Metabolic Influence of S. boulardii and S. cerevisiae in Cross-Kingdom Models of S. mutans and C. albicans
Abstract
:1. Introduction
2. Materials and Methods
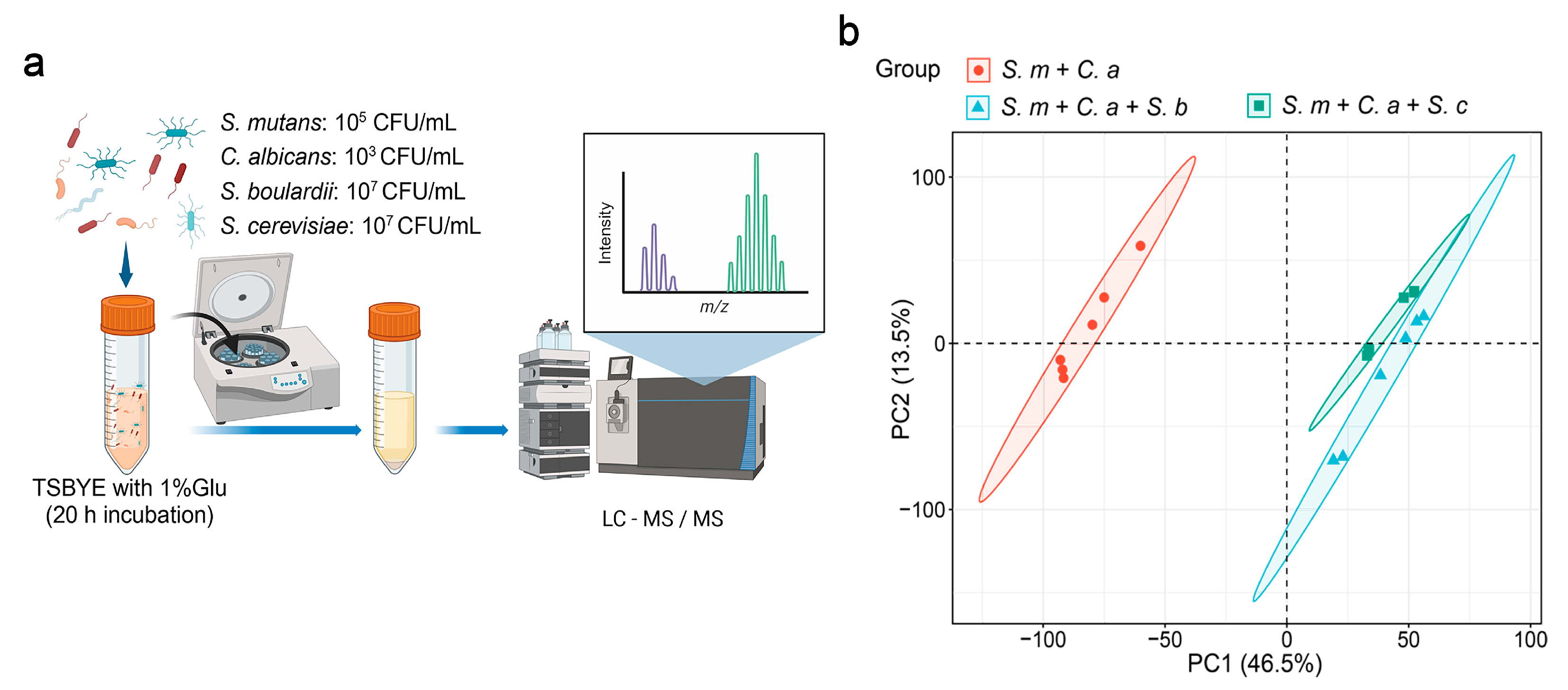
2.1. Bacteria and Yeast Strains and Starter Preparation
2.2. Planktonic Model
2.3. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.4. Statistical Analysis
2.5. Pathway Analysis
3. Results
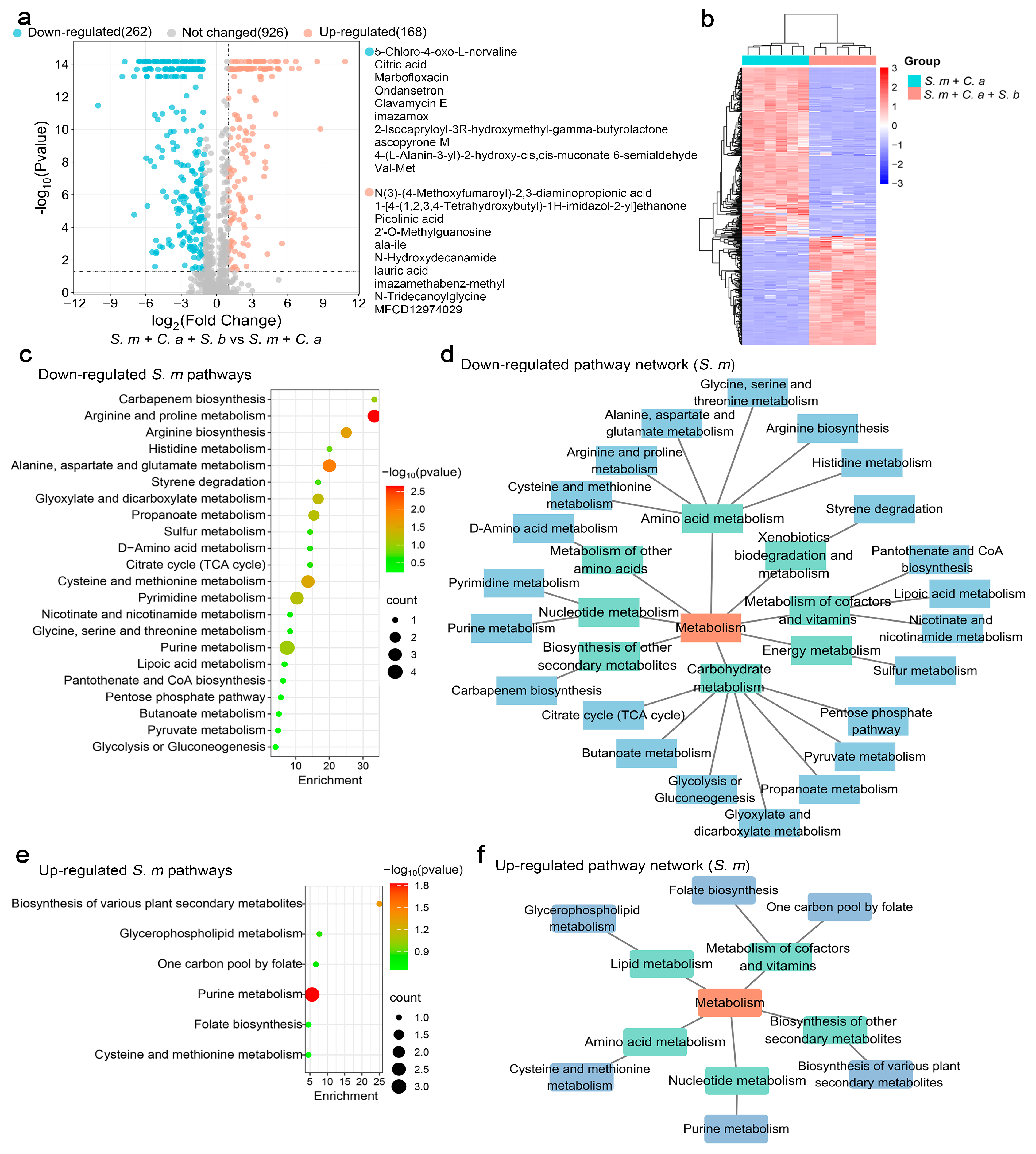
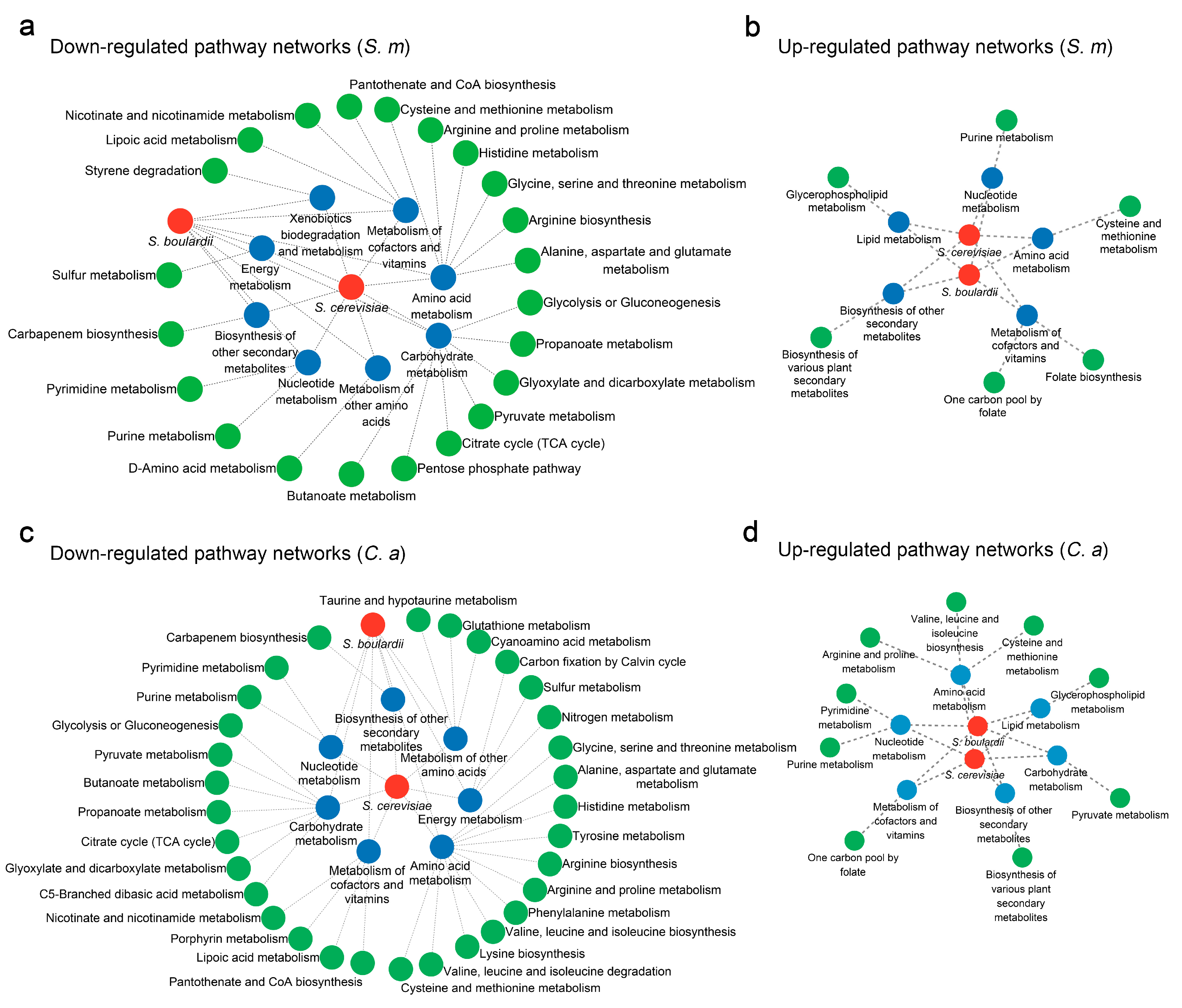
3.1. Impact of S. boulardii on S. mutans Metabolomics
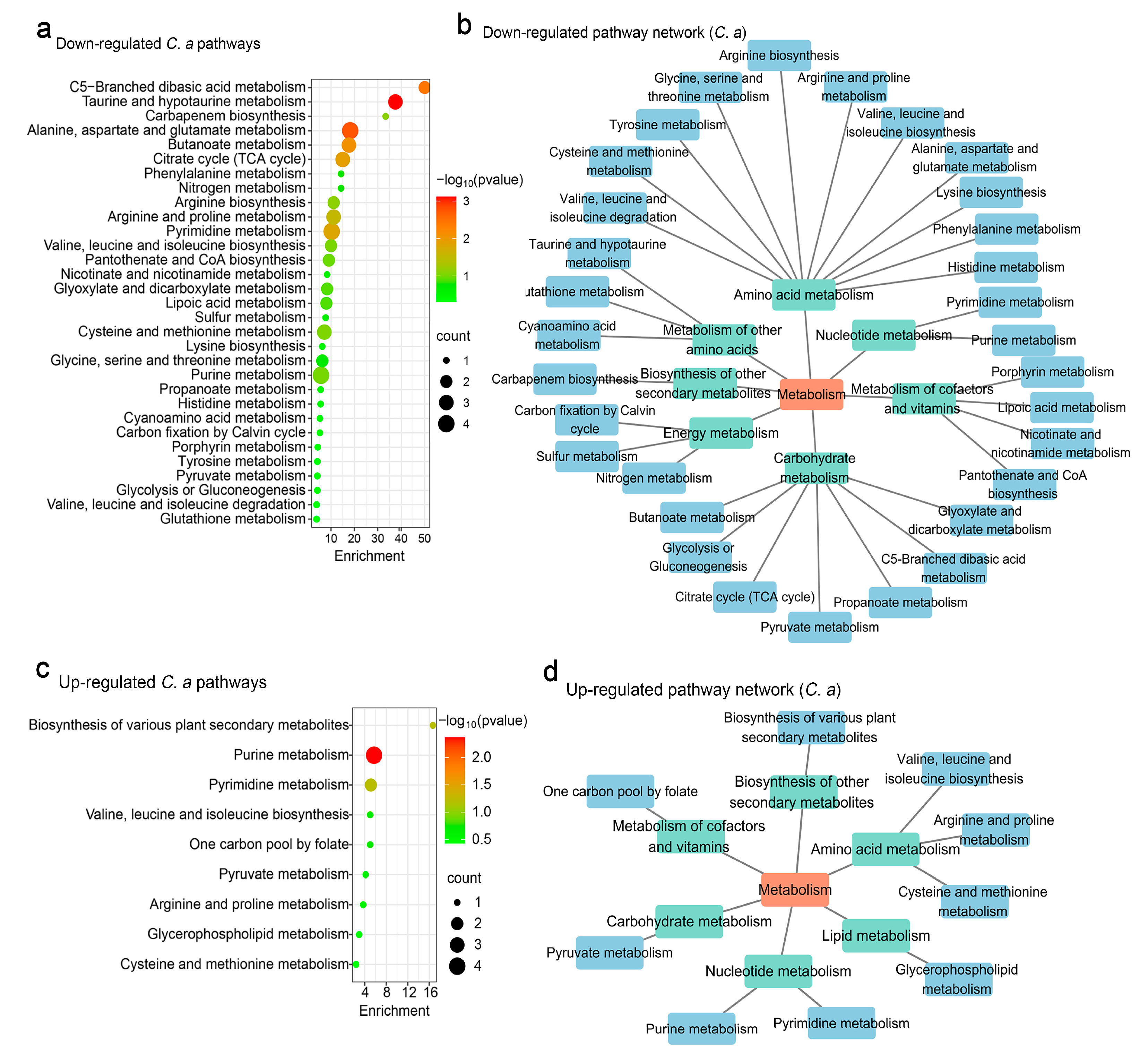
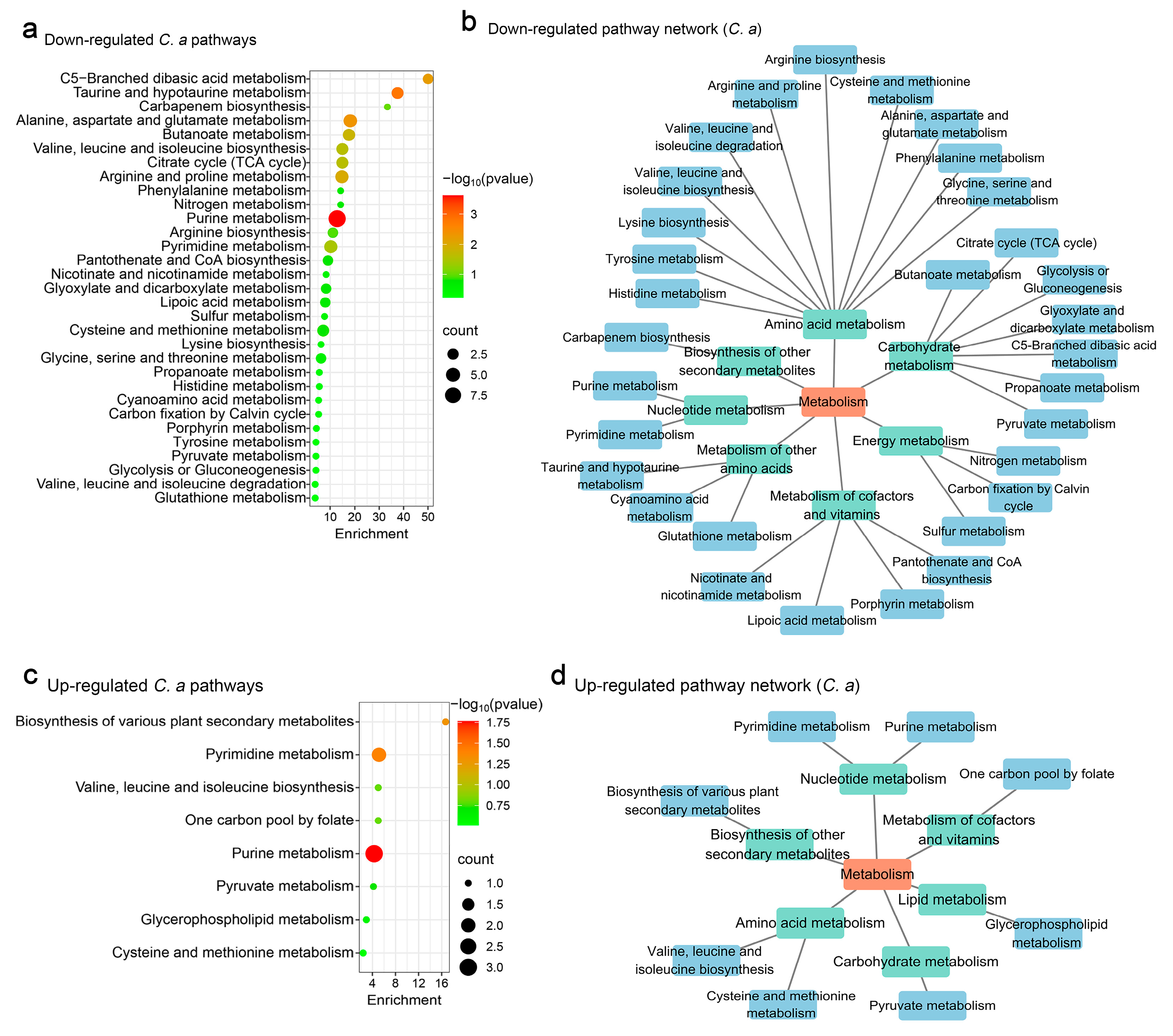
3.2. Influence of S. boulardii on C. albicans Metabolomics
3.3. Effects of S. cerevisiae on S. mutans Metabolism
3.4. Modulation of C. albicans Metabolism by S. cerevisiae
3.5. Cross-Species Metabolic Intersection of S. boulardii and S. cerevisiae
4. Discussion
4.1. Overview and Highlights
4.2. Metabolic Effects of S. boulardii and S. cerevisiae
4.3. Dual Regulatory Effects on Metabolic Pathways
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon-Soro, A.; Mira, A. Solving the etiology of dental caries. Trends Microbiol. 2015, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Astasov-Frauenhoffer, M.; Kulik, E.M. Cariogenic Biofilms and Caries from Birth to Old Age. Monogr. Oral Sci. 2021, 29, 53–64. [Google Scholar] [PubMed]
