Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens
Abstract
1. Introduction
2. New Patients at Risk for Fungal Disease
Immunotherapy: Medical Progress Begets New Risk Factors for Invasive Fungal Disease
3. Emerging Yeast Infections
3.1. Shift to Non-albicans Candida Species and the Emergence of Antifungal Resistance
3.2. The Global Emergence of Candida auris
3.3. Cryptic Speciation in Cryptococcus
4. Emerging Mould Infections
4.1. Emergence of Azole-Resistance in Aspergillus fumigatus
4.2. Influenza-Associated Invasive Pulmonary Aspergillosis
4.3. Non-Aspergillus Mould Infections
4.4. Indian Epidemic of Resistant Dermatophytosis
5. Emerging Dimorphic Fungal Infections
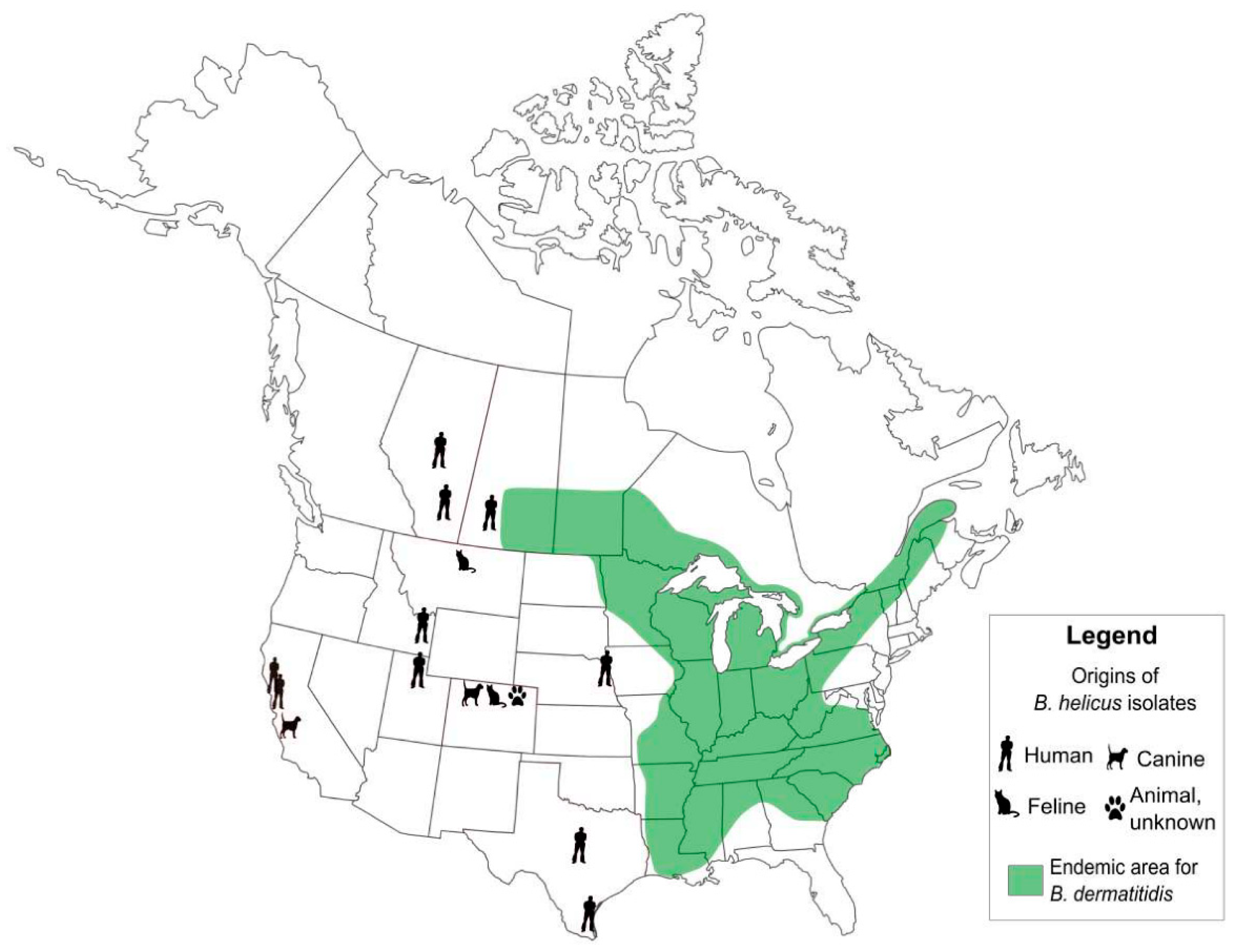
5.1. Emergence of Distinct, Novel Pathogens in Emergomyces and Blastomyces, and Cryptic Speciation in Histoplasma
5.2. Shifting Areas of Geographic Risks: Blastomycosis, Coccidioidomycosis, and Histoplasmosis
5.3. The Proliferation of Cases of Zoonotic Sporotrichosis in Brazil
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Clark, C.; Drummond, R. The hidden cost of modern medical interventions: How medical advances have shaped the prevalence of human fungal disease. Pathogens 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dukik, K.; Muñoz, J.F.; Sigler, L.; Schwartz, I.S.; Govender, N.P.; Kenyon, C.; Feng, P.; van den Ende, B.G.; Stielow, J.B.; et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018, 90, 245–291. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Zini, J.M.; Challan Belval, T.; Vignon, M.; Denis, B.; Alanio, A.; Malphettes, M. Fungal infections in patients treated with ibrutinib: Two unusual cases of invasive aspergillosis and cryptococcal meningoencephalitis. Leuk. Lymphoma 2017, 58, 2981–2982. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.-P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef] [PubMed]
- Achtnichts, L.; Obreja, O.; Conen, A.; Fux, C.A.; Nedeltchev, K. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated With Fingolimod. JAMA Neurol. 2015, 72, 1203–1205. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; Reder, A.T.; Bernard, J.T. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: A case report and review of literature. Mult. Scler. Relat. Disord. 2016, 9, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Veillet-Lemay, G.M.; Sawchuk, M.A.; Kanigsberg, N.D. Primary Cutaneous Histoplasma capsulatum Infection in a Patient Treated with Fingolimod: A Case Report. J. Cutan. Med. Surg. 2017, 21, 553–555. [Google Scholar] [CrossRef]
- Daver, N.; Kontoyiannis, D.P. Checkpoint inhibitors and aspergillosis in AML: The double hit hypothesis. Lancet Oncol. 2017, 18, 1571–1573. [Google Scholar] [CrossRef]
- Fujita, K.; Kim, Y.H.; Kanai, O.; Yoshida, H.; Mio, T.; Hirai, T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir. Med. 2019, 146, 66–70. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997–2016. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 1, i4–i13. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Fraser, M.; Borman, A.M.; Thorn, R.; Lawrance, L.M. Resistance to echinocandin antifungal agents in the United Kingdom in clinical isolates of Candida glabrata: Fifteen years of interpretation and assessment. Med. Mycol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, N.V.; Raush, E.R.; Rudneva, M.V.; Bogomolova, T.S.; Taraskina, A.E.; Fang, Y.; Zhang, F.; Klimko, N.N. Etiology of invasive candidosis agents in Russia: A multicenter epidemiological survey. Front. Med. 2018, 12, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.P.; Patel, J.; Magobo, R.E.; Naicker, S.; Wadula, J.; Whitelaw, A.; Coovadia, Y.; Kularatne, R.; Govind, C.; Lockhart, S.R.; et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: Results from laboratory-based sentinel surveillance in South Africa. J. Antimicrob. Chemother. 2016, 71, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, A.A.; Lackner, M.; Lass-Flörl, C.; Boekhout, T. The changing spectrum of Saccharomycotina yeasts causing candidemia: Phylogeny mirrors antifungal susceptibility patterns for azole drugs and amphothericin B. FEMS Yeast Res. 2019, 19, foz037. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov, a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tracking Candida auris|Candida auris|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html (accessed on 13 May 2019).
