Conserved and Divergent Functions of the cAMP/PKA Signaling Pathway in Candida albicans and Candida tropicalis
Abstract
:1. Introduction
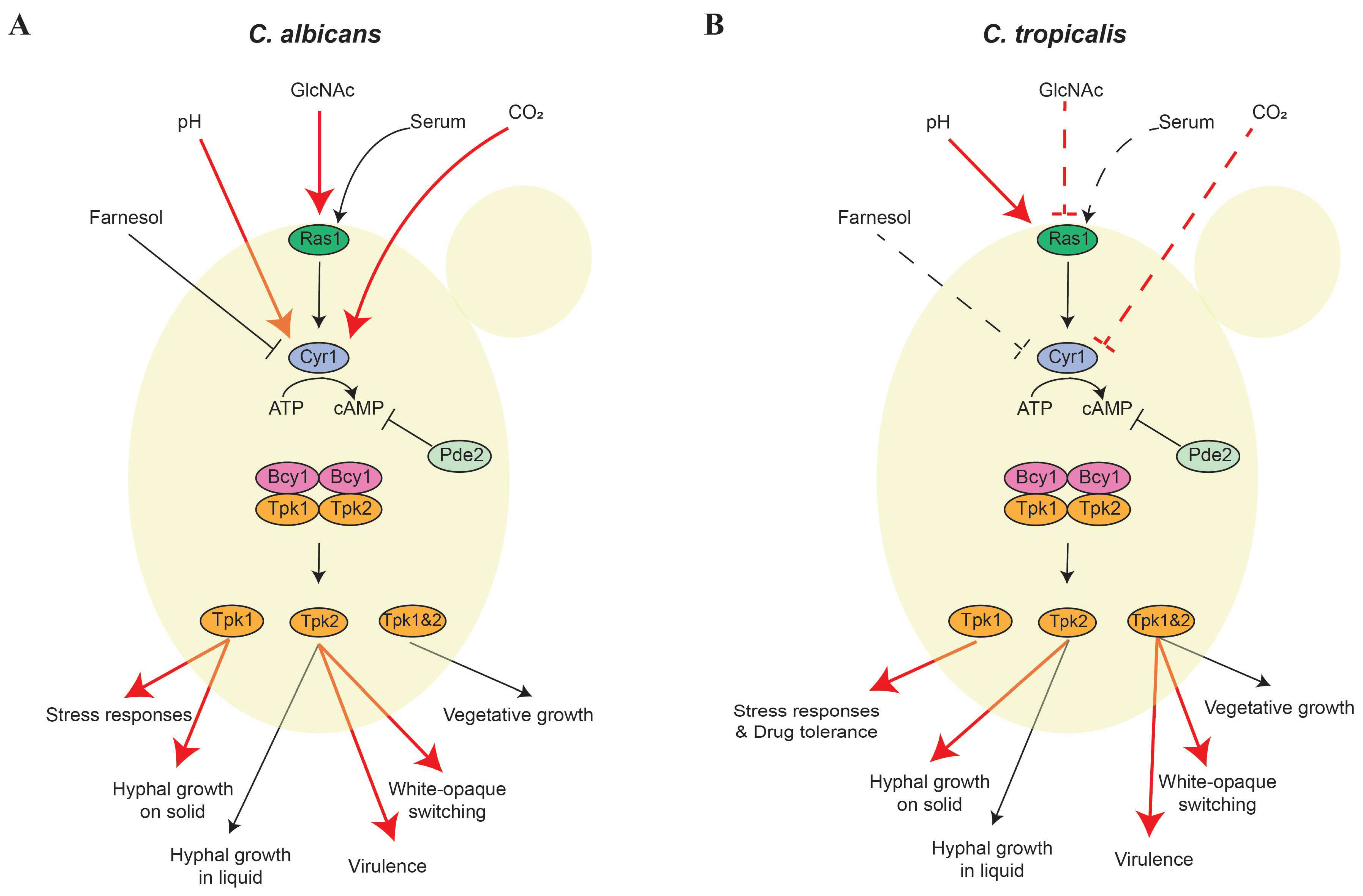
2. cAMP/PKA Signaling Pathway
2.1. cAMP/PKA Regulation of the Yeast-Hypha Transition and Virulence
2.2. Regulation of White-Opaque Switching through the cAMP/PKA Pathway
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv113. [Google Scholar] [CrossRef] [PubMed]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Diekema, D. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Colombo, A.L. Candidemia due to Candida tropicalis: Clinical, epidemiologic, and microbiologic characteristics of 188 episodes occurring in tertiary care hospitals. Diagn. Microbiol. Infect. Dis. 2007, 58, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, P.R.; Graybill, J.R.; Playford, E.G.; Watcharananan, S.P.; Oh, M.D.; Ja’alam, K.; Huang, S.; Nangia, V.; Kurup, A.; Padiglione, A.A. Consensus statement on the management of invasive candidiasis in Intensive Care Units in the Asia-Pacific Region. Int. J. Antimicrob. Agents 2009, 34, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Chakrabarti, A.; Li, R.Y.; Patel, A.K.; Watcharananan, S.P.; Liu, Z.; Chindamporn, A.; Tan, A.L.; Sun, P.L.; Wu, U.I.; et al. Incidence and species distribution of candidaemia in Asia: A laboratory-based surveillance study. Clin. Microbiol. Infect. 2015, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Guimaraes, T.; Silva, L.R.; de Almeida Monfardini, L.P.; Cunha, A.K.; Rady, P.; Alves, T.; Rosas, R.C. Prospective observational study of candidemia in Sao Paulo, Brazil: Incidence rate, epidemiology, and predictors of mortality. Infect. Control Hosp. Epidemiol. 2007, 28, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Zuza-Alves, D.L.; de Medeiros, S.S.; de Souza, L.B.; Silva-Rocha, W.P.; Francisco, E.C.; de Araujo, M.C.; Lima-Neto, R.G.; Neves, R.P.; Melo, A.S.; Chaves, G.M. Evaluation of virulence factors in vitro, resistance to osmotic stress and antifungal susceptibility of Candida tropicalis isolated from the coastal environment of Northeast Brazil. Front. Microbiol. 2016, 7, 1783. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Lin, C.C.; Chang, T.P.; Lauderdale, T.L.; Chen, H.T.; Lee, C.F.; Hsieh, C.W.; Chen, P.C.; Lo, H.J. Comparison of human and soil Candida tropicalis isolates with reduced susceptibility to fluconazole. PLoS ONE 2012, 7, e34609. [Google Scholar] [CrossRef] [PubMed]
- De Barros, P.P.; Rossoni, R.D.; Freire, F.; Ribeiro, F.C.; Lopes, L.; Junqueira, J.C.; Jorge, A.O.C. Candida tropicalis affects the virulence profile of Candida albicans: An in vitro and in vivo study. Pathog. Dis. 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.; van de Veerdonk, F.L.; Brown, A.J.; Netea, M.G. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Van Dijck, P.; Datta, A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007, 71, 348–376. [Google Scholar] [CrossRef] [PubMed]
- Huang, G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 2012, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; Gianetti, B.A.; Witchley, J.N. Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat. Rev. Microbiol. 2017, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, B.; Buffo, J.; Soll, D.R. High-frequency switching of colony morphology in Candida albicans. Science 1985, 230, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Daniels, K.J.; Srikantha, T.; Lockhart, S.R.; Pujol, C.; Soll, D.R. Opaque cells signal white cells to form biofilms in Candida albicans. EMBO J. 2006, 25, 2240–2252. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.J.; Kohler, J.R.; DiDomenico, B.; Loebenberg, D.; Cacciapuoti, A.; Fink, G.R. Nonfilamentous C. albicans mutants are avirulent. Cell 1997, 90, 939–949. [Google Scholar] [CrossRef]
