Abstract
Macrofungi are key decomposers of organic matter and play an active role in biogeochemical cycles, thereby contributing to carbon sequestration in forest ecosystems. Floodplain forests (várzeas) are characterized by the dynamics of rising and receding waters, which are rich in suspended material and influence species variation and adaptation. The knowledge about the distribution of macrofungi in várzea environments in the Brazilian Amazon is limited. This study aims to evaluate the diversity and composition of macrofungi on three várzea forest islands, while also examining differences in species richness and abundance between seasonal periods. A total of 88 macrofungal species that belong to the phylum Basidiomycota were identified. The findings revealed significant variations in species composition, yet no notable differences in species richness or abundance were observed between the seasonal periods. The environmental conditions and resources available to macrofungi appear to be consistent among the islands, which leads to a balanced diversity. However, additional research is essential to uncover the true distribution patterns of macrofungi in the várzeas of the Brazilian Amazon, an area under significant threat to its biodiversity.
1. Introduction
The Amazon is represented by different ecosystems that are characterized according to the type of predominant vegetation and the influence of seasonal regimes of rivers. In addition to ombrophilous forests, igapó and floodplain forests show a peculiarity: they are constantly fertilized by the rising and falling waters of the rivers, which determines the variation and adaptation of the species present [1]. Just as the level of the rivers interferes with the dynamics of organisms, the composition of their waters also plays an important role in the contribution of organic matter. While blackwater rivers, common in the igapó areas, have low levels of sediment and nutrients, whitewater rivers, rich in suspended material, fertilize the floodplain areas, which constitute the second largest plant formation in the Amazon [2,3,4].
Floodplain forests are ecosystems that store a large amount of carbon in the soil and actively participate in river dynamics and regulating biogeochemical cycles, thus making them a unique habitat for adapted species of animals, plants, and fungi [4]. Some species are strongly adapted and have a preference for certain flooded habitats and, in view of this, their unique ecological functions can be sensitive to climate and environmental changes [5]. Studies show that some species either tolerate or are resistant to disturbances such as severe floods or droughts, while others may be impacted or even lost [6,7].
In addition, due to the seasonal flow of rich nutrients from the sediments, the substrates of floodplain areas are highly fertile and become widely used by local communities in the Amazon in the cultivation, mainly of açaí palms (Euterpe oleraceae Mart.), cassava (Manihot esculenta Crantz), and cocoa (Theobroma cacao L.) [8]. Due to this dynamic ecosystem, these floodplain forests are subject to anthropogenic impacts caused by both agriculture and logging, which can cause losses in floodplain biodiversity [9]. Therefore, the floodplain is a naturally sensitive region, both in relation to temperature variations and rainfall levels and to human-induced impacts [10,11].
Macrofungi are members of the phyla Ascomycota and Basidiomycota and are part of the community of organisms present in floodplain ecosystems. The vast majority of these macrofungi belong to the class Agaricomycetes (Basidiomycota) and are known as mushrooms and wood ears (due to the characteristic shape of their basidiomes). The species can be parasitic, symbiotic, and/or saprophytic and are among nature’s main decomposers of organic matter [12]. Among the parasitic species, Moniliophthora perniciosa (Stahel) Aime and Phillips-Mora has a wide spectrum of hosts, including plants of economic importance such as cocoa, which causes serious economic and social impacts in areas that include floodplain plantations in the Amazon [13]. On the other hand, ectomycorrhizas establish symbiotic associations with plant roots and are essential for growth, protection, and tolerance in contaminated soils [14,15].
However, the vast majority of species act as saprophytes and actively participate in the cycling of elements. The conversion of the substrate and consequent release of carbon and nitrogen to the environment occurs in different ways, depending on the enzymatic arsenal of the fungus. In this way, organisms can be classified into different categories; for example, those that cause white rot are able to degrade lignin, cellulose, and hemicellulose, the three main polymers of wood. The species that cause brown rot, on the other hand, degrade only cellulose and hemicellulose, and those that cause soft rot secrete cellulase enzymes that cause a characteristic pattern of decomposition [16].
In addition to playing a fundamental ecological role in nature, research has shown that macrofungi, as well as plants, also present a wide variety of bioactive compounds with some type of action and/or health benefit. In fact, the demand for macrofungi has been increasing globally among consumers due to their outstanding taste and nutritional capacity as a result of the presence of polysaccharides, vitamins, flavones, and unsaturated fatty acids. In turn, the therapeutic capacities are associated with the immunomodulatory, antimicrobial, and antioxidant abilities of these bioactive substances, which have an important effect in the fight against cancer, diabetes, obesity, and hypertension, for example [17]. Depending on how they act in the ecosystem, biotic and abiotic factors exert selective pressures that interfere with the frequency and distribution of species. As important as temperature, seasonal variations in river levels and the contribution of organic matter, and the construction of housing and land use by riverine populations (mainly in monocultures) demand even more from the adaptive processes of fungi in the floodplain environment. Most studies in floodplain forests are related to floristic inventories, diversity, and composition [18,19,20,21], as well as effects of the flood pulse and soil organic matter on vegetation [22,23]. However, knowledge about macrofungi in these areas is still scarce. Since knowledge on the dynamics of the macrofungi species from the class Agaricomycetes in these flooded areas is scarce, this study aims to verify the diversity and composition of macrofungi in three floodplain forest islands, as well as the differences in richness and abundance between seasonal periods.
2. Materials and Methods
2.1. Study Area
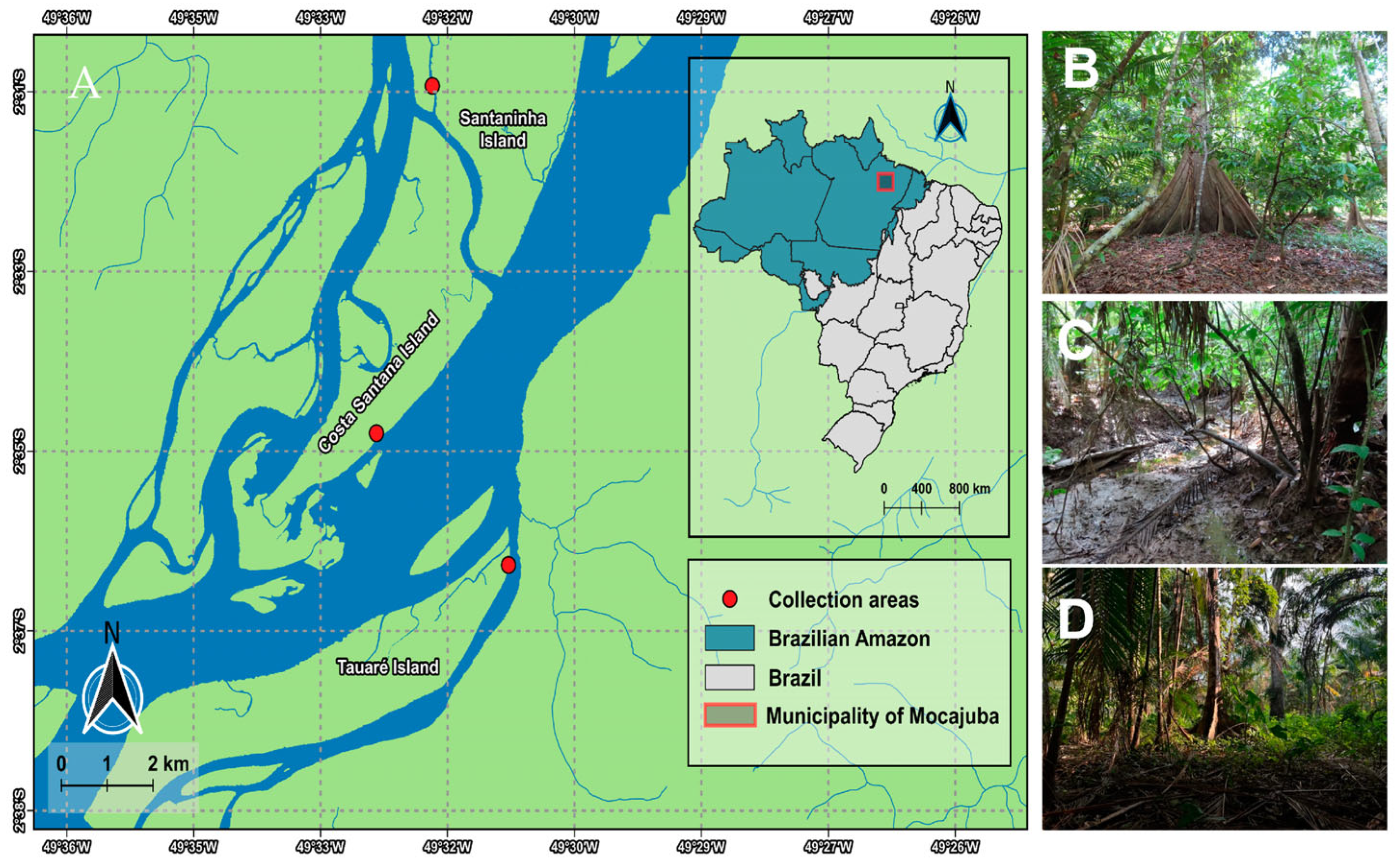
This study was conducted in the state of Pará, in the municipality of Mocajuba, located in the mesoregion of northeastern Pará and in the microregion of Cametá (02°35′31″ S, 49°28′60″ W). The predominant ecosystems are those of terra-firme—with the islands presenting a dense alluvial ombrophilous forest type vegetation—and the floodplain areas, managed and exploited by the riverine population in the form of monocultures and for agroforestry based on cocoa plantations [24,25].