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Chow, N.A.; de Groot, T.; Badali, H.; Abastabar, M.; Chiller, T.M.; Meis, J.F. Potential fifth clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019, 25, 190686. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Oh, B.J.; Shin, J.H.; Kim, M.-N.; Sung, H.; Lee, K.; Joo, M.Y.; Shin, M.G.; Suh, S.P.; Ryang, D.W. Biofilm formation and genotyping of Candida haemulonii, Candida pseudohaemulonii, and a proposed new species (Candida auris) isolates from Korea. Med. Mycol. 2011, 49, 98–102. [Google Scholar] [CrossRef]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017, 61, e02396-16. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A review of the literature. Clin. Microbiol. Rev. 2017, 31, e00029-17. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Shaikh, A.A.; Piedrahita, C.T.; Sankar, T.; Jencson, A.L.; Larkin, E.L.; Ghannoum, M.A.; Donskey, C.J. Effectiveness of disinfectants against Candida auris and other Candida species. Infect. Control Hosp. Epidemiol. 2017, 38, 1240–1243. [Google Scholar] [CrossRef]
- Colombo, A.L.; de Júnior, J.N.A.; Guinea, J. Emerging multidrug-resistant Candida species. Curr. Opin. Infect. Dis. 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef]
- Spivak, E.S.; Hanson, K.E. Candida auris: An Emerging Fungal Pathogen. J. Clin. Microbiol. 2017, 56, e01588-17. [Google Scholar] [CrossRef]
- Barantsevich, N.E.; Orlova, O.E.; Shlyakhto, E.V.; Johnson, E.M.; Woodford, N.; Lass-Floerl, C.; Churkina, I.V.; Mitrokhin, S.D.; Shkoda, A.S.; Barantsevich, E.P. Emergence of Candida auris in Russia. J. Hosp. Infect. 2019, 102, 445–448. [Google Scholar] [CrossRef]
- Belkin, A.; Gazit, Z.; Keller, N.; Ben-Ami, R.; Wieder-Finesod, A.; Novikov, A.; Rahav, G.; Brosh-Nissimov, T. Candida auris infection leading to nosocomial transmission, Israel, 2017. Emerg. Infect. Dis. 2018, 24, 801–804. [Google Scholar] [CrossRef]
- Kohlenberg, A.; Struelens, M.J.; Monnet, D.L.; Plachouras, D.; The Candida Auris Survey Collaborative Group. Candida auris: Epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Eurosurveillance 2018, 23, 18–0013. [Google Scholar]
- Lone, S.A.; Ahmad, A. Candida auris—The growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef]
- Riat, A.; Neofytos, D.; Coste, A.T.; Harbarth, S.; Bizzini, A.; Grandbastien, B.; Pugin, J.; Lamoth, F. First case of Candida auris in Switzerland: Discussion about preventive strategies. Swiss Med. Wkly. 2018, 148, w14622. [Google Scholar]
- Chew, S.M.; Sweeney, N.; Kidd, S.E.; Reed, C. Candida auris arriving on our shores: An Australian microbiology laboratory’s experience. Pathology 2019, 51, 431–433. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Joseph, L.; Alfouzan, W.; Asadzadeh, M. Increasing prevalence, molecular characterization and antifungal drug susceptibility of serial Candida auris isolates in Kuwait. PLoS ONE 2018, 13, e0195743. [Google Scholar] [CrossRef]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef]
- Schwartz, I.; Smith, S.; Dingle, T. Something wicked this way comes: What health care providers need to know about Candida auris. Can. Commun. Dis. Rep. 2018, 44, 271–276. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Emergence of Cryptococcus gattii—Pacific Northwest, 2004–2010. MMWR Morb. Mortal. Wkly. Rep. 2010, 59, 865–868. [Google Scholar]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, H.T.; Boekhout, T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef]
