First Report on Antifungal Activity of Metschnikowia pulcherrima Against Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honeybee (Apis mellifera L.) Colonies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Cultures
2.2. Isolation of Ascosphaera apis
2.3. Genotypic Identification
2.4. Growth Parameters of A. apis
2.5. Antifungal Activity
2.5.1. Preliminary Antifungal Screening
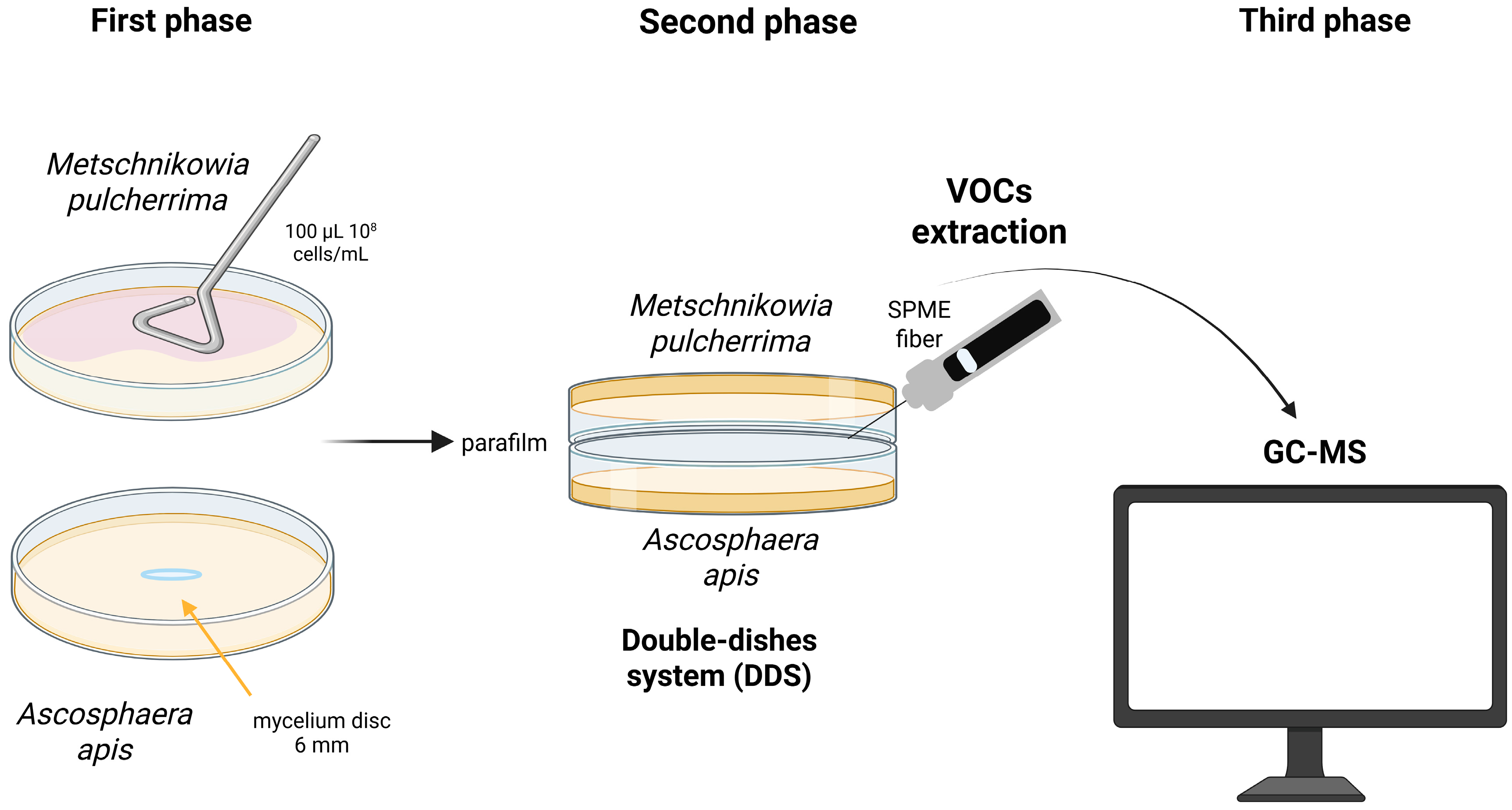
2.5.2. Evaluation of the Antifungal Activity of M. pulcherrima VOCs
2.6. Identification of Volatile Organic Compounds (VOCs)
2.6.1. Volatile Compounds Extraction