- Si, H.; Hernday, A.D.; Hirakawa, M.P.; Johnson, A.D.; Bennett, R.J. Candida albicans white and opaque cells undergo distinct programs of filamentous growth. PLoS Pathog. 2013, 9, e1003210. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.; Daniels, K.J.; Soll, D.R. Comparison of Switching and Biofilm Formation between MTL-Homozygous Strains of Candida albicans and Candida dubliniensis. Eukaryot. Cell 2015, 14, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.M.; Leng, P.; Straffon, M.; Wishart, J.; Macaskill, S.; MacCallum, D.; Schnell, N.; Talibi, D.; Marechal, D.; Tekaia, F.; et al. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2001, 20, 4742–4752. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.R.; Johnson, A.D. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 1997, 277, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Whiteway, M.; Bachewich, C. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 2007, 61, 529–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Summers, E.; Guo, B.; Fink, G. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 1999, 181, 6339–6346. [Google Scholar] [PubMed]
- Rocha, C.R.; Schroppel, K.; Harcus, D.; Marcil, A.; Dignard, D.; Taylor, B.N.; Thomas, D.Y.; Whiteway, M.; Leberer, E. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 2001, 12, 3631–3643. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Lane, S.; Raniga, P.P.; Lu, Y.; Zhou, Z.; Ramon, K.; Chen, J.; Liu, H. The Flo8 transcription factor is essential for hyphal development and virulence in Candida albicans. Mol. Biol. Cell 2006, 17, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; Uppuluri, P.; Zaas, A.K.; Collins, C.; Senn, H.; Perfect, J.R.; Heitman, J.; Cowen, L.E. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr. Biol. 2009, 19, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Liang, W.J.; Chaloupka, J.; Ruoff, C.; Schroppel, K.; Naglik, J.R.; Eckert, S.E.; Mogensen, E.G.; Haynes, K.; Tuite, M.F.; et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 2005, 15, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; Sullivan, P.A.; Shepherd, M.G. N-acetyl-d-glucosamine-induced morphogenesis in Candida albicans. Microbiologica 1985, 8, 85–99. [Google Scholar] [PubMed]
- Hollomon, J.M.; Grahl, N.; Willger, S.D.; Koeppen, K.; Hogan, D.A. Global Role of Cyclic AMP Signaling in pH-Dependent Responses in Candida albicans. mSphere 2016, 1, e00283-16. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Ischer, F.; Marchetti, O.; Entenza, J.; Bille, J. Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003, 48, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.R.; Fink, G.R. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in Hyphal development. Proc. Natl. Acad. Sci. USA 1996, 93, 13223–13228. [Google Scholar] [CrossRef] [PubMed]
- Leberer, E.; Harcus, D.; Broadbent, I.D.; Clark, K.L.; Dignard, D.; Ziegelbauer, K.; Schmidt, A.; Gow, N.A.; Brown, A.J.; Thomas, D.Y. Signal transduction through homologs of the Ste20p and Ste7p protein kinases can trigger hyphal formation in the pathogenic fungus Candida albicans. Proc. Natl. Acad. Sci. USA 1996, 93, 13217–13222. [Google Scholar] [CrossRef] [PubMed]