The patterns related to humidity, rainfall, and temperature in the municipality change according to the months of the year. The average temperatures range between 25 °C and 34 °C; when the temperature indices begin to increase in July, humidity and precipitation decrease, as do the water levels on the islands, determining the arrival of the dry season [26] and making it possible to access the trails on the islands (Figure 1). Three islands were selected as study areas: Tauaré, Santaninha, and Costa Santana. The selected areas are marked by native cocoa plantations and the predominance of vegetation is mainly characterized by Hevea brasiliensis (Willd. ex A. Juss.) Müll.Arg., Euterpe oleracea, Virola surinamensis (Rol. ex Rottb.) Warb., Pterocarpus santalinoides L’Hér. ex DC., and Carapa guianensis Aubl. (Table 1).
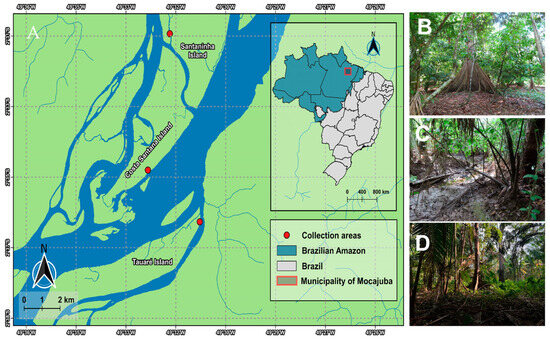
Figure 1.
(A) Map of the studied region of the municipality of Mocajuba. (B) Costa Santana Island; (C) Santaninha Island; (D) Tauré Island.

Table 1.
Description of researched areas.
2.2. Collection, Processing, and Identification
The collections were carried out in June and November of 2022 and 2023. The seasons chosen for the field trips mark the end of the rainy and dry periods, respectively, when the levels related to precipitation, humidity, and temperature vary significantly. In the selected floodplain areas, plots of two hectares were demarcated with the help of GPS, which could be covered in a period of two hours. The stages of collection, preservation, and herborization were based on the methodology of Fidalgo and Bononi [27] and Neves et al. [28]. Photographic records of the specimens were made, as well as the description of the substrate and notes on the main characteristics of the basidiomes (size, color, and consistency). The specimens were dehydrated in an electric oven.
Microscopic analyses were performed from freehand sections of the hymenial surface, the context, and the abhymenial surface with the help of steel blades. The fragments were placed on a slide with an aqueous solution of 3–5% potassium hydroxide and 1% phloxin. For cyanophilic observations, Amann’s Blue was used, which stains the walls of hyphae, basidiospores, and cystidia blue. Melzer’s reagent was used in the observation of amyloid (grayish, bluish, or purplish) or dextrinoid (reddish-brown) reactions of the walls of the basidiospores, hyphae, and other microstructures. The observation of the hyphalic system, shape, and size of fertile and sterile followed Teixeira [29]. The slides were observed under an optical microscope for the analysis and measurement of microscopic structures. For the identification, the works of Ryvarden [30,31,32,33,34], Zabin et al. [35], and Gomes-Silva et al. [36] and the specific literature for each taxonomic group were used.
2.3. Statistical Analysis
Species diversity was verified by means of relative frequency measurements between the different floodplain areas selected in this study, in addition to the estimation of richness indices. For the diversity analysis, the Shannon–Wiener index (represented by H’) was chosen, which was calculated based on the number of individuals of each species and the total number of specimens sampled. The higher the Shannon–Wiener index, the greater the diversity of the sample [37]. The relative frequency of the species was based on the number of specimens and determined by the following equation, F = n/N × 100, where n is the number of specimens of a species and N is the total number of individuals found [38]. The following frequency classes were considered regarding the occurrence of the species: 0.5 < F ≤ 1.5% = rare; 1.5 < F ≤ 5% = occasional; 5 < F ≤ 10% = frequent; F > 10% = abundant; while relative abundance used the equation [number of individuals of a species (Nspecimens)/total number of individuals on the island (Ntotal) times 100] [39].
Analyses involving species richness and composition between islands were based on the Jaccard index, which measures similarity between finite sample sets through the relationship between the intersection and the size of the union of these same sets. The hypothesis of heterogeneity between the three islands was investigated with the aid of PERMDISP (permutational analysis of multivariate dispersions) and PERMANOVA (permutational multivariate analysis of variance) tests. In addition, the species accumulation curve demonstrated the relationship between sampling effort and observed richness, while the bootstrap method was used to calculate the estimates of richness accompanied by a confidence interval, thus increasing the reliability of the results. All the statistical analyses were performed in R 3.5.2 [40].
To verify the influence of seasonality (dry and rainy periods) on the abundance of macrofungi (n = 88) on the three islands, we used the analysis of variance (ANOVA). This method was selected for its effectiveness in comparing means between groups and identifying possible significant differences related to seasonal conditions. To complement the analysis, the Tukey post hoc and Fisher tests were applied, which allowed for us to detail the differences between the groups, providing different levels of confidence in the results. The box plot graphs were generated using MINITAB software version 14.13 (OSB Software, Bela Vista, SP, Brazil).
3. Results
3.1. Macrofungal Diversity, Richness and Composition Among the Islands
In all, 223 specimens of fungi were identified, representing 88 species belonging to 18 families from the class Agaricomycetes, of the phylum Basidiomycota (Figure 2; Table 2). The order Polyporales presented eleven families, while Agaricales and Hymenochaetales presented three families each, and Russulalles only one. Of the 17 families of Agaricomycetes identified in morphological analyses, Polyporaceae had the highest number of species collected in the floodplain islands (38), followed by Meripilaceae and Hymenochaetaceae with 12 and 8, respectively. Regarding the genera, Rigidoporus, Lentinus, and Trametes were the most diverse, with 12, 10, and 9 species, respectively, while 24 genera presented only a single species. When the absolute number of species and specimens was compared among the islands, Costa Santana presented the highest number of identified species (50) and the highest number of specimens collected (90). Santaninha island occupied the second place, with 37 species and 66 specimens, which was closely followed by Tauaré, with 36 species and 67 specimens.

Figure 2.
Macrofungi from the flood forests studied in Brazilian Amazon: (A) Ganoderma stipitatum, (B) Trametes elegans, (C) Rigidoporus ulmarium, (D) Earliella scabrosa, (E) Flaviporus liebmannii, (F) Cerrena caperata, (G) Foraminispora rugosa, (H) Amauroderma exile, (I) Lentinus swartzii, (J) Lentinus sp., (K) Lentinus concavus, (L) Favolus trigonus, (M) Favolus tenuiculus, (N) Pleurotus djamor, (O) Truncospora tephropora.

Table 2.
List of species collected in Mocajuba. Quantity of specimens and the relative abundance (%) based on the number of specimens. Relative frequency [%] classified as follows: R = rare, O = occasional, F = frequent, and A = abundant.
Trametes elegans (Spreng.) Fr. (19 specimens) and Lentinus berteroi (Fr.) Fr. Rigidoporus lineatus (Pers.) Ryvarden (12 specimens) were considered to be frequent, while Earliella scabrosa (Pers.) Gilb. and Ryvarden was noted as being abundant (23 specimens). Each island analyzed in isolation showed at least one of these macrofungi in its list of abundant species.
Earliella scabrosa (12 specimens) was the most abundant species, while Trametes elegans (9 specimens) and Trametes maxima (Mont. A. David and Rajchenb (5 specimens) were the most frequent species in Costa Santana according to frequency classes. Rigidoporus lineatus, unlike on the other two islands, was one of the most representative species in Santaninha (with 8 specimens), with Trametes elegans (9) and Polyporus guianensis Mont. (4 specimens) occupying second place. As in Costa Santana, the most abundant species in Tauaré was Earliella scabrosa (10), followed by Podoscypha aculeata (Berk. and M. A. Curtis) Boidin (7) and Lentinus berteroi (7) (Table 2).
Analysis of the totality of the data provided a Shannon–Wiener index equivalent to 3.91 for the three islands. As evidenced by the absolute number of species and specimens collected, Costa Santana presented the greatest diversity among the three islands (3.54) (indicated by the respective Shannon–Wiener index), followed by Santaninha (3.30) and Tauaré (2.99).
