Juárez-Rodríguez, L.; Robles-Yerena, L.; Leyva-Mir, S.G.; Camacho-Tapia, M. Fusarium sp., causal agent of vascular wilt in citrus and its sensitivity to fungicides. Rev. Mex. De Fitopatol. 2022, 40, 1–17. [Google Scholar] [CrossRef]
- Kurt, Ş.; Uysal, A.; Soylu, E.M.; Kara, M.; Soylu, S. Characterization and pathogenicity of Fusarium solani associated with dry root rot of citrus in the eastern Mediterranean region of Turkey. J. Gen. Plant Pathol. 2020, 86, 326–332. [Google Scholar] [CrossRef]
- Garzón-Nivia, M.A.; Mártiz Mártiz, J.; Moya-Elizondo, E.A.; Ruiz, B.; Cornejo, J.C.; Valdés-Gómez, H.A. Characterization and Identification of Neocosmospora solani and Fusarium oxysporum Causing Root Necrosis and Wilting of Orange Trees in Chile. Plants 2025, 14, 376. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J. Fungi infecting woody plants: Emerging frontiers. Persoonia-Mol. Phylogeny Evol. Fungi 2018, 40, 1–3. [Google Scholar] [CrossRef]
- Hasan, M.F.; Islam, M.A.; Sikdar, B. First report on molecular identification of Fusarium species causing fruit rot of mandarin (Citrus reticulata) in Bangladesh. F1000Research 2020, 9, 1212. [Google Scholar] [CrossRef]
- Sukmawati, D.; Miarsyah, M. Pathogenic activity of Fusarium equiseti from plantation of citrus plants (Citrus nobilis) in the village Tegal Wangi, Jember Umbulsari, East Java, Indonesia. Asian J. Agric. Biol. 2017, 5, 202–213. [Google Scholar]
- Hannachi, I.; Rezgui, S.; Cherif, M. First report of mature citrus trees being affected by Fusarium wilt in Tunisia. Plant Dis. 2014, 98, 566. [Google Scholar] [CrossRef]
- Ezrari, S.; Radouane, N.; Tahiri, A.; Amiri, S.; Lazraq, A.; Lahlali, R. Environmental effects of temperature and water potential on mycelial growth of Neocosmospora solani and Fusarium spcausing dry root rot of citrus. Curr. Microbiol. 2021, 78, 3092–3103. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Lombard, L.; Crous, W. Back to the roots: A reappraisal of Neocosmospora. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 90–185. [Google Scholar] [CrossRef]
- Aguayo, J.; Mostert, D.; Fourrier-Jeandel, C.; Cerf-Wendling, I.; Hostachy, B.; Viljoen, A.; Ioos, R. Development of a hydrolysis probe-based real-time assay for the detection of tropical strains of Fusarium oxysporum f. sp. cubense race 4. PLoS ONE 2017, 12, e0171767. [Google Scholar] [CrossRef]
- Mishra, K.; Fox, R.T.; Culham, A. Development of a PCR-based assay for rapid and reliable identification of pathogenic Fusaria. FEMS Microbiol. Lett. 2003, 218, 329–332. [Google Scholar] [CrossRef]
- Arif, M.; Chawla, S.; Zaidi, M.W.; Rayar, J.K.; Variar, M.; Singh, U.S. Development of specific primers for genus Fusarium and F. solani using rDNA sub-unit and transcription elongation factor (TEF-1α) gene. Afr. J. Biotechnol. 2012, 11, 444–447. [Google Scholar]
- Azadvar, M.; Alizadeh, H.; Safarnejad, M.R.; Najafinia, M.; Atellio Bianco, P. Etiology of quick decline disease of Citrus on Bakraee (Citrus sp.) rootstock in Southern Kerman. Iran. J. Plant Prot. Sci. 2019, 50, 87–97. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar]
- Wurzbacher, C.; Larsson, E.; Bengtsson-Palme, J.; Van den Wyngaert, S.; Svantesson, S.; Kristiansson, E.; Kagami, M.; Nilsson, R.H. Introducing ribosomal tandem repeat barcoding for fungi. Mol. Ecol. Resour. 2019, 19, 118–127. [Google Scholar]
- Tedersoo, L.; Sánchez-Ramírez, S.; Koljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsaard, J.; Thangavel, R.; et al. Fungal Planet description sheets: 951–1041. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 223. [Google Scholar]
- Wang, Z.; Binder, M.; Schoch, C.L.; Johnston, R.; Spatafora, J.W.; Hibbett, D.S. New insights into fungal evolution from phylogenomic analysis of a taxonomically comprehensive dataset. Nat. Commun. 2022, 13, 1–13. [Google Scholar]
- Guan, Y.M.; Ma, Y.Y.; Jin, Q.; Wang, Q.X.; Liu, N.; Fu, Y.P.; Zhang, Y.Y.; Li, Y. Multi-locus phylogeny and taxonomy of the fungal complex associated with rusty root rot of Panax ginseng in China. Front. Microbiol. 2020, 11, 618942. [Google Scholar] [CrossRef]