- Wu, R.; Cui, G.; Cao, Y.; Zhao, W.; Lin, H. Streptococcus mutans Membrane Vesicles Enhance Candida albicans Pathogenicity and Carbohydrate Metabolism. Front. Cell Infect. Microbiol. 2022, 12, 940602. [Google Scholar] [CrossRef]
- Sztajer, H.; Szafranski, S.P.; Tomasch, J.; Reck, M.; Nimtz, M.; Rohde, M.; Wagner-Dobler, I. Cross-feeding and interkingdom communication in dual-species biofilms of Streptococcus mutans and Candida albicans. ISME J. 2014, 8, 2256–2271. [Google Scholar] [CrossRef]
- Chen, H.; Gu, L.; Liao, B.; Zhou, X.; Cheng, L.; Ren, B. Advances of Anti-Caries Nanomaterials. Molecules 2020, 25, 5047. [Google Scholar] [CrossRef] [PubMed]
- Panchbhai, A.S.; Khatib, M.N.; Borle, R.M.; Deolia, S.S.; Babar, V.M.; Vasistha, A.H.; Parida, R.P. Efficacy and Safety of Probiotics for Dental Caries in Preschool Children: A Systematic Review and Meta-analysis. Contemp. Clin. Dent. 2024, 15, 10–16. [Google Scholar] [CrossRef]
- Kazmierczyk-Winciorek, M.; Nedzi-Gora, M.; Slotwinska, S.M. The immunomodulating role of probiotics in the prevention and treatment of oral diseases. Cent. Eur. J. Immunol. 2021, 46, 99–104. [Google Scholar] [CrossRef]
- Waitzberg, D.; Guarner, F.; Hojsak, I.; Ianiro, G.; Polk, D.B.; Sokol, H. Can the Evidence-Based Use of Probiotics (Notably Saccharomyces boulardii CNCM I-745 and Lactobacillus rhamnosus GG) Mitigate the Clinical Effects of Antibiotic-Associated Dysbiosis? Adv. Ther. 2024, 41, 901–914. [Google Scholar] [CrossRef]
