Amphibian (Xenopus laevis) Macrophage Subsets Vary in Their Responses to the Chytrid Fungus Batrachochytrium dendrobatidis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Batrachochytrium Dendrobatidis JEL197
2.3. Recombinant Cytokine Production
2.4. Subcutaneous Administration of rCSF1 or rIL34 and In Vivo Bd Challenge
2.5. Bone Marrow-Derived Mϕ Cultures and In Vitro Mϕ-Bd Challenge
2.6. Quantitative Gene Expression and Bd Load Analyses
2.7. Electron Microscopy
2.8. Statistical Analyses
3. Results
3.1. X. laevis IL34-Mϕs, but Not CSF1-Mϕs, Confer Anti-Bd Resistance In Vivo
3.2. Both CSF1- and IL34-Mϕs Phagocytose Bd In Vitro
3.3. CSF1- and IL34-Mϕs Cocultured with Bd Exhibit Disparate Polarization States
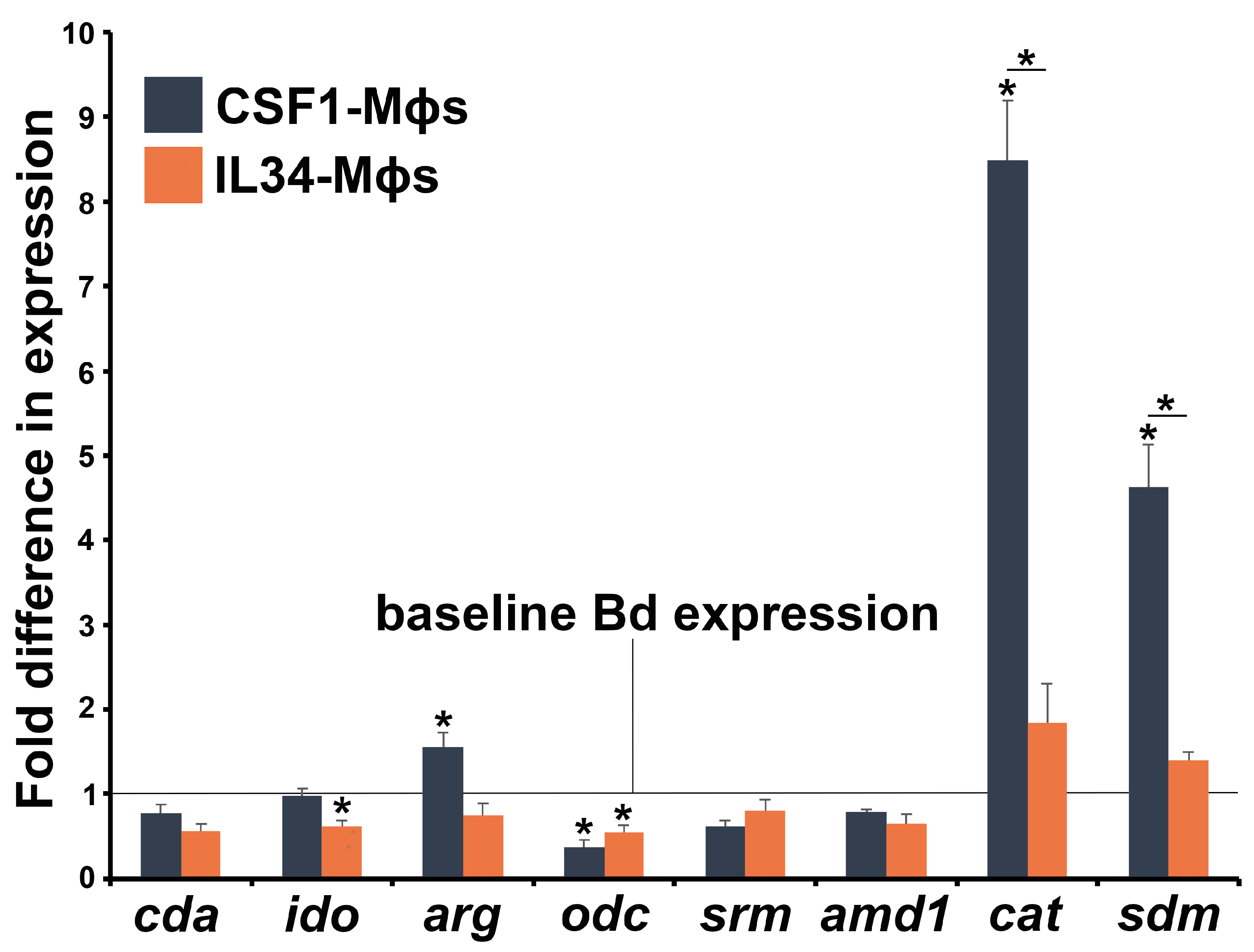
3.4. Bd Gene Expression Differs When in Coculture with CSF1- or IL34-Mϕs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| arg | arginase |
| amd | adenosylmethionine decarboxylase |
| Bd | Batrachochytrium dendrobatidis |
| cat | catalase |
| cmd | chitin deacetylase |
| CSF1 | colony-stimulating factor-1 |
| CSF1R | colony-stimulating factor-1 receptor |
| H&E | hematoxylin and eosin |
| ido | indoleamine 2,3 dioxygenase |
| il10 | interleukin-10 |
| IL34 | interleukin-34 |
| inos | inducible nitric oxide synthase |
| Mϕ | macrophage |
| odc | ornithine decarboxylase |
| rctrl | recombinant control |
| sdm | superoxide dismutase |
| SEM | scanning electron microscopy |
| srm | spermidine synthase |
| TEM | transmission electron microscopy |
| tnf | tumor necrosis factor |
References
- Berger, L.; Speare, R.; Daszak, P.; Green, D.E.; Cunningham, A.A.; Goggin, C.L.; Slocombe, R.; Ragan, M.A.; Hyatt, A.D.; McDonald, K.R.; et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc. Natl. Acad. Sci. USA 1998, 95, 9031–9036. [Google Scholar] [CrossRef]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Pessier, A.P.; Nichols, D.K.; Longcore, J.E.; Fuller, M.S. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea). J. Vet. Diagn. Investig. 1999, 11, 194–199. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Skerratt, L.F.; Berger, L.; Speare, R.; Cashins, S.; McDonald, K.R.; Phillott, A.D.; Hines, H.B.; Kenyon, N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 2007, 4, 125–134. [Google Scholar] [CrossRef]
- Berger, L.; Hyatt, A.D.; Speare, R.; Longcore, J.E. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis. Aquat. Organ. 2005, 68, 51–63. [Google Scholar] [CrossRef]
- Greenspan, S.E.; Longcore, J.E.; Calhoun, A.J. Host invasion by Batrachochytrium dendrobatidis: Fungal and epidermal ultrastructure in model anurans. Dis. Aquat. Organ. 2012, 100, 201–210. [Google Scholar] [CrossRef]
- Van Rooij, P.; Martel, A.; D’Herde, K.; Brutyn, M.; Croubels, S.; Ducatelle, R.; Haesebrouck, F.; Pasmans, F. Germ tube mediated invasion of Batrachochytrium dendrobatidis in amphibian skin is host dependent. PLoS ONE 2012, 7, e41481. [Google Scholar] [CrossRef]