- Swenie, R.A.; Looney, B.P.; Ke, Y.H.; Alejandro Rojas, J.; Cubeta, M.A.; Langer, G.J.; Vilgalys, R.; Matheny, B. PacBio high-throughput multi-locus sequencing reveals high genetic diversity in mushroom-forming fungi. Mol. Ecol. Resour. 2024, 24, e13885. [Google Scholar]
- Adesemoye, A.O.; Mayorquin, J.S.; Peacock, B.B.; Moreno, K.; Hajeri, S.; Yokomi, R.; Eskalen, A. Association of Neonectria macrodidyma with dry root rot of citrus in California. J. Plant Pathol. Microbiol. 2016, 8, 391–394. [Google Scholar]
- Hao, W.; Gray, M.A.; Förster, H.; Adaskaveg, J.E. Evaluation of new Oomycota fungicides for management of Phytophthora root rot of citrus in California. Plant Dis. 2019, 103, 619–628. [Google Scholar]
- Jackson, E.; Li, J.; Weerasinghe, T.; Li, X. The Ubiquitous Wilt-Inducing Pathogen Fusarium oxysporum—A Review of Genes Studied with Mutant Analysis. Pathogens 2024, 13, 823. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium fungi pathogens, identification, adverse effects, disease management, and global food security: A review of the latest research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Amby, D.B.; Thuy, T.T.T.; Ho, B.D.; Kosawang, C.; Son, T.B.; Jørgensen, H.J.L. First report of Fusarium lichenicola as a causal agent of fruit rot in pomelo (Citrus maxima). Plant Dis. 2015, 99, 1278. [Google Scholar]
- Dandurand, L.M.; Menge, J.A. Influence of Fusarium solani on citrus root rot caused by Phytophthora parasitica and Phytophthora citrophthora. Plant Soil 1992, 144, 13–21. [Google Scholar]
- Spina, S.; Coco, V.; Gentile, A.; Catara, A.; Cirvilleri, G. Association of Fusarium solani with rolABC and wild type Troyer Citrange. J. Plant Pathol. 2008, 90, 479–486. [Google Scholar]
- Parikh, L.; Kodati, S.; Eskelson, M.J.; Adesemoye, A.O. Identification and pathogenicity of Fusarium spin row crops in Nebraska. Crop Prot. 2018, 108, 120–127. [Google Scholar]
- Rivera-Jiménez, M.N.; Zavaleta-Mancera, H.A.; Rebollar-Alviter, A.; Aguilar-Rincón, V.H.; García-de-los-Santos, G.; Vaquera-Huerta, H.; Silva-Rojas, H.V. Phylogenetics and histology provide insight into damping-off infections of ‘Poblano’ pepper seedlings caused by Fusarium wilt in greenhouses. Mycol. Progress 2018, 17, 1237–1249. [Google Scholar]
- Joshi, R. A review of Fusarium oxysporum on its plant interaction and industrial use. J. Med. Plants Stud. 2018, 6, 112–115. [Google Scholar]
- Rana, A.; Sahgal, M.; Johri, B.N. Fusarium oxysporum: Genomics, diversity and plant–Host interaction. Dev. Fungal Biol. Appl. Mycol. 2017, 159–199. [Google Scholar] [CrossRef]
- Hannachi, I.; Poli, A.; Rezgui, S.; Prassad, R.D.; Cherif, M. Genetic and phenotypic differences of Fusarium oxysporum f. sp. citri isolated from sweet orange and tangerine. Eur. J. Plant Pathol. 2015, 142, 269–280. [Google Scholar]
- Parra-Cota, F.I.; García Pereyra, J.; Aviña Martínez, G.N.; Santos-Villalobos, S.D.L. First report of Fusarium wilt on Citrus sinensis var. Valencia in the Yaqui Valley, Mexico. Rev. Mex. Fitopatol. 2019, 37, 193–201. [Google Scholar]
- Kunta, M.; Salas, B.; Gonzales, M.; da Graça, J.V. First report on citrus dry rot in sour orange rootstock in Texas. J. Citrus Pathol. 2015, 2. [Google Scholar] [CrossRef]
- Usman, H.M.; Iffat, A.; Shakeel, Q.; Bhatti, A.M.; Karim, M.M.; Zafar, M.I.; Bajwa, R.T.; Rashid, I.; Kiptoo, J.J.; Shafique, T. Insights into Fusarium spp., Associated with Dry Root Rot of Citrus and its Management. Phytopathogenomics Dis. Control 2024, 3, 241–249. [Google Scholar]
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Labushagne, N.; van der Veote, P.A.; Kotze, J.M. Interaction between Fusarium solani and Tylenchulus semipenetrans on citrus roots. Phytophylactica 1989, 21, 29–34. [Google Scholar]
- Dandurand, L.M.; Menge, J.A. Influence of Fusarium solani on citrus root growth and population dynamics of Phytophthora parasitica and Phytophthora citrophthora. Phytopathology 1993, 83, 767–771. [Google Scholar]