- Dromer, F.; Mathoulin, S.; Dupont, B.; Letenneur, L.; Ronin, O.; French Cryptococcosis Study Group. Individual and Environmental Factors Associated with Infection Due to Cryptococcus neoformans Serotype D. Clin. Infect. Dis. 1996, 23, 91–96. [Google Scholar] [CrossRef]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole resistance in Aspergillus fumigatus: A consequence of antifungal use in agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Arikan-Akdagli, S.; Ghannoum, M.; Meis, J.F. Antifungal resistance: Specific focus on multidrug resistance in Candida auris and secondary azole resistance in Aspergillus fumigatus. J. Fungi 2018, 4, 129. [Google Scholar] [CrossRef]
- Rybak, J.M.; Ge, W.; Wiederhold, N.P.; Parker, J.E.; Kelly, S.L.; Rogers, P.D.; Fortwendel, J.R. Mutations in hmg1, challenging the paradigm of clinical triazole resistance in Aspergillus fumigatus. mBio 2019, 10, e00437-19. [Google Scholar] [CrossRef]
- Denning, D.W.; Venkateswarlu, K.; Oakley, K.L.; Anderson, M.J.; Manning, N.J.; Stevens, D.A.; Warnock, D.W.; Kelly, S.L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 1997, 41, 1364–1368. [Google Scholar] [CrossRef]
- Verweij, P.E.; Te Dorsthorst, D.T.A.; Rijs, A.J.M.M.; De Vries-Hospers, H.G.; Meis, J.F.G.M. Nationwide Survey of In Vitro Activities of Itraconazole and Voriconazole against Clinical Aspergillus fumigatus Isolates Cultured between 1945 and 1998. J. Clin. Microbiol. 2002, 40, 2648–2650. [Google Scholar] [CrossRef]
- Buil, J.B.; Snelders, E.; Denardi, L.B.; Melchers, W.J.G.; Verweij, P.E. Trends in azole resistance in Aspergillus fumigatus, the Netherlands, 1994–2016. Emerg. Infect. Dis. 2019, 25, 176–178. [Google Scholar] [CrossRef]
- Engel, T.G.P.; Slabbers, L.; de Jong, C.; Melchers, W.J.G.; Hagen, F.; Verweij, P.E.; Merkus, P.; Meis, J.F.; Dutch Cystic Fibrosis Fungal Collection Consortium. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients—A Dutch, multicentre study. J. Cyst. Fibros. 2019, 18, 221–226. [Google Scholar] [CrossRef]
- Snelders, E.; van der Lee, H.A.L.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of Azole Resistance in Aspergillus fumigatus and Spread of a Single Resistance Mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef]
- Lelièvre, L.; Groh, M.; Angebault, C.; Maherault, A.-C.; Didier, E.; Bougnoux, M.-E. Azole resistant Aspergillus fumigatus: An emerging problem. Méd. Mal. Infect. 2013, 43, 139–145. [Google Scholar] [CrossRef]
- Cui, N.; He, Y.; Yao, S.; Zhang, H.; Ren, J.; Fang, H.; Yu, Y. Tebuconazole induces triazole-resistance in Aspergillus fumigatus in liquid medium and soil. Sci. Total Environ. 2019, 648, 1237–1243. [Google Scholar] [CrossRef]
- Trovato, L.; Scalia, G.; Domina, M.; Oliveri, S. Environmental Isolates of Multi-Azole-Resistant Aspergillus spp. in Southern Italy. J. Fungi 2018, 4, 131. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole Fungicides Can Induce Cross-Resistance to Medical Triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef]
- Resendiz Sharpe, A.; Lagrou, K.; Meis, J.F.; Chowdhary, A.; Lockhart, S.R.; Verweij, P.E. Triazole resistance surveillance in Aspergillus fumigatus. Med. Mycol. 2018, 1, 83–92. [Google Scholar] [CrossRef]
- Berkow, E.L.; Nunnally, N.S.; Bandea, A.; Kuykendall, R.; Beer, K.; Lockhart, S.R. Detection of TR34/L98H CYP51A mutation through passive surveillance for azole-resistant Aspergillus fumigatus in the United States from 2015 to 2017. Antimicrob. Agents Chemother. 2018, 62, e02240-17. [Google Scholar] [CrossRef]
- Abdolrasouli, A.; Scourfield, A.; Rhodes, J.; Shah, A.; Elborn, J.S.; Fisher, M.C.; Schelenz, S.; Armstrong-James, D. High prevalence of triazole resistance in clinical Aspergillus fumigatus isolates in a specialist cardiothoracic centre. Int. J. Antimicrob. Agents 2018, 52, 637–642. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, Z.; Hagen, F.; Meis, J.F. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ. Res. 2014, 133, 20–26. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Rivero-Menendez, O.; Ayats, J.; Castro, C.; Garcia-Rodriguez, J.; Goterris-Bonet, L.; Ibanez-Martinez, E.; Linares-Sicilia, M.J.; Martin-Gomez, M.T.; et al. Molecular Identification and Susceptibility Testing of Molds Isolated in a Prospective Surveillance of Triazole Resistance in Spain (FILPOP2 Study). Antimicrob. Agents Chemother. 2018, 62, e00358-18. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.-A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Azole-resistant Aspergillus fumigatus harboring TR34/L98H, TR46/Y121F/T289A and TR53 mutations related to flower fields in Colombia. Sci. Rep. 2017, 7, 45631. [Google Scholar] [CrossRef]
