Gut Mycobiome: Latest Findings and Current Knowledge Regarding Its Significance in Human Health and Disease
Abstract
:1. Introduction
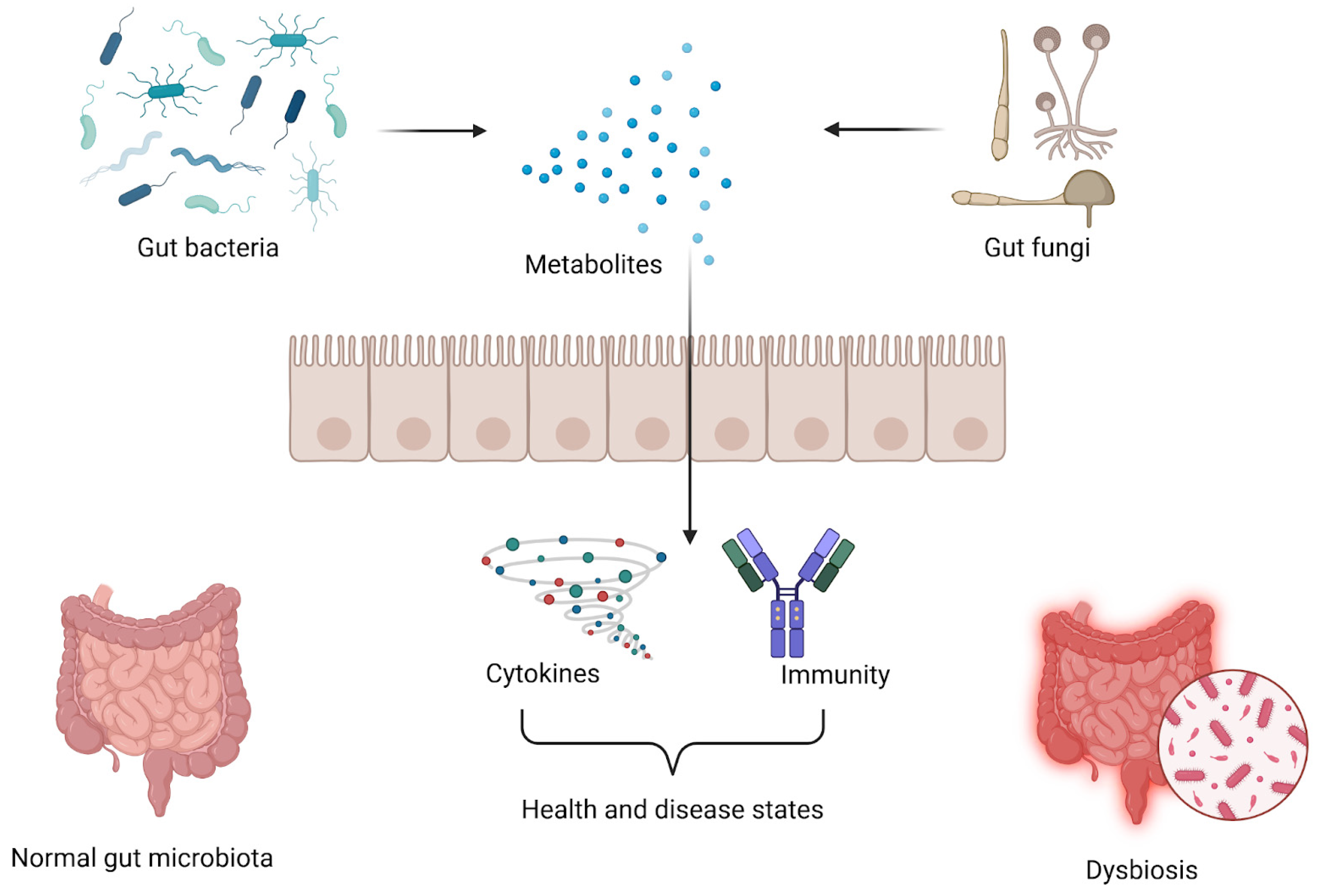
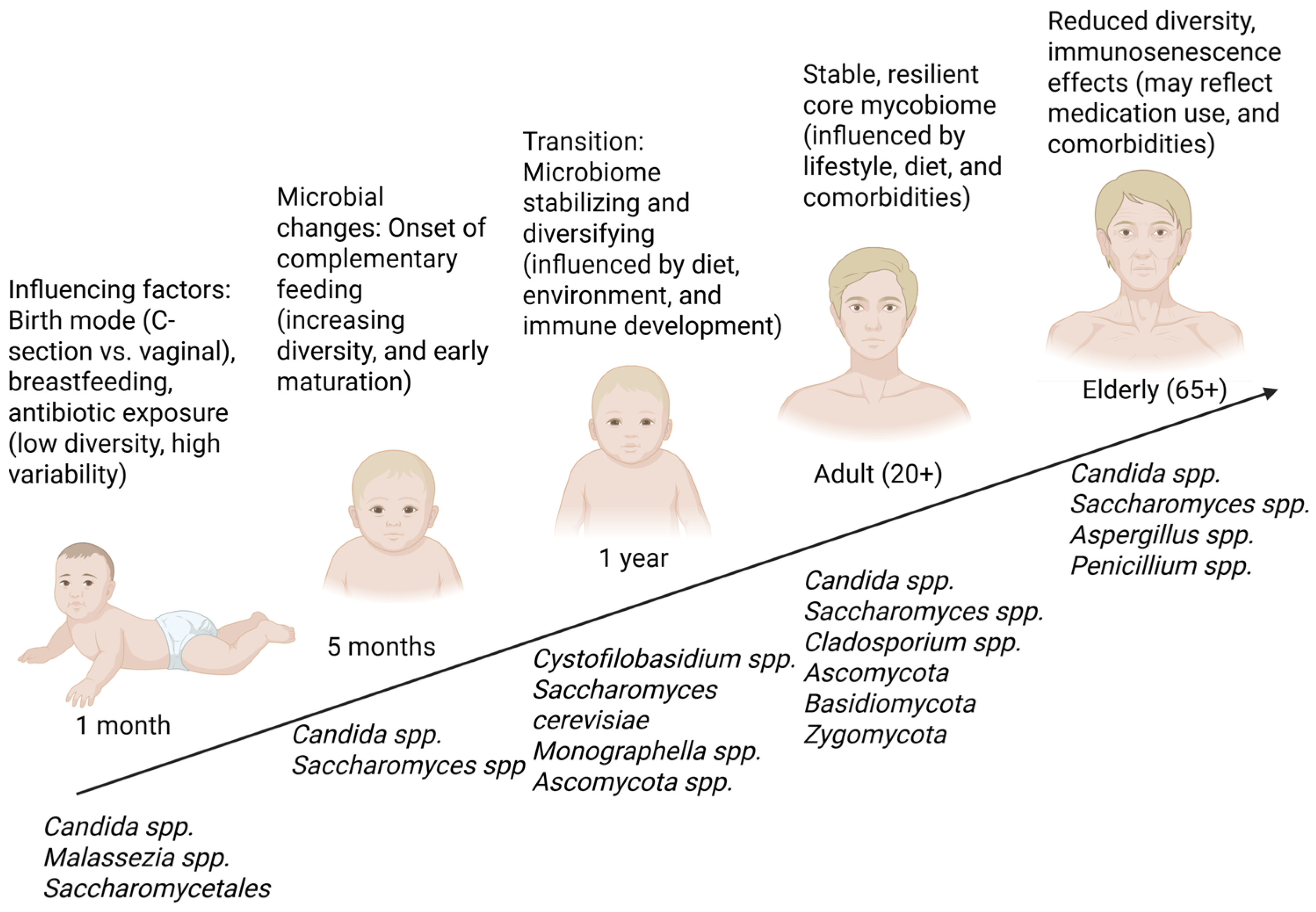
2. Gut Mycobiome: Important Fungi Residing in the Human Gut
3. The Balance Between Gut Fungi and Bacteria in Healthy Subjects
4. Gut Mycobiome Characterization in Diseases
5. Clinical Perspectives on the Possibilities for Calibrating the Gut Mycobiome
5.1. Antifungal Therapies
5.2. Probiotics
5.3. Fecal Microbiota Transplantation
5.4. Dietary Support
6. Technological Advances in Mycobiome Research and Future Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| CD | Crohn’s disease |
| CDI | Clostridium difficile infection |
| CRC | Colorectal cancer |
| FMT | Fecal microbiota transplantation |
| IBD | Inflammatory bowel disease |
| MS | Multiple sclerosis |
| RA | Rheumatoid arthritis |
| SCFAs | Short-chain fatty acids |
| SIFO | Small intestine fungal overgrowth |
| T2D | Type 2 diabetes |
| Tregs | Regulatory T cells |
| UC | Ulcerative colitis |
References
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Willis, K.A.; Purvis, J.H.; Myers, E.D.; Aziz, M.M.; Karabayir, I.; Gomes, C.K.; Peters, B.M.; Akbilgic, O.; Talati, A.J.; Pierre, J.F. Fungi form interkingdom microbial communities in the primordial human gut that develop with gestational age. Faseb J. 2019, 33, 12825–12837. [Google Scholar] [CrossRef]
- Blaser, M.J.; Devkota, S.; McCoy, K.D.; Relman, D.A.; Yassour, M.; Young, V.B. Lessons learned from the prenatal microbiome controversy. Microbiome 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef]
- Sun, Y.; Zuo, T.; Cheung, C.P.; Gu, W.; Wan, Y.; Zhang, F.; Chen, N.; Zhan, H.; Yeoh, Y.K.; Niu, J.; et al. Population-Level Configurations of Gut Mycobiome Across 6 Ethnicities in Urban and Rural China. Gastroenterology 2021, 160, 272–286.e211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhan, H.; Xu, W.; Yan, S.; Ng, S.C. The role of gut mycobiome in health and diseases. Ther. Adv. Gastroenterol. 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review article: Fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.V.; Leonardi, I.; Putzel, G.G.; Semon, A.; Fiers, W.D.; Kusakabe, T.; Lin, W.Y.; Gao, I.H.; Doron, I.; Gutierrez-Guerrero, A.; et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 2022, 603, 672–678. [Google Scholar] [CrossRef]
- Hoggard, M.; Vesty, A.; Wong, G.; Montgomery, J.M.; Fourie, C.; Douglas, R.G.; Biswas, K.; Taylor, M.W. Characterizing the Human Mycobiota: A Comparison of Small Subunit rRNA, ITS1, ITS2, and Large Subunit rRNA Genomic Targets. Front. Microbiol. 2018, 9, 2208. [Google Scholar] [CrossRef]
- Rao, C.; Coyte, K.Z.; Bainter, W.; Geha, R.S.; Martin, C.R.; Rakoff-Nahoum, S. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature 2021, 591, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wong, S.H.; Cheung, C.P.; Lam, K.; Lui, R.; Cheung, K.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Wu, J.C.Y.; et al. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat. Commun. 2018, 9, 3663. [Google Scholar] [CrossRef]
- Spatz, M.; Richard, M.L. Overview of the Potential Role of Malassezia in Gut Health and Disease. Front. Cell Infect. Microbiol. 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Limon, J.J.; Tang, J.; Li, D.; Wolf, A.J.; Michelsen, K.S.; Funari, V.; Gargus, M.; Nguyen, C.; Sharma, P.; Maymi, V.I.; et al. Malassezia Is Associated with Crohn’s Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe 2019, 25, 377–388.e6. [Google Scholar] [CrossRef]
- Kombrink, A.; Tayyrov, A.; Essig, A.; Stöckli, M.; Micheller, S.; Hintze, J.; van Heuvel, Y.; Dürig, N.; Lin, C.W.; Kallio, P.T.; et al. Induction of antibacterial proteins and peptides in the coprophilous mushroom Coprinopsis cinerea in response to bacteria. Isme J. 2019, 13, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Siavoshi, F.; Heydari, S.; Shafiee, M.; Ahmadi, S.; Saniee, P.; Sarrafnejad, A.; Kolahdoozan, S. Sequestration inside the yeast vacuole may enhance Helicobacter pylori survival against stressful condition. Infect. Genet. Evol. 2019, 69, 127–133. [Google Scholar] [CrossRef]
- Jain, U.; Ver Heul, A.M.; Xiong, S.; Gregory, M.H.; Demers, E.G.; Kern, J.T.; Lai, C.W.; Muegge, B.D.; Barisas, D.A.G.; Leal-Ekman, J.S.; et al. Debaryomyces is enriched in Crohn’s disease intestinal tissue and impairs healing in mice. Science 2021, 371, 1154–1159. [Google Scholar] [CrossRef]
- Zou, R.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zheng, H. Dysbiosis of Gut Fungal Microbiota in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2021, 51, 267–275. [Google Scholar] [CrossRef]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef]