- Adesemoye, A.; Eskalen, A.; Faber, B.; Bender, G.; O’connell, N.; Kallsen, C.; Shea, T. Current knowledge on Fusarium dry rot of citrus. Citrograph 2011, 2, 29–33. [Google Scholar]
- Srivastava, V.; Patra, K.; Pai, H.; Aguilar-Pontes, M.V.; Berasategui, A.; Kamble, A.; Di Pietro, A.; Redkar, A. Molecular dialogue during host manipulation by the vascular wilt fungus Fusarium oxysporum. Annu. Rev. Phytopathol. 2024, 62, 97–126. [Google Scholar]
- Lyu, X.; Shen, C.; Fu, Y.; Xie, J.; Jiang, D.; Li, G.; Cheng, J. Comparative genomic and transcriptional analyses of the carbohydrate-active enzymes and secretomes of phytopathogenic fungi reveal their significant roles during infection and development. Sci. Rep. 2015, 5, 15565. [Google Scholar]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research progress on phytopathogenic fungi and their role as biocontrol agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin in Serbian maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Kant, P.; Reinprecht, Y.; Martin, C.J.; Islam, R.; Pauls, K.P. Disease resistance. Compr. Biotechnol. 2019, 4, 789–805. [Google Scholar]
- Hou, S.; Liu, Z.; Shen, H.; Wu, D. Damage-associated molecular pattern-triggered immunity in plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar]
- Shcherbakova, L.A.; Dzhavakhiya, V.G.; Duan, Y.; Zhang, J. Microbial proteins as elicitors of plant resistance to pathogens and their potential for eco-friendly crop protection in sustainable agriculture. Agric. Biol. 2023, 58, 789–820. [Google Scholar]
- Gutiérrez-Sánchez, A.; Plasencia, J.; Monribot-Villanueva, J.L.; Rodríguez-Haas, B.; Ruíz-May, E.; Guerrero-Analco, J.A.; Sánchez-Rangel, D. Virulence factors of the genus Fusarium with targets in plants. Microbiol. Res. 2023, 277, 127506. [Google Scholar] [CrossRef]
- Chen, A.; Ju, Z.; Wang, J.; Wang, J.; Wang, H.; Wu, J.; Yin, Y.; Zhao, Y.; Ma, Z.; Chen, Y. The RasGEF FgCdc25 regulates fungal development and virulence in Fusarium graminearum via cAMP and MAPK signalling pathways. Environ. Microbiol. 2020, 22, 5109–5124. [Google Scholar]
- Yang, Y.; Huang, P.; Ma, Y.; Jiang, R.; Jiang, C.; Wang, G. Insights into intracellular signaling network in Fusarium species. Int. J. Biol. Macromol. 2022, 222, 1007–1014. [Google Scholar]
- El-Defrawy, M.M.; Hesham, A.E.L. G-protein-coupled receptors in fungi. Fungal Biotechnol. Bioeng. 2020, 37–126. [Google Scholar] [CrossRef]
- Reddy, V.P.; Verma, S.; Sharma, D.; Thakur, A. Role of resistant-proteins in plant innate immunity—A review. Agric. Rev. 2019, 40, 12–20. [Google Scholar]
- Essarioui, A.; Mokrini, F.; Afechtal, M. Molecular interactions between tomato and its wilt pathogen Fusarium oxysporum f. sp. Lycopersici—A review. Rev. Marocaine Des Sci. Agron. Vétérinaires 2016, 4, 66–74. [Google Scholar]
- Li, X.; Ke, Z.; Xu, S.; Tang, W.; Liu, Z. The G-protein alpha subunit CgGa1 mediates growth, sporulation, penetration and pathogenicity in Colletotrichum gloeosporioides. Microb. Pathog. 2021, 161, 105254. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, S.; Tang, W.; Wang, J.; Liu, H.; Zhang, Y.; Wang, L.; Li, X.; Liu, Z. The velvet proteins CsVosA and CsVelB coordinate growth, cell wall integrity, sporulation, conidial viability and pathogenicity in the rubber anthracnose fungus Colletotrichum siamense. Microbiol. Res. 2023, 268, 127290. [Google Scholar] [CrossRef]
- Stępień, Ł.; Lalak-Kańczugowska, J. Signaling pathways involved in virulence and stress response of plant-pathogenic Fusarium species. Fungal Biol. Rev. 2021, 35, 27–39. [Google Scholar]
- de Guillen, K.; Ortiz-Vallejo, D.; Gracy, J.; Fournier, E.; Kroj, T.; Padilla, A. Structure analysis uncovers a highly diverse but structurally conserved effector family in phytopathogenic fungi. PLoS Pathog. 2015, 11, e1005228. [Google Scholar]
- Tan, K.C.; Oliver, R.P. Regulation of proteinaceous effector expression in phytopathogenic fungi. PLoS Pathog. 2017, 13, e1006241. [Google Scholar]