- Arabatzis, M.; Kambouris, M.; Kyprianou, M.; Chrysaki, A.; Foustoukou, M.; Kanellopoulou, M.; Kondyli, L.; Kouppari, G.; Koutsia-Karouzou, C.; Lebessi, E.; et al. Polyphasic identification and susceptibility to seven antifungals of 102 Aspergillus isolates recovered from immunocompromised hosts in Greece. Antimicrob. Agents Chemother. 2011, 55, 3025–3030. [Google Scholar] [CrossRef]
- Bader, O.; Tünnermann, J.; Dudakova, A.; Tangwattanachuleeporn, M.; Weig, M.; Groß, U. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob. Agents Chemother. 2015, 59, 4356–4359. [Google Scholar] [CrossRef]
- Bader, O.; Weig, M.; Reichard, U.; Lugert, R.; Kuhns, M.; Christner, M.; Held, J.; Peter, S.; Schumacher, U.; Buchheidt, D.; et al. cyp51A-based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob. Agents Chemother. 2013, 57, 3513–3517. [Google Scholar] [CrossRef]
- Bedin Denardi, L.; Hoch Dalla-Lana, B.; de Pantella, F.K.J.; Bittencourt Severo, C.; Morais Santurio, J.; Zanette, R.A.; Hartz Alves, S. In vitro antifungal susceptibility of clinical and environmental isolates of Aspergillus fumigatus and Aspergillus flavus in Brazil. Braz. J. Infect. Dis. 2018, 22, 30–36. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Zhao, J.; Zou, Z.; Gong, Y.; Qu, F.; Bao, Z.; Qiu, G.; Song, M.; Zhang, Q.; et al. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob. Agents Chemother. 2016, 60, 5878–5884. [Google Scholar] [CrossRef]
- Choukri, F.; Botterel, F.; Sitterlé, E.; Bassinet, L.; Foulet, F.; Guillot, J.; Costa, J.M.; Fauchet, N.; Dannaoui, E. Prospective evaluation of azole resistance in Aspergillus fumigatus clinical isolates in France. Med. Mycol. 2015, 53, 593–596. [Google Scholar] [CrossRef][Green Version]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J.F. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front. Microbiol. 2015, 06, 428. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; van den Boom, M.; Yntema, J.B.; Hagen, F.; Verweij, P.E.; Meis, J.F. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J. Antimicrob. Chemother. 2014, 69, 2979–2983. [Google Scholar] [CrossRef]
- Escribano, P.; Peláez, T.; Muñoz, P.; Bouza, E.; Guinea, J. Is azole resistance in Aspergillus fumigatus a problem in Spain? Antimicrob. Agents Chemother. 2013, 57, 2815–2820. [Google Scholar] [CrossRef]
- Gungor, O.; Sampaio-Maia, B.; Amorim, A.; Araujo, R.; Erturan, Z. Determination of azole resistance and TR34/L98H mutations in Isolates of Aspergillus Section Fumigati from Turkish cystic fibrosis patients. Mycopathologia 2018, 183, 913–920. [Google Scholar] [CrossRef]
- Hurst, S.F.; Berkow, E.L.; Stevenson, K.L.; Litvintseva, A.P.; Lockhart, S.R. Isolation of azole-resistant Aspergillus fumigatus from the environment in the south-eastern USA. J. Antimicrob. Chemother. 2017, 72, 2443–2446. [Google Scholar] [CrossRef]
- Jensen, R.H.; Hagen, F.; Astvad, K.M.T.; Tyron, A.; Meis, J.F.; Arendrup, M.C. Azole-resistant Aspergillus fumigatus in Denmark: A laboratory-based study on resistance mechanisms and genotypes. Clin. Microbiol. Infect. 2016, 22, 570. [Google Scholar] [CrossRef]
- Lass-Florl, C.; Mayr, A.; Aigner, M.; Lackner, M.; Orth-Holler, D. A nationwide passive surveillance on fungal infections shows a low burden of azole resistance in molds and yeasts in Tyrol, Austria. Infection 2018, 46, 701–704. [Google Scholar] [CrossRef]
- Lazzarini, C.; Esposto, M.C.; Prigitano, A.; Cogliati, M.; De Lorenzis, G.; Tortorano, A.M. Azole resistance in Aspergillus fumigatus clinical isolates from an Italian culture collection. Antimicrob. Agents Chemother. 2016, 60, 682–685. [Google Scholar] [CrossRef]