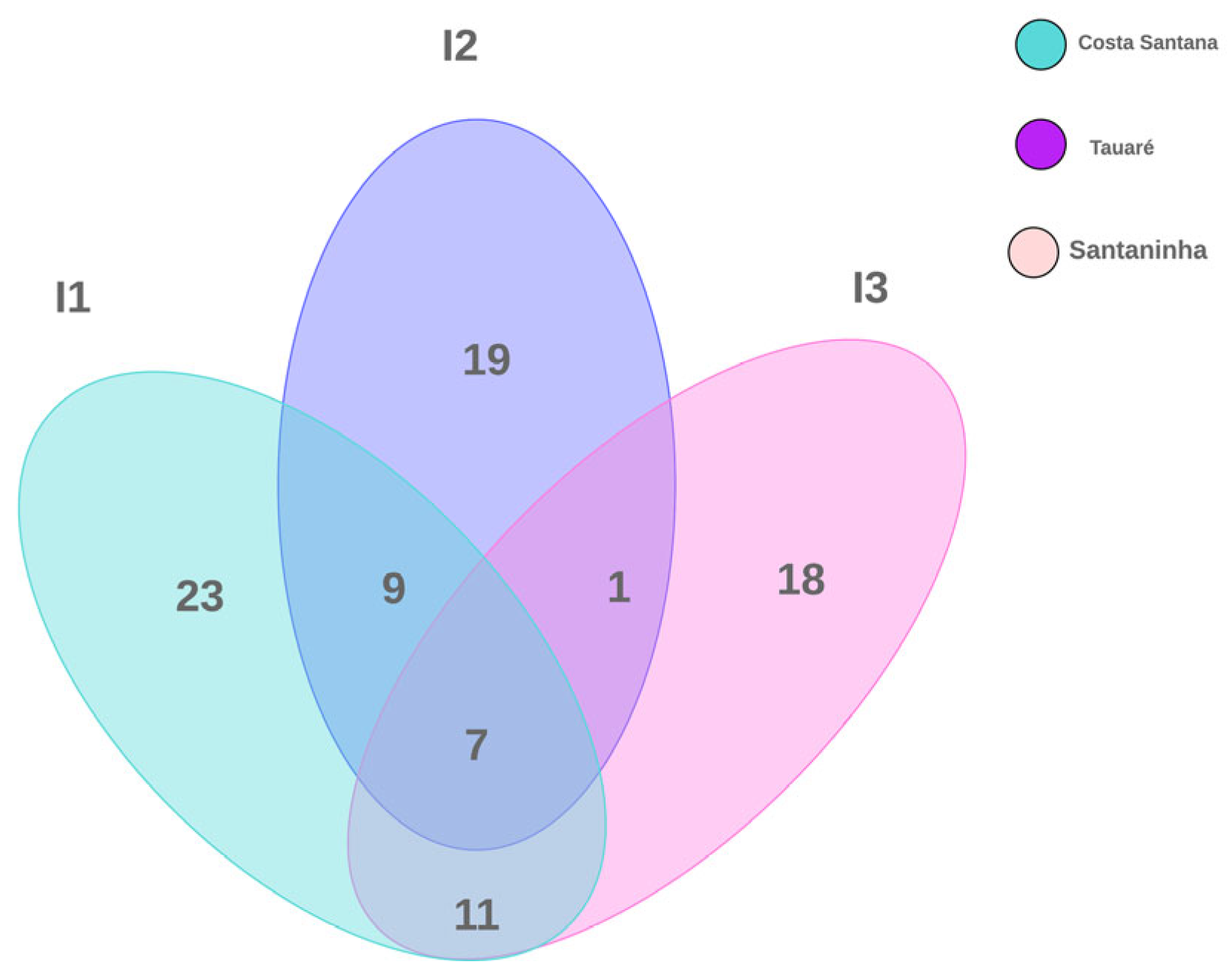
According to the composition of the species recorded on the islands, 23 were exclusively observed in Costa Santana, 18 in Santaninha, and 19 species in Tauaré. In turn, the three islands share seven species between them. The number of species shared between Costa Santana/Santaninha was equal to 11 and Costa Santana/Tauaré was equal to 9. Interestingly, Santaninha presented only one species associated with Tauaré, but shared nine of them with Costa Santana (Figure 3).
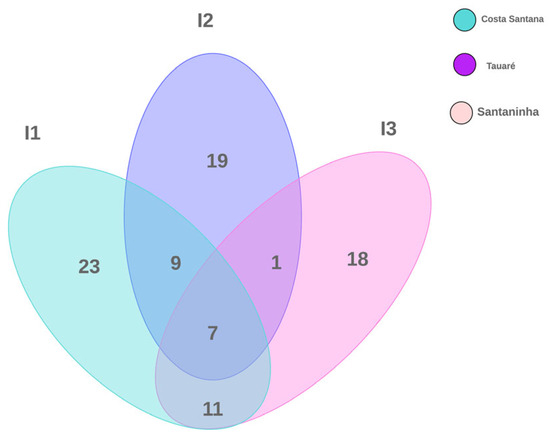
Figure 3.
Venn diagram showing the total number of macrofungal species in floodplain forests investigated on three islands in the Brazilian Amazon.
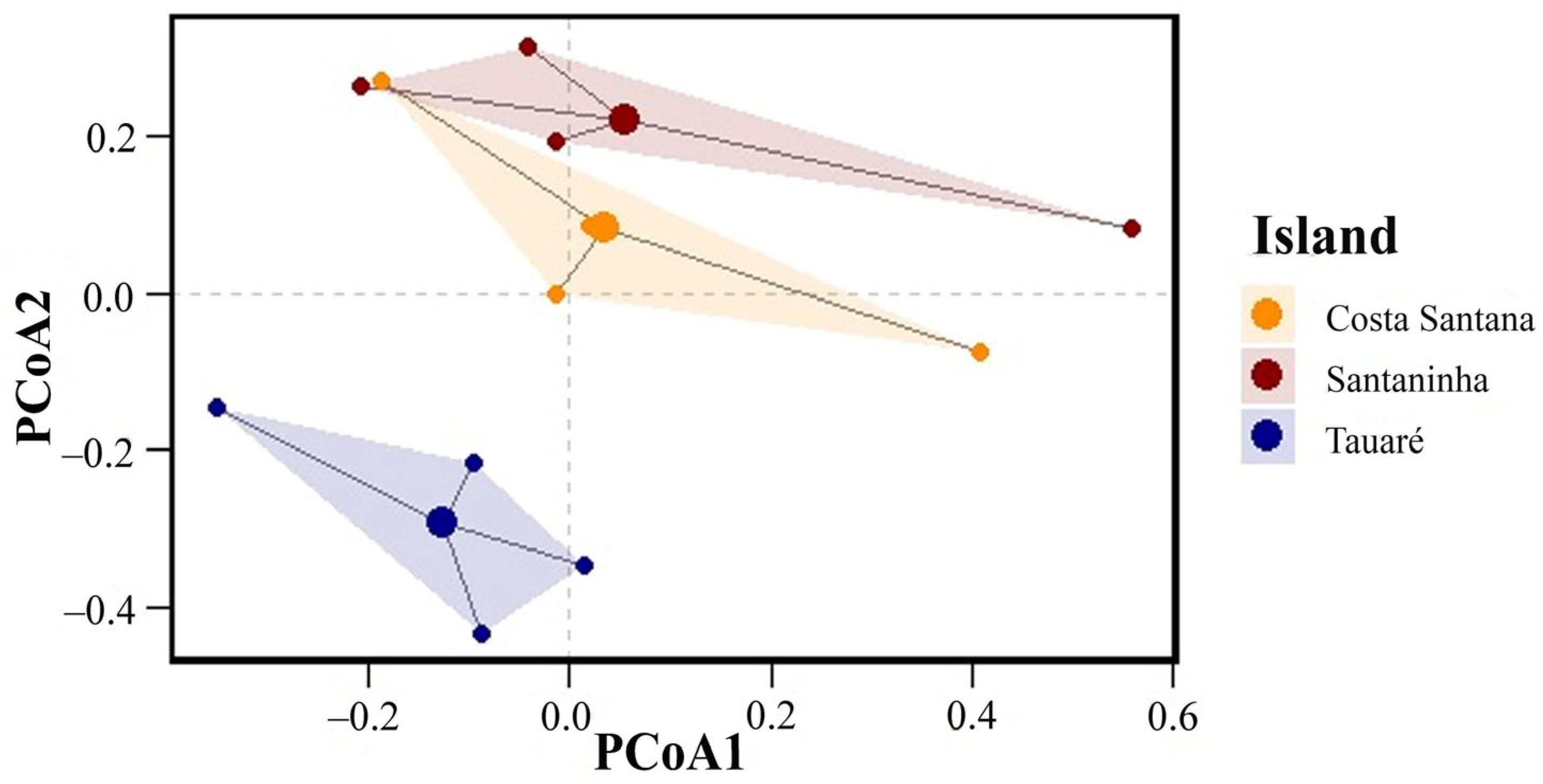
Regarding species composition, the PERMANOVA showed a significant difference in fungal species composition among the islands (F = 5.678, p = 0.045). With a value of p < 0.05, we can infer that the mean composition of fungal species varies statistically among the islands, indicating that each island has a distinct set of species, which suggests specific ecological or environmental factors may be shaping these communities differently. The total variation in species composition (R2 = 0.45) indicates that approximately 45% can be explained by the differences among the islands.