- Rauwane, M.E.; Ogugua, U.V.; Kalu, C.M.; Ledwaba, L.K.; Woldesemayat, A.A.; Ntushelo, K. Pathogenicity and virulence factors of Fusarium graminearum including factors discovered using next generation sequencing technologies and proteomics. Microorganisms 2020, 8, 305. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N.K. Microbial enzymes in biocontrol of phytopathogens. In Microbial Enzymes: Roles and Applications in Industries; Springer: Berlin, Germany, 2020; pp. 259–285. [Google Scholar]
- Perincherry, L.; Urbaniak, M.; Pawłowicz, I.; Kotowska, K.; Waśkiewicz, A.; Stępień, Ł. Dynamics of Fusarium mycotoxins and lytic enzymes during pea plants’ infection. Int. J. Mol. Sci. 2021, 22, 9888. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar]
- Feng, L.; Zhao, G.; Sun, R.; Wang, J.; Sun, T.; Xing, S.; Lian, W.; Zhao, Y. Dynamic changes in cell wall-degrading enzymes and physio-biochemistry of ginseng in response to Fusarium oxysporum infection. Eur. J. Plant Pathol. 2023, 165, 569–578. [Google Scholar] [CrossRef]
- Bagherieh, B.; Taheri, P. Characterization, pathogenicity, and extracellular enzymes of Fusarium solani f. sp. phaseoli associated with common bean in Khorasan Razavi Province of Iran. J. Adv. Plant Prot. 2024, 1, 53–64. [Google Scholar]
- Elagamey, E.; Abdellatef, M.A.; Arafat, M.Y. Proteomic insights of chitosan mediated inhibition of Fusarium oxysporum f. sp. cucumerinum. J. Proteom. 2022, 260, 104560. [Google Scholar] [CrossRef]
- Majeed, G.; Noor, W.; Lone, R.; Agrawal, S.; Alaklabi, A.; Shah, M.A.; Kamili, A.N. Modulation of plant defenses by Jasmonic acid and salicylic acid in Capsicum annuum L. against Fusarium wilt in response to Fusarium oxysporum pathogen. Plant Stress 2024, 14, 100571. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Liu, Z.; Qian, Y.; Li, Y.; Yang, L.; Liu, S.; Liang, W.; Li, J. The secreted FolAsp aspartic protease facilitates the virulence of Fusarium oxysporum f. sp. lycopersici. Front. Microbiol. 2023, 14, 1103418. [Google Scholar]
- Fernandes, L.B.; Ghag, S.B. Molecular insights into the jasmonate signaling and associated defense responses against wilt caused by Fusarium Oxysporum. Plant Physiol. Biochem. 2022, 174, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Nemec, S. Vessel blockage by myelin forms in citrus with and without rough-lemon decline symptoms. Can. J. Bot. 1975, 53, 102–108. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar]
- Appu, M.; Ramalingam, P.; Sathiyanarayanan, A.; Huang, J. An overview of plant defense-related enzymes responses to biotic stresses. Plant Gene 2021, 27, 100302. [Google Scholar] [CrossRef]
- Akbar, M.U.; Aqeel, M.; Shah, M.S.; Jeelani, G.; Iqbal, N.; Latif, A.; Elnour, R.O.; Hashem, M.; Alzoubi, O.M.; Habeeb, T.; et al. Molecular regulation of antioxidants and secondary metabolites act in conjunction to defend plants against pathogenic infection. S. Afr. J. Bot. 2023, 161, 247–257. [Google Scholar] [CrossRef]
- Iqbal, N.; Riyazuddin, R.; Nauman, M.; Czékus, Z.; Hayat, M.T.; Poór Ördög, A. Role of Plant Defence System in Crop Protection against Fusarium Pathogens. Fusarium-Recent Studies; IntechOpen: London, UK, 2024; p. 71. [Google Scholar]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed]
- Maithani, D.; Singh, H.; Sharma, A. Stress alleviation in plants using SAR and ISR: Current views on stress signaling network. In Microbes and Signaling Biomolecules Against Plant Stress: Strategies of Plant-Microbe Relationships for Better Survival; Springer: Cham, Switzerland, 2021; pp. 7–36. [Google Scholar]
- Riera, N.; Wang, H.; Li, Y.; Li, J.; Pelz-Stelinski, K.; Wang, N. Induced Systemic Resistance against Citrus Canker Disease by Rhizobacteria. Phytopathology 2018, 108, 1038–1045. [Google Scholar] [PubMed]