- Loeffert, S.T.; Hénaff, L.; Dupont, D.; Bienvenu, A.-L.; Dananché, C.; Cassier, P.; Bénet, T.; Wallon, M.; Gustin, M.-P.; Vanhems, P. Prospective survey of azole drug resistance among environmental and clinical isolates of Aspergillus fumigatus in a French University hospital during major demolition works. J. Mycol. Med. 2018, 28, 469–472. [Google Scholar] [CrossRef]
- Mohammadi, F.; Hashemi, S.J.; Seyedmousavi, S.M.; Akbarzade, D. Isolation and characterization of clinical triazole resistance Aspergillus fumigatus in Iran. Iran. J. Public Health 2018, 47, 994–1000. [Google Scholar]
- Monteiro, C.; Pinheiro, D.; Maia, M.; Faria, M.A.; Lameiras, C.; Pinto, E. Aspergillus species collected from environmental air samples in Portugal-molecular identification, antifungal susceptibility and sequencing of cyp51A gene on A. fumigatus sensu stricto itraconazole-resistant. J. Antimicrob. Chemother. 2019, 126, 1140–1148. [Google Scholar] [CrossRef]
- Mortensen, K.L.; Mellado, E.; Lass-Florl, C.; Rodriguez-Tudela, J.L.; Johansen, H.K.; Arendrup, M.C. Environmental Study of Azole-Resistant Aspergillus fumigatus and Other Aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 2010, 54, 4545–4549. [Google Scholar] [CrossRef]
- Mushi, M.F.; Buname, G.; Bader, O.; Groß, U.; Mshana, S.E. Aspergillus fumigatus carrying TR34/L98H resistance allele causing complicated suppurative otitis media in Tanzania: Call for improved diagnosis of fungi in sub-Saharan Africa. BMC Infect. Dis. 2016, 16, 464. [Google Scholar] [CrossRef]
- Nawrot, U.; Kurzyk, E.; Arendrup, M.C.; Mroczynska, M.; Wlodarczyk, K.; Sulik-Tyszka, B.; Wroblewska, M.; Ussowicz, M.; Zdziarski, P.; Niewinska, K.; et al. Detection of Polish clinical Aspergillus fumigatus isolates resistant to triazoles. Med. Mycol. 2018, 56, 121–124. [Google Scholar] [CrossRef]
- Özmerdiven, G.E.; Ak, S.; Ener, B.; Ağca, H.; Cilo, B.D.; Tunca, B.; Akalın, H. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J. Infect. Chemother. 2015, 21, 581–586. [Google Scholar] [CrossRef]
- Pham, C.D.; Reiss, E.; Hagen, F.; Meis, J.F.; Lockhart, S.R. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg. Infect. Dis. 2014, 20, 1498–1503. [Google Scholar] [CrossRef]
- Prigitano, A.; Esposto, M.C.; Romano, L.; Auxilia, F.; Tortorano, A.M. Azole-resistant Aspergillus fumigatus in the Italian environment. J. Glob. Antimicrob. Resist. 2018, 16, 220–224. [Google Scholar] [CrossRef]
- Reichert-Lima, F.; Lyra, L.; Pontes, L.; Moretti, M.L.; Pham, C.D.; Lockhart, S.R.; Schreiber, A.Z. Surveillance for azoles resistance in Aspergillus spp. highlights a high number of amphotericin B-resistant isolates. Mycoses 2018, 61, 360–365. [Google Scholar] [CrossRef]
- Riat, A.; Plojoux, J.; Gindro, K.; Schrenzel, J.; Sanglard, D. Azole resistance of environmental and clinical Aspergillus fumigatus isolates from Switzerland. Antimicrob. Agents Chemother. 2018, 62, e02088-17. [Google Scholar] [CrossRef]
- Seufert, R.; Sedlacek, L.; Kahl, B.; Hogardt, M.; Hamprecht, A.; Haase, G.; Gunzer, F.; Haas, A.; Grauling-Halama, S.; MacKenzie, C.R.; et al. Prevalence and characterization of azole-resistant Aspergillus fumigatus in patients with cystic fibrosis: A prospective multicentre study in Germany. J. Antimicrob. Chemother. 2018, 73, 2047–2053. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Hashemi, S.J.; Zibafar, E.; Zoll, J.; Hedayati, M.T.; Mouton, J.W.; Melchers, W.J.G.; Verweij, P.E. Azole-resistant Aspergillus fumigatus, Iran. Emerg. Infect. Dis. 2013, 19, 832–834. [Google Scholar] [CrossRef]
- Sharma, C.; Hagen, F.; Moroti, R.; Meis, J.F.; Chowdhary, A. Triazole-resistant Aspergillus fumigatus harbouring G54 mutation: Is it de novo or environmentally acquired? J. Glob. Antimicrob. Resist. 2015, 3, 69–74. [Google Scholar] [CrossRef]
- Talbot, J.J.; Subedi, S.; Halliday, C.L.; Hibbs, D.E.; Lai, F.; Lopez-Ruiz, F.J.; Harper, L.; Park, R.F.; Cuddy, W.S.; Biswas, C.; et al. Surveillance for azole resistance in clinical and environmental isolates of Aspergillus fumigatus in Australia and cyp51A homology modelling of azole-resistant isolates. J. Antimicrob. Chemother. 2018, 73, 2347–2351. [Google Scholar] [CrossRef]
