Antifungal Volatile Organic Compounds from Talaromyces purpureogenus CEF642N: Insights from One Strain Many Compounds (OSMAC) Strategy for Controlling Verticillium dahliae in Cotton
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Inhibitory Activity of Total VOCs from Different Media
2.3. Identification of VOCs Produced by CEF642N from Different Media
2.4. In Vitro Antifungal Activity Assay
2.5. Transcriptome Analysis of Different Solid Media
2.6. Statistical Analyses
3. Results
3.1. Inhibitory Effects of Total VOCs from Various Solid Media
3.2. Identification and Comparative of CEF642N VOCs from Different Solid Media
3.3. In Vitro Antifungal Activity
3.4. Transcriptome Analysis of Mycelium in Different Solid Media
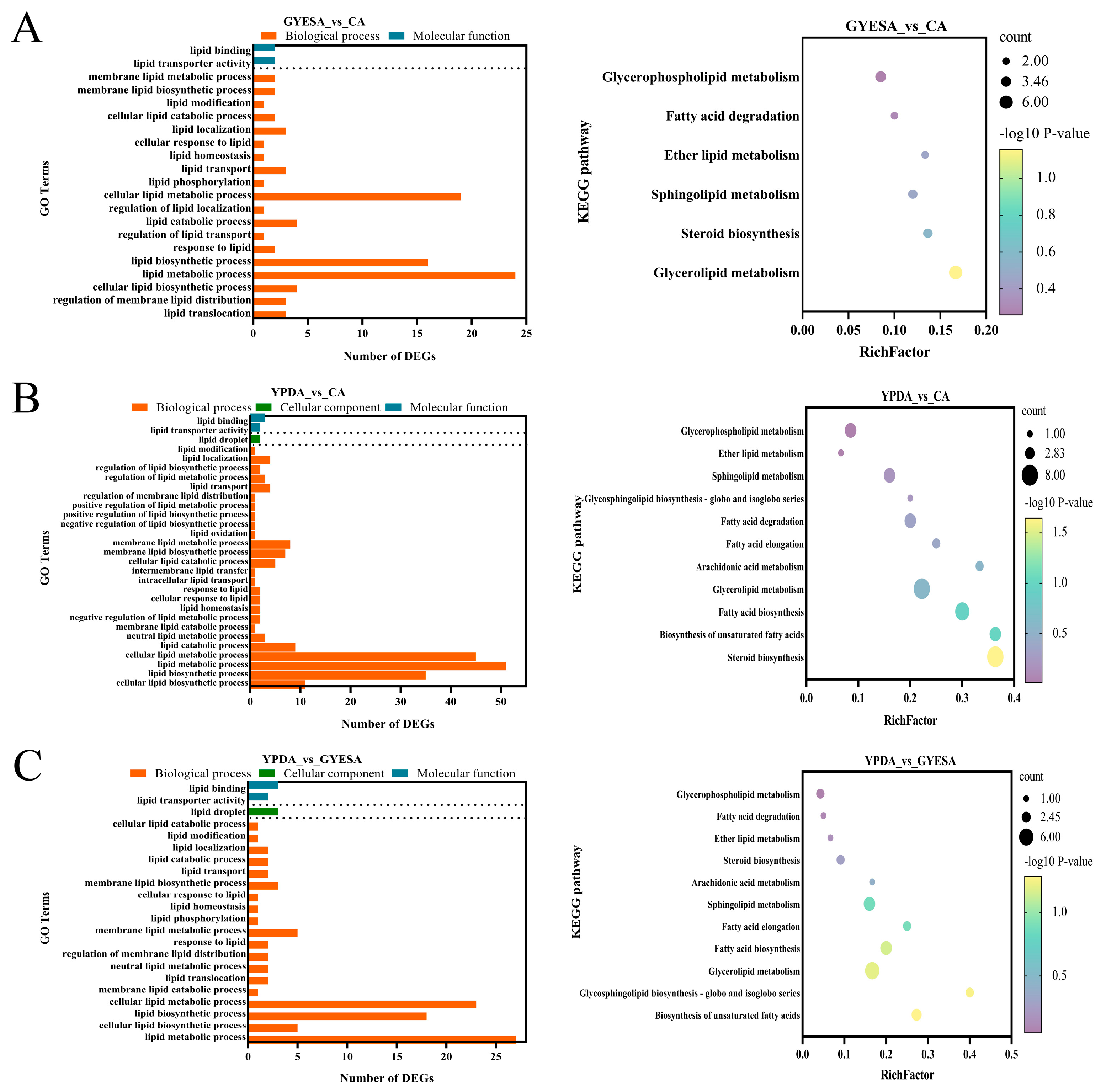
3.4.1. Enrichment Analysis of Fatty Acid Metabolism-Related Genes
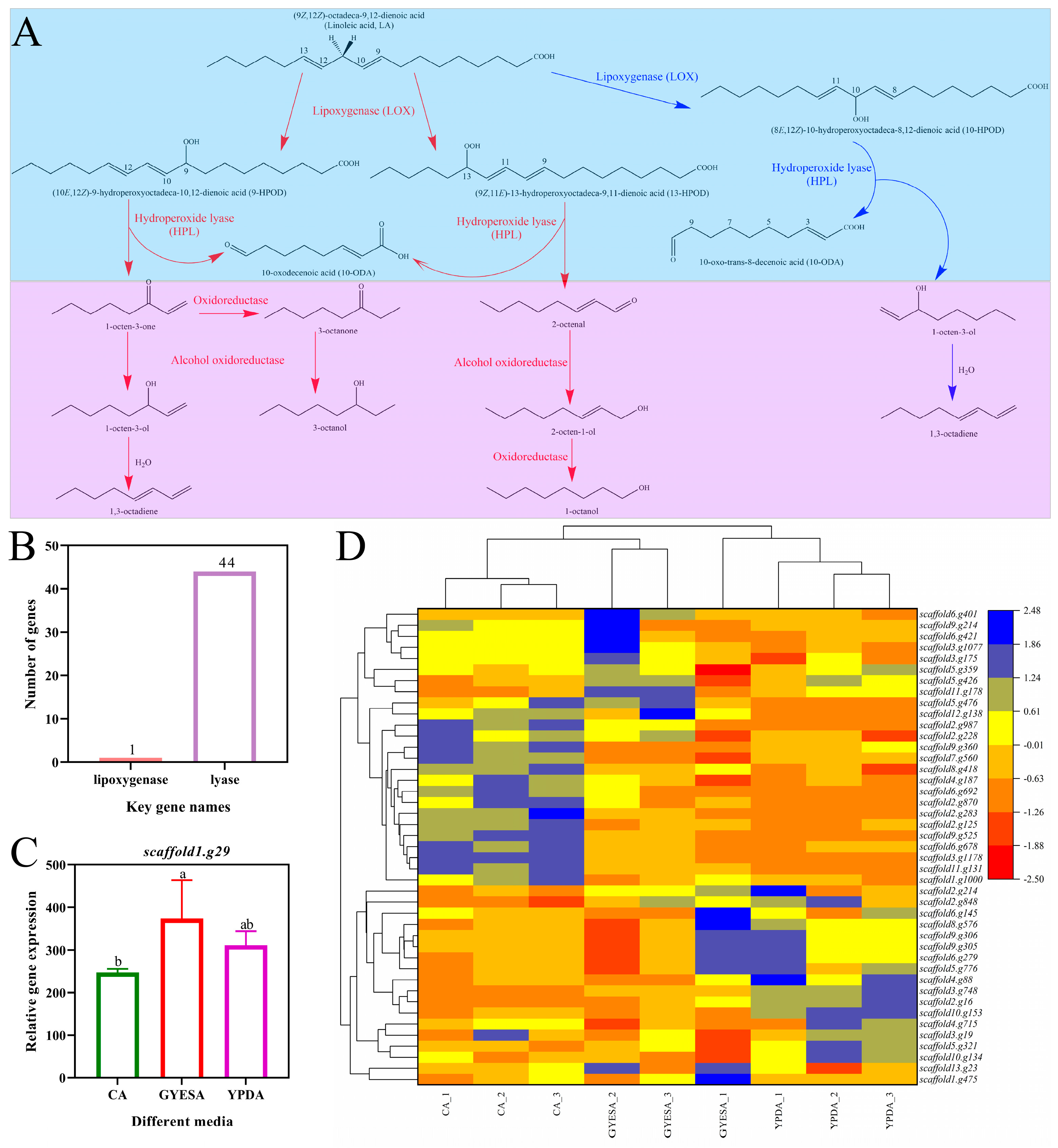
3.4.2. Expression of Genes Related to Eight-Carbon VOCs Biosynthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOCs | Volatile organic compounds |
| OSMAC | One strain many compounds |
| LOX | Lipoxygenase |
| SMs | Secondary metabolites |
| HS-SPME | Headspace solid-phase microextraction |
| PAL | Prep And Load |
| GC-MS | Gas chromatography–mass spectrometry |
| CA | Czapek agar |
| GYESA | Glucose yeast extract soluble starch agar |
| YPDA | Yeast extract peptone dextrose agar |
| PDA | Potato dextrose agar |
| LSD | Least significant difference |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
| PCA | Principal component analysis |
| EC50 | Median effect concentration |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| HPL | Hydroperoxide lyase |
| SSF | Solid-state fermentation |
References
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An overview of the molecular genetics of plant resistance to the Verticillium wilt pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Kaddes, A.; Fauconnier, M.; Sassi, K.; Nasraoui, B.; Jijakli, M. Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Razo-Belman, R.; Ozuna, C. Volatile organic compounds: A review of their current applications as pest biocontrol and disease management. Horticulturae 2023, 9, 441. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef]
- Xing, M.; Zhao, J.; Zhang, J.; Wu, Y.; Khan, R.A.A.; Li, X.; Wang, R.; Li, T.; Liu, T. 6-Pentyl-2H-pyran-2-one from Trichoderma erinaceum is fungicidal against litchi downy blight pathogen Peronophythora litchii and preservation of Litchi. J. Agric. Food Chem. 2023, 71, 19488–19500. [Google Scholar] [CrossRef]