- Nelson, A.; Stewart, C.J.; Kennedy, N.A.; Lodge, J.K.; Tremelling, M.; Probert, C.S.; Parkes, M.; Mansfield, J.C.; Smith, D.L.; Hold, G.L.; et al. The Impact of NOD2 Genetic Variants on the Gut Mycobiota in Crohn’s Disease Patients in Remission and in Individuals Without Gastrointestinal Inflammation. J. Crohns Colitis 2021, 15, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Duan, Y.; Liu, J.; Torralba, M.G.; Kuelbs, C.; Ventura-Cots, M.; Abraldes, J.G.; Bosques-Padilla, F.; Verna, E.C.; Brown, R.S., Jr.; et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients with Alcoholic Hepatitis. Hepatology 2020, 71, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.K.; Lui, G.C.Y.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.L.; et al. Alterations in Fecal Fungal Microbiome of Patients with COVID-19 During Time of Hospitalization until Discharge. Gastroenterology 2020, 159, 1302–1310.e5. [Google Scholar] [CrossRef] [PubMed]
- Uryu, H.; Hashimoto, D.; Kato, K.; Hayase, E.; Matsuoka, S.; Ogasawara, R.; Takahashi, S.; Maeda, Y.; Iwasaki, H.; Miyamoto, T.; et al. α-Mannan induces Th17-mediated pulmonary graft-versus-host disease in mice. Blood 2015, 125, 3014–3023. [Google Scholar] [CrossRef]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Röcker, M.; Blango, M.G.; Kaufmann, S.; Röhmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355.e15. [Google Scholar] [CrossRef]
- Gunsalus, K.T.; Tornberg-Belanger, S.N.; Matthan, N.R.; Lichtenstein, A.H.; Kumamoto, C.A. Manipulation of Host Diet To Reduce Gastrointestinal Colonization by the Opportunistic Pathogen Candida albicans. mSphere 2015, 1, e00020-15. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2015, 18, 489–500. [Google Scholar] [CrossRef]
- van Tilburg Bernardes, E.; Pettersen, V.K.; Gutierrez, M.W.; Laforest-Lapointe, I.; Jendzjowsky, N.G.; Cavin, J.-B.; Vicentini, F.A.; Keenan, C.M.; Ramay, H.R.; Samara, J.; et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 2020, 11, 2577. [Google Scholar] [CrossRef]
- Seelbinder, B.; Chen, J.; Brunke, S.; Vazquez-Uribe, R.; Santhaman, R.; Meyer, A.C.; de Oliveira Lino, F.S.; Chan, K.F.; Loos, D.; Imamovic, L.; et al. Antibiotics create a shift from mutualism to competition in human gut communities with a longer-lasting impact on fungi than bacteria. Microbiome 2020, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.F.; et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e3. [Google Scholar] [CrossRef]
- Stewart, D.B., Sr.; Wright, J.R.; Fowler, M.; McLimans, C.J.; Tokarev, V.; Amaniera, I.; Baker, O.; Wong, H.T.; Brabec, J.; Drucker, R.; et al. Integrated Meta-omics Reveals a Fungus-Associated Bacteriome and Distinct Functional Pathways in Clostridioides difficile Infection. mSphere 2019, 4, e00454-19. [Google Scholar] [CrossRef] [PubMed]
- Doron, I.; Leonardi, I.; Li, X.V.; Fiers, W.D.; Semon, A.; Bialt-DeCelie, M.; Migaud, M.; Gao, I.H.; Lin, W.Y.; Kusakabe, T.; et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 2021, 184, 1017–1031.e14. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Q.; Yang, Y.; Zhong, W.; He, F.; Li, J. The mycobiome as integral part of the gut microbiome: Crucial role of symbiotic fungi in health and disease. Gut Microbes 2024, 16, 2440111. [Google Scholar] [CrossRef]
- Mok, K.; Poolsawat, T.; Somnuk, S.; Wanikorn, B.; Patumcharoenpol, P.; Nitisinprasert, S.; Vongsangnak, W.; Nakphaichit, M. Preliminary characterization of gut mycobiome enterotypes reveals the correlation trends between host metabolic parameter and diet: A case study in the Thai Cohort. Sci. Rep. 2024, 14, 5805. [Google Scholar] [CrossRef]
- Auchtung, T.A.; Stewart, C.J.; Smith, D.P.; Triplett, E.W.; Agardh, D.; Hagopian, W.A.; Ziegler, A.G.; Rewers, M.J.; She, J.X.; Toppari, J.; et al. Temporal changes in gastrointestinal fungi and the risk of autoimmunity during early childhood: The TEDDY study. Nat. Commun. 2022, 13, 3151. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Schei, K.; Avershina, E.; Øien, T.; Rudi, K.; Follestad, T.; Salamati, S.; Ødegård, R.A. Early gut mycobiota and mother-offspring transfer. Microbiome 2017, 5, 107. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Kachman, S.D.; Kim, J.; Legge, R.M.; Martínez, I. Fungi inhabiting the healthy human gastrointestinal tract: A diverse and dynamic community. Fungal Ecol. 2015, 15, 9–17. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Deligios, M.; Milanesi, L.; Langille, M.G.I.; Zinellu, A.; Rubino, S.; Carru, C.; Kelvin, D.J. Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia. mSphere 2020, 5, e00558-19. [Google Scholar] [CrossRef]
- Iliev, I.D.; Leonardi, I. Fungal dysbiosis: Immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial-fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef]