- Varano, A.; Shirahigue, L.D.; Azevedo, F.A.; da Silva, M.A.; Ceccato-Antonini, S.R. Mandarin essential oil as an antimicrobial in ethanolic fermentation: Effects on Limosilactobacillus fermentum and Saccharomyces cerevisiae. Lett. Appl. Microbiol. 2022, 74, 981–991. [Google Scholar] [CrossRef]
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Oliver, S.G.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 2458–2467. [Google Scholar] [CrossRef]
- Yousif, D.; Wu, Y.; Gonzales, A.A.; Mathieu, C.; Zeng, Y.; Sample, L.; Terando, S.; Li, T.; Xiao, J. Anti-Cariogenic Effects of S. cerevisiae and S. boulardii in S. mutans-C. albicans Cross-Kingdom In Vitro Models. Pharmaceutics 2024, 16, 215. [Google Scholar] [CrossRef] [PubMed]
- More, M.I.; Swidsinski, A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis—A review. Clin. Exp. Gastroenterol. 2015, 8, 237–255. [Google Scholar] [CrossRef]
- van der Aa Kuhle, A.; Skovgaard, K.; Jespersen, L. In vitro screening of probiotic properties of Saccharomyces cerevisiae var. boulardii and food-borne Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2005, 101, 29–39. [Google Scholar]