- Ning, M.; Guo, Q.; Guo, P.; Cui, Y.; Wang, K.; Du, G.; Wang, Z.; Yuan, Y.; Yue, T. Biocontrol activity of Kluyveromyces marxianus YG-4 against Penicillium expansum LPH9 on apples. Int. J. Food Microbiol. 2025, 427, 110943. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Teles, A.P.C.; Bracarense, A.d.A.P.; Gomes, D.C. Classical and epigenetic approaches to metabolite diversification in filamentous fungi. Phytochem. Rev. 2013, 12, 773–789. [Google Scholar] [CrossRef]
- Schuller, A.; Studt-Reinhold, L.; Strauss, J. How to completely squeeze a fungus—Advanced genome mining tools for novel bioactive substances. Pharmaceutics 2022, 14, 1837. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Hofs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Rivilla, C.; Aleu, J.; Duran-Patron, R. Cryptic metabolites from marine-derived microorganisms using OSMAC and epigenetic approaches. Mar. Drugs 2022, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Li, X.M.; Liu, H.; Wang, B.G.; Meng, L.H. Mining new meroterpenoids from the marine red alga-derived endophytic Penicillium chermesinum EN-480 by comparative transcriptome analysis. Bioorg. Chem. 2022, 128, 106021. [Google Scholar] [CrossRef]
- Li, P.; Wei, F.; Feng, H.; Zhao, L.; Zhang, Y.; Zhou, J.; Feng, Z.; Zhu, H. Talaromyces purpuregenus CEF642N as a promising biocontrol agent for cotton disease control. J. Agric. Food Chem. 2025, 73, 2760–2772. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Liu, R.; Feng, Z.; Feng, H.; Zhou, J.; Zhao, L.; Wei, F.; Zhu, H. In the coevolution of cotton and pathogentic fungi, resistant cotton varieties lead to an escalation in the virulence of Verticillium dahliae. Ecotoxicol. Environ. Saf. 2025, 290, 117730. [Google Scholar] [CrossRef]
- Tang, T.; Wang, F.; Huang, H.; Xie, N.; Guo, J.; Guo, X.; Duan, Y.; Wang, X.; Wang, Q.; You, J. Antipathogenic activities of volatile organic compounds produced by Bacillus velezensis LT1 against Sclerotium rolfsii LC1, the pathogen of southern blight in Coptis chinensis. J. Agric. Food Chem. 2024, 722, 10282–10294. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, B.; Quan, X.; Zhao, G.; Zhang, Z.; Liu, W.; Tian, Y. Antagonistic potential of Trichoderma as a biocontrol agent against Sclerotinia asari. Front. Microbiol. 2022, 13, 997050. [Google Scholar] [CrossRef]
- Pei, Y.; Zhu, Y.; Jia, Y.; Ge, X.; Li, X.; Li, F.; Hou, Y. Molecular evidence for the involvement of cotton GhGLP2, in enhanced resistance to Verticillium and Fusarium Wilts and oxidative stress. Sci. Rep. 2020, 10, 12510. [Google Scholar] [CrossRef]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Pennerman, K.K.; Yin, G.; Bennett, J.W. Eight-carbon volatiles: Prominent fungal and plant interaction compounds. J. Exp. Bot. 2022, 73, 487–497. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific roles of lipoxygenases in development and responses to stress in plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Engelberthe, J. Green leaf volatiles—The forefront of plant responses against biotic attack. Plant Cell Physiol. 2022, 63, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Wurzenberger, M.; Grosch, W. Origin of the oxygen in the products of the enzymatic cleavage reaction of linoleic acid to 1-octen-3-ol and 10-oxo-trans-8-decenoic acid in mushrooms (Psalliota bispora). BBA-Lipid. Lipd Metab. 1984, 794, 18–24. [Google Scholar] [CrossRef]
- Wurzenberger, M.; Grosch, W. The formation of 1-octen-3-ol from the 10-hydroperoxide isomer of linoleic acid by a hydroperoxide lyase in mushrooms (Psalliota bispora). BBA-Lipid. Lipd Metab. 1984, 794, 25–30. [Google Scholar] [CrossRef]
- Wurzenberger, M.; Grosch, W. Stereochemistry of the cleavage of the 10-hydroperoxide isomer of linoleic acid to 1-octen-3-ol by a hydroperoxide lyase from mushrooms (Psalliota bispora). BBA-Lipid. Lipd Metab. 1984, 795, 163–165. [Google Scholar] [CrossRef]
- Ferrari, R.; Lacaze, I.; Faouder, P.L.; Bertrand-Michel, J.; Oger, C.; Galano, J.; Durand, T.; Moularat, S.; Tong, L.C.H.; Boucher, C.; et al. Cyclooxygenases and lipoxygenases are used by the fungus Podospora anserina to repel nematodes. BBA-Gen. Subjects 2018, 1862, 2174–2182. [Google Scholar] [CrossRef]
- Ibekwe, A.M. Effects of fumigants on non-target organisms in soils. Adv. Agron. 2004, 83, 1–35. [Google Scholar]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci. 2015, 20, 206–211. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Zhang, Z.; Tian, S. 3-Octanol controls gray mold on postharvest fruit by inducing autophagy of Botrytis cinerea. Postharvest Biol. Tec. 2023, 205, 112525. [Google Scholar] [CrossRef]
- Simone, N.D.; Lopez, L.; Ciudad, C.S.; Scauro, A.; Russo, P.; Rodriguez, J.; Spano, G.; Martinez, B. Antifungal activity of Lactiplantibacillus plantarum isolated from fruit and vegetables and detection of novel antifungal VOCs from fungal-LAB co-cultures. Food Biosci. 2024, 58, 103824. [Google Scholar] [CrossRef]
- Wang, T.; Li, W.; Wang, F.; Li, J.; Qin, J.; Song, Z.; Xu, J.; Qiu, H.; Cheng, Y. Biocontrol potential of Bacillus velezensis SEC-024A against southern blight of industrial hemp. Ind. Crop. Prod. 2024, 222, 119767. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, X.; Chen, J.X.; Zhang, Y.; Ouyang, Q.; Tao, N. (E)-2-Octenal suppresses the growth of a prochlora-resistant Penicillium italicum strain and its potential antifungal mechanisms. Postharvest Biol. Technol. 2023, 205, 112515. [Google Scholar] [CrossRef]