In contrast, the PERMDISP indicates that the dispersion of species compositions relative to their centroids (the mean or central point of species composition for each island) does not differ significantly between islands (F = 1.234, p = 0.561). This result suggests that although the islands have distinct mean species compositions, the variability or heterogeneity within each island (i.e., how species are distributed relative to the center of their mean composition) is similar. The dispersion of species compositions on each island is homogeneous.
The PCoA graph (Figure 4) illustrates these results visually. The dots represent individual samples, grouped by island, with different colors. The shaded areas reflect the dispersal of species compositions on each island. The separation of the groups indicates differences in the mean composition between the islands (confirmed by PERMANOVA), while the similar size and shape of the shaded areas suggest that the internal dispersion of the species within each island is homogeneous (corroborated by PERMDISP).
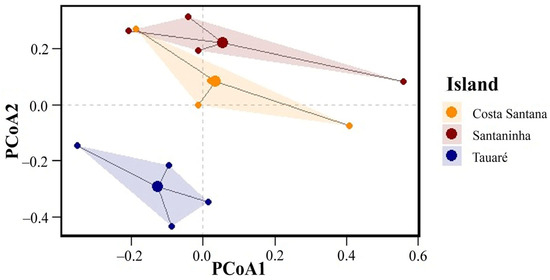
Figure 4.
Principal coordinate analyses (PCoAs) of macrofungi composition on the three islands.
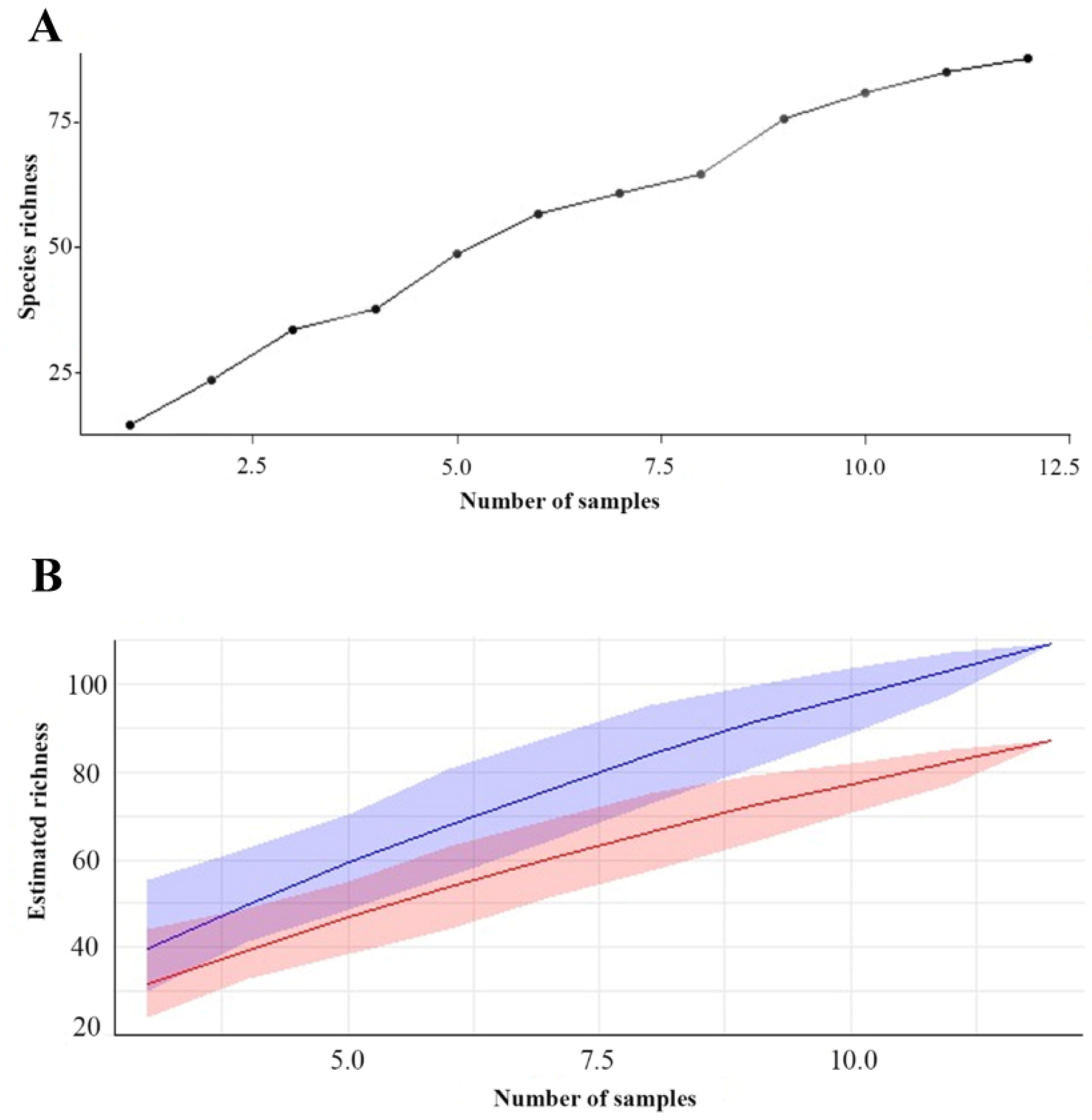
Regarding species richness, the rarefaction curve, obtained by the method of the collector, revealed that the estimated species richness increased rapidly at the beginning and later stabilized around 80 species after the collection of 10 samples. Bootstrap analysis complemented this assessment by providing robust estimates of richness, averaging approximately 83.46 species. The 95% confidence interval for the bootstrap richness estimate ranged from approximately 75.04 to 90.76 species, confirming the accuracy and robustness of the estimates and indicating a reliable species richness within this range of variation.
Combined analysis of the three islands over the twelve collections reveals an accumulation curve that indicates a rapid initial increase in species richness as more samples are collected. However, as sampling progresses, the curve shows signs of stabilization, suggesting that most of the species present on the three islands were identified during the collection period. This accumulation dynamic provides valuable information about biological diversity on the islands, demonstrating that the discovery of new species tends to decline as sampling progresses (Figure 5).
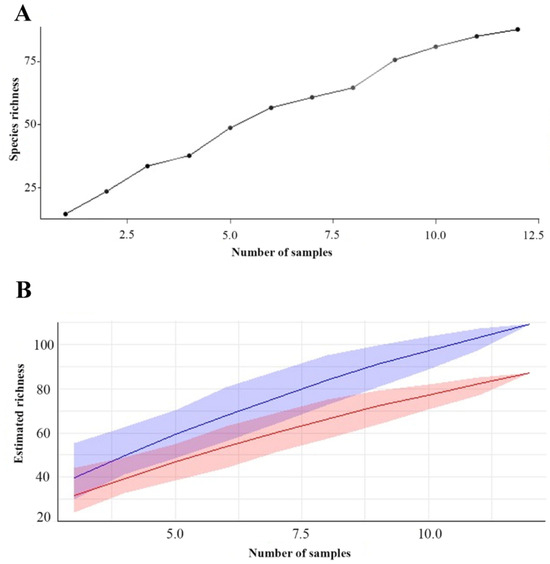
Figure 5.
Dynamic accumulation: (A) Species accumulation curve in the three islands of floodplain forest. (B) Bootstrap chart. The red line represents the richness estimate calculated using bootstrap, while the blue line represents the observed or estimated richness directly from the data. The shaded area indicates the confidence interval.
3.2. Seasonal Diversity on the Three Islands
In relation to the differences between the dry and rainy periods, 41 species were recorded in the rainy period, 25 in the dry period, and 22 in both. In the rainy season, the highest number of species was recorded on Costa Santana with 34 species, and both Tauaré and Santaninha presented 25 species each. In the dry period, the largest number of species was recorded on Costa Santana (32 species), while Tauaré presented 22 and Santaninha 18 species.