- Voyles, J.; Young, S.; Berger, L.; Campbell, C.; Voyles, W.F.; Dinudom, A.; Cook, D.; Webb, R.; Alford, R.A.; Skerratt, L.F.; et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 2009, 326, 582–585. [Google Scholar] [CrossRef]
- Voyles, J.; Berger, L.; Young, S.; Speare, R.; Webb, R.; Warner, J.; Rudd, D.; Campbell, R.; Skerratt, L.F. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis. Aquat. Organ. 2007, 77, 113–118. [Google Scholar] [CrossRef]
- Marcum, R.D.; St-Hilaire, S.; Murphy, P.J.; Rodnick, K.J. Effects of Batrachochytrium dendrobatidis infection on ion concentrations in the boreal toad Anaxyrus (Bufo) boreas boreas. Dis. Aquat. Organ. 2010, 91, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, M.; Deepe, G.S., Jr.; Klein, B. Adaptive immunity to fungi. Annu. Rev. Immunol. 2012, 30, 115–148. [Google Scholar] [CrossRef]
- Rizzetto, L.; Cavalieri, D. Friend or foe: Using systems biology to elucidate interactions between fungi and their hosts. Trends Microbiol. 2011, 19, 509–515. [Google Scholar] [CrossRef]
- Collette, J.R.; Lorenz, M.C. Mechanisms of immune evasion in fungal pathogens. Curr. Opin. Microbiol. 2011, 14, 668–675. [Google Scholar] [CrossRef]
- Cheng, S.C.; Joosten, L.A.; Kullberg, B.J.; Netea, M.G. Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 2012, 80, 1304–1313. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Bell, S.C.; Kenyon, N.; Alford, R.A.; Rollins-Smith, L.A. Immune evasion or avoidance: Fungal skin infection linked to reduced defence peptides in Australian green-eyed treefrogs, Litoria serrata. Fungal Biol. 2012, 116, 1203–1211. [Google Scholar] [CrossRef]
- Bataille, A.; Cashins, S.D.; Grogan, L.; Skerratt, L.F.; Hunter, D.; McFadden, M.; Scheele, B.; Brannelly, L.A.; Macris, A.; Harlow, P.S.; et al. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc. Biol. Sci. 2015, 282, 20143127. [Google Scholar] [CrossRef]
- Ellison, A.R.; Savage, A.E.; DiRenzo, G.V.; Lanhammer, P.; Lips, K.R.; Zumudio, K.R. Fighting a losing battle: Vigorous immune response countered by pathogen suppression of host defenses in the chytridiomycosis-susceptible frog Atelopus zeteki. G3 2014, 4, 1275–1289. [Google Scholar] [CrossRef]
- Ellison, A.R.; Tunstall, T.; DiRenzo, G.V.; Hughey, M.C.; Rebollar, E.A.; Belden, L.K.; Harris, R.N.; Ibanez, R.; Lips, K.R.; Zamudio, K.R. More than skin deep: Functional genomic basis for resistance to amphibian chytridiomycosis. Genome Biol. Evol. 2014, 7, 286–298. [Google Scholar] [CrossRef]
- Grogan, L.F.; Cashins, S.D.; Skerratt, L.F.; Berger, L.; McFadden, M.S.; Harlow, P.; Hunter, D.A.; Scheele, B.C.; Mulvenna, J. Evolution of resistance to chytridiomycosis is associated with a robust early immune response. Mol. Ecol. 2018, 27, 919–934. [Google Scholar] [CrossRef]