- Tan, X.; Jiang, X.; Reymick, O.O.; Zhu, C.; Tao, N. (E)-2-Octenal inhibits Neofusicoccum parvum growth by disrupting mitochondrial energy metabolism and is a potential preservative for postharvest mango. Food Res. Int. 2025, 201, 115639. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Gonzalez, J. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Karrer, D.; Weigel, V.; Hoberg, N.; Atamasov, A.; Ruhl, M. Biotransformation of [U-13C] linoleic acid suggests two independent ketonic- and aldehydic cycles with C8-oxylipin biosynthesis in Cyclocybe aegerita (V. Brig.) Vizzini. Mycol. Prog. 2021, 20, 929–940. [Google Scholar] [CrossRef]
- Haeggstrom, J.Z.; Funk, C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011, 111, 5866–5898. [Google Scholar] [CrossRef]
- Kihara, H.; Tanaka, M.; Yamato, K.T.; Horibata, A.; Yamada, A.; Kita, S.; Ishizaki, K.; Kajikawa, M.; Fukuzawa, H.; Kohchi, T.; et al. Arachidonic acid-dependent carbon-eight volatile synthesis from wounded liverwort (Marchantia polymorpha). Phytochemistry 2014, 107, 42–49. [Google Scholar] [CrossRef]
- Hajeyah, A.A.; Griffiths, W.J.; Wang, Y.; Finch, A.J.; O’Donnell, V.B. The biosynthesis of enzymatically oxidized lipids. Front. Endocrinol. 2020, 11, 591819. [Google Scholar] [CrossRef]
- Orban, A.; Weber, A.; Herzog, R.; Hennicke, F.; Ruhl, M. Transcriptome of different fruiting stages in the cultivated mushroom Cyclocybe aegerita suggests a complex regulation of fruiting and reveals enzymes putatively involved in fungal oxylipin biosynthesis. BMC Genomics 2021, 22, 324. [Google Scholar] [CrossRef]
- Tasaki, Y.; Kobayashi, D.; Sato, R.; Hayashi, S.; Joh, T. Variations in 1-octen-3-ol and lipoxygenase gene expression in the oyster mushroom Pleurotus ostreatus according to fruiting body development, tissue specificity, maturity, and postharvest storage. Mycoscience 2019, 60, 170–176. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Chen, W.; Wang, C. The regulatory functions of oxylipins in fungi: A review. J. Basic Microb. 2023, 63, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sasahara, S.; Akakabe, Y.; Kajiwara, T. Linoleic acid 10-hydroperoxide as an intermediate during formation of 1-octen-3-ol from linoleic acid in Lentinus decadetes. Biosci. Biotechnol. Bioch. 2003, 67, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- Stolterfoht, H.; Rinnofner, C.; Winkler, M.; Pichler, H. Recombinant lipoxygenases and hydroperoxide lyases for the synthesis of green leaf volatiles. J. Agric. Food Chem. 2019, 67, 13367–13392. [Google Scholar] [CrossRef]