- Kunwar, S.; Redondo, A.; Manker, D.; Lott, M.; Knobloch, T.; Brunet, S.; Dufour, J.; Batuman, O. Novel systemic acquired resistance (SAR) inducers for managing huanglongbing (citrus greening) and Citrus canker diseases. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Sustainable Control of Pests and Diseases, Angers, France, 14–20 August 2022; Volume 1378, pp. 133–142. [Google Scholar]
- Ilham, B.; Noureddine, C.; Philippe, G.; Mohammed, E.G.; Brahim, E.; Sophie, A.; Muriel, M. Induced systemic resistance (ISR) in Arabidopsis thaliana by Bacillus amyloliquefaciens and Trichoderma harzianum used as seed treatments. Agriculture 2019, 9, 166. [Google Scholar] [CrossRef]
- Karuppiah, V.; Vallikkannu, M.; Li, T.; Chen, J. Simultaneous and sequential based co-fermentations of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841: A strategy to enhance the gene expression and metabolites to improve the bio-control and plant growth promoting activity. Microb. Cell Factories 2019, 18, 1–16. [Google Scholar]
- Bagio, T.Z.; Canteri, M.G.; Barreto, T.P.; Júnior, R.P.L. Activation of systemic acquired resistance in citrus to control huanglongbing disease. Semin. Ciências Agrárias 2016, 37, 1757–1765. [Google Scholar]
- Frąckowiak, P.; Pospieszny, H.; Smiglak, M.; Obrępalska-Stęplowska, A. Assessment of the efficacy and mode of action of benzo (1, 2, 3)-thiadiazole-7-carbothioic acid s-methyl ester (bth) and its derivatives in plant protection against viral disease. Int. J. Mol. Sci. 2019, 20, 1598. [Google Scholar]
- Poveda, J.; Barquero, M.; González-Andrés, F. Insight into the microbiological control strategies against Botrytis cinerea using systemic plant resistance activation. Agronomy 2020, 10, 1822. [Google Scholar] [CrossRef]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: A review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar]
- Llorens, E.; García-Agustín, P.; Lapeña, L. Advances in induced resistance by natural compounds: Towards new options for woody crop protection. Sci. Agric. 2017, 74, 90–100. [Google Scholar]
- Yan, Z.L.; Chen, J.P.; Nong, X.X.; Li, X.; Luo, H.Y.; Wei, L.L.; Ruan, J.H.; Guan, X.Y.; Lu, S. Isolation and identification of endophytic fungi from citrus cultivars and their inhibitory activity against Xanthomonas citri subscitri causing citrus canker. Sci. Press 2021, 41, 1196–1208. [Google Scholar]
- Wang, X.; Liang, L.; Shao, H.; Ye, X.; Yang, X.; Chen, X.; Shi, Y.; Zhang, L.; Xu, L.; Wang, J. Isolation of the novel strain Bacillus amyloliquefaciens F9 and identification of lipopeptide extract components responsible for activity against Xanthomonas citri subscitri. Plants 2022, 11, 457. [Google Scholar]
- Rabelo, R.S.; Dyer, L.A.; Yamaguchi, L.F.; Diniz, I.; Simbaña, W.; Kussano, A.J.M.; Kato, M.J.; Massad, T.J. Plasticity in plant defense and the role of phytochemical dissimilarity in limiting specialist herbivory. Front. Ecol. Evol. 2023, 11, 1175590. [Google Scholar]
- Okwu, D.E.; Awurum, A.N.; Okoronkwo, J.I. Phytochemical composition and in vitro antifungal activity screening of extracts from citrus plants against Fusarium oxysporum of okra plant (Hibiscus esculentus). Pest. Technol. 2007, 1, 145–148. [Google Scholar]
- Umeoka, N. Phytochemical screening and antifungal properties of the peel extract of citrus sinensis. J. Health Metab. Nutr. Stud. 2024, 3, 40–43. [Google Scholar]
- Oikeh, E.I.; Oviasogie, F.E.; Omoregie, E.S. Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet Orange) peels. Clin. Phytoscience 2020, 6, 1–6. [Google Scholar]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; Asad, M.H.H.B. Citrus flavonoids as potential therapeutic agents: A review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar]
- Zahr, S.; Zahr, R.; El Hajj, R.; Khalil, M. Phytochemistry and biological activities of Citrus sinensis and Citrus limon: An update. J. Herbal. Med. 2023, 100737. [Google Scholar]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical characteristics of citrus peel and effect of conventional and nonconventional processing on phenolic compounds: A review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar]