2.6.2. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.7. Statistical Analysis
3. Results and Discussions
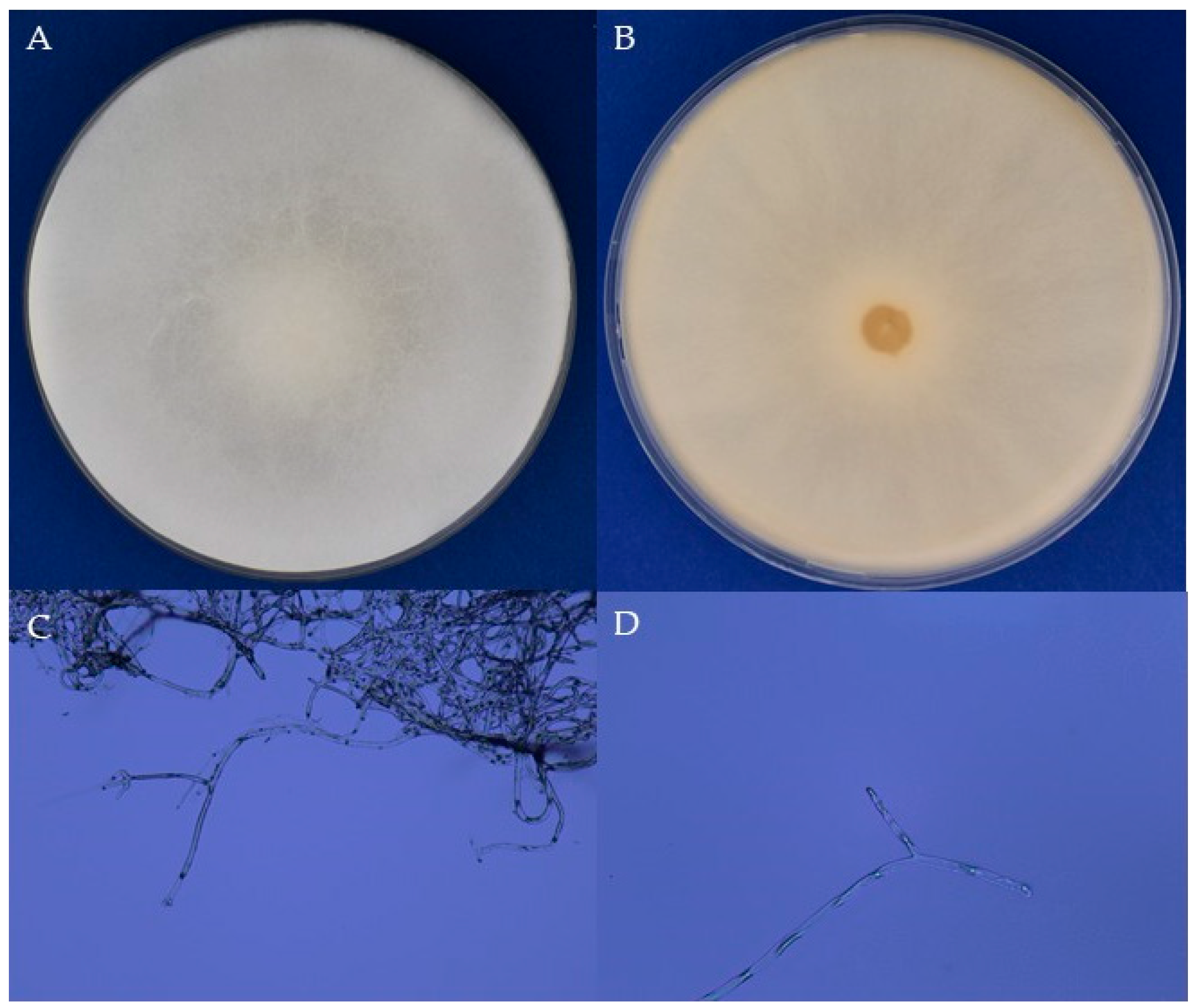
3.1. Taxonomical Identification
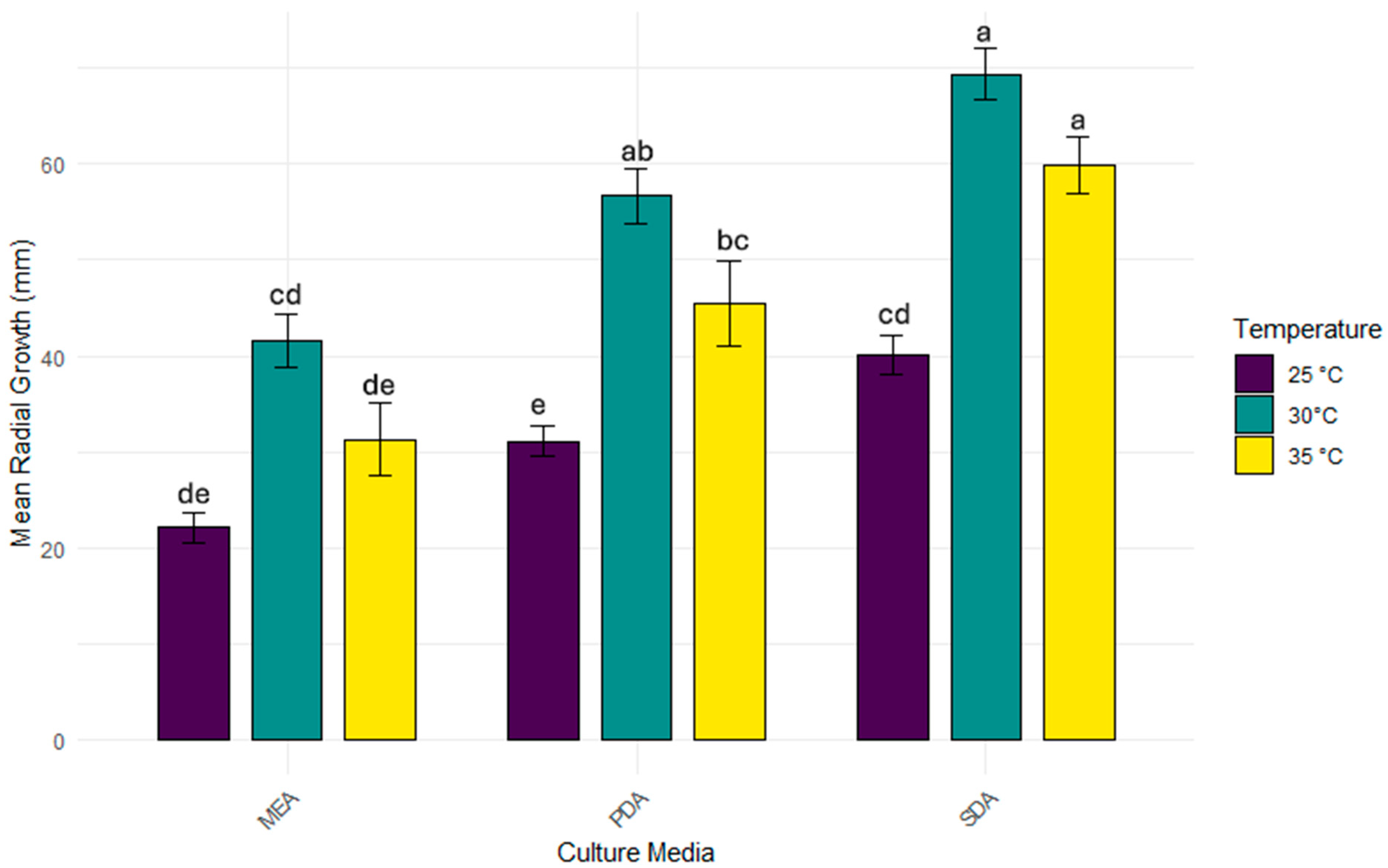
3.2. Growth Parameters of the A. apis
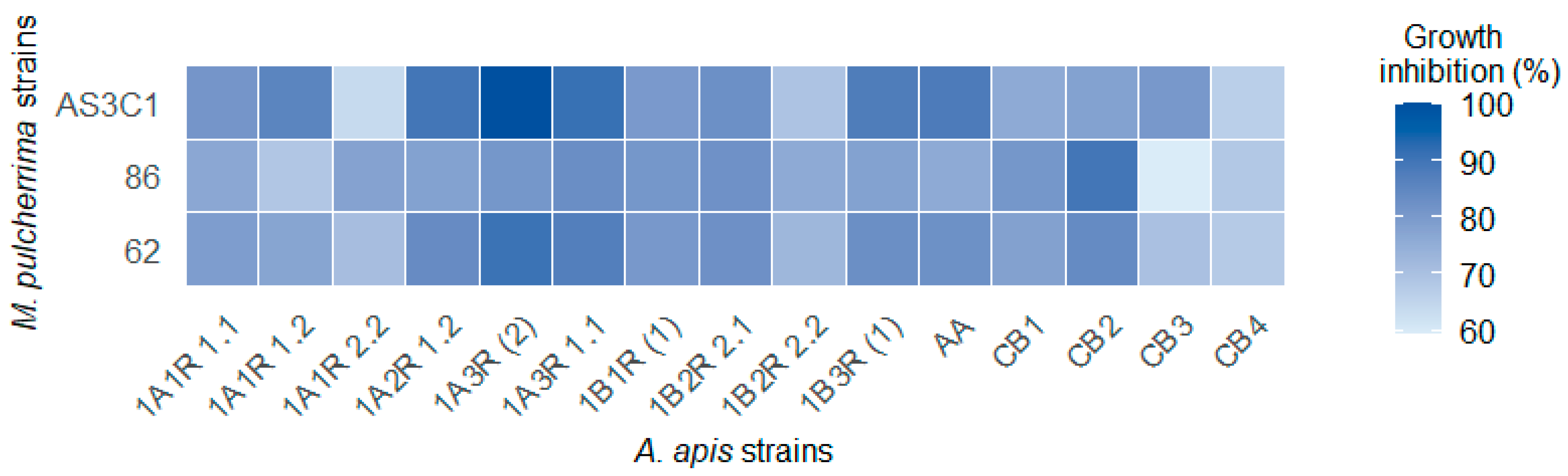
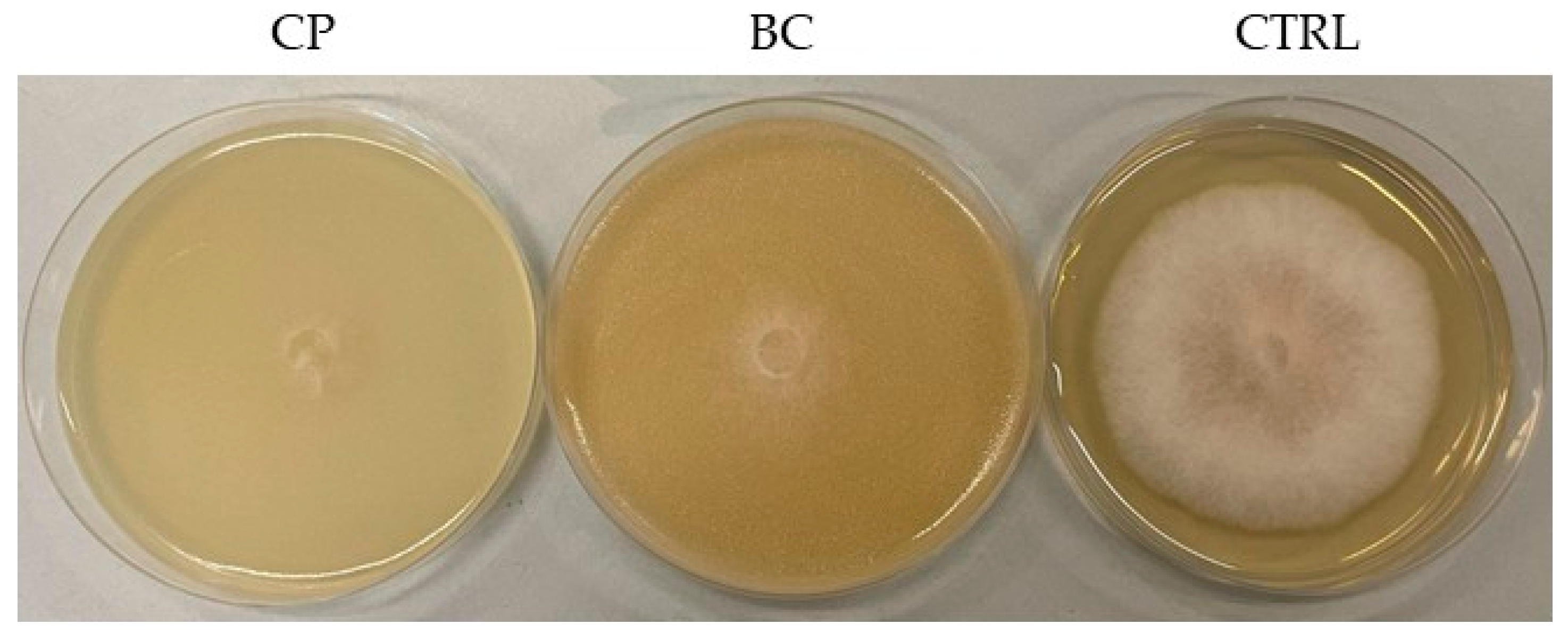
3.3. Preliminary Antifungal Screening and Evaluation of the Antifungal Activity of M. pulcherrima VOCs
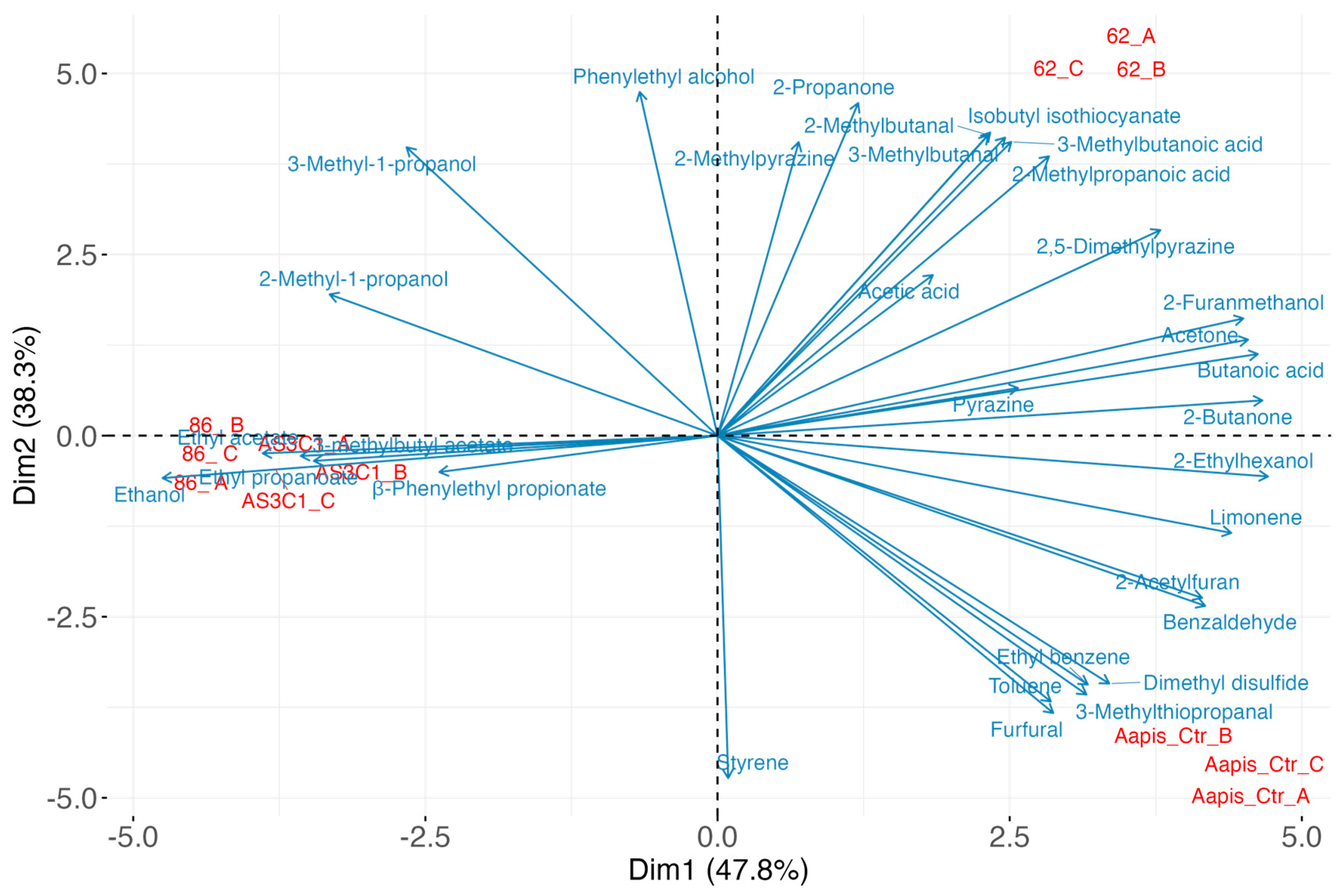
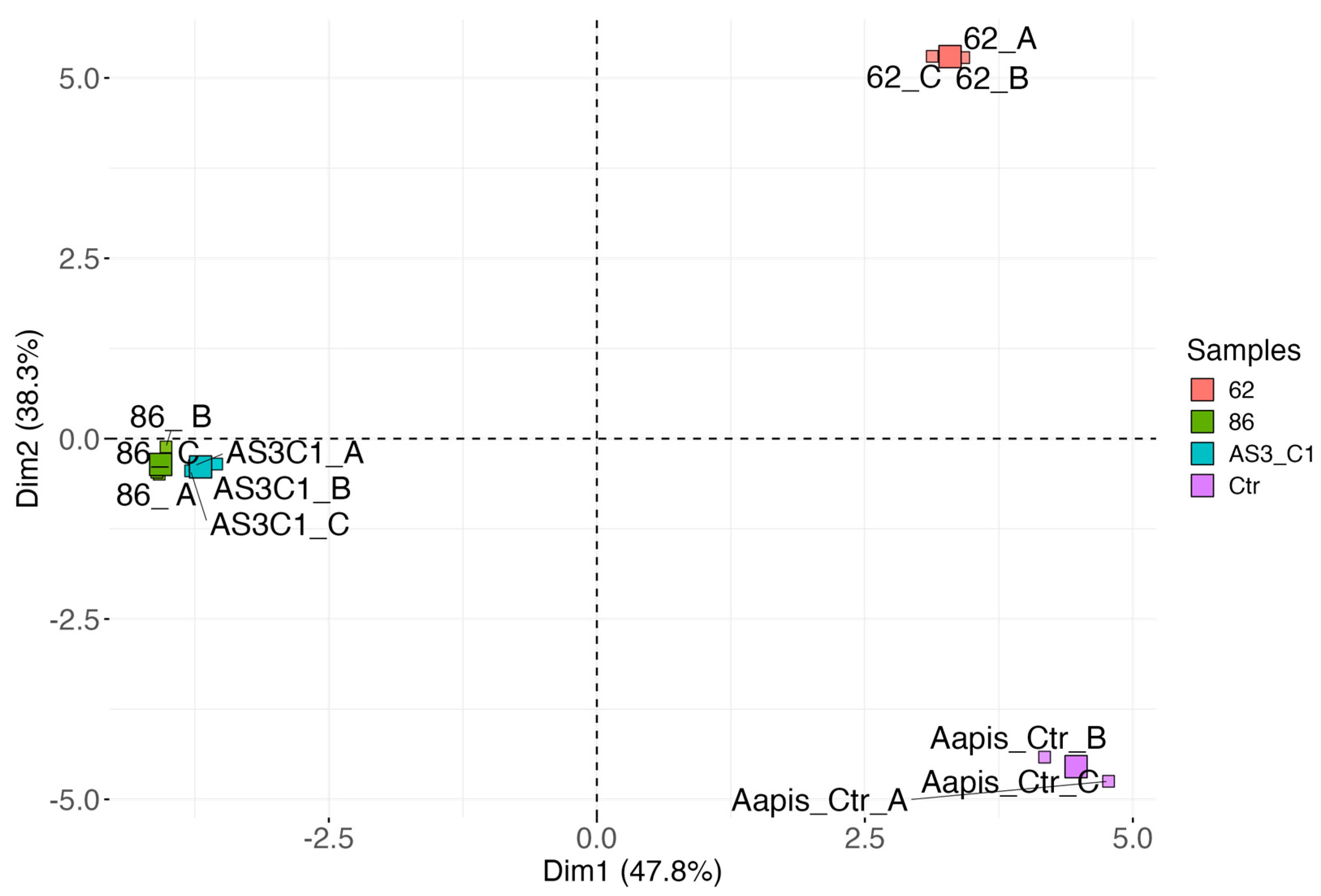
3.4. Identification of Antifungal Volatile Organic Compounds (VOCs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding Pollinators and Their Values to Human Well-Being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Smith, M.R.; Singh, G.M.; Mozaffarian, D.; Myers, S.S. Effects of Decreases of Animal Pollinators on Human Nutrition and Global Health: A Modelling Analysis. Lancet 2015, 386, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef]
- Home|Global Action on Pollination Services for Sustainable Agriculture|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/pollination/en/ (accessed on 17 March 2025).
