Plant–Soil Interactions Shape Arbuscular Mycorrhizal Fungal Diversity and Functionality in Eastern Tibetan Meadows
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Field Investigation and Soil Sampling
2.3. Measurement of Plant Traits and Calculation of Functional Diversity
2.4. Measurement of Soil Properties
2.5. Measurement of AM Fungal Biomass
2.6. Soil DNA Extraction, PCR, and MiSeq Sequencing
2.7. Bioinformatics
2.8. Statistical Analyses
3. Results
3.1. Responses of Soil Properties Across the Three Alpine Meadow Sites
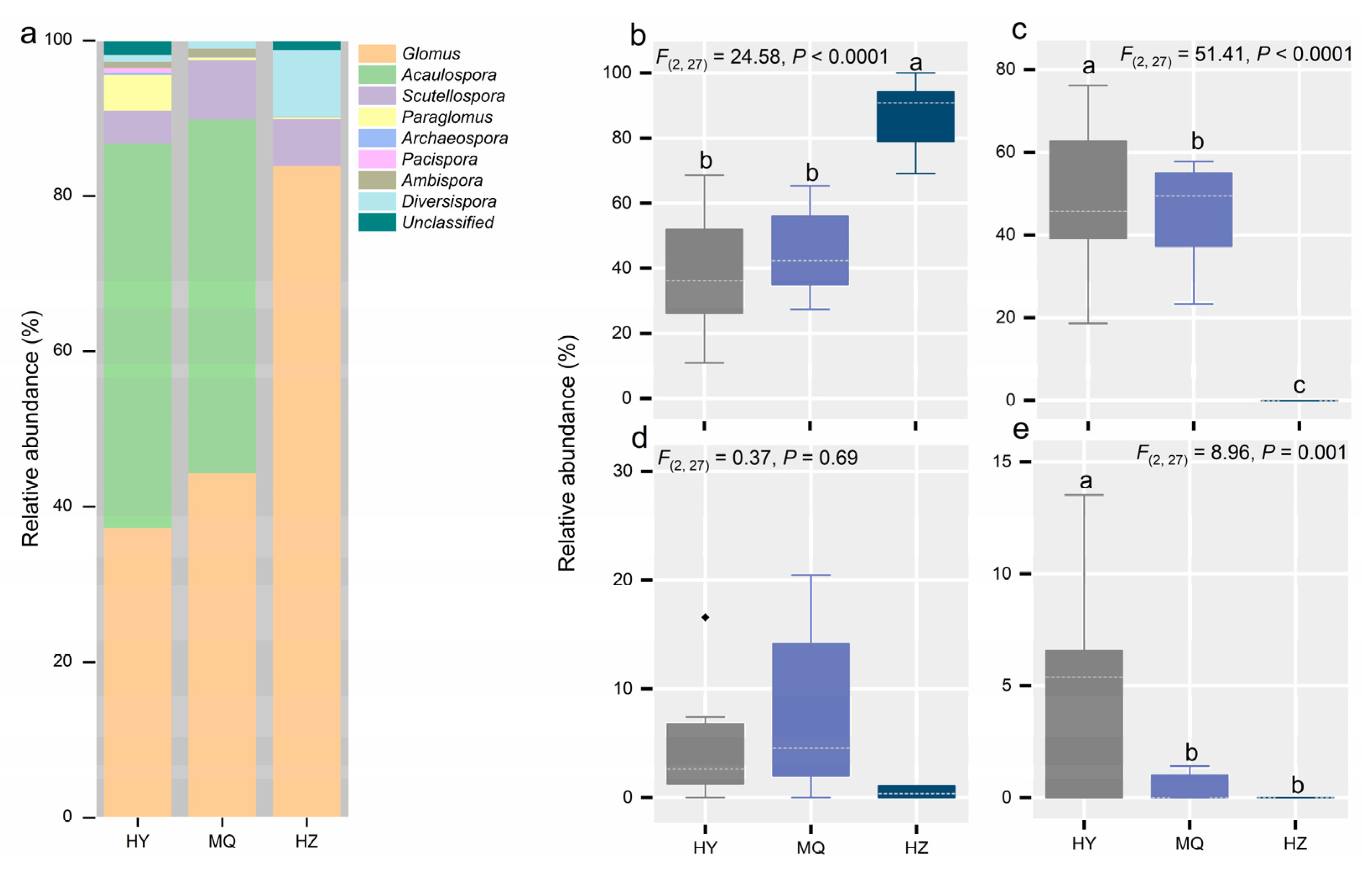
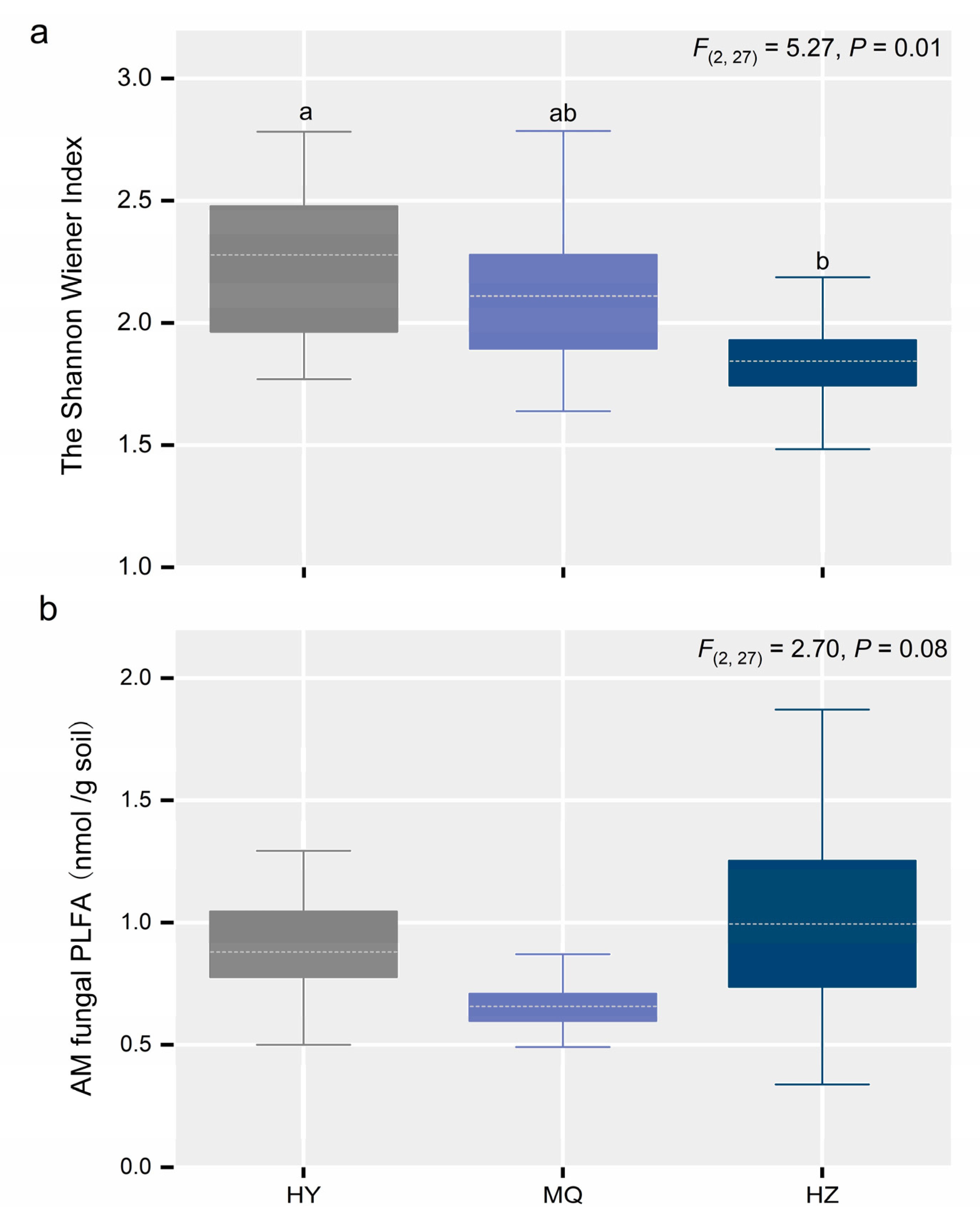
3.2. Responses of the AM Fungal Community Across the Three Sites
3.3. Plant Community Functional Diversity and Community-Weighed Trait Means
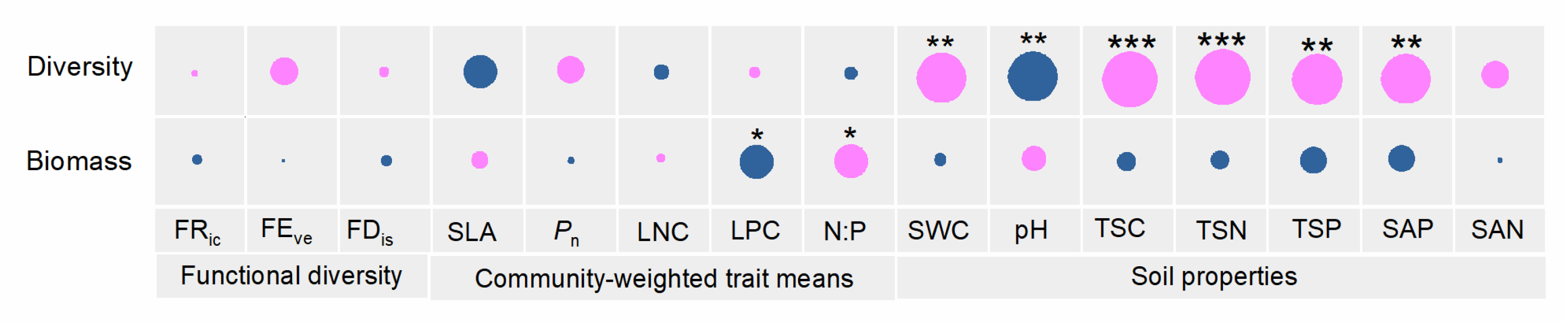
3.4. The AM Fungal Community in Relation to Plant Community Functional Diversity, Community-Weighed Trait Means, and Soil Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanasaki, N.; Fujimori, S.; Yamamoto, T.; Yoshikawa, S.; Masaki, Y.; Kainuma, M.; Kanamori, Y.; Masui, T.; Takahashi, K.; Kanae, S. A global water scarcity assessment under Shared Socio-economic Pathways 49—Part 2: Water availability and scarcity. Hydrol. Earth Syst. Sci. 2013, 17, 2375–2391. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Chen, X. Projection and uncertainty analysis of global precipitation-related 30 extremes using CMIP5 models. Int. J. Climatol. 2014, 34, 2730–2748. [Google Scholar] [CrossRef]
- Westra, S.L.V.; Alexander, L.V.; Zwiers, F.W. Global increasing trends in annual maximum daily precipitation. J. Clim. 2013, 26, 3904–3918. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, X.; Zwiers, F.; Westra, S.; Alexander, L. A global, continental and regional analysis of changes in extreme precipitation. J. Clim. 2021, 34, 243–258. [Google Scholar] [CrossRef]
- Knapp, A.K.; Hoover, D.L.; Wilcox, K.R.; Avolio, M.L.; Koerner, S.E.; La Pierre, K.J.; Loik, M.E.; Luo, Y.; Sala, O.E.; Smith, M.D. Characterizing differences in precipitation regimes of extreme wet and dry years: Implications for climate change experiments. Glob. Change Biol. 2015, 21, 2624–2633. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, Y.; Crowther, T.W.; Sun, W.; Chen, S.; Zhou, D.; Shangguan, Z.; Huang, J.; He, J.-S.; Wang, Y.; et al. Mycorrhiza increases plant diversity and soil carbon storage in grasslands. Proc. Natl. Acad. Sci. USA 2025, 122, e2412556122. [Google Scholar] [CrossRef]
- Yang, H.; Wu, M.; Liu, W.; Zhang, Z.; Zhang, N.; Wan, S. Community structure and composition in response to climate change in a temperate steppe. Global Change Biol. 2011, 17, 452–465. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Lin, L.; Wang, Y.; Zhang, Z.; Zhang, F.; Wang, T.; Liu, L.; Zhu, B.; Cao, G.; et al. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. USA 2018, 115, 4051–4056. [Google Scholar] [CrossRef]