- Tangwattanachuleeporn, M.; Minarin, N.; Saichan, S.; Sermsri, P.; Mitkornburee, R.; Groß, U.; Chindamporn, A.; Bader, O. Prevalence of azole-resistant Aspergillus fumigatus in the environment of Thailand. Med. Mycol. 2017, 55, 429–435. [Google Scholar]
- Tsuchido, Y.; Tanaka, M.; Nakano, S.; Yamamoto, M.; Matsumura, Y.; Nagao, M. Prospective multicenter surveillance of clinically isolated Aspergillus species revealed azole-resistant Aspergillus fumigatus isolates with TR34/L98H mutation in the Kyoto and Shiga regions of Japan. Med. Mycol. 2019. [Google Scholar] [CrossRef]
- Vermeulen, E.; Maertens, J.; De Bel, A.; Nulens, E.; Boelens, J.; Surmont, I.; Mertens, A.; Boel, A.; Lagrou, K. Nationwide surveillance of azole resistance in Aspergillus diseases. Antimicrob. Agents Chemother. 2015, 59, 4569–4576. [Google Scholar] [CrossRef]
- Wirmann, L.; Ross, B.; Reimann, O.; Steinmann, J.; Rath, P.-M. Airborne Aspergillus fumigatus spore concentration during demolition of a building on a hospital site, and patient risk determination for invasive aspergillosis including azole resistance. J. Hosp. Infect. 2018, 100, e91–e97. [Google Scholar] [CrossRef]
- Wu, C.-J.; Wang, H.-C.; Lee, J.-C.; Lo, H.-J.; Dai, C.-T.; Chou, P.-H.; Ko, W.-C.; Chen, Y.-C. Azole-resistant Aspergillus fumigatus isolates carrying TR34/L98H mutations in Taiwan. Mycoses 2015, 58, 544–549. [Google Scholar] [CrossRef]
- Isla, G.; Leonardelli, F.; Tiraboschi, I.N.; Refojo, N.; Hevia, A.; Vivot, W.; Szusz, W.; Cordoba, S.B.; Garcia-Effron, G. First clinical isolation of an azole-resistant Aspergillus fumigatus harboring a TR46 Y121F T289A mutation in South America. Antimicrob. Agents Chemother. 2018, 62, e00872-18. [Google Scholar] [CrossRef]
- Dunne, K.; Hagen, F.; Pomeroy, N.; Meis, J.F.; Rogers, T.R. Intercountry transfer of triazole-resistant Aspergillus fumigatus on plant bulbs. Clin. Infect. Dis. 2017, 65, 147–149. [Google Scholar] [CrossRef]
- Lee, H.-J.; Cho, S.-Y.; Lee, D.-G.; Park, C.; Chun, H.-S.; Park, Y.-J. TR34/L98H mutation in CYP51A gene in Aspergillus fumigatus clinical isolates during posaconazole prophylaxis: First case in Korea. Mycopathologia 2018, 183, 731–736. [Google Scholar] [CrossRef]
- Fuller, J.; Bull, A.; Shokoples, S.; Dingle, T.C.; Adam, H.; Baxter, M.; Hoban, D.J.; Zhanel, G.G. Antifungal Susceptibility of Aspergillus Isolates from the Respiratory Tract of Patients in Canadian Hospitals: Results of the CANWARD 2016 Study. In Proceedings of the Oral Presentation at: AMMI Canada—Canadian Association for Clinical Microbiology and Infectious Diseases Annual Conference, Vancouver, BC, Canada, 2–5 May 2018. [Google Scholar]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive Fungal Infections among Organ Transplant Recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Pasqualotto, A.C. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 2009, 47, S261–S270. [Google Scholar] [CrossRef]
- Sharma, C.; Kumar, R.; Kumar, N.; Masih, A.; Gupta, D.; Chowdhary, A. Investigation of Multiple Resistance Mechanisms in Voriconazole-Resistant Aspergillus flavus Clinical Isolates from a Chest Hospital Surveillance in Delhi, India. Antimicrob. Agents Chemother. 2018, 62, e01928-17. [Google Scholar] [CrossRef]
- Paul, R.A.; Rudramurthy, S.M.; Dhaliwal, M.; Singh, P.; Ghosh, A.K.; Kaur, H.; Varma, S.; Agarwal, R.; Chakrabarti, A. Magnitude of Voriconazole Resistance in Clinical and Environmental Isolates of Aspergillus flavus and Investigation into the Role of Multidrug Efflux Pumps. Antimicrob. Agents Chemother. 2018, 62, e01022-18. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Chakrabarti, A.; Geertsen, E.; Mouton, J.W.; Meis, J.F. In vitro activity of isavuconazole against 208 Aspergillus flavus isolates in comparison with 7 other antifungal agents: Assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Diagn. Microbiol. Infect. Dis. 2011, 71, 370–377. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Castanheira, M.; Messer, S.A.; Jones, R.N. In vitro antifungal susceptibilities of isolates of Candida spp. and Aspergillus spp. from China to nine systemically active antifungal agents: Data from the SENTRY antifungal surveillance program, 2010 through 2012. Mycoses 2015, 58, 209–214. [Google Scholar] [CrossRef]