- Csank, C.; Schroppel, K.; Leberer, E.; Harcus, D.; Mohamed, O.; Meloche, S.; Thomas, D.Y.; Whiteway, M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 1998, 66, 2713–2721. [Google Scholar] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Negri, M.; Silva, S.; Henriques, M.; Oliveira, R. Insights into Candida tropicalis nosocomial infections and virulence factors. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1399–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Du, H.; Guan, G.; Tong, Y.; Kourkoumpetis, T.K.; Zhang, L.; Bai, F.Y.; Huang, G. N-acetylglucosamine induces white-to-opaque switching and mating in Candida tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot. Cell 2012, 11, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tao, L.; Guan, G.; Yue, H.; Liang, W.; Cao, C.; Dai, Y.; Huang, G. Regulation of filamentation in the human fungal pathogen Candida tropicalis. Mol. Microbiol. 2016, 99, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Yu, S.J.; Huang, H.Y.; Chang, Y.L.; Lehman, V.N.; Silao, F.G.; Bigol, U.G.; Bungay, A.A.; Averette, A.; Heitman, J. Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis. Eukaryot. Cell 2014, 13, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, Q.; Bing, J.; Ding, X.; Huang, G. Environmental and genetic regulation of white-opaque switching in Candida tropicalis. Mol. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.K.; Rhodes, J.C. Protein kinase A and fungal virulence: A sinister side to a conserved nutrient sensing pathway. Virulence 2012, 3, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogan, D.A.; Sundstrom, P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 2009, 4, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Kozubowski, L.; Lee, S.C.; Heitman, J. Signalling pathways in the pathogenesis of Cryptococcus. Cell. Microbiol. 2009, 11, 370–380. [Google Scholar] [CrossRef] [PubMed]
- McDonough, K.A.; Rodriguez, A. The myriad roles of cyclic AMP in microbial pathogens: From signal to sword. Nat. Rev. Microbiol. 2012, 10, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, B.; Müller, M.; Braun, A.; Brakhage, A.A. The cyclic AMP-dependent protein kinase a network regulates development and virulence in Aspergillus fumigatus. Infect. Immun. 2004, 72, 5193–5203. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, C.A.; Alspaugh, J.A.; Yue, C.; Harashima, T.; Cox, G.M.; Perfect, J.R.; Heitman, J. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 2001, 21, 3179–3191. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Vogl, A.W.; Kronstad, J.W. Regulated expression of cyclic AMP-dependent protein kinase A reveals an influence on cell size and the secretion of virulence factors in Cryptococcus neoformans. Mol. Microbiol. 2012, 85, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, X.; Gu, X.; Cao, S.; Wang, C.; Xu, J.R. The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 2014, 27, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Hamer, J.E. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 1998, 10, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Hu, S.; Liu, H.; Xu, J.R. PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. PLoS Genet. 2017, 13, e1006954. [Google Scholar] [CrossRef] [PubMed]