- Griffin-Nolan, R.J.; Blumenthal, D.M.; Collins, S.L.; Farkas, T.E.; Hoffman, A.M.; Mueller, K.E.; Ocheltree, T.W.; Smith, M.D.; Whitney, K.D.; Knapp, A.K. Shifts in plant functional composition following long-term drought in grasslands. J. Ecol. 2019, 107, 2133–2148. [Google Scholar] [CrossRef]
- Luo, W.; Griffin-Nolan, R.J.; Ma, W.; Liu, B.; Zuo, X.; Xu, C.; Yu, Q.; Luo, Y.; Mariotte, P.; Smith, M.D.; et al. Plant traits and soil fertility mediate productivity losses under extreme drought in C3 grasslands. Ecology 2021, 102, e03465. [Google Scholar] [CrossRef]
- Zuo, X.; Zhao, S.; Cheng, S.; Hu, Y.; Wang, S.; Yue, P.; Liu, R.; Knapp, A.K.; Smith, M.D.; Yu, Q.; et al. Functional diversity response to geographic and experimental precipitation gradients varies with plant community type. Funct. Ecol. 2021, 35, 2119–2132. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S.; Roumet, C. Complementarity as a mechanism of coexistence between functional groups of grasses. J. Ecol. 2007, 95, 1296–1305. [Google Scholar] [CrossRef]
- Valencia, E.; Maestre, F.T.; Le Bagousse-Pinguet, Y.; Quero, J.L.; Tamme, R.; Borger, L.; García-Gómez, M.; Gross, N. Functional diversity enhances the resistance of ecosystem muntifunctionality to acridity in Mediterranean drylands. New Phytol. 2015, 206, 660–671. [Google Scholar] [CrossRef]
- Gross, N.; Robson, T.M.; Lavorel, S.; Albert, C.; Le Bagousse-Pinguet, Y.; Guillemin, R. Plant response traits mediate the effects of subalpine grasslands on soil moisture. New Phytol. 2008, 180, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Faucon, M.P.; Houben, D.; Lambers, H. Plant functional traits: Soil and ecosystem services. Trends Plant Sci. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Soudzilovskaia, N.A.; van Bodegom, P.M.; Terrer, C.; van’t Zelfde, M.; McCallum, I.; Luke McCormack, M.; Fisher, J.B.; Brundrett, M.C.; de Sá, N.C.; Tedersoo, L. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat Commun. 2019, 10, 5077. [Google Scholar] [CrossRef]
- Hawkins, H.J.; Cargill, R.I.M.; Van Nuland, M.E.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal mycelium as a global carbon pool. Curr. Biol. 2023, 33, R560–R573. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Andrew Smith, F. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Delavaux, C.; Smtth-Ramesh, L.M.; Kuebbng, S.E. Beyond nutrients: A meta-analysis of the diverse effects of arbuscular mycorrhizal fungi on plants and soils. Ecology 2017, 98, 2111–2119. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, M.; Ainsaar, L.; Bâ, A.; Diedhiou, A.G.; Hiiesalu, I.; Jairus, T.; Johnson, N.C.; et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef]