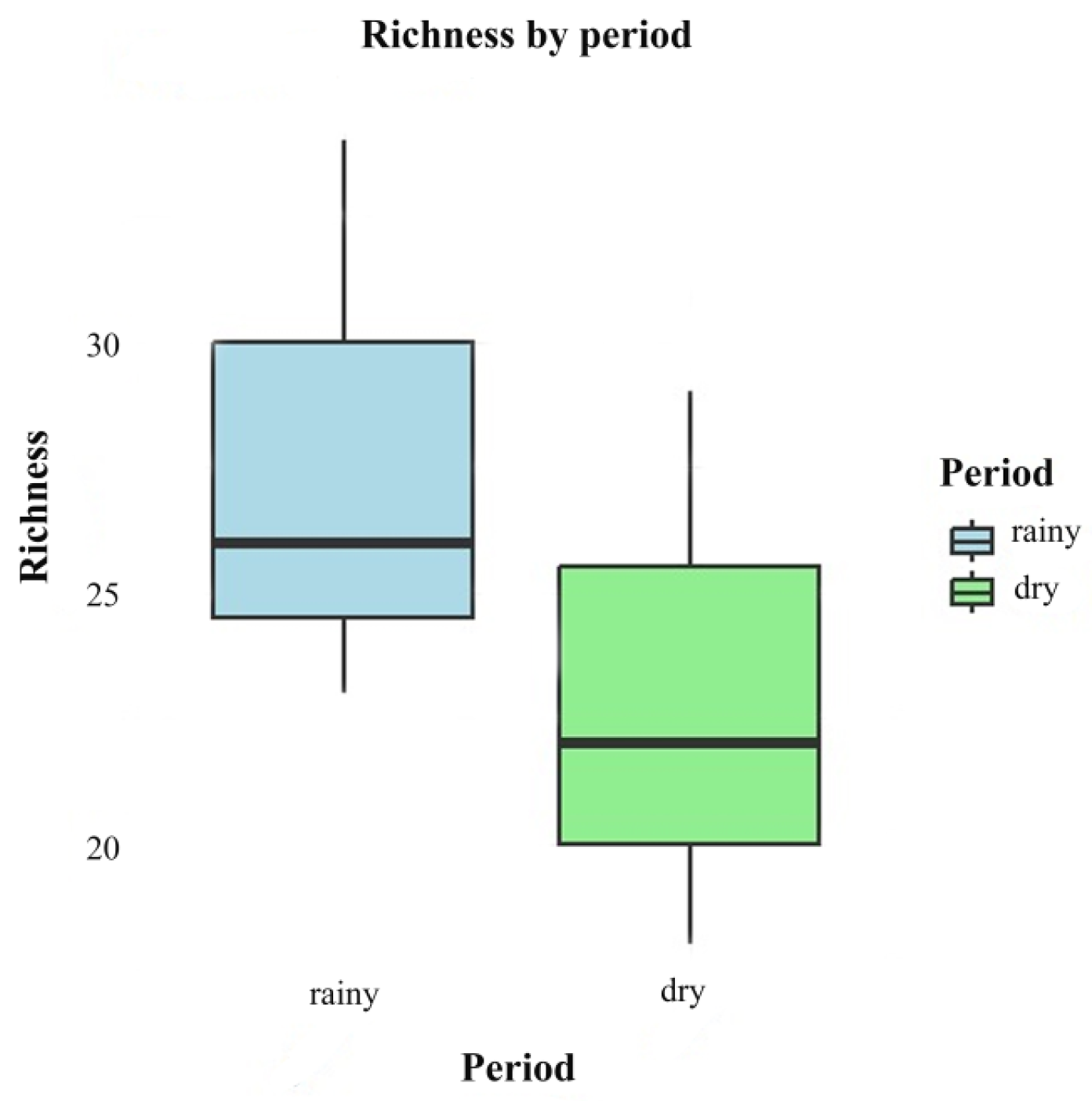
There was no significant difference between the rainy season (June 2022 and 2023) and dry season (November 2022 and 2023) in relation to species richness (F = 1.032; p = 0.367). Although the means indicate that wealth is higher in the rainy season (mean = 27.7, standard deviation (SD) = 5.69) compared to the dry season (mean = 23.0, standard deviation (SD) = 5.57), the ANOVA indicated that this difference is not statistically significant (Figure 6).
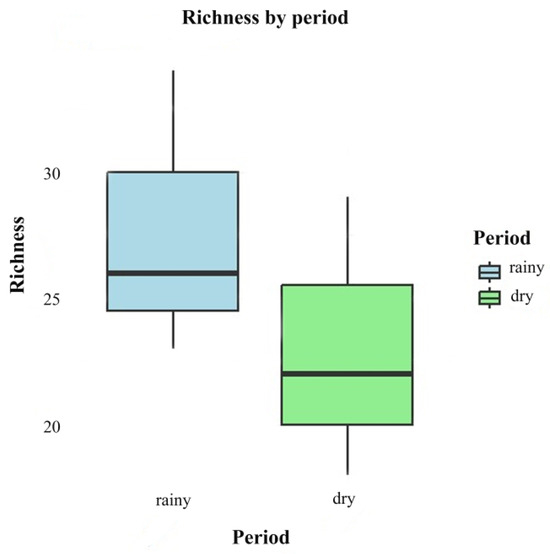
Figure 6.
Box plots comparing the richness of fungi in different periods (rainy and dry).
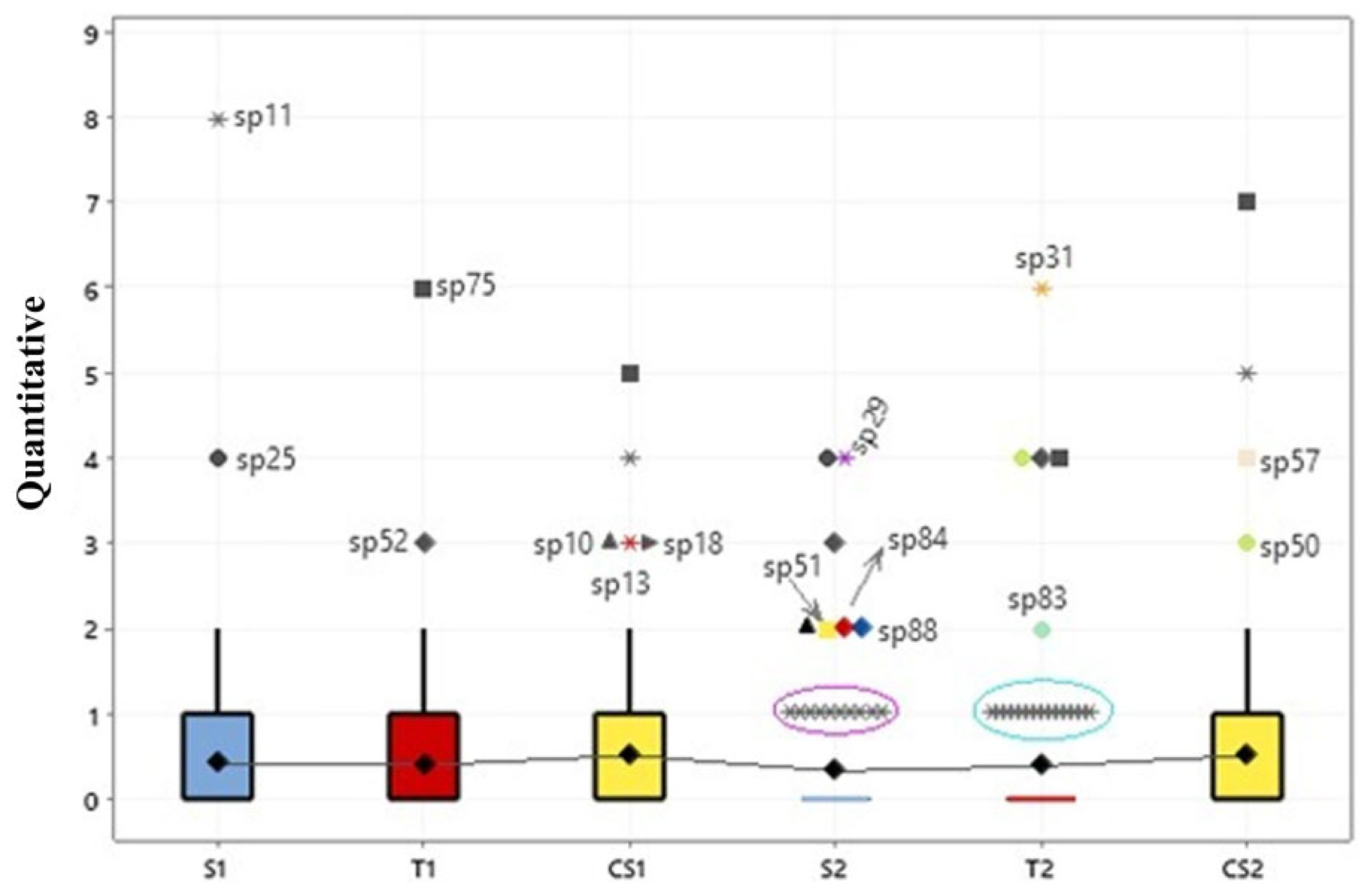
An ANOVA was also applied to evaluate species abundance (n = 88) in relation to seasonality and there were no significant differences in the quantity of specimens of macrofungi on the islands (p < 0.001) (Figure 7). Nonetheless, the abundance is noticeable within each group reported via the outliers.