- Bashir, L.U.; Abdulkadir, A.; Shah, M.M.; Hamisu, A.; Sharif, U.; Kamalu, A.A. Phytochemical screening and antifungal potentials of Citrus limon peels against Fusarium oxysporum and Rhizopus stolonifer causing rots in watermelon (Citrullus lanatus L.). J. Exp. Sci. 2020, 11, 1–5. [Google Scholar]
- Hernández, A.; Ruiz-Moyano, S.; Galván, A.I.; Merchán, A.V.; Nevado, F.P.; Aranda, E.; Serradilla, M.J.; de Guía Córdoba, M.; Martín, A. Anti-fungal activity of phenolic sweet orange peel extract for controlling fungi responsible for post-harvest fruit decay. Fungal Biol. 2021, 125, 143–152. [Google Scholar] [PubMed]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Chemical composition, antimicrobial and antibiotic potentiating activity of essential oils from 10 tropical medicinal plants from Mauritius. J. Herbal. Med. 2016, 6, 88–95. [Google Scholar]
- Chen, Y.; Xing, M.; Chen, T.; Tian, S.; Li, B. Effects and mechanisms of plant bioactive compounds in preventing fungal spoilage and mycotoxin contamination in postharvest fruits: A review. Food Chem. 2023, 415, 135787. [Google Scholar]
- Zhou, X.; Zeng, M.; Huang, F.; Qin, G.; Song, Z.; Liu, F. The potential role of plant secondary metabolites on antifungal and immunomodulatory effect. Appl. Microbiol. Biotechnol. 2023, 107, 4471–4492. [Google Scholar] [PubMed]
- Duan, B.; Reymick, O.O.; Liu, Z.; Zhou, Y.; Wang, X.; Feng, Z.; Tao, N. Citral enhances disease resistance in postharvest citrus fruit through inducing jasmonic acid pathway and accumulating phenylpropanoid compounds. Postharvest Biol. Technol. 2024, 207, 112633. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Ahsen, M.A.; Naqvi, S.A.; Jaskani, M.J.; Waseem, M.; Khan, I.A.; Hussnain, K.; Mahmood, K.; Khan, M.M. Evaluation of exotic citrus rootstocks against Fusarium spp. J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 151–156. [Google Scholar]
- Chiesa, M.A.; Roeschlin, R.A.; Favaro, M.A.; Uviedo, F.; Campos-Beneyto, L.; D’Andrea, R.; Gadea, J.; Marano, M.R. Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris. Mol. Plant Pathol. 2019, 20, 254–269. [Google Scholar]
- Krueger, R.R.; Bender, G.S. Screening a core collection of citrus genetic resources for resistance to Fusarium solani. Acta Hortic. 2015, 1065, 155–164. [Google Scholar]
- Wang, N.; Sundin, G.W.; Fuente, L.D.L.; Cubero, J.; Tatineni, S.; Brewer, M.T.; Zeng, Q.; Bock, C.H.; Cunniffe, N.J.; Wang, C.; et al. Key challenges in plant pathology in the next decade. Phytopathology 2024, 114, 837–842. [Google Scholar]
- Ezrari, S.; Mhidra, O.; Radouane, N.; Tahiri, A.; Polizzi, G.; Lazraq, A.; Lahlali, R. Potential role of rhizobacteria isolated from Citrus rhizosphere for biological control of Citrus dry root rot. Plants 2021, 10, 872. [Google Scholar] [CrossRef]
- Narayanasamy, P. Soilborne Microbial Plant Pathogens and Disease Management, Volume Two: Management of Crop Diseases; CRC Press: Boca Raton, FL, USA, 2019; Volume 2, pp. 1–10. [Google Scholar]
- Dubey, A.K.; Rao, K.K.; Kumar, S.; Tamta, M.; Dwivedi, S.K.; Kumar, R.; Mishra, J.S. Disease management in major field crops. In Conservation Agriculture for Climate Resilient Farming & Doubling Farmers’ Income; ICAR Research Complex for Eastern Region: Patna, India, 2019; p. 246. [Google Scholar]
- Pakdaman Sardrood, B.; Mohammadi Goltapeh, E. Effect of agricultural chemicals and organic amendments on biological control fungi. In Sustainable Agriculture Reviews 31: Biocontrol; Springer: Cham, Switzerland, 2018; pp. 217–359. [Google Scholar]
- Khanzada, M.A.; Tanveer, M.; Maitlo, S.A.; Hajano, J.; Ujjan, A.A.; Syed, R.N.; Lodhi, A.M.; Rajput, A.Q. Comparative efficacy of chemical fungicides, plant extracts and bio-control agents against Fusarium solani under laboratory conditions. Pak. J. Phytopathol. 2016, 28, 133–139. [Google Scholar]
- Marais, L.J. Efficacy of water-soluble silicon in managing Fusarium dry root rot of citrus. Acta Hortic. 2015, 1065, 993–1000. [Google Scholar]