- Wu, S.; Fu, W.; Rillig, M.C.; Chen, B.; Zhu, Y.; Huang, L. Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi—An updated conceptual framework. New Phytol. 2024, 242, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Schweigert, M.; Herrmann, S.; Miltner, A.; Fester, T.; Kästner, M. Fate of ectomycorrhizal fungal biomass in a soil bioreactor system and its contribution to soil organic matter formation. Soil Biol. Biochem. 2015, 88, 120–127. [Google Scholar] [CrossRef]
- Gavito, M.E.; Jakobsen, I.; Mikkelsen, T.N.; Mora, F. Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol. 2019, 223, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Shachar-Hill, Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 2005, 165, 899–912. [Google Scholar] [CrossRef]
- Kakouridis, A.; Hagen, J.A.; Kan, M.P.; Feldman, L.J.; Herman, D.J.; Weber, P.K.; Pett-Ridge, J.; Firestone, M.K. Routes to roots: Direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 2022, 236, 210–221. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Chen, Y.; Xu, Z.; Xu, T.; Veresoglou, S.; Yang, G.; Chen, B. Nitrogen deposition and precipitation induced phylogenetic clustering of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2017, 115, 233–242. [Google Scholar] [CrossRef]
- Li, X.; Zhu, T.; Peng, F.; Chen, Q.; Lin, S.; Christie, P.; Zhang, J. Inner mongolian steppe arbuscular mycorrhizal fungal communities respond more strongly to water availability than to nitrogen fertilization. Environ. Microbiol. 2015, 17, 3051–3068. [Google Scholar] [CrossRef]
- House, G.L.; Bever, J.D. Disturbance reduces the differentiation of mycorrhizal fungal communities in grasslands along a precipitation gradient. Ecol. App. 2018, 28, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Deveautour, C.; Donn, S.; Power, S.A.; Bennett, A.E.; Powell, J.R. Experimentally altered precipitation regimes and host root traits affect grassland arbuscular mycorrhizal fungal communities. Mol. Ecol. 2018, 27, 2152–2163. [Google Scholar] [CrossRef]
- Deveautour, C.; Power, S.; Barnett, K.; Ochoa-Hueso, R.; Powell, J. Temporal dynamics of mycorrhizal fungal communities and co-associations with grassland plant communities following experimental manipulation of precipitation. J. Ecol. 2019, 108, 515–527. [Google Scholar] [CrossRef]
- Staddon, P.L.; Thompso, K.; Jakobsen, I.; Grime, J.P.; Askew, A.P.; Fitter, A.H. Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob. Change Biol. 2003, 9, 186–194. [Google Scholar] [CrossRef]