- Zattara, E.E.; Aizen, M.A. Worldwide Occurrence Records Suggest a Global Decline in Bee Species Richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee Declines Driven by Combined Stress from Parasites, Pesticides, and Lack of Flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Siviter, H.; Bailes, E.J.; Martin, C.D.; Oliver, T.R.; Koricheva, J.; Leadbeater, E.; Brown, M.J.F. Agrochemicals Interact Synergistically to Increase Bee Mortality. Nature 2021, 596, 389–392. [Google Scholar] [CrossRef]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P.; vanEngelsdorp, D. Drivers of Colony Losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural Intensification and Climate Change Are Rapidly Decreasing Insect Biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Meeus, I.; Pisman, M.; Smagghe, G.; Piot, N. Interaction Effects of Different Drivers of Wild Bee Decline and Their Influence on Host-Pathogen Dynamics. Curr. Opin. Insect Sci. 2018, 26, 136–141. [Google Scholar] [CrossRef]
- Drossart, M.; Gérard, M. Beyond the Decline of Wild Bees: Optimizing Conservation Measures and Bringing Together the Actors. Insects 2020, 11, 649. [Google Scholar] [CrossRef]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Maxfield-Taylor, S.A.; Mujic, A.B.; Rao, S. First Detection of the Larval Chalkbrood Disease Pathogen Ascosphaera apis (Ascomycota: Eurotiomycetes: Ascosphaerales) in Adult Bumble Bees. PLoS ONE 2015, 10, e0124868. [Google Scholar] [CrossRef]
- Reynaldi, F.J.; Lucia, M.; Genchi Garcia, M.L. Ascosphaera apis, the Entomopathogenic Fungus Affecting Larvae of Native Bees (Xylocopa augusti): First Report in South America. Rev. Iberoam. Micol. 2015, 32, 261–264. [Google Scholar] [CrossRef]
- Deneke, Y. Review on Chalkbrood Disease of Honey Bee. Vet. Med. Open J. 2023, 8, 47–55. [Google Scholar] [CrossRef]
- Chen, D.; Guo, R.; Xiong, C.; Zheng, Y.; Hou, C.; Fu, Z. Morphological and Molecular Identification of Chalkbrood Disease Pathogen Ascosphaera apis in Apis Cerana Cerana. J. Apic. Res. 2018, 57, 516–521. [Google Scholar] [CrossRef]
- Tejerina, M.R.; Benitez-Ahrendts, M.R. Pathogenicity Bioassays of Ascosphaera apis Strains from Spanish Provinces in Bee Larvae from Northern Argentina. J. Apic. Res. 2023, 62, 488–495. [Google Scholar] [CrossRef]
- Karthik, V.; Srinivasan, M.R.; Saminathan, V.R.; Karthikeyan, S.; Balasubramani, V. Morphological and Molecular Identification and Mating Type Detection of Chalkbrood Fungal Pathogen Ascosphaera apis in Apis mellifera L. in Southern India. Indian J. Entomol. 2024, 1–7. [Google Scholar] [CrossRef]
- Sevim, A.; Akpınar, R.; Karaoğlu, Ş.A.; Bozdeveci, A.; Sevim, E. Prevalence and Phylogenetic Analysis of Ascosphaera apis (Maassen Ex Claussen) LS Olive & Spiltoir (1955) Isolates from Honeybee Colonies in Turkey. Biologia 2022, 77, 2689–2699. [Google Scholar] [CrossRef]
- Das, R.; Kumar, R.; Kunal, G.; Goldar, S.; Dutta, S.; Jha, S. Detection of Ascosphaera apis, Causing Chalkbrood Disease in the Colonies of European Honey Bee, Apis Mellifera in West Bengal, India. Sociobiology 2023, 70, e9192. [Google Scholar] [CrossRef]
- Pereira, K.D.S.; Meeus, I.; Smagghe, G. Honey Bee-Collected Pollen Is a Potential Source of Ascosphaera apis Infection in Managed Bumble Bees. Sci. Rep. 2019, 9, 4241. [Google Scholar] [CrossRef]
- Pavlović, R.; Brodschneider, R.; Goessler, W.; Stanisavljević, L.; Vujčić, Z.; Zarić, N.M. Micronutrient Deficiency May Be Associated with the Onset of Chalkbrood Disease in Honey Bees. Insects 2024, 15, 269. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, S.; Hamilton, M.; Yan, L.; Chen, Y. The Ability to Cause Infection in a Pathogenic Fungus Uncovers a New Biological Feature of Honey Bee Viruses. J. Invertebr. Pathol. 2014, 120, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, L.; Luo, J.; Yang, S.; Deng, Y.; Li, J.; Hou, C. Two Pathogenic Fungi Isolated from Chalkbrood Samples and Honey Bee Viruses They Carried. Front. Microbiol. 2022, 13, 843842. [Google Scholar] [CrossRef]
- Yoder, J.A.; Jajack, A.J.; Cornacchione, W.S.; Dunn, A.L.; Cunningham, E.G.; Matchett, C.L.; Rosselot, A.E. In Vitro Evaluation of Sugar Syrups, Antibiotics, and Miticides on Growth of Honey Bee Pathogen, Ascosphaera apis: Emphasis for Chalkbrood Prevention Is on Keeping Bees Healthy. Apidologie 2014, 45, 568–578. [Google Scholar] [CrossRef]
- Castagnino, G.L.B.; Mateos, A.; Meana, A.; Montejo, L.; Zamorano Iturralde, L.V.; Cutuli De Simón, M.T. Etiology, Symptoms and Prevention of Chalkbrood Disease: A Literature Review. Rev. Bras. Saúde E Produção Anim. 2020, 21, e210332020. [Google Scholar] [CrossRef]
- Kim, D.Y.; Maeng, S.; Cho, S.-J.; Park, H.J.; Kim, K.; Lee, J.K.; Srinivasan, S. The Ascosphaera apis Infection (Chalkbrood Disease) Alters the Gut Bacteriome Composition of the Honeybee. Pathogens 2023, 12, 734. [Google Scholar] [CrossRef]
- Khan, S.; Somerville, D.; Frese, M.; Nayudu, M. Environmental Gut Bacteria in European Honey Bees (Apis mellifera) from Australia and Their Relationship to the Chalkbrood Disease. PLoS ONE 2020, 15, e0238252. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Antagonistic Activity against Ascosphaera apis and Functional Properties of Lactobacillus Kunkeei Strains. Antibiotics 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Testa, B.; Ganassi, S.; Lombardi, S.J.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Probiotic Properties and Potentiality of Lactiplantibacillus Plantarum Strains for the Biological Control of Chalkbrood Disease. J. Fungi. 2021, 7, 379. [Google Scholar] [CrossRef]
- von Knoblauch, T.; Jensen, A.B.; Mülling, C.K.W.; Aupperle-Lellbach, H.; Genersch, E. Chalkbrood Disease Caused by Ascosphaera apis in Honey Bees (Apis mellifera)—Morphological and Histological Changes in Infected Larvae. Vet. Sci. 2024, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Boonmee, T.; Sinpoo, C.; Thayatham, K.; Suanpoot, P.; Disayathanoowat, T.; Pettis, J.S.; Chaimanee, V. Atmospheric Non-Thermal Plasma Inactivation of Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honeybee. Sci. Rep. 2024, 14, 1831. [Google Scholar] [CrossRef]