- Brückner, S.; Mösch, H.U. Choosing the right lifestyle: Adhesion and development in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2012, 36, 25–58. [Google Scholar] [CrossRef] [PubMed]
- Mancera, E.; Porman, A.M.; Cuomo, C.A.; Bennett, R.J.; Johnson, A.D. Finding a Missing Gene: EFG1 Regulates Morphogenesis in Candida tropicalis. G3 Genes Genomes Genet. 2015, 5, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Bockmühl, D.P.; Ernst, J.F. A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics 2001, 157, 1523–1530. [Google Scholar] [PubMed]
- Saputo, S.; Kumar, A.; Krysan, D.J. Efg1 directly regulates ACE2 expression to mediate cross talk between the cAMP/PKA and RAM pathways during Candida albicans morphogenesis. Eukaryot. Cell 2014, 13, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Thevelein, J.M.; de Winde, J.H. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 1999, 33, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Renault, G.; Garreau, H.; Jacquet, M. Stress induces depletion of Cdc25p and decreases the cAMP producing capability in Saccharomyces cerevisiae. Microbiology 2004, 150, 3383–3391. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Sprague, G.F., Jr. Glucose depletion causes haploid invasive growth in yeast. Proc. Natl. Acad. Sci. USA 2000, 97, 13619–13624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, P.J.; Sprague, G.F., Jr. The regulation of filamentous growth in yeast. Genetics 2012, 190, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Heitman, J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999, 19, 4874–4887. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.S.; Fink, G.R. The three yeast A kinases have specific signaling functions in pseudohyphal growth. Proc. Natl. Acad. Sci. USA 1998, 95, 13783–13787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglis, D.O.; Sherlock, G. Ras signaling gets fine-tuned: Regulation of multiple pathogenic traits of Candida albicans. Eukaryot. Cell 2013, 12, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassola, A.; Parrot, M.; Silberstein, S.; Magee, B.B.; Passeron, S.; Giasson, L.; Cantore, M.L. Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit. Eukaryot. Cell 2004, 3, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Bockmühl, D.P.; Krishnamurthy, S.; Gerads, M.; Sonneborn, A.; Ernst, J.F. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 2001, 42, 1243–1257. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, C.; Zheng, Q.; Huang, G. The Regulatory Subunit of Protein Kinase A (Bcy1) in Candida albicans Plays Critical Roles in Filamentation and White-Opaque Switching but Is Not Essential for Cell Growth. Front. Microbiol. 2016, 7, 2127. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wu, M.; Bing, J.; Tao, L.; Ding, X.; Liu, X.; Huang, G. Global regulatory roles of the cAMP/PKA pathway revealed by phenotypic, transcriptomic and phosphoproteomic analyses in a null mutant of the PKA catalytic subunit in Candida albicans. Mol. Microbiol. 2017, 105, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.K.; Richie, D.L.; Feng, X.; Krishnan, K.; Stephens, T.J.; Wikenheiser-Brokamp, K.A.; Askew, D.S.; Rhodes, J.C. Divergent Protein Kinase A isoforms co-ordinately regulate conidial germination, carbohydrate metabolism and virulence in Aspergillus fumigatus. Mol. Microbiol. 2011, 79, 1045–1062. [Google Scholar] [CrossRef] [PubMed]
- Sonneborn, A.; Bockmuhl, D.P.; Ernst, J.F. Chlamydospore formation in Candida albicans requires the Efg1p morphogenetic regulator. Infect. Immun. 1999, 67, 5514–5517. [Google Scholar] [PubMed]