- Shen, N.T.; Tmanova, L.L.; Pino, A.; Ancy, K.M.; Simon, M.S.; Crawford, C.V.; Bosworth, B.P.; Maw, A.M. The Use of Probiotics for the Prevention of Clostridium difficile Infection (CDI) in Hospitalized Adults Receiving Antibiotics: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 150, S134. [Google Scholar] [CrossRef]
- Zanello, G.; Meurens, F.; Berri, M.; Salmon, H. Saccharomyces boulardii effects on gastrointestinal diseases. Curr. Issues Mol. Biol. 2009, 11, 47–58. [Google Scholar]
- Micklefield, G. Saccharomyces boulardii in the treatment and prevention of antibiotic-associated diarrhea. MMW Fortschr. Med. 2014, 156, 61. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, X.; Liang, L.; Wang, X.; Bai, X.; Zhu, L.; He, Q.; Liang, H.; Xin, X.; Wang, L.; et al. Lactic Acid-Producing Probiotic Saccharomyces cerevisiae Attenuates Ulcerative Colitis via Suppressing Macrophage Pyroptosis and Modulating Gut Microbiota. Front. Immunol. 2021, 12, 777665. [Google Scholar] [CrossRef]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biot. 2015, 99, 6563–6570. [Google Scholar] [CrossRef]
- Kim, K.; Abramishvili, D.; Du, S.; Papadopoulos, Z.; Cao, J.; Herz, J.; Smirnov, I.; Thomas, J.L.; Colonna, M.; Kipnis, J. Meningeal lymphatics-microglia axis regulates synaptic physiology. Cell 2025. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, Y.; Chen, J.; Han, Q.; Wang, Z.; Peng, X.; Cheng, L. Lacticaseibacillus rhamnosus inhibits the development of dental caries in rat caries model and in vitro. J. Dent. 2024, 149, 105278. [Google Scholar] [CrossRef]