- Ansari, M.J.; Al-Ghamdi, A.; Usmani, S.; Khan, K.A.; Alqarni, A.S.; Kaur, M.; Al-Waili, N. In Vitro Evaluation of the Effects of Some Plant Essential Oils on Ascosphaera apis, the Causative Agent of Chalkbrood Disease. Saudi J. Biol. Sci. 2017, 24, 1001–1006. [Google Scholar] [CrossRef]
- Usta, M. Biological Control of Honey Bee Diseases and Pests. In Melittology—New Advances; IntechOpen: London, UK, 2023; ISBN 978-1-83769-370-2. [Google Scholar]
- Krutmuang, P.; Rajula, J.; Pittarate, S.; Chatima, C.; Thungrabeab, M.; Mekchay, S.; Senthil-Nathan, S. The Inhibitory Action of Plant Extracts on the Mycelial Growth of Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honey Bee. Toxicol. Rep. 2022, 9, 713–719. [Google Scholar] [CrossRef]
- Pusceddu, M.; Floris, I.; Mangia, N.P.; Angioni, A.; Satta, A. In Vitro Activity of Several Essential Oils Extracted from Aromatic Plants against Ascosphaera apis. Vet. Sci. 2021, 8, 80. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Ansari, M.J.; Khan, M.H.U.; Kamal, S.; Rahman, K.; Shoaib, M.; Man, S.; Khan, A.J.; Khan, S.U.; et al. Antimicrobial Potentials of Medicinal Plant’s Extract and Their Derived Silver Nanoparticles: A Focus on Honey Bee Pathogen. Saudi J. Biol. Sci. 2019, 26, 1815–1834. [Google Scholar] [CrossRef]
- Abdi, K.; Ben Said, M.; Crotti, E.; Masmoudi, A.S.; Cherif, A. The Promise of Probiotics in Honeybee Health and Disease Management. Arch. Microbiol. 2023, 205, 73. [Google Scholar] [CrossRef]
- Iorizzo, M.; Letizia, F.; Ganassi, S.; Testa, B.; Petrarca, S.; Albanese, G.; Di Criscio, D.; De Cristofaro, A. Functional Properties and Antimicrobial Activity from Lactic Acid Bacteria as Resources to Improve the Health and Welfare of Honey Bees. Insects 2022, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.A.; Fernández, L.A.; Díaz, M.L.; Pérez, M.; Corona, M.; Reynaldi, F.J. Microbiological and Chemical Characterization of Water Kefir: An Innovative Source of Potential Probiotics for Bee Nutrition. Rev. Argent. Microbiol. 2023, 55, 176–180. [Google Scholar] [CrossRef]
- Tejerina, M.R.; Benítez-Ahrendts, M.R.; Audisio, M.C. Lactobacillus salivarius A3iob Reduces the Incidence of Varroa destructor and Nosema Spp. in Commercial Apiaries Located in the Northwest of Argentina. Probiotics Antimicrob. Proteins 2020, 12, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Motta, E.V.; Moran, N.A. The Honeybee Microbiota and Its Impact on Health and Disease. Nat. Rev. Microbiol. 2024, 22, 122–137. [Google Scholar] [CrossRef]
- Kwong, W.K.; Moran, N.A. Gut Microbial Communities of Social Bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Nishida, A.; Kwong, W.K.; Koch, H.; Engel, P.; Steele, M.I.; Moran, N.A. Metabolism of Toxic Sugars by Strains of the Bee Gut Symbiont Gilliamella Apicola. mBio 2016, 7, e01326-16. [Google Scholar] [CrossRef]
- Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Succi, M.; Sorrentino, E.; Petrarca, S.; De Cristofaro, A.; Coppola, R.; et al. Inter- and Intra-Species Diversity of Lactic Acid Bacteria in Apis mellifera ligustica Colonies. Microorganisms 2020, 8, 1578. [Google Scholar] [CrossRef]
- Stefanini, I. Yeast-Insect Associations: It Takes Guts. Yeast Chichester Engl. 2018, 35, 315–330. [Google Scholar] [CrossRef]
- Malassigné, S.; Minard, G.; Vallon, L.; Martin, E.; Valiente Moro, C.; Luis, P. Diversity and Functions of Yeast Communities Associated with Insects. Microorganisms 2021, 9, 1552. [Google Scholar] [CrossRef]
- Vega, F.E.; Blackwell, M. Insect-Fungal Associations: Ecology and Evolution; Oxford University Press: Oxford, UK, 2005; ISBN 978-0-19-803727-9. [Google Scholar]
- Callegari, M.; Crotti, E.; Fusi, M.; Marasco, R.; Gonella, E.; De Noni, I.; Romano, D.; Borin, S.; Tsiamis, G.; Cherif, A.; et al. Compartmentalization of Bacterial and Fungal Microbiomes in the Gut of Adult Honeybees. Npj Biofilms Microbiomes 2021, 7, 42. [Google Scholar] [CrossRef]
- Rutkowski, D.; Weston, M.; Vannette, R.L. Bees Just Wanna Have Fungi: A Review of Bee Associations with Nonpathogenic Fungi. FEMS Microbiol. Ecol. 2023, 99, fiad077. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Gattucci, S.; Canonico, L.; Ciani, M.; Comitini, F. Yeast Communities Related to Honeybees: Occurrence and Distribution in Flowers, Gut Mycobiota, and Bee Products. Appl. Microbiol. Biotechnol. 2024, 108, 175. [Google Scholar] [CrossRef]
- Agarbati, A.; Moretti, L.; Canonico, L.; Ciani, M.; Comitini, F. Agro-Ecosystem of Honeybees as Source for Native Probiotic Yeasts. World J. Microbiol. Biotechnol. 2024, 40, 147. [Google Scholar] [CrossRef]
- Detry, R.; Simon-Delso, N.; Bruneau, E.; Daniel, H.-M. Specialisation of Yeast Genera in Different Phases of Bee Bread Maturation. Microorganisms 2020, 8, 1789. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.-A.; Starmer, W.T.; Rosa, C.A.; Bowles, J.M.; Barker, J.S.F.; Janzen, D.H. Biogeography of the Yeasts of Ephemeral Flowers and Their Insects. FEMS Yeast Res. 2001, 1, 1–8. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Al-Nijir, M.; Henk, D.A.; Bedford, M.R.; Chuck, C.J. Assessing Metschnikowia Pulcherrima as a Potential Probiotic Yeast for Animal Feed. Sustain. Microbiol. 2024, 1, qvae008. [Google Scholar] [CrossRef]
- Hicks, R.H.; Moreno-Beltrán, M.; Gore-Lloyd, D.; Chuck, C.J.; Henk, D.A. The Oleaginous Yeast Metschnikowia Pulcherrima Displays Killer Activity against Avian-Derived Pathogenic Bacteria. Biology 2021, 10, 1227. [Google Scholar] [CrossRef]
- Cristina Vergara Alvarez, S.; José Leiva Alaniz, M.; Victoria Mestre Furlani, M.; Vazquez, F.; Mancha Agresti, P.; Cristina Nally, M.; Paola Maturano, Y. Bioprospecting of the Probiotic Potential of Yeasts Isolated from a Wine Environment. Fungal Genet. Biol. 2023, 164, 103767. [Google Scholar] [CrossRef]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic Yeasts and How to Find Them—Polish Wines of Spontaneous Fermentation as Source for Potentially Probiotic Yeasts. Foods 2023, 12, 3392. [Google Scholar] [CrossRef]
- Diguță, C.F.; Mihai, C.; Toma, R.C.; Cîmpeanu, C.; Matei, F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods 2023, 12, 124. [Google Scholar] [CrossRef]