- McMahon, T.A.; Sears, B.F.; Venesky, M.D.; Bessler, S.M.; Brown, J.M.; Deutsch, K.; Halstead, N.T.; Lentz, G.; Tenouri, N.; Young, S.; et al. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 2014, 511, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Pask, J.D.; Woodhams, D.C.; Rollins-Smith, L.A. The ebb and flow of antimicrobial skin peptides defends northern leopard frogs (Rana pipiens) against chytridiomycosis. Glob. Change Biol. 2012, 18, 1231–1238. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Carey, C.; Longcore, J.; Doersam, J.K.; Boutte, A.; Bruzgal, J.E.; Conlon, J.M. Activity of antimicrobial skin peptides from ranid frogs against Batrachochytrium dendrobatidis, the chytrid fungus associated with global amphibian declines. Dev. Comp. Immunol. 2002, 26, 471–479. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Ramsey, J.P.; Reinert, L.K.; Woodhams, D.C.; Livo, L.J.; Carey, C. Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front. Biosci. 2009, 1, 68–91. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Woodhams, D.C.; Reinert, L.K.; Vredenburg, V.T.; Briggs, C.J.; Nielsen, P.F.; Conlon, J.M. Antimicrobial peptide defenses of the mountain yellow-legged frog (Rana muscosa). Dev. Comp. Immunol. 2006, 30, 831–842. [Google Scholar] [CrossRef]
- Savage, A.E.; Zamudio, K.R. MHC genotypes associate with resistance to a frog-killing fungus. Proc. Natl. Acad. Sci. USA 2011, 108, 16705–16710. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Vredenburg, V.T.; Simon, M.-A.; Billheimer, D.; Shakhtour, B.; Shyr, Y.; Briggs, C.J.; Rollins-Smith, L.A.; Harris, R.N. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol. Conserv. 2007, 138, 390–398. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Ardipradja, K.; Alford, R.A.; Marantelli, G.; Reinert, L.K.; Rollins-Smith, L.A. Resistance to chytridiomycosis varies by amphibian species and is correlated with skin peptide defenses. Anim. Conserv. 2007, 10, 409–417. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Rollins-Smith, L.A.; Carey, C.; Reinert, L.; Tyler, M.J.; Alford, R.A. Population trends associated with skin peptide defenses against chytridiomycosis in Australian frogs. Oecologia 2006, 146, 531–540. [Google Scholar] [CrossRef]
- Brucker, R.M.; Baylor, C.M.; Walters, R.L.; Lauer, A.; Harris, R.N.; Minbiole, K.P. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 2008, 34, 39–43. [Google Scholar] [CrossRef]
- Brucker, R.M.; Harris, R.N.; Schwantes, C.R.; Gallaher, T.N.; Flaherty, D.C.; Lam, B.A.; Minbiole, K.P. Amphibian chemical defense: Antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J. Chem. Ecol. 2008, 34, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.N.; James, T.Y.; Lauer, A.; Simon, M.A.; Patel, A. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth 2006, 3, 53–56. [Google Scholar] [CrossRef]