- Johnson, N.C.; Rowland, D.L.; Corkidi, L.; Egerton-Warburton, L.M.; Allen, E.B. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 2003, 84, 1895–1908. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol. Lett. 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Q.; Peng, C.; Wu, L.; Wang, Y.; Fang, X.; Wu, J. The impacts of climate change and human activities on biogeochemical cycles on the Qinghai-Tibetan Plateau. Glob. Change Biol. 2013, 19, 2940–2955. [Google Scholar] [CrossRef]
- Ding, D.; Yang, T.; Zhao, Y.; Liu, D.; Wang, X.; Yao, Y.; Piao, S. Increasingly important role of atmospheric aridity on Tibetan alpine grasslands. Geophys. Res. Lett. 2018, 45, 2852–2859. [Google Scholar] [CrossRef]
- He, J.; Dong, S.; Shang, Z.; Sundqvist, M.K.; Wu, G.; Yang, Y. Above-belowground interactions in alpine ecosystems on the roof of the world. Plant Soil 2021, 458, 1–6. [Google Scholar] [CrossRef]
- Gai, J.; Feng, G.; Cai, X.; Christie, P.; Li, L. A preliminary survey of the arbuscular mycorrhizal status of grassland plants in southern Tibet. Mycorrhiza 2006, 16, 191–196. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Collins, C.D.; Foster, B.L. Community-level consequences of mycorrhizae depend on phosphorus availability. Ecology 2009, 90, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, R.G.; Marcos, F.C.C.; Ribeiro, R.V.; de Andrade, S.A.L. Mycorrhizae enhance nitrogen fixation and photosynthesis in phosphorus starved soybean (L. Merrill). Environ. Exp. Bot. 2017, 140, 26–33. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Xu, H. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Liu, Y.; Reich, P.B.; Li, G.; Sun, S. Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology 2011, 92, 1201–1207. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.; Sun, D.; Wang, X.; Smith, J.; Du, G. Impacts of altitude and position on the rates of soil nitrogen mineralization and nitrification in alpine meadows on the eastern Qinghai-Tibetan Plateau, China. Biol. Fert. Soils 2012, 48, 393–400. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Xiao, R.; Zhang, H.; Ma, J.; Du, Z.; Li, G.; Jiang, L. Losses of low-germinating, slow-growing species prevent grassland composition recovery from nutrient amendment. Global Change Biol. 2024, 30, e17264. [Google Scholar] [CrossRef]
- An, H.; Zhao, Y.; Ma, M. Precipitation controls seed bank size and its role in alpine meadow community regeneration with increasing altitude. Glob. Change Biol. 2020, 26, 5767–5777. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, X.; Zhang, L.; Song, Z.; Zhou, S. The allometry of plant height explains species loss under nitrogen addition. Ecol. Lett. 2021, 24, 553–562. [Google Scholar] [CrossRef]