- Yildiran, H.; Başyiğit Kiliç, G.; Karahan Çakmakçi, A.G. Characterization and Comparison of Yeasts from Different Sources for Some Probiotic Properties and Exopolysaccharide Production. Food Sci. Technol. 2019, 39, 646–653. [Google Scholar] [CrossRef]
- MendonçA-Hagler, L.C.; Hagler, A.N.; Kurtzman, C.P. Phylogeny of Metschnikowia Species Estimated from Partial rRNA Sequences. Int. J. Syst. Evol. Microbiol. 1993, 43, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Metschnikowia Pulcherrima and Related Pulcherrimin-Producing Yeasts: Fuzzy Species Boundaries and Complex Antimicrobial Antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Kregiel, D.; Nowacka, M.; Rygala, A.; Vadkertiová, R. Biological Activity of Pulcherrimin from the Meschnikowia Pulcherrima Clade. Molecules 2022, 27, 1855. [Google Scholar] [CrossRef]
- Commenges, A.; Lessard, M.-H.; Coucheney, F.; Labrie, S.; Drider, D. The Biopreservative Properties of Metschnikowia pulcherrima LMA 2038 and Trichosporon asahii LMA 810 in a Model Fresh Cheese, Are Presented. Food Biosci. 2024, 58, 103458. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Biocontrol of Postharvest Brown Rot of Sweet Cherries by Saccharomyces cerevisiae Disva 599, Metschnikowia pulcherrima Disva 267 and Wickerhamomyces anomalus Disva 2 Strains. Postharvest Biol. Technol. 2014, 96, 64–68. [Google Scholar] [CrossRef]
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile Organic Compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae Inhibit Growth of Decay Causing Fungi and Control Postharvest Diseases of Strawberries. Int. J. Food Microbiol. 2018, 265, 18–22. [Google Scholar] [CrossRef]
- Settier-Ramírez, L.; López-Carballo, G.; Hernández-Muñoz, P.; Fontana, A.; Strub, C.; Schorr-Galindo, S. New Isolated Metschnikowia Pulcherrima Strains from Apples for Postharvest Biocontrol of Penicillium Expansum and Patulin Accumulation. Toxins 2021, 13, 397. [Google Scholar] [CrossRef]
- Lombardo, M.F.; Panebianco, S.; Restuccia, C.; Cirvilleri, G. Biocontrol Efficacy of Metschnikowia spp. Yeasts in Organic Vineyards against Major Airborne Diseases of Table Grapes in the Field and in Postharvest. Foods 2023, 12, 3508. [Google Scholar] [CrossRef]
- Puyo, M.; Simonin, S.; Bach, B.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bio-Protection in Oenology by Metschnikowia Pulcherrima: From Field Results to Scientific Inquiry. Front. Microbiol. 2023, 14, 1252973. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, A.; Kammerecker, S.; Müller, L.; Hilber-Bodmer, M.; Perren, S.; Freimoser, F.M. Stability of Dry and Liquid Metschnikowia Pulcherrima Formulations for Biocontrol Applications against Apple Postharvest Diseases. Horticulturae 2021, 7, 459. [Google Scholar] [CrossRef]
- Steglińska, A.; Kołtuniak, A.; Berłowska, J.; Czyżowska, A.; Szulc, J.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Metschnikowia Pulcherrima as a Biocontrol Agent against Potato (Solanum tuberosum) Pathogens. Agronomy 2022, 12, 2546. [Google Scholar] [CrossRef]
- Fernandez-San Millan, A.; Fernandez-Irigoyen, J.; Santamaria, E.; Larraya, L.; Farran, I.; Veramendi, J. Metschnikowia pulcherrima as an Efficient Biocontrol Agent of Botrytis cinerea Infection in Apples: Unraveling Protection Mechanisms through Yeast Proteomics. Biol. Control 2023, 183, 105266. [Google Scholar] [CrossRef]
- Oztekin, S.; Karbancioglu-Guler, F. Bioprospection of Metschnikowia sp. Isolates as Biocontrol Agents against Postharvest Fungal Decays on Lemons with Their Potential Modes of Action. Postharvest Biol. Technol. 2021, 181, 111634. [Google Scholar] [CrossRef]
- Türkel, S.; Korukluoğlu, M.; Yavuz, M. Biocontrol Activity of the Local Strain of Metschnikowia Pulcherrima on Different Postharvest Pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima as Biocontrol Agent and Wine Aroma Enhancer in Combination with a Native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- De Curtis, F.; de Felice, D.V.; Ianiri, G.; De Cicco, V.; Castoria, R. Environmental Factors Affect the Activity of Biocontrol Agents against Ochratoxigenic Aspergillus Carbonarius on Wine Grape. Int. J. Food Microbiol. 2012, 159, 17–24. [Google Scholar] [CrossRef]
- Gross, S.; Kunz, L.; Müller, D.C.; Santos Kron, A.; Freimoser, F.M. Characterization of Antagonistic Yeasts for Biocontrol Applications on Apples or in Soil by Quantitative Analyses of Synthetic Yeast Communities. Yeast 2018, 35, 559–566. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Wu, C.; Yuan, Y.; Ni, X.; Wu, T.; Chang, R.; Wang, Y. Volatile Organic Compounds Produced by Metschnikowia pulcherrima Yeast T-2 Inhibited the Growth of Botrytis cinerea in Postharvest Blueberry Fruits. Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamçe, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia Pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary Characterisation of Metschnikowia Pulcherrima to Be Used as a Starter Culture in Red Winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
- Iorizzo, M.; Bagnoli, D.; Vergalito, F.; Testa, B.; Tremonte, P.; Succi, M.; Pannella, G.; Letizia, F.; Albanese, G.; Lombardi, S.J.; et al. Diversity of Fungal Communities on Cabernet and Aglianico Grapes from Vineyards Located in Southern Italy. Front. Microbiol. 2024, 15, 1399968. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.B.; Aronstein, K.; Flores, J.M.; Vojvodic, S.; Palacio, M.A.; Spivak, M. Standard Methods for Fungal Brood Disease Research. J. Apic. Res. 2013, 52, 1–20. [Google Scholar] [CrossRef]
- Mbareche, H.; Veillette, M.; Bilodeau, G.; Duchaine, C. Comparison of the Performance of ITS1 and ITS2 as Barcodes in Amplicon-Based Sequencing of Bioaerosols. PeerJ 2020, 8, e8523. [Google Scholar] [CrossRef]
- Nucleotide BLAST. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome (accessed on 19 March 2024).
- NCBI Resource Coordinators. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- MM18: DNA Target Sequencing & Bacteria & Fungi ID—CLSI. Available online: https://clsi.org/standards/products/molecular-diagnostics/documents/mm18/ (accessed on 3 March 2025).