- Hill, J.H.; Round, J.L. Intestinal fungal-host interactions in promoting and maintaining health. Cell Host Microbe 2024, 32, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef]
- Fan, D.; Coughlin, L.A.; Neubauer, M.M.; Kim, J.; Kim, M.S.; Zhan, X.; Simms-Waldrip, T.R.; Xie, Y.; Hooper, L.V.; Koh, A.Y. Activation of HIF-1α and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat. Med. 2015, 21, 808–814. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef]
- Li, H.; Miao, M.X.; Jia, C.L.; Cao, Y.B.; Yan, T.H.; Jiang, Y.Y.; Yang, F. Interactions between Candida albicans and the resident microbiota. Front. Microbiol. 2022, 13, 930495. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef]
- Charlet, R.; Pruvost, Y.; Tumba, G.; Istel, F.; Poulain, D.; Kuchler, K.; Sendid, B.; Jawhara, S. Remodeling of the Candida glabrata cell wall in the gastrointestinal tract affects the gut microbiota and the immune response. Sci. Rep. 2018, 8, 3316. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, L.; De Salvo, C.; Buela, K.A.; Hager, C.; Ghannoum, M.; Osme, A.; Buttò, L.; Bamias, G.; Pizarro, T.T.; Cominelli, F. Candida tropicalis Infection Modulates the Gut Microbiome and Confers Enhanced Susceptibility to Colitis in Mice. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 901–923. [Google Scholar] [CrossRef]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio 2016, 7, e01250-16. [Google Scholar] [CrossRef]
- Sun, S.; Sun, L.; Wang, K.; Qiao, S.; Zhao, X.; Hu, X.; Chen, W.; Zhang, S.; Li, H.; Dai, H.; et al. The gut commensal fungus, Candida parapsilosis, promotes high fat-diet induced obesity in mice. Commun. Biol. 2021, 4, 1220. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.R. Chapter 2—Yeasts Pathogenic to Humans. In The Yeasts, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: London, UK, 2011; pp. 9–19. [Google Scholar] [CrossRef]
- Noor-Ul, H.; Liu, H.; Jin, J.; Zhu, X.; Han, D.; Yang, Y.; Xie, S. Dietary supplementation of Geotrichum candidum improves growth, gut microbiota, immune-related gene expression and disease resistance in gibel carp CAS III (Carassius auratus gibelio). Fish Shellfish Immunol. 2020, 99, 144–153. [Google Scholar] [CrossRef]
- Farid, F.; Sideeq, O.; Khan, F.; Niaz, K. Chapter 5.1—Saccharomyces cerevisiae. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 501–508. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Muhammad Ali, G.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Iancu, M.A.; Profir, M.; Roşu, O.A.; Ionescu, R.F.; Cretoiu, S.M.; Gaspar, B.S. Revisiting the Intestinal Microbiome and Its Role in Diarrhea and Constipation. Microorganisms 2023, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fruehauf, J.; Goldsmith, J.D.; Xu, H.; Katchar, K.K.; Koon, H.W.; Zhao, D.; Kokkotou, E.G.; Pothoulakis, C.; Kelly, C.P. Saccharomyces boulardii inhibits EGF receptor signaling and intestinal tumor growth in Apc(min) mice. Gastroenterology 2009, 137, 914–923. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Guo, R.; Chen, N.; Lu, H.; Huang, S.; Wang, J.; Li, L. Correlation between gastrointestinal fungi and varying degrees of chronic hepatitis B virus infection. Diagn. Microbiol. Infect. Dis. 2011, 70, 492–498. [Google Scholar] [CrossRef]
- Hamad, I.; Sokhna, C.; Raoult, D.; Bittar, F. Molecular detection of eukaryotes in a single human stool sample from Senegal. PLoS ONE 2012, 7, e40888. [Google Scholar] [CrossRef]
- Gouba, N.; Raoult, D.; Drancourt, M. Plant and fungal diversity in gut microbiota as revealed by molecular and culture investigations. PLoS ONE 2013, 8, e59474. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef]
- Gao, R.; Kong, C.; Li, H.; Huang, L.; Qu, X.; Qin, N.; Qin, H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Nakatsu, G.; Dai, R.Z.; Wu, W.K.K.; Wong, S.H.; Ng, S.C.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 2019, 68, 654–662. [Google Scholar] [CrossRef]
- Profir, M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. Friend or Foe: Exploring the Relationship between the Gut Microbiota and the Pathogenesis and Treatment of Digestive Cancers. Microorganisms 2024, 12, 955. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ouyang, J.; Pi, D.; Feng, L.; Yang, J. Malassezia in Inflammatory Bowel Disease: Accomplice of Evoking Tumorigenesis. Front. Immunol. 2022, 13, 846469. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Velegraki, A.; Magiatis, P.; Pappas, P.; Bassukas, I.D. Could Malassezia yeasts be implicated in skin carcinogenesis through the production of aryl-hydrocarbon receptor ligands? Med. Hypotheses 2011, 77, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Aflatoxins. 3 July 2024. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/aflatoxins (accessed on 23 February 2025).