- Harris, R.N.; Brucker, R.M.; Walke, J.B.; Becker, M.H.; Schwantes, C.R.; Flaherty, D.C.; Lam, B.A.; Woodhams, D.C.; Briggs, C.J.; Vredenburg, V.T.; et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009, 3, 818–824. [Google Scholar] [CrossRef]
- Kueneman, J.G.; Bletz, M.C.; McKenzie, V.J.; Becker, C.G.; Joseph, M.B.; Abarca, J.G.; Archer, H.; Arellano, A.L.; Bataille, A.; Becker, M.; et al. Community richness of amphibian skin bacteria correlates with bioclimate at the global scale. Nat. Ecol. Evol. 2019, 3, 381–389. [Google Scholar] [CrossRef]
- Loudon, A.H.; Holland, J.A.; Umile, T.P.; Burzynski, E.A.; Minbiole, K.P.; Harris, R.N. Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front. Microbiol. 2014, 5, 441. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Bridges, T.; Hughey, M.C.; Medina, D.; Belden, L.K.; Harris, R.N. Integrating the role of antifungal bacteria into skin symbiotic communities of three Neotropical frog species. ISME J. 2019, 13, 1763–1775. [Google Scholar] [CrossRef]
- Ramsey, J.P.; Reinert, L.K.; Harper, L.K.; Woodhams, D.C.; Rollins-Smith, L.A. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 2010, 78, 3981–3992. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim. Biophys. Acta 2009, 1788, 1593–1599. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. The importance of antimicrobial peptides (AMPs) in amphibian skin defense. Dev. Comp. Immunol. 2023, 142, 104657. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Doersam, J.K.; Longcore, J.E.; Taylor, S.K.; Shamblin, J.C.; Carey, C.; Zasloff, M.A. Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev. Comp. Immunol. 2002, 26, 63–72. [Google Scholar] [CrossRef]
- Daly, J.W.; Spande, T.F.; Garraffo, H.M. Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 2005, 68, 1556–1575. [Google Scholar] [CrossRef]
- Macfoy, C.; Danosus, D.; Sandit, R.; Jones, T.H.; Garraffo, H.M.; Spande, T.F.; Daly, J.W. Alkaloids of anuran skin: Antimicrobial function? Z. Naturforsch. C J. Biosci. 2005, 60, 932–937. [Google Scholar] [CrossRef]
- Rodriguez, C.; Rollins-Smith, L.; Ibanez, R.; Durant-Archibold, A.A.; Gutierrez, M. Toxins and pharmacologically active compounds from species of the family Bufonidae (Amphibia, Anura). J. Ethnopharmacol. 2017, 198, 235–254. [Google Scholar] [CrossRef]
- Ostrovsky, D.S.; Snyder, J.A.; Iwata, T.; Izaka, K.I.; Maglott, D.S.; Nace, G.W. Frog lysozyme. I. Its identification, occurrence as isozymes, and quantitative distribution in tissues of the leopard frog, Rana pipiens. J. Exp. Zool. 1976, 195, 279–290. [Google Scholar] [CrossRef]
- Savage, A.E.; Kiemnec-Tyburczy, K.M.; Ellison, A.R.; Fleischer, R.C.; Zamudio, K.R. Conservation and divergence in the frog immunome: Pyrosequencing and de novo assembly of immune tissue transcriptomes. Gene 2014, 542, 98–108. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Hovav, A.H. Mucosal and Skin Langerhans Cells—Nurture Calls. Trends Immunol. 2018, 39, 788–800. [Google Scholar] [CrossRef]