- Kimura, M. A Simple Method for Estimating Evolutionary Rates of Base Substitutions through Comparative Studies of Nucleotide Sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol Ability and Action Mechanism of Food-Isolated Yeast Strains against Botrytis cinerea Causing Post-Harvest Bunch Rot of Table Grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Ruiz-Moyano, S.; Hernández, A.; Galvan, A.I.; Córdoba, M.G.; Casquete, R.; Serradilla, M.J.; Martín, A. Selection and Application of Antifungal VOCs-Producing Yeasts as Biocontrol Agents of Grey Mould in Fruits. Food Microbiol. 2020, 92, 103556. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martín, A.; Hernandez, A.; López-Corrales, M.; Lozano, M.; Córdoba, M. de G. Effect of the Commercial Ripening Stage and Postharvest Storage on Microbial and Aroma Changes of ‘Ambrunés’ Sweet Cherries. J. Agric. Food Chem. 2010, 58, 9157–9163. [Google Scholar] [CrossRef] [PubMed]
- Rieppel, O. Species Monophyly. J. Zool. Syst. Evol. Res. 2010, 48, 1–8. [Google Scholar] [CrossRef]
- Mráz, P.; Hýbl, M.; Kopecký, M.; Bohatá, A.; Konopická, J.; Hoštičková, I.; Konvalina, P.; Šipoš, J.; Rost, M.; Čurn, V. The Effect of Artificial Media and Temperature on the Growth and Development of the Honey Bee Brood Pathogen Ascosphaera apis. Biology 2021, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Srinivasan, M.R.; Saminathan, V.R.; Karthikeyan, S.; Balasubramani, V. Effect of Media on the Mycelial Growth of Honey Bee Fungal Pathogen Ascosphaera apis Mating Types. Indian J. Entomol. 2024, 1–4. [Google Scholar] [CrossRef]
- Huber, J. Untersuchungen zur Physiologie insektentötender Pilze. Arch. Für Mikrobiol. 1958, 29, 257–276. [Google Scholar] [CrossRef]
- Anderson, D.L.; Gibson, N.L. New Species and Isolates of Spore-Cyst Fungi (Plectomycetes: Ascosphaerales) from Australia. Aust. Syst. Bot. 1998, 11, 53–72. [Google Scholar] [CrossRef]
- Stabentheiner, A.; Kovac, H.; Mandl, M.; Käfer, H. Coping with the Cold and Fighting the Heat: Thermal Homeostasis of a Superorganism, the Honeybee Colony. J. Comp. Physiol. A 2021, 207, 337–351. [Google Scholar] [CrossRef]
- Flores, J.M.; Ruiz, J.A.; Ruz, J.M.; Puerta, F.; Bustos, M.; Padilla, F.; Campano, F. Effect of Temperature and Humidity of Sealed Brood on Chalkbrood Development under Controlled Conditions. Apidologie 1996, 27, 185–192. [Google Scholar] [CrossRef]
- Medrzycki, P.; Sgolastra, F.; Bortolotti, L.; Bogo, G.; Tosi, S.; Padovani, E.; Porrini, C.; Sabatini, A.G. Influence of Brood Rearing Temperature on Honey Bee Development and Susceptibility to Poisoning by Pesticides. J. Apic. Res. 2010, 49, 52–59. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, X.; Zhu, X.; Chen, L.; Zhou, S.; Huang, Z.Y.; Zhou, B. Low-Temperature Stress during Capped Brood Stage Increases Pupal Mortality, Misorientation and Adult Mortality in Honey Bees. PLoS ONE 2016, 11, e0154547. [Google Scholar] [CrossRef]
- Pawlikowska, E.; Kolesińska, B.; Nowacka, M.; Kregiel, D. A New Approach to Producing High Yields of Pulcherrimin from Metschnikowia Yeasts. Fermentation 2020, 6, 114. [Google Scholar] [CrossRef]
- Monnet, C.; Back, A.; Irlinger, F. Growth of Aerobic Ripening Bacteria at the Cheese Surface Is Limited by the Availability of Iron. Appl. Environ. Microbiol. 2012, 78, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, E. Enzymatic Profiles and Antimicrobial Activity of the Yeast Metschnikowia Pulcherrima. Acta Innov. 2017, 17–24. [Google Scholar]
- Yang, H.; Wang, L.; Li, S.; Gao, X.; Wu, N.; Zhao, Y.; Sun, W. Control of Postharvest Grey Spot Rot of Loquat Fruit with Metschnikowia pulcherrima E1 and Potential Mechanisms of Action. Biol. Control 2021, 152, 104406. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms Underlying the Protective Effects of Beneficial Fungi against Plant Diseases. Biol. Control 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety Traits, Genetic and Technological Characterization of Lacticaseibacillus Rhamnosus Strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Coppola, F.; Abdalrazeq, M.; Fratianni, F.; Ombra, M.N.; Testa, B.; Zengin, G.; Ayala Zavala, J.F.; Nazzaro, F. Rosaceae Honey: Antimicrobial Activity and Prebiotic Properties. Antibiotics 2025, 14, 298. [Google Scholar] [CrossRef]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef]
- Parafati, L.; Cirvilleri, G.; Restuccia, C.; Wisniewski, M. Potential Role of Exoglucanase Genes (WaEXG1 and WaEXG2) in the Biocontrol Activity of Wickerhamomyces anomalus. Microb. Ecol. 2017, 73, 876–884. [Google Scholar] [CrossRef]
- Millan, A.F.-S.; Gamir, J.; Farran, I.; Larraya, L.; Veramendi, J. Identification of New Antifungal Metabolites Produced by the Yeast Metschnikowia pulcherrima Involved in the Biocontrol of Postharvest Plant Pathogenic Fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.F.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile Organic Compounds from Starmerella Bacillaris to Control Gray Mold on Apples and Modulate Cider Aroma Profile. Food Microbiol. 2020, 89, 103446. [Google Scholar] [CrossRef]
- Mari, M.; Bautista-Baños, S.; Sivakumar, D. Decay Control in the Postharvest System: Role of Microbial and Plant Volatile Organic Compounds. Postharvest Biol. Technol. 2016, 122, 70–81. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, J.; Tian, R.; Liu, Y. Microbial Volatile Organic Compounds: Antifungal Mechanisms, Applications, and Challenges. Front. Microbiol. 2022, 13, 922450. [Google Scholar] [CrossRef]
- Korpi, A.; Järnberg, J.; Pasanen, A.-L. Microbial Volatile Organic Compounds. Crit. Rev. Toxicol. 2009, 39, 139–193. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Bohm, K.; Martín-Sánchez, L.; Garbeva, P. Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Veselova, M.A.; Plyuta, V.A.; Khmel, I.A. Volatile Compounds of Bacterial Origin: Structure, Biosynthesis, and Biological Activity. Microbiology 2019, 88, 261–274. [Google Scholar] [CrossRef]
- Contarino, R.; Brighina, S.; Fallico, B.; Cirvilleri, G.; Parafati, L.; Restuccia, C. Volatile Organic Compounds (VOCs) Produced by Biocontrol Yeasts. Food Microbiol. 2019, 82, 70–74. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Performance Evaluation of Volatile Organic Compounds by Antagonistic Yeasts Immobilized on Hydrogel Spheres against Gray, Green and Blue Postharvest Decays. Food Microbiol. 2017, 63, 191–198. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Martínez-Checa, F.; Montaño, A.; Cortés-Delgado, A.; Smolinska, A.; Llamas, I.; Sampedro, I. Corrigendum: Identification of Volatile Organic Compounds in Extremophilic Bacteria and Their Effective Use in Biocontrol of Postharvest Fungal Phytopathogens. Front. Microbiol. 2023, 14, 1267324. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of Food-Borne Bacillus Volatile Compounds and Influence on Fungal Growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Moret, E.; Cignola, R.; Garagozzo, L.; Torelli, E.; Di Foggia, M. Yeasts Volatile Organic Compounds (VOCs) as Potential Growth Enhancers and Molds Biocontrol Agents of Mushrooms Mycelia. Fungal Biol. 2024, 128, 1859–1867. [Google Scholar] [CrossRef]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Volatile Organic Compounds Produced by Aureobasidium pullulans Induce Electrolyte Loss and Oxidative Stress in Botrytis cinerea and Alternaria aternata. Res. Microbiol. 2021, 172, 103788. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, J.; Yan, H.; Li, D.; Wang, X.; Liang, W.; Wang, G. Ethyl Acetate Produced by Hanseniaspora Uvarum Is a Potential Biocontrol Agent Against Tomato Fruit Rot Caused by Phytophthora Nicotianae. Front. Microbiol. 2022, 13, 978920. [Google Scholar] [CrossRef] [PubMed]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans Volatilome Identified by a Novel, Quantitative Approach Employing SPME-GC-MS, Suppressed Botrytis cinerea and Alternaria aternata In Vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of Volatile Organic Compounds by Aureobasidium pullulans as a Potential Mechanism of Action against Postharvest Fruit Pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, H.; He, S.; Duan, S.; Xing, S.; Li, X.; Huang, Q. Antifungal Activity of the Volatile Organic Compounds Produced by Ceratocystis Fimbriata Strains WSJK-1 and Mby. Front. Microbiol. 2022, 13, 1034939. [Google Scholar] [CrossRef]