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; Samsudin, N.I.P.; Azri, F.A. Aspergillus section Flavi and Aflatoxins: Occurrence, Detection, and Identification in Raw Peanuts and Peanut-Based Products Along the Supply Chain. Front. Microbiol. 2019, 10, 2602. [Google Scholar] [CrossRef]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef]
- Maas, E.; Penders, J.; Venema, K. Fungal-Bacterial Interactions in the Human Gut of Healthy Individuals. J. Fungi 2023, 9, 139. [Google Scholar] [CrossRef]
- MacAlpine, J.; Robbins, N.; Cowen, L.E. Bacterial-fungal interactions and their impact on microbial pathogenesis. Mol. Ecol. 2023, 32, 2565–2581. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, T.E. Symbioses between fungi and bacteria: From mechanisms to impacts on biodiversity. Curr. Opin. Microbiol. 2024, 80, 102496. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Tang, J. The interactions of Candida albicans with gut bacteria: A new strategy to prevent and treat invasive intestinal candidiasis. Gut Pathog. 2023, 15, 30. [Google Scholar] [CrossRef]
- Gill, P.A.; Inniss, S.; Kumagai, T.; Rahman, F.Z.; Smith, A.M. The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease. Front. Immunol. 2022, 13, 866059. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Victoria Obayomi, O.; Folakemi Olaniran, A.; Olugbemiga Owa, S. Unveiling the role of functional foods with emphasis on prebiotics and probiotics in human health: A review. J. Funct. Foods 2024, 119, 106337. [Google Scholar] [CrossRef]
- Facchin, S.; Bertin, L.; Bonazzi, E.; Lorenzon, G.; De Barba, C.; Barberio, B.; Zingone, F.; Maniero, D.; Scarpa, M.; Ruffolo, C.; et al. Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications. Life 2024, 14, 559. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed. Pharmacother. 2021, 141, 111817. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- Enache, R.M.; Profir, M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. The Role of Gut Microbiota in the Onset and Progression of Obesity and Associated Comorbidities. Int. J. Mol. Sci. 2024, 25, 12321. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. (Encinitas) 2018, 17, 28–32. [Google Scholar] [PubMed]
- Fekete, M.; Lehoczki, A.; Major, D.; Fazekas-Pongor, V.; Csípő, T.; Tarantini, S.; Csizmadia, Z.; Varga, J.T. Exploring the Influence of Gut-Brain Axis Modulation on Cognitive Health: A Comprehensive Review of Prebiotics, Probiotics, and Symbiotics. Nutrients 2024, 16, 789. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Nutsch, K.M.; Hsieh, C.S. T cell tolerance and immunity to commensal bacteria. Curr. Opin. Immunol. 2012, 24, 385–391. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, J.F.; Romo-Martínez, E.J.; Durán-Avelar Mde, J.; García-Magallanes, N.; Vibanco-Pérez, N. Th17 cells in autoimmune and infectious diseases. Int. J. Inflam. 2014, 2014, 651503. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Skoufou, M.; Tsigalou, C.; Vradelis, S.; Bezirtzoglou, E. The Networked Interaction between Probiotics and Intestine in Health and Disease: A Promising Success Story. Microorganisms 2024, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Antibiotic-Therapy-Induced Gut Dysbiosis Affecting Gut Microbiota-Brain Axis and Cognition: Restoration by Intake of Probiotics and Synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Selegato, D.M.; Castro-Gamboa, I. Enhancing chemical and biological diversity by co-cultivation. Front. Microbiol. 2023, 14, 1117559. [Google Scholar] [CrossRef]
- Maas, E.; Penders, J.; Venema, K. Studying Fungal-Bacterial Relationships in the Human Gut Using an In Vitro Model (TIM-2). J. Fungi 2023, 9, 174. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef]
- Lundberg, R.; Toft, M.F.; Metzdorff, S.B.; Hansen, C.H.F.; Licht, T.R.; Bahl, M.I.; Hansen, A.K. Human microbiota-transplanted C57BL/6 mice and offspring display reduced establishment of key bacteria and reduced immune stimulation compared to mouse microbiota-transplantation. Sci. Rep. 2020, 10, 7805. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Walter, J.; Finlay, B.B. Human Microbiota-Associated Mice: A Model with Challenges. Cell Host Microbe 2016, 19, 575–578. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Lundberg, R.; Krych, L.; Metzdorff, S.B.; Kot, W.; Sørensen, D.B.; Nielsen, D.S.; Hansen, C.H.F.; Hansen, A.K. A Humanized Diet Profile May Facilitate Colonization and Immune Stimulation in Human Microbiota-Colonized Mice. Front. Microbiol. 2020, 11, 1336. [Google Scholar] [CrossRef]
- Fox, J.D.; Sims, A.; Ross, M.; Bettag, J.; Wilder, A.; Natrop, D.; Borsotti, A.; Kolli, S.; Mehta, S.; Verma, H.; et al. Bioinformatic Methodologies in Assessing Gut Microbiota. Microbiol. Res. 2024, 15, 2554–2574. [Google Scholar] [CrossRef]
- Hemmati, M.A.; Monemi, M.; Asli, S.; Mohammadi, S.; Foroozanmehr, B.; Haghmorad, D.; Oksenych, V.; Eslami, M. Using New Technologies to Analyze Gut Microbiota and Predict Cancer Risk. Cells 2024, 13, 1987. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A.; Blekhman, R. Multi-omic approaches for host-microbiome data integration. Gut Microbes 2024, 16, 2297860. [Google Scholar] [CrossRef] [PubMed]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-Bacterial Interactions in Health and Disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Li, S.; Ogamune, K.J.; Ahmed, A.A.; Kim, I.H.; Zhang, Y.; Cai, D. Fungi in the Gut Microbiota: Interactions, Homeostasis, and Host Physiology. Microorganisms 2025, 13, 70. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Ashbee, H.R.; Evans, E.G. Immunology of diseases associated with Malassezia species. Clin. Microbiol. Rev. 2002, 15, 21–57. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans-The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Carlson, S.L.; Mathew, L.; Savage, M.; Kok, K.; Lindsay, J.O.; Munro, C.A.; McCarthy, N.E. Mucosal Immunity to Gut Fungi in Health and Inflammatory Bowel Disease. J. Fungi 2023, 9, 1105. [Google Scholar] [CrossRef]
- Sun, M.; Ju, J.; Xu, H.; Wang, Y. Intestinal fungi and antifungal secretory immunoglobulin A in Crohn’s disease. Front. Immunol. 2023, 14, 1177504. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Durairajan, S.S.K.; Singh, A.K.; Krishnamoorthi, S.; Iyaswamy, A.; Mandavi, S.P.; Jeewon, R.; Williams, L.L. Role of Candida species in pathogenesis, immune regulation, and prognostic tools for managing ulcerative colitis and Crohn’s disease. World J. Gastroenterol. 2024, 30, 5212–5220. [Google Scholar] [CrossRef] [PubMed]
- Yiallouris, A.; Pana, Z.D.; Marangos, G.; Tzyrka, I.; Karanasios, S.; Georgiou, I.; Kontopyrgia, K.; Triantafyllou, E.; Seidel, D.; Cornely, O.A.; et al. Fungal diversity in the soil Mycobiome: Implications for ONE health. One Health 2024, 18, 100720. [Google Scholar] [CrossRef] [PubMed]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of antimicrobial resistance in biofilms. NPJ Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Profir, M.; Roşu, O.A.; Ionescu, R.F.; Pavelescu, L.A.; Cretoiu, S.M. Chapter 11—Benefits and safety of probiotics in gastrointestinal diseases. In Antidotes to Toxins and Drugs; Găman, M.-A., Egbuna, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 279–328. [Google Scholar] [CrossRef]
- Kreulen, I.A.M.; de Jonge, W.J.; van den Wijngaard, R.M.; van Thiel, I.A.M. Candida spp. in Human Intestinal Health and Disease: More than a Gut Feeling. Mycopathologia 2023, 188, 845–862. [Google Scholar] [CrossRef]
- Balderramo, D.C.; Romagnoli, P.A.; Granlund, A.V.B.; Catalan-Serra, I. Fecal Fungal Microbiota (Mycobiome) Study as a Potential Tool for Precision Medicine in Inflammatory Bowel Disease. Gut Liver 2023, 17, 505–515. [Google Scholar] [CrossRef]
- Lagomarsino, V.N.; Kostic, A.D.; Chiu, I.M. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol. Pain. 2021, 9, 100056. [Google Scholar] [CrossRef]
- Botschuijver, S.; Roeselers, G.; Levin, E.; Jonkers, D.M.; Welting, O.; Heinsbroek, S.E.M.; de Weerd, H.H.; Boekhout, T.; Fornai, M.; Masclee, A.A.; et al. Intestinal Fungal Dysbiosis Is Associated with Visceral Hypersensitivity in Patients with Irritable Bowel Syndrome and Rats. Gastroenterology 2017, 153, 1026–1039. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Zeng, Y.; Luo, Y.; Peng, J.; Zhang, J.; Kuang, T.; Fan, G. Gut mycobiome and metabolic diseases: The known, the unknown, and the future. Pharmacol. Res. 2023, 193, 106807. [Google Scholar] [CrossRef]
- Nikolic, D.M.; Dimitrijevic-Sreckovic, V.; Ranin, L.T.; Stojanovic, M.M.; Ilic, I.D.; Gostiljac, D.M.; Soldatovic, I.A. Homeostatic microbiome disruption as a cause of insulin secretion disorders Candida albicans, a new factor in pathogenesis of diabetes: A STROBE compliant cross-sectional study. Medicine 2022, 101, e31291. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Sencio, V.; Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: A Closer Look at the Lungs. Infect. Immun. 2021, 89, e0018821. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Jandu, J.S.; Brent, L.H.; Al-Dhahir, M.A. Rheumatoid Arthritis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441999/ (accessed on 3 March 2025).