- Cheng, Y.; Rutten, G.; Liu, X.; Ma, M.; Song, Z.; Maaroufi, N.I.; Zhou, S. Host plant height explains the effect of nitrogen enrichment on arbuscular mycorrhizal fungal communities. New Phytol. 2023, 240, 399–411. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Song, F.; Zhou, S.; Cadotte, M.W.; Bradshaw, C.J. Explaining maximum variation in productivity requires phylogenetic diversity and single functional traits. Ecology 2015, 96, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.; Choler, P.; Zhao, B.; Du, G. The allometry of reproductive biomass in response to land use in Tibetan alpine grasslands. Funct. Ecol. 2009, 23, 274–283. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Mouillot, D.; Mason, W.H.N.; Dumay, O.; Wilson, J.B. Functional regularity: A neglected aspect of functional diversity. Oecologia 2005, 142, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Cortez, J.; Billes, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegard, A. Changes in soil fungal: Bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef]
- Olsson, P.A.; Baath, E.; Jakobsen, I.; Soderstrom, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, P.; Li, J.; Ren, L.; Bai, W.; Tian, Q.; Sun, W.; Zhang, W. Arbuscular mycorrhizal fungal communities associated with two dominant species differ in their responses to long-term nitrogen addition in temperate grasslands. Funct. Ecol. 2018, 32, 1575–1588. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Öpik, M.; Metsis, M.; Daniell, T.J.; Zobel, M.; Moora, M. Large-scale parallel 454 sequencing reveals ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol. 2009, 184, 424–437. [Google Scholar] [CrossRef]
- Öpik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, M.J.; Reier, Ü.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 18, 223–241. [Google Scholar] [CrossRef]
- Harman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar]
- Laliberte, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.47.1. 2024. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 April 2024.).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Zhang, J.; Wang, F.; Che, R.; Wang, P.; Liu, H.; Ji, B.; Cui, X. Precipitation shapes communities of arbuscular mycorrhizal fungi in Tibetan alpine steppe. Sci. Rep. 2016, 6, 23488. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Zhang, Y.; Liu, Y.; Zhang, S. Soil properties and plant community-level traits mediate arbuscular mycorrhizal fungal response to nitrogen enrichment and altered precipitation. Appl. Soil Ecol. 2022, 169, 104246. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, G.; Mao, L.; Cheng, G.; Jiang, S.; Ma, X.; An, L.; Du, G.; Johnson, N.C.; Feng, H. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol. 2012, 194, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K.; Allen, M.F. Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: A model and field test. New Phytol. 2002, 155, 507–515. [Google Scholar] [CrossRef]