- Sonneborn, A.; Bockmühl, D.P.; Gerads, M.; Kurpanek, K.; Sanglard, D.; Ernst, J.F. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol. Microbiol. 2000, 35, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Wu, C.Y.; Yu, S.J.; Chen, Y.L. Protein kinase A governs growth and virulence in Candida tropicalis. Virulence 2018, 9, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Lee, R.T.; Fang, H.M.; Wang, Y.M.; Li, R.; Zou, H.; Zhu, Y.; Wang, Y. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 2008, 4, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Tutulan-Cunita, A.; Jung, W.; Hauser, N.C.; Hernandez, R.; Williamson, T.; Piekarska, K.; Rupp, S.; Young, T.; Stateva, L. Deletion of the high-affinity cAMP phosphodiesterase encoded by PDE2 affects stress responses and virulence in Candida albicans. Mol. Microbiol. 2007, 65, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.H.; Stateva, L.I. The cAMP phosphodiesterase encoded by CaPDE2 is required for hyphal development in Candida albicans. Microbiology 2003, 149, 2961–2976. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, M.; Castilla, R.; Bolduc, N.; Zelada, A.; Martineau, P.; Bouillon, M.; Magee, B.B.; Passeron, S.; Giasson, L.; Cantore, M.L. The two isoforms of the cAMP-dependent protein kinase catalytic subunit are involved in the control of dimorphism in the human fungal pathogen Candida albicans. Fungal Genet. Biol. 2003, 38, 133–141. [Google Scholar] [CrossRef]
- Giacometti, R.; Kronberg, F.; Biondi, R.M.; Passeron, S. Candida albicans Tpk1p and Tpk2p isoforms differentially regulate pseudohyphal development, biofilm structure, cell aggregation and adhesins expression. Yeast 2011, 28, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, R.; Kronberg, F.; Biondi, R.M.; Passeron, S. Catalytic isoforms Tpk1 and Tpk2 of Candida albicans PKA have non-redundant roles in stress response and glycogen storage. Yeast 2009, 26, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Slutsky, B.; Staebell, M.; Anderson, J.; Risen, L.; Pfaller, M.; Soll, D.R. “White-opaque transition”: A second high-frequency switching system in Candida albicans. J. Bacteriol. 1987, 169, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Soll, D.R. Why does Candida albicans switch? FEMS Yeast Res. 2009, 9, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Porman, A.M.; Alby, K.; Hirakawa, M.P.; Bennett, R.J. Discovery of a phenotypic switch regulating sexual mating in the opportunistic fungal pathogen Candida tropicalis. Proc. Natl. Acad. Sci. USA 2011, 108, 21158–21163. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Johnson, A.D. White-opaque switching in Candida albicans. Curr. Opin. Microbiol. 2009, 12, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Soll, D.R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J. Bacteriol. 1987, 169, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Cao, C.; Xie, J.; Ni, J.; Guan, G.; Tao, L.; Zhang, L.; Huang, G. N-acetylglucosamine-induced white-to-opaque switching in Candida albicans is independent of the Wor2 transcription factor. Fungal Genet. Biol. 2014, 62, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Li, M.; Lu, X.L.; She, X.D.; Hu, S.Q.; Chen, W.; Liu, W.D. Higher concentration of CO2 and 37 degrees C stabilize the less virulent opaque cell of Candida albicans. Chin. Med. J. 2010, 123, 2446–2450. [Google Scholar] [PubMed]