- Bishu, S.; Su, E.W.; Wilkerson, E.R.; Reckley, K.A.; Jones, D.M.; McGeachy, M.J.; Gaffen, S.L.; Levesque, M.C. Rheumatoid arthritis patients exhibit impaired Candida albicans-specific Th17 responses. Arthritis Res. Ther. 2014, 16, R50. [Google Scholar] [CrossRef]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal Fungi in Health and Disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef]
- Schinnerling, K.; Rosas, C.; Soto, L.; Thomas, R.; Aguillón, J.C. Humanized Mouse Models of Rheumatoid Arthritis for Studies on Immunopathogenesis and Preclinical Testing of Cell-Based Therapies. Front. Immunol. 2019, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Romero-Figueroa, M.D.S.; Ramírez-Durán, N.; Montiel-Jarquín, A.J.; Horta-Baas, G. Gut-joint axis: Gut dysbiosis can contribute to the onset of rheumatoid arthritis via multiple pathways. Front. Cell Infect. Microbiol. 2023, 13, 1092118. [Google Scholar] [CrossRef]
- Altieri, C.; Speranza, B.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Gut-Microbiota, and Multiple Sclerosis: Background, Evidence, and Perspectives. Nutrients 2023, 15, 942. [Google Scholar] [CrossRef]
- Yadav, M.; Ali, S.; Shrode, R.L.; Shahi, S.K.; Jensen, S.N.; Hoang, J.; Cassidy, S.; Olalde, H.; Guseva, N.; Paullus, M.; et al. Multiple sclerosis patients have an altered gut mycobiome and increased fungal to bacterial richness. PLoS ONE 2022, 17, e0264556. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflammation 2022, 19, 154. [Google Scholar] [CrossRef]
- Buga, A.M.; Padureanu, V.; Riza, A.L.; Oancea, C.N.; Albu, C.V.; Nica, A.D. The Gut-Brain Axis as a Therapeutic Target in Multiple Sclerosis. Cells 2023, 12, 1872. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Marr, K.A. Impact of Aspergillus fumigatus in allergic airway diseases. Clin. Transl. Allergy 2011, 1, 4. [Google Scholar] [CrossRef]
- Kanj, A.N.; Skalski, J.H. Gut Mycobiome and Asthma. J. Fungi 2024, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg Bernardes, E.; Gutierrez, M.W.; Arrieta, M.-C. The Fungal Microbiome and Asthma. Front. Cell. Infect. Microbiol. 2020, 10, 583418. [Google Scholar] [CrossRef]
- Kanj, A.N.; Kottom, T.J.; Schaefbauer, K.J.; Choudhury, M.; Limper, A.H.; Skalski, J.H. Dysbiosis of the intestinal fungal microbiota increases lung resident group 2 innate lymphoid cells and is associated with enhanced asthma severity in mice and humans. Respir. Res. 2023, 24, 144. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Dhakad, M.S.; Goyal, R.; Bhalla, P.; Dewan, R. Spectrum of Opportunistic Fungal Infections in HIV/AIDS Patients in Tertiary Care Hospital in India. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 2373424. [Google Scholar] [CrossRef]
- Jerez Puebla, L.E. Fungal Infections in Immunosuppressed Patients. In Immunodeficiency; Metodiev, K., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian. J. Med. Res. 2014, 139, 195–204. [Google Scholar]
- Anju, V.T.; Busi, S.; Imchen, M.; Kumavath, R.; Mohan, M.S.; Salim, S.A.; Subhaswaraj, P.; Dyavaiah, M. Polymicrobial Infections and Biofilms: Clinical Significance and Eradication Strategies. Antibiotics 2022, 11, 1731. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Lou, E.; Zhao, X.; Chen, X. The role of gut fungi in Clostridioides difficile infection. Biomed. J. 2024, 47, 100686. [Google Scholar] [CrossRef]
- Smits, W.K.; Lyras, D.; Lacy, D.B.; Wilcox, M.H.; Kuijper, E.J. Clostridium difficile infection. Nat. Rev. Dis. Primers 2016, 2, 16020. [Google Scholar] [CrossRef]
- Pérez, J.C. The interplay between gut bacteria and the yeast Candida albicans. Gut Microbes 2021, 13, 1979877. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Almendares, O.M.; Farley, M.M.; Reno, J.; Smith, Z.; Stein, B.; Magill, S.S.; Smith, R.M.; Cleveland, A.; Lessa, F.C. Candida Co-infection Among Adults with Clostridium difficile Infection in Metropolitan Atlanta, 2009–2013. Open Forum Infect. Dis. 2015, 2 (Suppl. 1), 921. [Google Scholar] [CrossRef]
- van Leeuwen, P.T.; van der Peet, J.M.; Bikker, F.J.; Hoogenkamp, M.A.; Oliveira Paiva, A.M.; Kostidis, S.; Mayboroda, O.A.; Smits, W.K.; Krom, B.P. Interspecies Interactions between Clostridium difficile and Candida albicans. mSphere 2016, 1, e00187-16. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, A. Exploring the Link Between the Gut Mycobiome and Neurological Disorders. Adv. Gut Microbiome Res. 2024, 2024, 9965893. [Google Scholar] [CrossRef]
- Hadrich, I.; Turki, M.; Chaari, I.; Abdelmoula, B.; Gargouri, R.; Khemakhem, N.; Elatoui, D.; Abid, F.; Kammoun, S.; Rekik, M.; et al. Gut mycobiome and neuropsychiatric disorders: Insights and therapeutic potential. Front. Cell. Neurosci. 2025, 18, 1495224. [Google Scholar] [CrossRef]
- Gamal, A.; Elshaer, M.; Alabdely, M.; Kadry, A.; McCormick, T.S.; Ghannoum, M. The Mycobiome: Cancer Pathogenesis, Diagnosis, and Therapy. Cancers 2022, 14, 2875. [Google Scholar] [CrossRef]
- Yunus, A.; Mokhtar, N.M.; Raja Ali, R.A.; Ahmad Kendong, S.M.; Ahmad, H.F. Methods for identification of the opportunistic gut mycobiome from colorectal adenocarcinoma biopsy tissues. MethodsX 2024, 12, 102623. [Google Scholar] [CrossRef]
- Cheng, W.; Li, F.; Gao, Y.; Yang, R. Fungi and tumors: The role of fungi in tumorigenesis (Review). Int. J. Oncol. 2024, 64, 52. [Google Scholar] [CrossRef]
- Prakash, V.; Singh, R.K.; Saurabh, K.; Kumar, V.; Kumari, R.; Kumar, S.; Rajpal, K.; Sinha, D.K.; Parwez, A. Spectrum of chemo-radiotherapy induced fungal infection in head and neck cancer patients at tertiary care centre of Eastern India. Oral Oncol. Rep. 2023, 6, 100039. [Google Scholar] [CrossRef]
- Reynolds, S. Gut Microbes May Influence How Well Radiation Therapy Works Against Cancer. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2021/cancer-fungi-gut-radiation-therapy (accessed on 23 February 2025).