- Husson, F.; Thomas, M.; Kermasha, S.; Belin, J. Effect of linoleic acid induction on the production of 1-octen-3-ol by the lipoxygenase and hydroperoxide lyase activities of Penicillium camemberti. J. Mol. Catal. B Enzym. 2002, 19–20, 363–369. [Google Scholar] [CrossRef]
- Husson, F.; Bompas, D.; Kermasha, S.; Belin, J.M. Biogeneration of 1-octen-3-ol by lipoxygenase and hydroperoxide lyase activities of Agaricus bisporus. Process Biochem. 2001, 37, 177–182. [Google Scholar] [CrossRef]
- Su, T.; Chen, Y.; Liu, H.; Gao, Y.; Guo, J.; Li, Y.; Qi, Y.; Qiu, L. The biosynthesis of 1-octene-3-ol by a multifunctional fatty acid dioxygenase and hydroperoxide lyase in Agaricus bisporus. J. Fungi 2022, 8, 827. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Wang, J.; Feng, J.; Wu, D.; Zhang, Z.; Zhang, J.; Yang, Y. Effects of enzymatic reaction on the generation of key aroma volatiles in shiitake mushroom at different cultivation substrates. Food Sci. Nutr. 2021, 9, 2247–2256. [Google Scholar] [CrossRef]
- Orban, A.M.; Ruhl, M. Identification of volatile producing enzymes in higher fungi: Combining analytical and bioinformatic methods. Method. Enzymol. 2022, 664, 221–242. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, Y.; Feng, H.; Zhou, J.; Zhao, L.; Zhu, H.; Wei, F.; Feng, Z. Antifungal Volatile Organic Compounds from Talaromyces purpureogenus CEF642N: Insights from One Strain Many Compounds (OSMAC) Strategy for Controlling Verticillium dahliae in Cotton. J. Fungi 2025, 11, 332. https://doi.org/10.3390/jof11050332
Li P, Zhang Y, Feng H, Zhou J, Zhao L, Zhu H, Wei F, Feng Z. Antifungal Volatile Organic Compounds from Talaromyces purpureogenus CEF642N: Insights from One Strain Many Compounds (OSMAC) Strategy for Controlling Verticillium dahliae in Cotton. Journal of Fungi. 2025; 11(5):332. https://doi.org/10.3390/jof11050332
Chicago/Turabian StyleLi, Peng, Yalin Zhang, Hongjie Feng, Jinglong Zhou, Lihong Zhao, Heqin Zhu, Feng Wei, and Zili Feng. 2025. "Antifungal Volatile Organic Compounds from Talaromyces purpureogenus CEF642N: Insights from One Strain Many Compounds (OSMAC) Strategy for Controlling Verticillium dahliae in Cotton" Journal of Fungi 11, no. 5: 332. https://doi.org/10.3390/jof11050332
APA StyleLi, P., Zhang, Y., Feng, H., Zhou, J., Zhao, L., Zhu, H., Wei, F., & Feng, Z. (2025). Antifungal Volatile Organic Compounds from Talaromyces purpureogenus CEF642N: Insights from One Strain Many Compounds (OSMAC) Strategy for Controlling Verticillium dahliae in Cotton. Journal of Fungi, 11(5), 332. https://doi.org/10.3390/jof11050332