- Carrillo-Farga, J.; Castell, A.; Perez, A.; Rondan, A. Langerhans-like cells in amphibian epidermis. J. Anat. 1990, 172, 39–45. [Google Scholar]
- Guiducci, C.; Vicari, A.P.; Sangaletti, S.; Trinchieri, G.; Colombo, M.P. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005, 65, 3437–3446. [Google Scholar] [CrossRef]
- Saccani, A.; Schioppa, T.; Porta, C.; Biswas, S.K.; Nebuloni, M.; Vago, L.; Bottazzi, B.; Colombo, M.P.; Mantovani, A.; Sica, A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006, 66, 11432–11440. [Google Scholar] [CrossRef]
- Stanley, E.R.; Berg, K.L.; Einstein, D.B.; Lee, P.S.; Pixley, F.J.; Wang, Y.; Yeung, Y.G. Biology and action of colony--stimulating factor-1. Mol. Reprod. Dev. 1997, 46, 4–10. [Google Scholar] [CrossRef]
- Freuchet, A.; Salama, A.; Remy, S.; Guillonneau, C.; Anegon, I. IL-34 and CSF-1, deciphering similarities and differences at steady state and in diseases. J. Leukoc. Biol. 2021, 110, 771–796. [Google Scholar] [CrossRef]
- Munoz-Garcia, J.; Cochonneau, D.; Teletchea, S.; Moranton, E.; Lanoe, D.; Brion, R.; Lezot, F.; Heymann, M.F.; Heymann, D. The twin cytokines interleukin-34 and CSF-1: Masterful conductors of macrophage homeostasis. Theranostics 2021, 11, 1568–1593. [Google Scholar] [CrossRef] [PubMed]
- Yaparla, A.; Docter-Loeb, H.; Melnyk, M.L.S.; Batheja, A.; Grayfer, L. The amphibian (Xenopus laevis) colony-stimulating factor-1 and interleukin-34-derived macrophages possess disparate pathogen recognition capacities. Dev. Comp. Immunol. 2019, 98, 89–97. [Google Scholar] [CrossRef]
- Yaparla, A.; Popovic, M.; Grayfer, L. Differentiation-dependent antiviral capacities of amphibian (Xenopus laevis) macrophages. J. Biol. Chem. 2018, 293, 1736–1744. [Google Scholar] [CrossRef]
- Grayfer, L.; Robert, J. Divergent antiviral roles of amphibian (Xenopus laevis) macrophages elicited by colony-stimulating factor-1 and interleukin-34. J. Leukoc. Biol. 2014, 96, 1143–1153. [Google Scholar] [CrossRef]
- Robert, J.; Abramowitz, L.; Gantress, J.; Morales, H.D. Xenopus laevis: A possible vector of Ranavirus infection? J. Wildl. Dis. 2007, 43, 645–652. [Google Scholar] [CrossRef]
- Grayfer, L.; Robert, J. Distinct functional roles of amphibian (Xenopus laevis) colony-stimulating factor-1- and interleukin-34-derived macrophages. J. Leukoc. Biol. 2015, 98, 641–649. [Google Scholar] [CrossRef]
- Hauser, K.A.; Garvey, C.N.; Crow, R.S.; Hossainey, M.R.H.; Howard, D.T.; Ranganathan, N.; Gentry, L.K.; Yaparla, A.; Kalia, N.; Zelle, M.; et al. Amphibian mast cells serve as barriers to chytrid fungus infections. Elife 2024, 12, RP92168. [Google Scholar] [CrossRef]
- Yaparla, A.; Koubourli, D.; Popovic, M.; Grayfer, L. Exploring the relationships between amphibian (Xenopus laevis) myeloid cell subsets. Dev. Comp. Immunol. 2020, 113, 103798. [Google Scholar] [CrossRef]