- Cong, L.; Chen, C.; Mao, S.; Han, Z.; Zhu, Z.; Li, Y. Intestinal bacteria-a powerful weapon for fungal infections treatment. Front. Cell Infect. Microbiol. 2023, 13, 1187831. [Google Scholar] [CrossRef] [PubMed]
- Lemoinne, S.; Kemgang, A.; Ben Belkacem, K.; Straube, M.; Jegou, S.; Corpechot, C.; Chazouillères, O.; Housset, C.; Sokol, H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut 2020, 69, 92–102. [Google Scholar] [CrossRef]
- Krohn, S.; Zeller, K.; Böhm, S.; Chatzinotas, A.; Harms, H.; Hartmann, J.; Heidtmann, A.; Herber, A.; Kaiser, T.; Treuheit, M.; et al. Molecular quantification and differentiation of Candida species in biological specimens of patients with liver cirrhosis. PLoS ONE 2018, 13, e0197319. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.; Martina-Lingua, M.N.; Bandapalle, S.; Pluznick, J.; Hamad, A.R.; Peterson, D.A.; Rabb, H. Intestinal microbiota-kidney cross talk in acute kidney injury and chronic kidney disease. Nephron Clin. Pract. 2014, 127, 139–143. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Jeong, D.; Kang, I.B.; Chon, J.W.; Kim, H.S.; Song, K.Y.; Seo, K.H. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: Targeted and untargeted community analysis with correlation of biomarkers. J. Nutr. Biochem. 2017, 44, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xia, Y.; He, F.; Zhu, C.; Ren, W. Intestinal mycobiota in health and diseases: From a disrupted equilibrium to clinical opportunities. Microbiome 2021, 9, 60. [Google Scholar] [CrossRef]
- Fakhim, H.; Vaezi, A.; Dannaoui, E.; Chowdhary, A.; Nasiry, D.; Faeli, L.; Meis, J.F.; Badali, H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses 2018, 61, 377–382. [Google Scholar] [CrossRef]
- Gosiewski, T.; Salamon, D.; Szopa, M.; Sroka, A.; Malecki, M.T.; Bulanda, M. Quantitative evaluation of fungi of the genus Candida in the feces of adult patients with type 1 and 2 diabetes—A pilot study. Gut Pathog. 2014, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef]
- Yang, J.; Yao, J.L.; Wu, Z.Q.; Zeng, D.L.; Zheng, L.Y.; Chen, D.; Guo, Z.D.; Peng, L. Current opinions on the mechanism, classification, imaging diagnosis and treatment of post-traumatic osteomyelitis. Chin. J. Traumatol. 2021, 24, 320–327. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: A systematic review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Pruthi, V.; Al-Janabi, A.; Pereira, B.M. Characterization of biofilm formed on intrauterine devices. Indian J. Med. Microbiol. 2003, 21, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Dahlen, G. Biofilms in Dental Unit Water Lines. Monogr. Oral Sci. 2021, 29, 12–18. [Google Scholar] [CrossRef]
- Stickler, D.J.; King, J.B.; Winters, C.; Morris, S.L. Blockage of urethral catheters by bacterial biofilms. J. Infect. 1993, 27, 133–135. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Gallant, C.V.; Daniels, C.; Leung, J.M.; Ghosh, A.S.; Young, K.D.; Kotra, L.P.; Burrows, L.L. Common beta-lactamases inhibit bacterial biofilm formation. Mol. Microbiol. 2005, 58, 1012–1024. [Google Scholar] [CrossRef]
- Ortíz-Pérez, A.; Martín-de-Hijas, N.; Alonso-Rodríguez, N.; Molina-Manso, D.; Fernández-Roblas, R.; Esteban, J. Importance of antibiotic penetration in the antimicrobial resistance of biofilm formed by non-pigmented rapidly growing mycobacteria against amikacin, ciprofloxacin and clarithromycin. Enferm. Infecc. Microbiol. Clin. 2011, 29, 79–84. [Google Scholar] [CrossRef]
- Muñoz-Egea, M.C.; García-Pedrazuela, M.; Mahillo-Fernandez, I.; Esteban, J. Effect of Antibiotics and Antibiofilm Agents in the Ultrastructure and Development of Biofilms Developed by Nonpigmented Rapidly Growing Mycobacteria. Microb. Drug Resist. 2016, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Martín-de-Hijas, N.Z.; García-Almeida, D.; Bodas-Sánchez, A.; Gadea, I.; Fernández-Roblas, R. Prevalence of erm methylase genes in clinical isolates of non-pigmented, rapidly growing mycobacteria. Clin. Microbiol. Infect. 2009, 15, 919–923. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Jiang, Q.; Jin, Z.; Sun, B. MgrA Negatively Regulates Biofilm Formation and Detachment by Repressing the Expression of psm Operons in Staphylococcus aureus. Appl. Environ. Microbiol. 2018, 84, e01008-18. [Google Scholar] [CrossRef]
- Sambanthamoorthy, K.; Gokhale, A.A.; Lao, W.; Parashar, V.; Neiditch, M.B.; Semmelhack, M.F.; Lee, I.; Waters, C.M. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrob. Agents Chemother. 2011, 55, 4369–4378. [Google Scholar] [CrossRef]
- Dinicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. H 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Zaongo, S.D.; Ouyang, J.; Isnard, S.; Zhou, X.; Harypursat, V.; Cui, H.; Routy, J.P.; Chen, Y. Candida albicans can foster gut dysbiosis and systemic inflammation during HIV infection. Gut Microbes 2023, 15, 2167171. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Osset-Trénor, P.; Pascual-Ahuir, A.; Proft, M. Fungal Drug Response and Antimicrobial Resistance. J. Fungi 2023, 9, 565. [Google Scholar] [CrossRef]
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.D.S.; Filho, A.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Heng, X.; Jiang, Y.; Chu, W. Influence of Fluconazole Administration on Gut Microbiome, Intestinal Barrier, and Immune Response in Mice. Antimicrob. Agents Chemother. 2021, 65, e02552-20. [Google Scholar] [CrossRef]
- Hof, H. Is there a serious risk of resistance development to azoles among fungi due to the widespread use and long-term application of azole antifungals in medicine? Drug Resist. Updat. 2008, 11, 25–31. [Google Scholar] [CrossRef]
- Birch, M.; Sibley, G. 5.22—Antifungal Chemistry Review. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Oxford, UK, 2017; pp. 703–716. [Google Scholar] [CrossRef]
- Denning, D.W.; Hope, W.W. Therapy for fungal diseases: Opportunities and priorities. Trends Microbiol. 2010, 18, 195–204. [Google Scholar] [CrossRef]
- Gallagher, J.J.; Williams-Bouyer, N.; Villarreal, C.; Heggers, J.P.; Herndon, D.N. Chapter 12—Treatment of infection in burns. In Total Burn Care, 3rd ed.; Herndon, D.N., Ed.; W.B. Saunders: Edinburgh, UK, 2007; pp. 136–176. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms 2023, 11, 573. [Google Scholar] [CrossRef]
- Sousa, F.; Nascimento, C.; Ferreira, D.; Reis, S.; Costa, P. Reviving the interest in the versatile drug nystatin: A multitude of strategies to increase its potential as an effective and safe antifungal agent. Adv. Drug Deliv. Rev. 2023, 199, 114969. [Google Scholar] [CrossRef]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Drummond, R.A.; Hohl, T.M. Immune responses to human fungal pathogens and therapeutic prospects. Nat. Rev. Immunol. 2023, 23, 433–452. [Google Scholar] [CrossRef]
- Jawhara, S. How Gut Bacterial Dysbiosis Can Promote Candida albicans Overgrowth during Colonic Inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef]
- da Silva, A.R.; de Andrade Neto, J.B.; da Silva, C.R.; Campos Rde, S.; Costa Silva, R.A.; Freitas, D.D.; do Nascimento, F.B.; de Andrade, L.N.; Sampaio, L.S.; Grangeiro, T.B.; et al. Berberine Antifungal Activity in Fluconazole-Resistant Pathogenic Yeasts: Action Mechanism Evaluated by Flow Cytometry and Biofilm Growth Inhibition in Candida spp. Antimicrob. Agents Chemother. 2016, 60, 3551–3557. [Google Scholar] [CrossRef]
- Simo, L.O.T. An In-Vitro Study to Determine the Antimicrobial Properties of Artostaphylos uva-ursi on the Growth of Aspergillus, Candida albicans and Escherichia coli. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2018. [Google Scholar]
- Gupta, S. Review on Uva-Ursi—A miracle herb for urinary tract disorders. World J. Pharm. Life Sci. 2017, 3, 51–54. [Google Scholar]
- Shi, D.; Zhao, Y.; Yan, H.; Fu, H.; Shen, Y.; Lu, G.; Mei, H.; Qiu, Y.; Li, D.; Liu, W. Antifungal effects of undecylenic acid on the biofilm formation of Candida albicans. Int. J. Clin. Pharmacol. Ther. 2016, 54, 343–353. [Google Scholar] [CrossRef]
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Ioannou, P.; Petrikkos, G.; Tsioutis, C. Fungal infections in patients with inflammatory bowel disease: A systematic review. Mycoses 2018, 61, 366–376. [Google Scholar] [CrossRef]
- Erdogan, A.; Rao, S.S. Small Intestinal Fungal Overgrowth. Curr. Gastroenterol. Rep. 2015, 17, 16. [Google Scholar] [CrossRef]