- Egerton-Warburton, L.M.; Johnson, N.C.; Allen, E.B. Mycorrhizal community dynamics following nitrogen fertilization: A cross-site test in five grasslands. Ecol. Monogr. 2007, 77, 527–544. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Herrera, J.; Natvig, D.O.; Sinsabaugh, R.L. Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 2007, 296, 65–75. [Google Scholar] [CrossRef]
- Johnson, N.C.; Rowland, D.L.; Corkidi, L.; Allen, E.B. Plant winners and losers during grassland N-eutrophication differ in biomass allocation and mycorrhizas. Ecology 2008, 89, 2868–2878. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 135, 575–585. [Google Scholar] [CrossRef]
- Grman, E.; Robinson, T.M.P. Resource availability and imbalance affect plant-mycorrhizal interactions: A field test of three hypotheses. Ecology 2013, 94, 62–71. [Google Scholar] [CrossRef]
- Hetrick, B.A.D.; Wilson, G.W.T.; Todd, T.C. Relationships of mycorrhizal symbiosis, rooting strategy, and phenology. Can. J. Bot. 1992, 70, 1521–1528. [Google Scholar] [CrossRef]
- Tian, Q.; Lu, P.; Ma, P.; Zhou, H.; Yang, M.; Zhai, X.; Chen, M.; Wang, H.; Li, W.; Bai, W.; et al. Processes at the soil-root interface determine the different responses of nutrient limitation and metal toxicity in forbs and grasses to nitrogen enrichment. J. Ecol. 2021, 109, 927–938. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, L.; Xu, L.; Lu, Z.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N.A. trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Douds, D.D.; Schenck, N.C. Relationship of colonization and sporulation by VA mycorrhizal fungi to plant nutrient and carbohydrate contents. New Phytol. 1990, 116, 621–627. [Google Scholar] [CrossRef]
- Fuchslueger, L.; Bahn, M.; Fritz, K.; Hasibeder, R.; Richter, A. Experimental drought reduces the transfer of recently fixed plant carbon to soil microbes and alters the bacterial community composition in a mountain meadow. New Phytol. 2014, 201, 916–927. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, E.B.; Egerton-Warburton, L.M.; Hart, M.M.; Klironomos, J.N.; Maherali, H.; Tedersoo, L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. J. Ecol. 2018, 106, 480–489. [Google Scholar] [CrossRef]
- Dueñas, J.F.; Camenzind, T.; Roy, J.; Hempel, S.; Homeier, J.; Suárez, J.P.; Rillig, M.C. Moderate phosphorus additions consistently affect community composition of arbuscular mycorrhizal fungi in tropical montane forests in southern Ecuador. New Phytol. 2020, 227, 1505–1518. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Bradley, R.L. Evidence that soil nutrient stoichiometry controls the competitive abilities of arbuscular mycorrhizal vs. root-borne non-mycorrhizal fungi. Fungal Ecol. 2013, 6, 557–560. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef]