- Hossainey, M.R.H.; Hauser, K.A.; Garvey, C.N.; Kalia, N.; Garvey, J.M.; Grayfer, L. A perspective into the relationships between amphibian (Xenopus laevis) myeloid cell subsets. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220124. [Google Scholar] [CrossRef] [PubMed]
- Grayfer, L.; Kerimoglu, B.; Yaparla, A.; Hodgkinson, J.W.; Xie, J.; Belosevic, M. Mechanisms of Fish Macrophage Antimicrobial Immunity. Front. Immunol. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A.; Fites, J.S.; Reinert, L.K.; Shiakolas, A.R.; Umile, T.P.; Minbiole, K.P. Immunomodulatory metabolites released by the frog-killing fungus Batrachochytrium dendrobatidis. Infect. Immun. 2015, 83, 4565–4570. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Shayan, M.; Niazi Shahraki, F.; Hosseini, Y.; Momtaz, S.; Abdolghaffari, A.H. IDO/Kynurenine; novel insight for treatment of inflammatory diseases. Cytokine 2023, 166, 156206. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Ruzzini, A.C.; Fites, J.S.; Reinert, L.K.; Hall, E.M.; Joosse, B.A.; Ravikumar, V.I.; Huebner, M.I.; Aka, A.; Kehs, M.H.; et al. Metabolites Involved in Immune Evasion by Batrachochytrium dendrobatidis Include the Polyamine Spermidine. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef]
- Deng, K.; He, H.; Qiu, J.; Lorber, B.; Bryson, J.B.; Filbin, M.T. Increased synthesis of spermidine as a result of upregulation of arginase I promotes axonal regeneration in culture and in vivo. J. Neurosci. 2009, 29, 9545–9552. [Google Scholar] [CrossRef]
- Brown, G.D. Innate antifungal immunity: The key role of phagocytes. Annu. Rev. Immunol. 2011, 29, 1–21. [Google Scholar] [CrossRef]
- Wang, Y.; Colonna, M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur. J. Immunol. 2014, 44, 1575–1581. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Udagawa, N.; Takahashi, N. IL-34 and CSF-1: Similarities and differences. J. Bone Miner. Metab. 2013, 31, 486–495. [Google Scholar] [CrossRef]
- Castell-Rodriguez, A.E.; Hernandez-Penaloza, A.; Sampedro-Carrillo, E.A.; Herrera-Enriquez, M.A.; Alvarez-Perez, S.J.; Rondan-Zarate, A. ATPase and MHC class II molecules co-expression in Rana pipiens dendritic cells. Dev. Comp. Immunol. 1999, 23, 473–485. [Google Scholar] [CrossRef]
- Lombardo, G.P.; Miller, A.; Aragona, M.; Messina, E.; Fumia, A.; Kuciel, M.; Alesci, A.; Pergolizzi, S.; Lauriano, E.R. Immunohistochemical Characterization of Langerhans Cells in the Skin of Three Amphibian Species. Biology 2024, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef]
- Idoyaga, J.; Suda, N.; Suda, K.; Park, C.G.; Steinman, R.M. Antibody to Langerin/CD207 localizes large numbers of CD8alpha+ dendritic cells to the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA 2009, 106, 1524–1529. [Google Scholar] [CrossRef]