- Zangl, I.; Pap, I.J.; Aspöck, C.; Schüller, C. The role of Lactobacillus species in the control of Candida via biotrophic interactions. Microb. Cell 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Abdel-Nasser, M.; Abdel-Maksoud, G.; Eid, A.M.; Hassan, S.E.; Abdel-Nasser, A.; Alharbi, M.; Elkelish, A.; Fouda, A. Antifungal Activity of Cell-Free Filtrate of Probiotic Bacteria Lactobacillus rhamnosus ATCC-7469 against Fungal Strains Isolated from a Historical Manuscript. Microorganisms 2023, 11, 1104. [Google Scholar] [CrossRef]
- Montiel, R.; Martín-Cabrejas, I.; Langa, S.; El Aouad, N.; Arqués, J.L.; Reyes, F.; Medina, M. Antimicrobial activity of reuterin produced by Lactobacillus reuteri on Listeria monocytogenes in cold-smoked salmon. Food Microbiol. 2014, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Mackie, J.; Donachie, G.E.; Chapuis, A.; Mezerová, K.; Lenardon, M.D.; Brown, A.J.P.; Duncan, S.H.; Walker, A.W. Human gut bifidobacteria inhibit the growth of the opportunistic fungal pathogen Candida albicans. FEMS Microbiol. Ecol. 2022, 98, fiac095. [Google Scholar] [CrossRef]
- Mendonça, F.H.; Santos, S.S.; Faria Ida, S.; Gonçalves e Silva, C.R.; Jorge, A.O.; Leão, M.V. Effects of probiotic bacteria on Candida presence and IgA anti-Candida in the oral cavity of elderly. Braz. Dent. J. 2012, 23, 534–538. [Google Scholar] [CrossRef]
- Alonso-Roman, R.; Last, A.; Mirhakkak, M.H.; Sprague, J.L.; Möller, L.; Großmann, P.; Graf, K.; Gratz, R.; Mogavero, S.; Vylkova, S.; et al. Lactobacillus rhamnosus colonisation antagonizes Candida albicans by forcing metabolic adaptations that compromise pathogenicity. Nat. Commun. 2022, 13, 3192. [Google Scholar] [CrossRef]
- Guo, H.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. The Potential Therapeutic Role of Lactobacillaceae rhamnosus for Treatment of Inflammatory Bowel Disease. Foods 2023, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Kunyeit, L.; K A, A.A.; Rao, R.P. Application of Probiotic Yeasts on Candida Species Associated Infection. J. Fungi 2020, 6, 189. [Google Scholar] [CrossRef]
- Krasowska, A.; Murzyn, A.; Dyjankiewicz, A.; Łukaszewicz, M.; Dziadkowiec, D. The antagonistic effect of Saccharomyces boulardii on Candida albicans filamentation, adhesion and biofilm formation. FEMS Yeast Res. 2009, 9, 1312–1321. [Google Scholar] [CrossRef]
- McFarland, L.V.; Huang, Y.; Wang, L.; Malfertheiner, P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United Eur. Gastroenterol. J. 2016, 4, 546–561. [Google Scholar] [CrossRef]
- Gul, S.; Durante-Mangoni, E. Unraveling the Puzzle: Health Benefits of Probiotics—A Comprehensive Review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef] [PubMed]
- Sahle, Z.; Engidaye, G.; Shenkute Gebreyes, D.; Adenew, B.; Abebe, T.A. Fecal microbiota transplantation and next-generation therapies: A review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. 2024, 12, 20503121241257486. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Salehi Omran, H.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Chen, Q.; Fan, Y.; Zhang, B.; Yan, C.; Chen, Z.; Wang, L.; Hu, Y.; Huang, Q.; Su, J.; Ren, J.; et al. Specific fungi associated with response to capsulized fecal microbiota transplantation in patients with active ulcerative colitis. Front. Cell Infect. Microbiol. 2022, 12, 1086885. [Google Scholar] [CrossRef]
- Park, S.Y.; Seo, G.S. Fecal Microbiota Transplantation: Is It Safe? Clin. Endosc. 2021, 54, 157–160. [Google Scholar] [CrossRef]
- van Lier, Y.F.; Rolling, T.; Armijo, G.K.; Zhai, B.; Haverkate, N.J.E.; Meijer, E.; Nur, E.; Blom, B.; Peled, J.U.; van den Brink, M.R.M.; et al. Profiling the Fungal Microbiome after Fecal Microbiota Transplantation for Graft-versus-Host Disease: Insights from a Phase 1 Interventional Study. Transplant. Cell. Ther. 2023, 29, e61–e63. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Jawhara, S. How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms 2024, 12, 1570. [Google Scholar] [CrossRef]
- Shankar, J. Food Habit Associated Mycobiota Composition and Their Impact on Human Health. Front. Nutr. 2021, 8, 773577. [Google Scholar] [CrossRef] [PubMed]
- Soldán, M.; Argalášová, Ľ.; Hadvinová, L.; Galileo, B.; Babjaková, J. The Effect of Dietary Types on Gut Microbiota Composition and Development of Non-Communicable Diseases: A Narrative Review. Nutrients 2024, 16, 3134. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.J.; Seo, L.; Macias, A.; Price, D.K.; Yew, J.Y. Bacterial and fungal components of the gut microbiome have distinct, sex-specific roles in Hawaiian Drosophila reproduction. bioRxiv 2023. [Google Scholar] [CrossRef]
- Guo, W.-L.; Deng, J.-C.; Pan, Y.-Y.; Xu, J.-X.; Hong, J.-L.; Shi, F.-F.; Liu, G.-L.; Qian, M.; Bai, W.-D.; Zhang, W.; et al. Hypoglycemic and hypolipidemic activities of Grifola frondosa polysaccharides and their relationships with the modulation of intestinal microflora in diabetic mice induced by high-fat diet and streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Buttar, J.; Kon, E.; Lee, A.; Kaur, G.; Lunken, G. Effect of diet on the gut mycobiome and potential implications in inflammatory bowel disease. Gut Microbes 2024, 16, 2399360. [Google Scholar] [CrossRef]
- Van Ende, M.; Wijnants, S.; Van Dijck, P. Sugar Sensing and Signaling in Candida albicans and Candida glabrata. Front. Microbiol. 2019, 10, 99. [Google Scholar] [CrossRef]
- Mohammed, L.; Jha, G.; Malasevskaia, I.; Goud, H.K.; Hassan, A. The Interplay Between Sugar and Yeast Infections: Do Diabetics Have a Greater Predisposition to Develop Oral and Vulvovaginal Candidiasis? Cureus 2021, 13, e13407. [Google Scholar] [CrossRef] [PubMed]
- Palmucci, J.R.; Sells, B.E.; Giamberardino, C.D.; Toffaletti, D.L.; Dai, B.; Asfaw, Y.G.; Dubois, L.G.; Li, Z.; Theriot, B.; Schell, W.A.; et al. A ketogenic diet enhances fluconazole efficacy in murine models of systemic fungal infection. mBio 2024, 15, e0064924. [Google Scholar] [CrossRef]
- Jeziorek, M.; Frej-Mądrzak, M.; Choroszy-Król, I. The influence of diet on gastrointestinal Candida spp. colonization and the susceptibility of Candida spp. to antifungal drugs. Rocz. Panstw. Zakl. Hig. 2019, 70, 195–200. [Google Scholar] [CrossRef]
- Jawhara, S. Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection. Microorganisms 2023, 11, 1556. [Google Scholar] [CrossRef] [PubMed]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, V.; Salvo, A.; Pacinella, G.; Tuttolomondo, A. The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4942. [Google Scholar] [CrossRef]
- Santana, I.L.; Gonçalves, L.M.; de Vasconcellos, A.A.; da Silva, W.J.; Cury, J.A.; Del Bel Cury, A.A. Dietary carbohydrates modulate Candida albicans biofilm development on the denture surface. PLoS ONE 2013, 8, e64645. [Google Scholar] [CrossRef]
- Ribeiro, N.C.B.V.; Ramer-Tait, A.E.; Cazarin, C.B.B. Resistant starch: A promising ingredient and health promoter. PharmaNutrition 2022, 21, 100304. [Google Scholar] [CrossRef]
- Mirabelli, M.; Shehab, R. Chapter 19—Nutrition. In Clinical Men’s Health; Heidelbaugh, J.J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2008; pp. 349–385. [Google Scholar] [CrossRef]
- Mandal, S.M.; Mahata, D.; Migliolo, L.; Parekh, A.; Addy, P.S.; Mandal, M.; Basak, A. Glucose directly promotes antifungal resistance in the fungal pathogen, Candida spp. J. Biol. Chem. 2014, 289, 25468–25473. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- Peng, Z.; Tang, J. Intestinal Infection of Candida albicans: Preventing the Formation of Biofilm by C. albicans and Protecting the Intestinal Epithelial Barrier. Front. Microbiol. 2021, 12, 783010. [Google Scholar] [CrossRef] [PubMed]
- Basmaciyan, L.; Bon, F.; Paradis, T.; Lapaquette, P.; Dalle, F. Candida albicans Interactions with The Host: Crossing The Intestinal Epithelial Barrier. Tissue Barriers 2019, 7, 1612661. [Google Scholar] [CrossRef]
- Escalante, J.; Artaiz, O.; Diwakarla, S.; McQuade, R.M. Leaky gut in systemic inflammation: Exploring the link between gastrointestinal disorders and age-related diseases. GeroScience 2024, 47, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Seo, Y.S.; Lee, H.B.; Kim, Y.; Park, H.Y. Dietary Carbohydrate Constituents Related to Gut Dysbiosis and Health. Microorganisms 2020, 8, 427. [Google Scholar] [CrossRef]
- What’s the Difference Between Starchy and Non-Starchy Vegetables? Available online: https://www.healthline.com/nutrition/starchy-vs-non-starchy-vegetables (accessed on 23 February 2025).