- Huang, G.; Srikantha, T.; Sahni, N.; Yi, S.; Soll, D.R. CO2 regulates white-to-opaque switching in Candida albicans. Curr. Biol. 2009, 19, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yi, S.; Sahni, N.; Daniels, K.J.; Srikantha, T.; Soll, D.R. N-acetylglucosamine induces white to opaque switching, a mating prerequisite in Candida albicans. PLoS Pathog. 2010, 6, e1000806. [Google Scholar] [CrossRef]
- Grahl, N.; Demers, E.G.; Lindsay, A.K.; Harty, C.E.; Willger, S.D.; Piispanen, A.E.; Hogan, D.A. Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of C. albicans Virulence Pathways. PLoS Pathog. 2015, 11, e1005133. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.S.; Zaas, A.K.; Betancourt-Quiroz, M.; Perfect, J.R.; Cowen, L.E. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PLoS ONE 2012, 7, e44734. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; O’Meara, T.R.; Polvi, E.J.; Robbins, N.; Cowen, L.E. Staurosporine Induces Filamentation in the Human Fungal Pathogen Candida albicans via Signaling through Cyr1 and Protein Kinase A. mSphere 2017. [Google Scholar] [CrossRef] [PubMed]
- Hornby, J.M.; Jensen, E.C.; Lisec, A.D.; Tasto, J.J.; Jahnke, B.; Shoemaker, R.; Dussault, P.; Nickerson, K.W. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 2001, 67, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Schrevens, S.; Van Zeebroeck, G.; Riedelberger, M.; Tournu, H.; Kuchler, K.; Van Dijck, P. Methionine is required for cAMP-PKA-mediated morphogenesis and virulence of Candida albicans. Mol. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Reedy, J.L.; Cramer, R.A., Jr.; Perfect, J.R.; Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007, 5, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Gupta, K.L.; John, G.T.; Del Busto, R.; Klintmalm, G.B.; Somani, J.; et al. Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: Correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob. Agents Chemother. 2008, 52, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Gupta, K.L.; John, G.T.; del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Cryptococcus neoformans in organ transplant recipients: Impact of calcineurin-inhibitor agents on mortality. J. Infect. Dis. 2007, 195, 756–764. [Google Scholar] [CrossRef] [PubMed]
| Genotypes | cyr1/cyr1 | bcy1/bcy1 | tpk1/tpk1 | tpk2/tpk2 | tpk1/tpk1 tpk2/tpk2 | pde2/pde2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotypes | Ca | Ct | Ca | Ct | Ca | Ct | Ca | Ct | Ca | Ct | Ca | Ct | |
| Vegetative growth | A | A | A | - | WT | WT | WT | WT | A | A | A | WT | |
| Hyphal development | A | A | E/A | - | WT | WT | WT/A | WT/E | A | A | A | E | |
| White-opaque switch | A | A | E | - | WT | WT | A | WT | A | A | E | E | |
| Virulence | A | - | - | - | WT | WT | A | WT | - | A | A | - | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-J.; Chen, Y.-L. Conserved and Divergent Functions of the cAMP/PKA Signaling Pathway in Candida albicans and Candida tropicalis. J. Fungi 2018, 4, 68. https://doi.org/10.3390/jof4020068
Lin C-J, Chen Y-L. Conserved and Divergent Functions of the cAMP/PKA Signaling Pathway in Candida albicans and Candida tropicalis. Journal of Fungi. 2018; 4(2):68. https://doi.org/10.3390/jof4020068
Chicago/Turabian StyleLin, Chi-Jan, and Ying-Lien Chen. 2018. "Conserved and Divergent Functions of the cAMP/PKA Signaling Pathway in Candida albicans and Candida tropicalis" Journal of Fungi 4, no. 2: 68. https://doi.org/10.3390/jof4020068