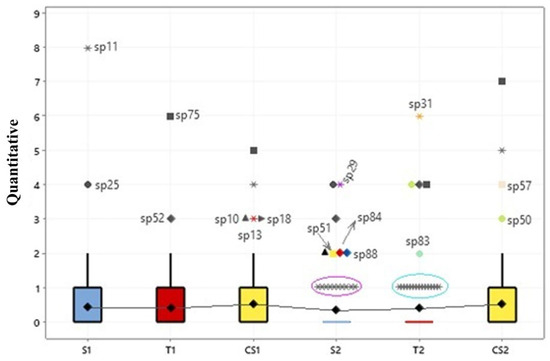
Figure 7.
Box plots comparing the abundance of fungi on the three floodplain islands, in relation to seasonality. S = Santaninha; T = Tauaré; CS = Costa Santana; 1 = rainy season; 2 = dry season. Symbols represent the species, for example: * sp11= Rigidoporus lineatus.
In Figure 7, the outliers detected in the model demonstrated the most abundant groups of macrofungi in the different seasons. In the rainy season, on Santaninha island (S1), there was the highest occurrence of the species Trametes elegans (sp. 25) with eight specimens and Rigidoporus lineatus (sp. 11) with four specimens. On the island of Tauaré (T1), the species Earliella scabrosa (sp. 75) and Lentinus berteroi (sp. 52) were the most abundant, with seven and three specimens, respectively. Finally, on the island of Costa Santana (CS1), in addition to E. scabrosa (five specimens) and T. elegans (four specimens), already recorded in S1 and T1, Trametes maxima (sp. 10), Tinctoporellus isabellinus (sp. 13), and R. ulmarius (sp. 18) were also the most common.
In contrast, in the dry season, the variability of these macrofungi was intensified. In Santaninha (S2), there was an increase in Polyporus guianensis (sp.29), L. berteroi, L. concavus (sp. 51), T. maxima, Cymatoderma caperatum (sp. 84), and Fomes fasciatus (sp. 88). On the island of Tauaré (T2), the most predominant macrofungi were the species Podoscypha aculeata (sp. 31), L. crinitus (sp. 50), L. berteroi, E. scabrosa, and Lentinula raphanica (sp. 83). On Costa Santana (CS2), the most frequent were E. scabrosa, T. elegans, Cerrena hydnoides (sp. 50), and L. crinitus.
The results of the Tukey and Fisher tests indicate a homogeneous distribution of these organisms, with certain highlights. This implies that the environmental conditions and resources available to macrofungi are similar among the islands and this reflects a balanced diversity.
4. Discussion
4.1. Composition and Richness Among the Islands
Geographical and edaphic attributes may explain the difference found in the diversity indices of the islands. The soil of Costa Santana presents high fertility in all its layers, which is fundamental for the development of plant species [41]. It has been a managed area for more than 50 years and is formed by native palms and tree species such as rubber trees, açaí palms, cocoa, and andiroba (Carapa guianensis Aubl.) trees and was the only one that presented the samaúma species [Ceiba pentandra (L.) Gaertn.—Malvaceae], a plant that is native to the Amazon. On the other hand, unlike Costa Santana, there are streams along the plantations of the Tauaré and Santaninha, characterizing a typically alluvial forest. In addition to the action of the water in the high-water period, it probably ends up selecting the plant and fungal species most adapted to the seasonal variations of the ecosystem. In fact, the specialization assumed by organisms along the floodplains causes the richness and diversity to be lower compared to the dry-land ecosystem [11,42].
In fact, the value related to species richness of macrofungi in the floodplain islands of this study was lower compared to that presented in regions of dry land in the Brazilian Amazon. Collections carried out in the Caxiuanã National Forest, located in the northwest of the state of Pará, showed indices of up to 130 species of Agaricomycetes against 88 on the floodplain islands [43,44]. Significant numbers of species were also found in Serra do Navio (100), and in Amapá National Forest (97), in the state of Amapá [45,46], as well as in a fragment of dense ombrophilous forest in the surroundings of HPP Silvio Braga, located in western Pará (91 species) [47]. However, a standardized comparative study is needed to confirm this hypothesis.
López-Quintero et al. [48] analyzed the diversity of macrofungi in two areas of the Colombian Amazon, one area being terra-firme and the other floodplains. The exclusive richness in each area was similar (n = 128), but the abundance was higher in the floodplain areas (804 sporocarps) than on dry land (741 sporocarps). The authors attributed this result to the nutrient-rich alluvial soils of the floodplains, which are due to the regular deposition of detritus in floodplain areas, and nutrients and organic matter during high/rising water, which occur on average twice a year. The authors stated that, despite the significant number of species, the rarefaction curve did not reach the asymptote, i.e., it did not saturate. This is different from the results found in this study, in which the accumulation curve stabilized from 80 species onwards.
Even if decomposing organisms are favored by organic matter and woody residues brought by river waters, it is necessary to check the richness in an area of floodplain primary forest for a more accurate assessment. Amazonian studies involving fungi associated with the floodplain ecosystem in the Brazilian Amazon, in addition to conidial microfungi [49], are scarce, especially when they report the richness and diversity of macrofungi. The data collected in this study are pioneering and fundamental for the increase in knowledge and understanding of the diversity of Agaricomycetes in floodplain forest areas, where the lack of studies involving the taxonomy of macroscopic fungi does not reflect the ecological importance of this ecosystem.
4.2. Seasonal Diversity
Since the field trips took place in two different periods of the year, climatological aspects probably interfered with the numbers of specimens observed in the floodplain ecosystem. In June 2022, 70 specimens of macrofungi of the class Agaricomycetes were collected, a value 25% higher when compared to November of the same year, which totaled 56. Traditionally, June presents a higher index of precipitation and humidity in the northern region in contrast to the month of November (174.4 mm/78.9%) [26]. Although there are differences in absolute numbers, statistically, this difference was not significant, as demonstrated in the ANOVA. This is different from the findings of Putra et al. [50], who evaluated the seasonal distribution of macrofungi in three forest communities in Indonesia and found significant differences. For example, of the 130 species recorded by the authors, 81 occurred in the rainy season, 25 in the dry season, and 22 in both periods. In this study, the species that were common in both periods were mainly from the Polyporaceae family (E. scabrosa, L. berteroi, T. elegans). Such species have a leathery and resistant basidiome and are often observed on decaying wood in open areas and/or those with intense sunlight.
Despite this, factors unrelated to climate have the potential to alter the richness and diversity of organisms in a given area. In June 2023, there was a decrease of approximately 49% in the number of specimens compared to the same period of the previous year, with 47 individuals collected. The Tauaré islands suffered the impact of the felling of its native vegetation and removal of woody material in the clearing of the land, thus reducing the supply of substrate for the growth of macrofungi. Combined with abiotic aspects, the availability of organic matter is in a relationship that is directly proportional to the number of fungi observed in the ecosystem. In other words, the greater the quantity and quality of the substrate, the greater the quantity and diversity of the fungal community [51].
Rustøen et al. [52] investigated the affinity of wood decomposing fungi in relation to the substrate of angiosperms and conifers in the United Kingdom and the effects of climate on a 40-year time scale to ascertain whether there would be compositional changes related to temporal changes and identified that the composition of wood fungi was mainly structured by the properties of the substrate, and that climatic effects were the least significant.
Although the values obtained in the diversity and richness analyses were practically the same for the three islands of Mocajuba, Costa Santana showed itself to have the highest indices, followed by Santaninha and Tauaré.
4.3. Future Research Directions
Floodplain forests are huge, and seasonal flood regimes, those that reach the maximum level in the wettest months, such as those that occur in the Tocantins River, limit research in these areas [53]. Although the research was not carried out in a primary floodplain area, it was carried out in an agroforestry system, which is an economic model of sustainability for preserving tree species [54]. Such preserved tree species provide ecological conditions for the maintenance of macrofungal species and, since they play a fundamental role in nutrient cycling, it is important to understand and identify diversity for the conservation and management of species for ecosystem health.
Li et al. [55] compared the diversity of macrofungi in native forests and managed plantation areas in China and noticed that the diversity was significantly higher in native forests. However, the composition of the fungal community was different between the areas. The authors point out that although diversity was the highest in native forests, the two management systems harbor distinct groups of macrofungi, suggesting that the two management methods together could provide a complementary range of macrofungi habitats.
Nonetheless, it is still necessary to compare areas of primary forests in order to discover the real distribution patterns of the species. This is a challenge in floodplain areas since they are forests that suffer from many types of anthropogenic pressures, by logging, livestock rearing and agriculture involving plants of economic interest, both for human survival and for commercialization [56]. In addition, few areas are under environmental protection, which makes it difficult to separate the effects of the impacts on populations of species.