- Laing, M.D.; Basdew, L. Sustainable Control of Pre-and Postharvest Diseases of Citrus Fruit in South Africa; University of KwaZulu-Natal: Durban, South Africa, 2018; pp. 34–36. [Google Scholar]
- Grahovac, J.; Pajčin, I.; Vlajkov, V. Bacillus VOCs in the context of biological control. Antibiotics 2023, 12, 581. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lee, P.C.; Huang, T.P. Biological control of collar rot on passion fruits via induction of apoptosis in the collar rot pathogen by Bacillus subtilis. Phytopathology 2021, 111, 627–638. [Google Scholar]
- Kim, Y.G.; Kang, H.K.; Kwon, K.D.; Seo, C.H.; Lee, H.B.; Park, Y. Antagonistic activities of novel peptides from Bacillus amyloliquefaciens PT14 against Fusarium solani and Fusarium oxysporum. J. Agric. Food Chem. 2015, 63, 10380–10387. [Google Scholar]
- Hammam, M.M.; El-Mohamedy, R.S.; Abd-El-Kareem, F.; Abd-Elgawad, M.M. Evaluation of soil amended with bio-agents and compost alone or in combination for controlling citrus nematode Tylenchulus semipenetrans and Fusarium dry root rot on Volkamer lime under greenhouse conditions. Int. J. ChemTech Res. 2016, 9, 86–96. [Google Scholar]
- El-Mohamedy, R.S.; Hammam, M.M.; Abd-El-Kareem, F.; Abd-Elgawad, M.M. Biological soil treatment to control Fusarium solani and Tylenchulus semipenetrans on sour orange seedlings under greenhouse conditions. Int. J. ChemTech Res. 2016, 9, 73–85. [Google Scholar]
- Shinde, S.B.; Sadgir, M.D. Efficiency of Pseudomonas fluorescens and Bacillus subtilis against Phytophthora spin Citrus. Int. J. Plant Prot. 2016, 9, 15–20. [Google Scholar]
- Altinok, H.H.; Erdogan, O. Determination of the In vitro Effect of Trichoderma harzianum on Phytopathogenic Strains of Fusarium oxysporum. Not. Bot. Horti Agrobot. 2015, 43, 494–500. [Google Scholar]
- Dewi, O.K.M.; Abadi, A.L.; Widyaningsih, S. Fusarium Rot Biological Control of Citrus caused by Fusarium Oxysporum. Res. J. Life Sci. 2022, 9, 91–98. [Google Scholar] [CrossRef]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; et al. Trichoderma spgenes involved in the biocontrol activity against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar]
- Juby, S.; Radhakrishnan, E.K.; Jayachandran, K. Exploring Mechanisms of Disease Suppression using Endophytic Fungi as Biocontrol Agents. In Biofungicides: Eco-Safety and Future Trends; CRC Press: Boca Raton, FL, USA, 2023; pp. 159–190. [Google Scholar]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and perspectives in the use of biocontrol agents against fungal plant diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Pellan, L.; Dieye, C.A.T.; Durand, N.; Fontana, A.; Strub, C.; Schorr-Galindo, S. Biocontrol agents: Toolbox for the screening of weapons against mycotoxigenic Fusarium. J. Fungi 2021, 7, 446. [Google Scholar] [CrossRef]
- Sharma, M.; Guleria, S.; Singh, K.; Chauhan, A.; Kulshrestha, S. Mycovirus associated hypovirulence, a potential method for biological control of Fusarium species. Virusdisease 2018, 29, 134–140. [Google Scholar] [CrossRef]
- García-Pedrajas, M.D.; Cañizares, M.C.; Sarmiento-Villamil, J.L.; Jacquat, A.G.; Dambolena, J.S. Mycoviruses in biological control: From basic research to field implementation. Phytopathology 2019, 109, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Adalia, E.J.; Fernández, M.M.; Diez, J.J. The use of mycoviruses in the control of forest diseases. Biocontrol Sci. Technol. 2016, 26, 577–604. [Google Scholar] [CrossRef]
- Li, P.; Bhattacharjee, P.; Wang, S.; Zhang, L.; Ahmed, I.; Guo, L. Mycoviruses in Fusarium species: An update. Front. Cell. Infect. Microbiol. 2019, 9, 257. [Google Scholar]
- Ćurković-Perica, M.; Ježić, M.; Rigling, D. Mycoviruses as antivirulence elements of fungal pathogens. In The Biological Role of a Virus; Springer International Publishing: Cham, Switzerland, 2022; pp. 209–249. [Google Scholar]
- Bocos-Asenjo, I.T.; Niño-Sánchez, J.; Ginésy, M.; Diez, J.J. New insights on the integrated management of plant diseases by RNA strategies: Mycoviruses and RNA interference. Int. J. Mol. Sci. 2022, 23, 9236. [Google Scholar] [CrossRef]