- Turganbay, S.; Kenesheva, S.; Jumagaziyeva, A.; Ilin, A.; Askarova, D.; Azembayev, A.; Kurmanaliyeva, A. Synthesis, physicochemical properties and antimicrobial activity of a di-aminopropionic acid hydrogen tri-iodide coordination compound. BMC Res. Notes 2024, 17, 384. [Google Scholar] [CrossRef]
- Collins, J.; Cilibrizzi, A.; Fedorova, M.; Whyte, G.; Mak, L.H.; Guterman, I.; Leatherbarrow, R.; Woscholski, R.; Vilar, R. Vanadyl complexes with dansyl-labelled di-picolinic acid ligands: Synthesis, phosphatase inhibition activity and cellular uptake studies. Dalton Trans. 2016, 45, 7104–7113. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chen, M.T.; Stedman, F.; Hernandez, J.; Kumble, G.; Kang, X.; Zhang, C.R.; Tang, G.C.; Daugherty, I.; Liu, W.Q.; et al. How Unnatural Amino Acids in Antimicrobial Peptides Change Interactions with Lipid Model Membranes. J. Phys. Chem. B 2024, 128, 9772–9784. [Google Scholar] [CrossRef]
- Miyauchi, M.; El Garch, F.; Theriault, W.; Leclerc, B.G.; Lepine, E.; Giboin, H.; Rhouma, M. Effect of single parenteral administration of marbofloxacin on bacterial load and selection of resistant Enterobacteriaceae in the fecal microbiota of healthy pigs. BMC Vet. Res. 2024, 20, 492. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, H.U.; Loosli, H.R.; Nussbaumer, A. Clavamycins, new clavam antibiotics from two variants of Streptomyces hygroscopicus. II. Isolation and structures of clavamycins A, B and C from Streptomyces hygroscopicus NRRL 15846, and of clavamycins D, E and F from Streptomyces hygroscopicus NRRL 15879. J. Antibiot. 1986, 39, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, X.L.; Xie, L.Y.; Tang, J.; Liu, L.X.; Wang, D.; Wu, X.; Liu, T.H.; Yu, Y.; Wang, Y.; et al. Comparative transcriptomics and metabolomics provide insight into degeneration-related physiological mechanisms of Morchella importuna after long-term preservation. Microb. Biotechnol. 2025, 18, e70045. [Google Scholar] [CrossRef]
- Stasolla, C.; Thorpe, T.A. Purine and pyrimidine nucleotide synthesis and degradation during in vitro morphogenesis of white spruce (Picea glauca). Front. Biosci. 2004, 9, 1506–1519. [Google Scholar] [CrossRef]
- Bauer, N.C.; Corbett, A.H.; Doetsch, P.W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015, 43, 10083–10101. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, Y.; Ding, N.; Fang, Y. Recent Advances in Metabolic Engineering for the Biosynthesis of Phosphoenol Pyruvate-Oxaloacetate-Pyruvate-Derived Amino Acids. Molecules 2024, 29, 2893. [Google Scholar] [CrossRef]