5. Conclusions
Amazonian floodplain forests are rich and unique ecosystems, but they remain poorly explored regarding macrofungal diversity. Costa Santana island exhibited the highest diversity, likely associated with edaphic factors. In this study, significant differences in species composition were observed among the islands. However, no significant differences were found in the richness and abundance of the macrofungal species in relation to seasonality. Nevertheless, further investigation is needed to understand the true distribution patterns of species in the Amazon, a region severely threatened by loss of biodiversity.
Author Contributions
Conceptualization, V.P.F., M.d.P.S.P.V., A.W.G.-V., R.B.M.-F. and A.M.d.S.S.; methodology—formal analysis, R.D.P. and B.S.F.d.S.; data curation and review, A.W.G.-V., R.B.M.-F. and A.M.d.S.S.; funding acquisition, M.d.P.S.P.V.; writing—original draft preparation, V.P.F.; writing—review and editing, A.M.d.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by “Fundação Amazônia de Amparo a Estudos e Pesquisas, grant number FAPESPA, No. 016/2021” and Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Museu Paraense Emílio Goeldi (MPEG), Universidade Federal Rural da Amazônia (UFRA) for the support and availability of the infrastructure to carry out this research and research project “Beneficiamento primário, propriedades físicas e químicas das amêndoas de cacau nativo (Theobroma cacao) e sistema informativo geográfico das ilhas de várzea”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zappi, D.C. Tipos de Vegetação da Amazônia com ênfase no Pará. In Abelhas Sem Ferrão do Pará: A Partir das Expedições Científicas de João M. F. Camargo; Imperatriz-Fonseca, V.L., Alves, D.A., Eds.; Instituto Tecnolégico Vale: Belém, Brazil, 2020; pp. 17–31. [Google Scholar]
- Prance, G.T. Notes on the Vegetation of Amazonia III. The Terminology of Amazonian Forest Types Subject to Inundation. Brittonia 1979, 31, 26. [Google Scholar] [CrossRef]
- Maués, B.A.R.; Jardim, M.A.G.; Batista, F.d.J.; Medeiros, T.D.S.; Quaresma, A.C. Composição florística e estrutura do estrato inferior da Floresta de Várzea na Área de Proteção Ambiental Ilha do Combu, município de Belém, Estado do Pará. Rev. Árvore 2011, 35, 669–677. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, J.E.; Piedade, M.T.F.; Schöngart, J.; Demarchi, L.O.; Quaresma, A.C.; Junk, W.J. A Review of the Ecological and Biogeographic Differences of Amazonian Floodplain Forests. Water 2022, 14, 3360. [Google Scholar] [CrossRef]
- Householder, J.E.; Wittmann, F.; Schöngart, J.; Piedade, M.T.F.; Junk, W.J.; Latrubesse, E.M.; Quaresma, A.C.; Demarchi, L.O.; de S. Lobo, G.; Aguiar, D.P.P.; et al. Author Correction: One Sixth of Amazonian Tree Diversity Is Dependent on River Floodplains. Nat. Ecol. Evol. 2024, 8, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.F.d.S.; Silva, S.C.P.; Vasconcelos, M.A.; Mendes, G.S.; Pereira, H.S. A Morte Catastrófica de Árvores Por Inundação Na Ilha Do Careiro Da Várzea-AM. Terceira Margem Amaz. 2019, 5, 13. [Google Scholar] [CrossRef]
- Ávila, J.V.C.; Ticktin, T.; Steward, A.M.; Giehl, E.L.H.; Cantor, M.; Clement, C.R. Recovery of Local Agrobiodiversity after an Extreme Flood in Amazon Floodplains. Biol. Conserv. 2024, 292, 110536. [Google Scholar] [CrossRef]
- Freitas, M.A.B.; Vieira, I.C.G.; Albernaz, A.L.K.M.; Magalhães, J.L.L.; Lees, A.C. Floristic Impoverishment of Amazonian Floodplain Forests Managed for Açaí Fruit Production. For. Ecol. Manag. 2015, 351, 20–27. [Google Scholar] [CrossRef]
- Junk, W.J.; Piedade, M.T.F. An Introduction to South American Wetland Forests: Distribution, Definitions and General Characterization. In Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management; Springer: Berlin/Heidelberg, Germany, 2010; p. 210. [Google Scholar]
- Choueri, R.B.; Azevedo, J.A.R. Biodiversidade e Impacto de Grandes Empreendimentos Hidrelétricos na Bacia Tocantins-Araguaia: Uma Análise Sistêmica. Soc. Nat. 2017, 29, 439–453. [Google Scholar] [CrossRef]
- Kowalska, N.; Šigut, L.; Stojanović, M.; Fischer, M.; Kyselova, I.; Pavelka, M. Analysis of Floodplain Forest Sensitivity to Drought. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190518. [Google Scholar] [CrossRef]
- Niego, A.G.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Walker, A.; Hyde, K.D.; et al. The Contribution of Fungi to the Global Economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Lisboa, D.O.; Evans, H.C.; Araújo, J.P.M.; Elias, S.G.; Barreto, R.W. Moniliophthora perniciosa, the Mushroom Causing Witches’ Broom Disease of Cacao: Insights into Its Taxonomy, Ecology and Host Range in Brazil. Fungal Biol. 2020, 124, 983–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Zhou, Y.; Zou, X.; Yin, Y.; Li, H.; Liu, L.; Zhang, S. Ectomycorrhizal Symbioses Increase Soil Calcium Availability and Water Use Efficiency of Quercus Acutissima Seedlings under Drought Stress. Eur. J. For. Res. 2021, 140, 1039–1048. [Google Scholar] [CrossRef]
- Chot, E.; Reddy, M.S. Role of Ectomycorrhizal Symbiosis Behind the Host Plants Ameliorated Tolerance Against Heavy Metal Stress. Front. Microbiol. 2022, 13, 855473. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Qian, Y.; Jellison, J. Fungal Decay of Wood: Soft Rot-Brown Rot-White Rot. In ACS Symposium Series; Schultz, T.P., Militz, H., Freeman, M.H., Goodell, B., Nicholas, D.D., Eds.; ACS Publications: Aurora, IL, USA, 2008; Volume 982, pp. 9–31. ISBN 9780841239517. [Google Scholar]
- Mazumder, M.; Roy, S.; Sarkar, A.K. Pharmacological and Therapeutic Value of Bamboo Mushroom Phallus Indusiatus (Agaricomycetes). Ital. J. Mycol. 2022, 51, 47–57. [Google Scholar]
- Cattanio, J.H.; Anderson, A.B.; Carvalho, M.S. Floristic Composition and Topographic Variation in a Tidal Floodplain Forest in the Amazon Estuary. Rev. Bras. Botânica 2002, 25, 419–430. [Google Scholar] [CrossRef]
- Almeida, S.S.; Amaral, D.D.; Silva, A.S.L. da Análise Florística e Estrutura de Florestas de Várzea No Estuário Amazônico. Acta Amaz. 2004, 34, 513–524. [Google Scholar] [CrossRef]
- Assis, R.L.; Wittmann, F. Forest Structure and Tree Species Composition of the Understory of Two Central Amazonian Várzea Forests of Contrasting Flood Heights. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 251–260. [Google Scholar] [CrossRef]
- Assis, R.L.; Wittmann, F.; Piedade, M.T.F.; Haugaasen, T. Effects of Hydroperiod and Substrate Properties on Tree Alpha Diversity and Composition in Amazonian Floodplain Forests. Plant Ecol. 2015, 216, 41–54. [Google Scholar] [CrossRef]
- Assis, R.L.; Wittmann, F.; Bredin, Y.K.; Schöngart, J.; Nobre Quesada, C.A.; Piedade, M.T.F.; Haugaasen, T. Above-Ground Woody Biomass Distribution in Amazonian Floodplain Forests: Effects of Hydroperiod and Substrate Properties. For. Ecol. Manag. 2019, 432, 365–375. [Google Scholar] [CrossRef]
- Zocatelli, R.; Moreira-Turcq, P.; Bernardes, M.; Turcq, B.; Cordeiro, R.C.; Gogo, S.; Disnar, J.R.; Boussafir, M. Sedimentary Evidence of Soil Organic Matter Input to the Curuai Amazonian Floodplain. Org. Geochem. 2013, 63, 40–47. [Google Scholar] [CrossRef]
- Nogueira, K.N.S.; Costa, F.D.A.; Adami, M. Território e Trabalho: Análise Geoeconômica com base em Trajetórias Camponesas. Novos Cad. NAEA 2018, 21, 1. [Google Scholar] [CrossRef][Green Version]
- Barros, D.J.; Carvalho, G.A.; Chaves, M.G.; Vanzela, L.S.; Kozusny-Andreani, D.I.; Guarda, E.A.; Neu, V.; Morais, P.B.; Tsai, S.M.; Navarrete, A.A. Microbial Metabolic Activity in Amazon Floodplain Forest and Agricultural Soils. Front. Microbiol. 2023, 14, 1144062. [Google Scholar] [CrossRef] [PubMed]
- INMET—Instituto Nacional de Metereologia. Gráficos Climátológicos; Ministério Da Agricultura e Pecuária: Brasília, DF. Available online: www.inmet.gov.br (accessed on 20 December 2024).