- Lambers, H.; Bishop, J.G.; Hopper, S.D.; Laliberte, E.; Zuniga-Feest, A. Phosphorus-mobilization ecosystem engineering: The roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann. Bot. 2012, 110, 959–968. [Google Scholar] [CrossRef]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Buecking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, B.; Rillig, M.C.; Jansa, J.; Ma, W.; Xu, C.; Luo, W.; Wu, H.; Hao, Z.; Wu, H.; et al. Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. New Phytol. 2022, 234, 2003–2017. [Google Scholar] [CrossRef] [PubMed]



| Soil Properties | Sites | ||
|---|---|---|---|
| HY | MQ | HZ | |
| Soil water content (%) | 55.47 ± 1.70 a | 35.65 ± 0.99 b | 28.57 ± 0.48 c |
| Soil pH | 5.97 ± 0.10 b | 6.34 ± 0.13 b | 6.93 ± 0.09 a |
| Total soil carbon (g Kg−1) | 64.06 ± 1.54 a | 53.14 ± 2.76 b | 35.78 ± 0.77 c |
| Total soil nitrogen (g Kg−1) | 5.74 ± 0.13 a | 4.96 ± 0.26 b | 3.47 ± 0.07 c |
| Total soil phosphorus (g Kg−1) | 0.84 ± 0.08 a | 0.78 ± 0.07 ab | 0.56 ± 0.05 b |
| Soil available phosphorus (mg Kg−1) | 3.6 ± 0.36 a | 3.33 ± 0.27 ab | 2.42 ± 0.20 b |
| Soil available nitrogen (mg Kg−1) | 4.59 ± 0.37 a | 4.05 ± 0.39 ab | 3.22 ± 0.35 b |
| Model | Selected Variables | df | R2 | ALCc | ΔAICc | Weight (wi) |
|---|---|---|---|---|---|---|
| Diversity | Soil available P, Soil total N | 4 | 0.47 | −106.4 | 0.00 | 0.08 |
| Biomass | Leaf P concentration | 3 | 0.15 | −66.6 | 0.00 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yang, Z.; Yang, X.; Ma, X.; Ma, Q.; Ma, M.; Zhang, J. Plant–Soil Interactions Shape Arbuscular Mycorrhizal Fungal Diversity and Functionality in Eastern Tibetan Meadows. J. Fungi 2025, 11, 337. https://doi.org/10.3390/jof11050337
Zhang S, Yang Z, Yang X, Ma X, Ma Q, Ma M, Zhang J. Plant–Soil Interactions Shape Arbuscular Mycorrhizal Fungal Diversity and Functionality in Eastern Tibetan Meadows. Journal of Fungi. 2025; 11(5):337. https://doi.org/10.3390/jof11050337
Chicago/Turabian StyleZhang, Shihu, Zhengying Yang, Xuechun Yang, Xiaoyu Ma, Qun Ma, Miaojun Ma, and Jiajia Zhang. 2025. "Plant–Soil Interactions Shape Arbuscular Mycorrhizal Fungal Diversity and Functionality in Eastern Tibetan Meadows" Journal of Fungi 11, no. 5: 337. https://doi.org/10.3390/jof11050337
APA StyleZhang, S., Yang, Z., Yang, X., Ma, X., Ma, Q., Ma, M., & Zhang, J. (2025). Plant–Soil Interactions Shape Arbuscular Mycorrhizal Fungal Diversity and Functionality in Eastern Tibetan Meadows. Journal of Fungi, 11(5), 337. https://doi.org/10.3390/jof11050337