- Hedin, K.A.; Mirhakkak, M.H.; Vaaben, T.H.; Sands, C.; Pedersen, M.; Baker, A.; Vazquez-Uribe, R.; Schauble, S.; Panagiotou, G.; Wellejus, A.; et al. Saccharomyces boulardii enhances anti-inflammatory effectors and AhR activation via metabolic interactions in probiotic communities. ISME J. 2024, 18, wrae212. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.Y.; Li, Y.L.; Jin, H.; Kwok, L.Y.; Zhang, H.P.; Sun, Z.H. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Lu, X.; Wu, Y.; Wu, T.; Xiao, J. Metabolic Influence of S. boulardii and S. cerevisiae in Cross-Kingdom Models of S. mutans and C. albicans. J. Fungi 2025, 11, 325. https://doi.org/10.3390/jof11040325
Li T, Lu X, Wu Y, Wu T, Xiao J. Metabolic Influence of S. boulardii and S. cerevisiae in Cross-Kingdom Models of S. mutans and C. albicans. Journal of Fungi. 2025; 11(4):325. https://doi.org/10.3390/jof11040325
Chicago/Turabian StyleLi, Ting, Xingyi Lu, Yan Wu, Tongtong Wu, and Jin Xiao. 2025. "Metabolic Influence of S. boulardii and S. cerevisiae in Cross-Kingdom Models of S. mutans and C. albicans" Journal of Fungi 11, no. 4: 325. https://doi.org/10.3390/jof11040325
APA StyleLi, T., Lu, X., Wu, Y., Wu, T., & Xiao, J. (2025). Metabolic Influence of S. boulardii and S. cerevisiae in Cross-Kingdom Models of S. mutans and C. albicans. Journal of Fungi, 11(4), 325. https://doi.org/10.3390/jof11040325