- Fidalgo, O.; Bononi, V.L.R. Técnicas de Coleta, Preservação e Herborização de Material Botânico; Instituto de Botânica: São Paulo, Brazil, 1989; p. 62. [Google Scholar]
- Neves, M.A.; Baseia, I.G.; Drechsler-Santos, E.R.; Góes-Neto, A. Guide to the Common Fungi of the Semiarid Region of Brazil; TECC Editora: Florianópolis, Brazil, 2013. [Google Scholar]
- Teixeira, A.R. Métodos Para Estudos Das Hifas Do Basidiocarpo de Fungos Poliporáceos; Instituto de Botânica: São Paulo, Brazil, 1995. [Google Scholar]
- Ryvarden, L. A Note on the Genus Hydnodon Banker. Synopsis Fungorum 2002, 15, 31–33. [Google Scholar]
- Ryvarden, L. Neotropical Polypores. Part 1. Introduction Ganodermataceae & Hymenochaetaceae. Synop. Fungorum 2004, 19, 238. [Google Scholar]
- Ryvarden, L. The Genus Inonotus—A Synopsis; Fungiflora: Oslo, Norway, 2005. [Google Scholar]
- Ryvarden, L. Studies in Neotropical Polypores 37. Some New and Interesting Species from Tropical America. Synopsis Fungorum 2014, 32, 58–67. [Google Scholar]
- Ryvarden, L. Neotropical Polypores. Part 3, Polyporaceae, Obba-Wrightoporia; Fungiflora: Oslo, Norway, 2016; Volume 46. [Google Scholar]
- Zabin, D.A.; Spirin, V.; Vlasák, J.; Coelho-Nascimento, C.; Menolli, N. Taxonomic Reinvestigation of Favolus in the Neotropics Utilizing Morphological and Multigene Phylogenetic Analyses. Mycol. Prog. 2024, 23, 44. [Google Scholar] [CrossRef]
- Gomes-Silva, A.C.; Medeiros, P.S.; Soares, A.M.S.; Sotão, H.M.P.; Ryvarden, L.; Gobertoni, T.B. Two New Species of Rigidoporus (Agaricomycetes) from Brazil and New Records from the Brazilian Amazonia. Phytotaxa 2014, 156, 191–200. [Google Scholar] [CrossRef]
- Nolan, K.A.; Callahan, J.E. Beachcomber Biology: The Shannon-Weiner Species Diversity Index. In Tested Studies for Laboratory Teaching: Proceedings of the 27th Workshop/Conference of the Association for Biology Laboratory Education (ABLE); Association for Biology Laboratory Education: Claremont, CA, Canada, 2006; pp. 334–338. [Google Scholar]
- Lindblad, I. Host Specificity of Some Wood-Inhabiting Fungi in a Tropical Forest. Mycologia 2000, 92, 399–405. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Zhong, Z.; Xiao, L.; Wang, Y.; Wang, H. Relating Macrofungal Diversity and Forest Characteristics in Boreal Forests in China: Conservation Effects, Inter-forest-type Variations, and Association Decoupling. Ecol. Evol. 2021, 11, 13268–13282. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 10 June 2023).
- Botelho, E.V.D. Avaliação Da Fertilidade de Solos de Cacau de Várzeas, Mocajuba–PA. In Proceedings of the 62nd Congresso Brasileiro de Química; UFRN: Natal, Brazil, 2023; pp. 1–18. [Google Scholar]
- Yu, Y.; Liu, H.; Zhang, L.; Sun, Z.; Lei, B.; Miao, Y.; Chu, H.; Han, S.; Shi, Y.; Zheng, J. Distinct Response Patterns of Plants and Soil Microorganisms to Agronomic Practices and Seasonal Variation in a Floodplain Ecosystem. Front. Microbiol. 2023, 14, 1094750. [Google Scholar] [CrossRef]
- Gibertoni, T.B.; Ryvarden, L.; Bernicchia, A.; Savino, E. Poroid Fungi (Agaricomycetes, Basidiomycota) in the National Caxiuanã Forest. In Caxiuanã, Paraíso Ainda Preservado; Lisboa, P.L.B., Ed.; Museu Paraense Emílio Goeldi: Pará, Brazil, 2013; pp. 397–409. [Google Scholar]
- Sotão, H.M.P.; Medeiros, P.S.; Gibertoni, T.B. Fungos Macroscópicos Da Floresta Nacional de Caxiuanã, Pará, Brasil: Basidiomycota (Agaricomycetes). In Caxiuanã: Desafios para Conservação de uma Floresta Nacional na Amazônia; Lisboa, P.L.B., Ed.; Museu Paraense Emílio Goeldi: Pará, Brazil, 2009; pp. 383–396. [Google Scholar]
- Soares, A.M.S.; Medeiros, P.S.; Gibertoni, T.B. Riqueza de Fungos Poliporoides (Agaricomycetes, Basidiomycota) Em Uma Floresta Ombrófila Densa No Amapá, Amazônia Brasileira. Bol. Mus. Biol. Mello Leitão 2014, 35, 5–18. [Google Scholar]
- Xavier, W.K.S.; Sotão, H.M.P.; Soares, A.M.D.S.; Gibertoni, T.B.; Rodrigues, F.D.J.; Ryvarden, L. Riqueza de Agaricomycetes Poroides Da Serra Do Navio, Amazônia Oriental, Com Novo Registro de Oxyporus Lacera Para o Brasil. Bol. do Mus. Para. Emílio Goeldi-Ciências Nat. 2018, 13, 303–315. [Google Scholar] [CrossRef]
- Couceiro, D.d.M.; Soares, A.M.S.; Couceiro, S.R.M. Contribution to the Knowledge of Polypores (Agaricomycetes) in the Amazonian Forest, with 16 New Records for the State of Pará, Brazil. Res. Soc. Dev. 2022, 11, e35111436024. [Google Scholar] [CrossRef]
- Lopez-Quintero, C.A.; Straatsma, G.; Franco-Molano, A.E.; Boekhout, T. Macrofungal Diversity in Colombian Amazon Forests Varies with Regions and Regimes of Disturbance. Res. Soc. Dev. 2012, 21, 2221–2243. [Google Scholar] [CrossRef]
- De Castro, C.C.; Gutiérrez, A.H.; Sotão, H.M.P. Fungos Conidiais em Euterpe Oleracea Mart. (Açaizeiro) Na Ilha Do Combu, Pará-Brasil. Acta Bot. Brasilica 2012, 26, 761–771. [Google Scholar] [CrossRef]
- Putra, P.S.; Supriadi; Achmad, A.; Yamada, T.; Ngakan, P.O. Seasonal Diversity and Distribution of Decomposing Macrofungi in Three Forest Communities: Why Do They Differ? IOP Conf. Ser. Earth Environ. Sci. 2023, 1230, 012059. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Hayata, M.A.; Lopes, C.A.; Pinheiro, J.S.; Souza, A. Composição e Riqueza de Macrofungos Da Madeira Em Três Estruturas Vegetacionais de Uma Área de Floresta Ombrófila Mista Impactada. In Ecologia de Campo: Ambientes Costeiros e Montanos; Hayata, M.A., Souza, A.R., Lopes, C., Eds.; Programa de Pós-Graduação em Ecologia, Universidade Federal de Santa Catarina: Florianópolis, Brazil, 2018; pp. 189–201. [Google Scholar]
- Rustøen, F.; Høiland, K.; Heegaard, E.; Boddy, L.; Gange, A.C.; Kauserud, H.; Andrew, C. Substrate Affinities of Wood Decay Fungi Are Foremost Structured by Wood Properties Not Climate. Fungal Ecol. 2023, 63, 101231. [Google Scholar] [CrossRef]
- Melack, J.M.; Hess, L.L. Remote Sensing of the Distribution and Extent of Wetlands in the Amazon Basin. Amaz. Floodplain For. Ecophysiol. Biodivers. Sustain. Manag. 2010, 210, 43–59. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Müller, M.W.; Gama-Rodrigues, E.F.; Mendes, F.A.T. Cacao-Based Agroforestry Systems in the Atlantic Forest and Amazon Biomes: An Ecoregional Analysis of Land Use. Agric. Syst. 2021, 194, 103270. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Karunarathna, S.C.; Ye, L.; Xu, J.; Hyde, K.D.; Mortimer, P.E. Native Forests Have a Higher Diversity of Macrofungi Than Comparable Plantation Forests in the Greater Mekong Subregion. Forests 2018, 9, 402. [Google Scholar] [CrossRef]
- Magalhães, J.L.L.; Lopes, M.A.; Queiroz, H.L. de Development of a Flooded Forest Anthropization Index (FFAI) Applied to Amazonian Areas under Pressure from Different Human Activities. Ecol. Indic. 2015, 48, 440–447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).