- Pedersen, C.; Marzano, S.Y.L. Mechanisms of primed defense: Plant immunity induced by endophytic colonization of a mycovirus-induced hypovirulent fungal pathogen. Mol. Plant-Microbe Interact. 2023, 36, 726–736. [Google Scholar] [PubMed]
- Wang, L.; He, H.; Wang, S.; Chen, X.; Qiu, D.; Kondo, H.; Guo, L. Evidence for a novel negative-stranded RNA mycovirus isolated from the plant pathogenic fungus Fusarium graminearum. Virology 2018, 518, 232–240. [Google Scholar] [PubMed]
- Umer, M.; Mubeen, M.; Shakeel, Q.; Ali, S.; Iftikhar, Y.; Bajwa, R.T.; Anwar, N.; Rao, M.J.; He, Y. Mycoviruses: Antagonistic potential, fungal pathogenesis, and their interaction with Rhizoctonia Solani. Microorganisms 2023, 11, 2515. [Google Scholar] [CrossRef]
- Urayama, S.I.; Zhao, Y.J.; Kuroki, M.; Chiba, Y.; Ninomiya, A.; Hagiwara, D. Greetings from virologists to mycologists: A review outlining viruses that live in fungi. Mycoscience 2024, 65, 1–11. [Google Scholar]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major biological control strategies for plant pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Vero, S.; Garmendia, G.; Allori, E.; Sanz, J.M.; Gonda, M.; Alconada, T.; Cavello, I.; Dib, J.R.; Diaz, M.A.; Nally, C.; et al. Microbial biopesticides: Diversity, scope, and mechanisms involved in plant disease control. Diversity 2023, 15, 457. [Google Scholar] [CrossRef]
- Galli, M.; Sede, A.; Heinlein, M.; Kogel, K.H. A biocontrol perspective on mycoviruses in fungal pathogen management. J. Plant Dis. Prot. 2025, 132, 1–15. [Google Scholar]
- Khan, H.A.; Nerva, L.; Bhatti, M.F. The good, the bad and the cryptic: The multifaceted roles of mycoviruses and their potential applications for a sustainable agriculture. Virology 2023, 585, 259–269. [Google Scholar] [CrossRef]
- Guo, L. Mycoviruses in Fusarium Species: An Update. In Frontiers in Fungal Virus Research; Frontiers Media S.A.: Lausanne, Switzerland, 2020; Volume 9, p. 257. [Google Scholar]
- Ma, G.; Zhang, Y.; Ma, L.; Cui, K.; Zhang, B.; Jiang, H.; Qi, K.; Qi, J. Molecular and Biological Characterization of an Isolate of Fusarium graminearum dsRNA mycovirus 4 (FgV4) from a New Host Fusarium pseudograminearum. Microorganisms 2025, 13, 418. [Google Scholar] [CrossRef]
- Jacquat, A.G.; Theumer, M.G.; Cañizares, M.C.; Debat, H.J.; Iglesias, J.; García Pedrajas, M.D.; Dambolena, J.S. A survey of mycoviral infection in Fusarium spisolated from maize and sorghum in Argentina identifies the first mycovirus from Fusarium verticillioides. Viruses 2020, 12, 1161. [Google Scholar]
- Yu, J.; Kim, K.H. Exploration of the interactions between mycoviruses and Fusarium Graminearum. Adv. Virus Res. 2020, 106, 123–144. [Google Scholar] [PubMed]
- Lee, Y.; Son, H.; Shin, J.Y.; Choi, G.J.; Lee, Y.W. Genome-wide functional characterization of putative peroxidases in the head blight fungus Fusarium Graminearum. Mol. Plant Pathol. 2018, 19, 715–730. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badiwe, M.; Fialho, R.O.; Stevens, C.; Lombard, P.-H.; van Niekerk, J. Fusarium Species Associated with Diseases of Citrus: A Comprehensive Review. J. Fungi 2025, 11, 263. https://doi.org/10.3390/jof11040263
Badiwe M, Fialho RO, Stevens C, Lombard P-H, van Niekerk J. Fusarium Species Associated with Diseases of Citrus: A Comprehensive Review. Journal of Fungi. 2025; 11(4):263. https://doi.org/10.3390/jof11040263
Chicago/Turabian StyleBadiwe, Mihlali, Régis Oliveira Fialho, Charles Stevens, Paul-Henri Lombard, and Jan van Niekerk. 2025. "Fusarium Species Associated with Diseases of Citrus: A Comprehensive Review" Journal of Fungi 11, no. 4: 263. https://doi.org/10.3390/jof11040263
APA StyleBadiwe, M., Fialho, R. O., Stevens, C., Lombard, P.-H., & van Niekerk, J. (2025). Fusarium Species Associated with Diseases of Citrus: A Comprehensive Review. Journal of Fungi, 11(4), 263. https://doi.org/10.3390/jof11040263