- Sugizaki, C.S.A.; Naves, M.M.V. Potential Prebiotic Properties of Nuts and Edible Seeds and Their Relationship to Obesity. Nutrients 2018, 10, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ugural, A.; Akyol, A. Can pseudocereals modulate microbiota by functioning as probiotics or prebiotics? Crit. Rev. Food Sci. Nutr. 2022, 62, 1725–1739. [Google Scholar] [CrossRef]
- Sonia, S.; Witjaksono, F.; Ridwan, R. Effect of cooling of cooked white rice on resistant starch content and glycemic response. Asia Pac. J. Clin. Nutr. 2015, 24, 620–625. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Argüelles, J.C.; Sánchez-Fresneda, R.; Argüelles, A.; Solano, F. Natural Substances as Valuable Alternative for Improving Conventional Antifungal Chemotherapy: Lights and Shadows. J. Fungi 2024, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Han, L.; Lu, R.Y.; Wang, Y. Antifungal and Immunomodulatory Ingredients from Traditional Chinese Medicine. Antibiotics 2022, 12, 48. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Aitzhanova, A.; Oleinikova, Y.; Mounier, J.; Hymery, N.; Leyva Salas, M.; Amangeldi, A.; Saubenova, M.; Alimzhanova, M.; Ashimuly, K.; Sadanov, A. Dairy associations for the targeted control of opportunistic Candida. World J. Microbiol. Biotechnol. 2021, 37, 143. [Google Scholar] [CrossRef]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the Gut: A Multiperspective Review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, H.; Hildebrand, F.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Amend, A.S.; Seifert, K.A.; Samson, R.; Bruns, T.D. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 13748–13753. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.H.; Larsson, K.H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Usyk, M.; Zolnik, C.P.; Patel, H.; Levi, M.H.; Burk, R.D. Novel ITS1 Fungal Primers for Characterization of the Mycobiome. mSphere 2017, 2, e00488-17. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Ghedin, E. The human mycobiome in health and disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef]
- Vaz, A.B.; Fonseca, P.L.; Leite, L.R.; Badotti, F.; Salim, A.C.; Araujo, F.M.; Cuadros-Orellana, S.; Duarte Ângelo, A.; Rosa, C.A.; Oliveira, G.; et al. USING Next-Generation Sequencing (NGS) TO UNCOVER DIVERSITY OF WOOD-DECAYING FUNGI IN NEOTROPICAL ATLANTIC FORESTS. Phytotaxa 2017, 295, 1–21. [Google Scholar] [CrossRef]
- Halwachs, B.; Madhusudhan, N.; Krause, R.; Nilsson, R.H.; Moissl-Eichinger, C.; Högenauer, C.; Thallinger, G.G.; Gorkiewicz, G. Critical Issues in Mycobiota Analysis. Front. Microbiol. 2017, 8, 180. [Google Scholar] [CrossRef]
- Gou, W.; Wang, H.; Su, C.; Fu, Y.; Wang, X.; Gao, C.; Shuai, M.; Miao, Z.; Zhang, J.; Jia, X.; et al. The temporal dynamics of the gut mycobiome and its association with cardiometabolic health in a nationwide cohort of 12,641 Chinese adults. Cell Rep. Med. 2024, 5, 101775. [Google Scholar] [CrossRef]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef]
- Borrel, G.; Brugère, J.F.; Gribaldo, S.; Schmitz, R.A.; Moissl-Eichinger, C. The host-associated archaeome. Nat. Rev. Microbiol. 2020, 18, 622–636. [Google Scholar] [CrossRef]
- Zuo, T.; Sun, Y.; Wan, Y.; Yeoh, Y.K.; Zhang, F.; Cheung, C.P.; Chen, N.; Luo, J.; Wang, W.; Sung, J.J.Y.; et al. Human-Gut-DNA Virome Variations across Geography, Ethnicity, and Urbanization. Cell Host Microbe 2020, 28, 741–751.e4. [Google Scholar] [CrossRef]
- Enaud, R.; Vandenborght, L.E.; Coron, N.; Bazin, T.; Prevel, R.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Lamireau, T.; Delhaes, L. The Mycobiome: A Neglected Component in the Microbiota-Gut-Brain Axis. Microorganisms 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Scarpa, M.; Marchiori, C.; Sarasin, G.; Caputi, V.; Porzionato, A.; Giron, M.C.; Palù, G.; Castagliuolo, I. Saccharomyces boulardii CNCM I-745 supplementation reduces gastrointestinal dysfunction in an animal model of IBS. PLoS ONE 2017, 12, e0181863. [Google Scholar] [CrossRef]
- Takata, K.; Tomita, T.; Okuno, T.; Kinoshita, M.; Koda, T.; Honorat, J.A.; Takei, M.; Hagihara, K.; Sugimoto, T.; Mochizuki, H.; et al. Dietary Yeasts Reduce Inflammation in Central Nerve System via Microflora. Ann. Clin. Transl. Neurol. 2015, 2, 56–66. [Google Scholar] [CrossRef] [PubMed]


| Study | Fungi | Disease | Effect | Ref. |
|---|---|---|---|---|
| Pfaller et al. | C. albicans | Invasive fungal infection | Exacerbates | [46] |
| Li et al. | C. albicans | Antibiotic-associated diarrhea | Exacerbates | [47] |
| Charlet et al. | C. glabrata | Colitis | Exacerbates | [51] |
| Martino et al. | C. tropicalis | Colitis | Exacerbates | [52] |
| Hoarau et al. | C. tropicalis | CD | Exacerbates | [53] |
| Sun et al. | C. parapsilosis | Diet-related obesity | Exacerbates | [54] |
| Sokol et al. | C. albicans | CD and UC | Exacerbates | [48] |
| Chester et al. | Clavispora lusitaniae (formerly Candida lusitaniae) | Digestive and urinary infections Amphotericin B resistance | Induces | [55] |
| Noor-UI et al. | Geotrichum candidum | Probiotic that enhances feed utilization, improves immunity, and reduces disease resistance | [56] | |
| Abid et al. | Saccharomyces | Traveler’s diarrhea and CDI | Protects | [58] |
| Chen et al. | Saccharomyces | Colon cancer | Antineoplastic effects | [60] |
| Spatz et al. | Malassezia | Dermatitis and pityriasis | Exacerbates | [13] |
| Limon et al. | Malassezia | Inflammatory bowel disease | Exacerbates | [15] |
| Sokol et al. | Malassezia | Inflammatory bowel disease | Exacerbates | [48] |
| Yang et al. | Malassezia | CRC | Unclear—leads to tumorigenesis | [68] |
| Norlia et al. | Aspergillus | Hepatocellular carcinoma | Hepatotoxic effect | [71] |
| Malir et al. | Penicillium | Urothelial carcinoma | Nephrotoxic effect | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, B.S.; Roşu, O.A.; Enache, R.-M.; Manciulea, M.; Pavelescu, L.A.; Creţoiu, S.M. Gut Mycobiome: Latest Findings and Current Knowledge Regarding Its Significance in Human Health and Disease. J. Fungi 2025, 11, 333. https://doi.org/10.3390/jof11050333
Gaspar BS, Roşu OA, Enache R-M, Manciulea M, Pavelescu LA, Creţoiu SM. Gut Mycobiome: Latest Findings and Current Knowledge Regarding Its Significance in Human Health and Disease. Journal of Fungi. 2025; 11(5):333. https://doi.org/10.3390/jof11050333
Chicago/Turabian StyleGaspar, Bogdan Severus, Oana Alexandra Roşu, Robert-Mihai Enache, Monica Manciulea (Profir), Luciana Alexandra Pavelescu, and Sanda Maria Creţoiu. 2025. "Gut Mycobiome: Latest Findings and Current Knowledge Regarding Its Significance in Human Health and Disease" Journal of Fungi 11, no. 5: 333. https://doi.org/10.3390/jof11050333
APA StyleGaspar, B. S., Roşu, O. A., Enache, R.-M., Manciulea, M., Pavelescu, L. A., & Creţoiu, S. M. (2025). Gut Mycobiome: Latest Findings and Current Knowledge Regarding Its Significance in Human Health and Disease. Journal of Fungi, 11(5), 333. https://doi.org/10.3390/jof11050333