- Choi, M.J.; Won, E.J.; Joo, M.Y.; Park, Y.-J.; Kim, S.H.; Shin, M.G.; Shin, J.H. Microsatellite Typing and Resistance Mechanism Analysis of Voriconazole-Resistant Aspergillus flavus Isolates in South Korean Hospitals. Antimicrob. Agents Chemother. 2019, 63, e01610-18. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Barba, P.; Arnan, M.; Moreno, A.; Ruiz-Camps, I.; Gudiol, C.; Ayats, J.; Orti, G.; Carratala, J. Invasive aspergillosis complicating pandemic influenza A (H1N1) infection in severely immunocompromised patients. Clin. Infect. Dis. 2011, 53, e16–e19. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Kolwijck, E.; Lestrade, P.P.A.; Hodiamont, C.J.; Rijnders, B.J.A.; van Paassen, J.; Haas, P.-J.; Oliveira dos Santos, C.; Kampinga, G.A.; Bergmans, D.C.J.J.; et al. Influenza-associated Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2017, 196, 524–527. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Auberger, J.; Lass-Florl, C.; Aigner, M.; Clausen, J.; Gastl, G.; Nachbaur, D. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: Real-life data from a single-centre institutional retrospective observational study. J. Antimicrob. Chemother. 2012, 67, 2268–2273. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Singh, R. Mucormycosis in India: Unique features. Mycoses 2014, 57, 85–90. [Google Scholar] [CrossRef]
- Bala, K.; Chander, J.; Handa, U.; Punia, R.S.; Attri, A.K. A prospective study of mucormycosis in north India: Experience from a tertiary care hospital. Med. Mycol. 2015, 53, 248–257. [Google Scholar] [CrossRef]
- Jenks, J.D.; Reed, S.L.; Seidel, D.; Koehler, P.; Cornely, O.A.; Mehta, S.R.; Hoenigl, M. Rare mould infections caused by Mucorales, Lomentospora prolificans and Fusarium, in San Diego, CA: The role of antifungal combination therapy. Int. J. Antimicrob. Agents 2018, 52, 706–712. [Google Scholar] [CrossRef]
- Dalyan Cilo, B.; Al-Hatmi, A.M.S.; Seyedmousavi, S.; Rijs, A.J.M.M.; Verweij, P.E.; Ener, B.; de Hoog, G.S.; van Diepeningen, A.D. Emergence of fusarioses in a university hospital in Turkey during a 20-year period. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1683–1691. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Prigitano, A.; Arsic Arsenijevic, V.; Kolarovic, J.; Ivanovic, D.; Paripovic, L.; Klingspor, L.; Nordøy, I.; Hamal, P.; Arikan Akdagli, S.; et al. European Confederation of Medical Mycology (ECMM) epidemiological survey on invasive infections due to Fusarium species in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1623–1630. [Google Scholar] [CrossRef]
- Seidel, D.; Meißner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-García, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Durán Graeff, L.; Köhler, P.; et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit. Rev. Microbiol. 2019, 45, 1–21. [Google Scholar] [CrossRef]
- McCarty, T.P.; Baddley, J.W.; Walsh, T.J.; Alexander, B.D.; Kontoyiannis, D.P.; Perl, T.M.; Walker, R.; Patterson, T.F.; Schuster, M.G.; Lyon, G.M.; et al. Phaeohyphomycosis in transplant recipients: Results from the Transplant Associated Infection Surveillance Network (TRANSNET). Med. Mycol. 2015, 53, 440–446. [Google Scholar] [CrossRef]
- Neofytos, D.; Fishman, J.A.; Horn, D.; Anaissie, E.; Chang, C.-H.; Olyaei, A.; Pfaller, M.; Steinbach, W.J.; Webster, K.M.; Marr, K.A. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 220–229. [Google Scholar] [CrossRef]
- Penteado, F.D.; Litvinov, N.; Sztajnbok, J.; Thomaz, D.Y.; dos Santos, A.M.; Vasconcelos, D.M.; Motta, A.L.; Rossi, F.; Fernandes, J.F.; Marques, H.H.S.; et al. Lomentospora prolificans fungemia in hematopoietic stem cell transplant patients: First report in South America and literature review. Transpl. Infect. Dis. 2018, 20, e12908. [Google Scholar] [CrossRef]