- Sparber, F.; Dolowschiak, T.; Mertens, S.; Lauener, L.; Clausen, B.E.; Joller, N.; Stoitzner, P.; Tussiwand, R.; LeibundGut-Landmann, S. Langerin+ DCs regulate innate IL-17 production in the oral mucosa during Candida albicans-mediated infection. PLoS Pathog. 2018, 14, e1007069. [Google Scholar] [CrossRef]
- Xuan, S.; Li, Y.; Wu, Y.; Adcock, I.M.; Zeng, X.; Yao, X. Langerin-expressing dendritic cells in pulmonary immune-related diseases. Front. Med. 2022, 9, 909057. [Google Scholar] [CrossRef]
- Yaparla, A.; Grayfer, L. Isolation and Culture of Amphibian (Xenopus laevis) Sub-Capsular Liver and Bone Marrow Cells. Methods Mol. Biol. 2018, 1865, 275–281. [Google Scholar] [CrossRef]
- Ni, B.; Zhang, D.; Zhou, H.; Zheng, M.; Wang, Z.; Tao, J.; Han, Z.; Ju, X.; Tan, R.; Gu, M. IL-34 attenuates acute T cell-mediated rejection following renal transplantation by upregulating M2 macrophages polarization. Heliyon 2024, 10, e24028. [Google Scholar] [CrossRef]
- Yeung, A.W.; Terentis, A.C.; King, N.J.; Thomas, S.R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 2015, 129, 601–672. [Google Scholar] [CrossRef]
- Fei, M.; Bhatia, S.; Oriss, T.B.; Yarlagadda, M.; Khare, A.; Akira, S.; Saijo, S.; Iwakura, Y.; Fallert Junecko, B.A.; Reinhart, T.A.; et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5360–5365. [Google Scholar] [CrossRef]
- Popovic, M.; Yaparla, A.; Paquin-Proulx, D.; Koubourli, D.V.; Webb, R.; Firmani, M.; Grayfer, L. Colony-stimulating factor-1- and interleukin-34-derived macrophages differ in their susceptibility to Mycobacterium marinum. J. Leukoc. Biol. 2019, 106, 1257–1269. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Le Sage, E.H. Batrachochytrium fungi: Stealth invaders in amphibian skin. Curr. Opin. Microbiol. 2021, 61, 124–132. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaparla, A.; Popovic, M.; Hauser, K.A.; Rollins-Smith, L.A.; Grayfer, L. Amphibian (Xenopus laevis) Macrophage Subsets Vary in Their Responses to the Chytrid Fungus Batrachochytrium dendrobatidis. J. Fungi 2025, 11, 311. https://doi.org/10.3390/jof11040311
Yaparla A, Popovic M, Hauser KA, Rollins-Smith LA, Grayfer L. Amphibian (Xenopus laevis) Macrophage Subsets Vary in Their Responses to the Chytrid Fungus Batrachochytrium dendrobatidis. Journal of Fungi. 2025; 11(4):311. https://doi.org/10.3390/jof11040311
Chicago/Turabian StyleYaparla, Amulya, Milan Popovic, Kelsey A. Hauser, Louise A. Rollins-Smith, and Leon Grayfer. 2025. "Amphibian (Xenopus laevis) Macrophage Subsets Vary in Their Responses to the Chytrid Fungus Batrachochytrium dendrobatidis" Journal of Fungi 11, no. 4: 311. https://doi.org/10.3390/jof11040311
APA StyleYaparla, A., Popovic, M., Hauser, K. A., Rollins-Smith, L. A., & Grayfer, L. (2025). Amphibian (Xenopus laevis) Macrophage Subsets Vary in Their Responses to the Chytrid Fungus Batrachochytrium dendrobatidis. Journal of Fungi, 11(4), 311. https://doi.org/10.3390/jof11040311