- Zou, X.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Zhan, P.; Shao, X. ROS Stress and Cell Membrane Disruption Are the Main Antifungal Mechanisms of 2-Phenylethanol against Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 14468–14479. [Google Scholar] [CrossRef]
- Dev, U.; Devakumar, C.; Mohan, J.; Agarwal, P.C. Antifungal Activity of Aroma Chemicals Against Seed-Borne Fungi. J. Essent. Oil Res. 2004, 16, 496–499. [Google Scholar] [CrossRef]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Dufour, V.; Stahl, M.; Baysse, C. The Antibacterial Properties of Isothiocyanates. Microbiol. Read. Engl. 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Romeo, L.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. Isothiocyanates: An Overview of Their Antimicrobial Activity against Human Infections. Molecules 2018, 23, 624. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Bi, Y.; Wang, T.; Dong, Y.; Yang, Q.; Zhang, T. 2-Phenylethyl Isothiocyanate Exerts Antifungal Activity against Alternaria aternata by Affecting Membrane Integrity and Mycotoxin Production. Toxins 2020, 12, 124. [Google Scholar] [CrossRef]
- Saladino, F.; Bordin, K.; Luciano, F.B.; Franzón, M.F.; Mañes, J.; Meca, G. Antimicrobial Activity of the Glucosinolates. In Glucosinolates; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switerland, 2017; pp. 249–274. ISBN 978-3-319-25462-3. [Google Scholar]
- Yu, H.; Jia, W.; Zhao, M.; Li, L.; Liu, J.; Chen, J.; Pan, H.; Zhang, X. Antifungal Mechanism of Isothiocyanates Against. Pest Manag. Sci. 2022, 78, 5133–5141. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, J.B.; Bozic, D.; Naidoo, U.; Sadik, O.A. A Comparative Review of Key Isothiocyanates and Their Health Benefits. Nutrients 2024, 16, 757. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Bi, Y.; Zhang, M.; Zhang, T.; Zheng, X.; Dong, Y.; Huang, Y. Benzyl Isothiocyanate Fumigation Inhibits Growth, Membrane Integrity and Mycotoxin Production in Alternaria aternata. RSC Adv. 2020, 10, 1829–1837. [Google Scholar] [CrossRef]
- Patil, S.B.; Basrani, S.T.; Chougule, S.A.; Gavandi, T.C.; Karuppayil, S.M.; Jadhav, A.K. Butyl Isothiocyanate Exhibits Antifungal and Anti-Biofilm Activity against Candida Albicans by Targeting Cell Membrane Integrity, Cell Cycle Progression and Oxidative Stress. Arch. Microbiol. 2024, 206, 251. [Google Scholar] [CrossRef]
- Jeschke, V.; Kearney, E.E.; Schramm, K.; Kunert, G.; Shekhov, A.; Gershenzon, J.; Vassão, D.G. How Glucosinolates Affect Generalist Lepidopteran Larvae: Growth, Development and Glucosinolate Metabolism. Front. Plant Sci. 2017, 8, 1995. [Google Scholar] [CrossRef]
- Sontowski, R.; Guyomar, C.; Poeschl, Y.; Weinhold, A.; van Dam, N.M.; Vassão, D.G. Mechanisms of Isothiocyanate Detoxification in Larvae of Two Belowground Herbivores, Delia Radicum and D. Floralis (Diptera: Anthomyiidae). Front. Physiol. 2022, 13, 874527. [Google Scholar] [CrossRef]
- Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Capano, V.; Guerra, I.; Zavatta, L.; Albertazzi, S.; Matteo, R.; Lazzeri, L.; et al. Glucosinolate Bioactivation by Apis Mellifera Workers and Its Impact on Nosema Ceranae Infection at the Colony Level. Biomolecules 2021, 11, 1657. [Google Scholar] [CrossRef]
- Adams, T.B.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Munro, I.C.; Newberne, P.M.; Portoghese, P.S.; Smith, R.L.; Waddell, W.J.; et al. The FEMA GRAS Assessment of Pyrazine Derivatives Used as Flavor Ingredients. Flavor and Extract Manufacturers Association. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002, 40, 429–451. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J. Pyrazine Derivatives: A Patent Review (June 2012—Present). Expert Opin. Ther. Pat. 2015, 25, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kucerova-Chlupacova, M.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity. Molecules 2015, 20, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Su, Z.; Zhao, Q.; Zhao, Z.; Wu, Z.; Zhao, M.; Lai, M. Synthesis, Thermal Property and Antifungal Evaluation of Pyrazine Esters. Arab. J. Chem. 2022, 15, 104351. [Google Scholar] [CrossRef]
- Guazzotti, V.; Hendrich, V.; Gruner, A.; Störmer, A.; Welle, F. Styrene Monomer Levels in Polystyrene-Packed Dairy Products from the Market versus Simulated Migration Testing. Foods 2023, 12, 2609. [Google Scholar] [CrossRef]
- Kubica, D.; Kaczmarczyk, M.; Kaszuba, A. Determination of Volatile Organic Compounds in Materials from Polystyrene Intended for Contact with Food: Comparison of HS-GS/MS and SPME-GC/MS Techniques. Rocz. Panstw. Zakl. Hig. 2018, 69, 235–242. [Google Scholar]
- Pagot, Y.; Belin, J.-M.; Husson, F.; Spinnler, H.-E. Metabolism of Phenylalanine and Biosynthesis of Styrene in Penicillium Camemberti. J. Dairy Res. 2007, 74, 180–185. [Google Scholar] [CrossRef]
- Lafeuille, J.-L.; Buniak, M.-L.; Vioujas, M.-C.; Lefevre, S. Natural Formation of Styrene by Cinnamon Mold Flora. J. Food Sci. 2009, 74, M276–M283. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorizzo, M.; Coppola, F.; Pannella, G.; Ganassi, S.; Matarazzo, C.; Albanese, G.; Tedino, C.; Di Donato, L.M.; Iacovino, V.P.; Cozzolino, R.; et al. First Report on Antifungal Activity of Metschnikowia pulcherrima Against Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honeybee (Apis mellifera L.) Colonies. J. Fungi 2025, 11, 336. https://doi.org/10.3390/jof11050336
Iorizzo M, Coppola F, Pannella G, Ganassi S, Matarazzo C, Albanese G, Tedino C, Di Donato LM, Iacovino VP, Cozzolino R, et al. First Report on Antifungal Activity of Metschnikowia pulcherrima Against Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honeybee (Apis mellifera L.) Colonies. Journal of Fungi. 2025; 11(5):336. https://doi.org/10.3390/jof11050336
Chicago/Turabian StyleIorizzo, Massimo, Francesca Coppola, Gianfranco Pannella, Sonia Ganassi, Cristina Matarazzo, Gianluca Albanese, Cosimo Tedino, Licia Maria Di Donato, Vincenzo Pio Iacovino, Rosaria Cozzolino, and et al. 2025. "First Report on Antifungal Activity of Metschnikowia pulcherrima Against Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honeybee (Apis mellifera L.) Colonies" Journal of Fungi 11, no. 5: 336. https://doi.org/10.3390/jof11050336
APA StyleIorizzo, M., Coppola, F., Pannella, G., Ganassi, S., Matarazzo, C., Albanese, G., Tedino, C., Di Donato, L. M., Iacovino, V. P., Cozzolino, R., & De Cristofaro, A. (2025). First Report on Antifungal Activity of Metschnikowia pulcherrima Against Ascosphaera apis, the Causative Agent of Chalkbrood Disease in Honeybee (Apis mellifera L.) Colonies. Journal of Fungi, 11(5), 336. https://doi.org/10.3390/jof11050336