- Verma, S.; Madhu, R. The Great Indian Epidemic of Superficial Dermatophytosis: An Appraisal. Indian J. Dermatol. 2017, 62, 227. [Google Scholar]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.-C.; Wittig, F.; Krüger, C.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses 2019, 62, 336–356. [Google Scholar] [CrossRef]
- Panda, S.; Verma, S. The menace of dermatophytosis in India: The evidence that we need. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 281–284. [Google Scholar] [CrossRef]
- Upadhyay, V.; Kumar, A.; Singh, A.K.; Pandey, J. Epidemiological characterization of dermatophytes at a tertiary care hospital in Eastern Uttar Pradesh, India. Curr. Med. Mycol. 2019, 5, 1. [Google Scholar]
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine Resistance of Trichophyton Clinical Isolates Caused by Specific Point Mutations in the Squalene Epoxidase Gene. Antimicrob. Agents Chemother. 2017, 61, e00115-17. [Google Scholar] [CrossRef]
- Suzuki, S.; Mano, Y.; Furuya, N.; Fujitani, K. Discovery of Terbinafine Low Susceptibility Trichophyton rubrum strain in Japan. Biocontrol. Sci. 2018, 23, 151–154. [Google Scholar] [CrossRef]
- Dukik, K.; Muñoz, J.F.; Jiang, Y.; Feng, P.; Sigler, L.; Stielow, J.B.; Freeke, J.; Jamalian, A.; van den Ende, B.G.; McEwen, J.G.; et al. Novel taxa of thermally dimorphic systemic pathogens in the Ajellomycetaceae (Onygenales). Mycose 2017, 60, 296–309. [Google Scholar] [CrossRef]
- Kenyon, C.; Bonorchis, K.; Corcoran, C.; Meintjes, G.; Locketz, M.; Lehloenya, R.; Vismer, H.F.; Naicker, P.; Prozesky, H.; van Wyk, M.; et al. A dimorphic fungus causing disseminated infection in South Africa. N. Engl. J. Med. 2013, 369, 1416–1424. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Govender, N.P.; Corcoran, C.; Dlamini, S.; Prozesky, H.; Burton, R.; Mendelson, M.; Taljaard, J.; Lehloenya, R.; Calligaro, G.; et al. Clinical Characteristics, Diagnosis, Management, and Outcomes of Disseminated Emmonsiosis: A Retrospective Case Series. Clin. Infect. Dis. 2015, 61, 1004–1012. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Kenyon, C.; Feng, P.; Govender, N.P.; Dukik, K.; Sigler, L.; Jiang, Y.; Stielow, J.B.; Muñoz, J.F.; Cuomo, C.A.; et al. 50 years of Emmonsia disease in Humans: The dramatic emergence of a cluster of novel fungal pathogens. PLoS Pathog. 2015, 11, e1005198. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Maphanga, T.G.; Govender, N.P. Emergomyces: A new genus of dimorphic fungal pathogens causing disseminated disease among immunocomprised persons globally. Curr. Fungal Infect. Rep. 2018, 12, 44–50. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Sanche, S.; Wiederhold, N.P.; Patterson, T.F.; Sigler, L. Emergomyces canadensis, a dimorphic fungus causing fatal systemic human disease in North America. Emerg. Infect. Dis. 2018, 24, 758–761. [Google Scholar] [CrossRef]
- Wang, P.; Kenyon, C.; de Hoog, S.; Guo, L.; Fan, H.; Liu, H.; Li, Z.; Sheng, R.; Yang, Y.; Jiang, Y.; et al. A novel dimorphic pathogen, Emergomyces orientalis (Onygenales), agent of disseminated infection. Mycoses 2017, 60, 310–319. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Kenyon, C.; Lehloenya, R.; Claasens, S.; Spengane, Z.; Prozesky, H.; Burton, R.; Parker, A.; Wasserman, S.; Meintjes, G.; et al. AIDS-related endemic mycoses in Western Cape, South Africa, and clinical mimics: A cross-sectional study of adults with advanced HIV and recent-onset, widespread skin lesions. Open Forum Infect. Dis. 2017, 4, ofx186. [Google Scholar] [CrossRef]
- Dukik, K.; Al-Hatmi, A.M.S.; Curfs-Breuker, I.; Faro, D.; de Hoog, S.; Meis, J.F. Antifungal susceptibility of emerging dimorphic pathogens in the family Ajellomycetaceae. Antimicrob. Agents Chemother. 2017, 62, e01886-17. [Google Scholar] [CrossRef]
- Maphanga, T.G.; Britz, E.; Zulu, T.G.; Mpembe, R.S.; Naicker, S.D.; Schwartz, I.S.; Govender, N.P. In Vitro antifungal susceptibility of yeast and mold phases of isolates of dimorphic fungal pathogen Emergomyces africanus (formerly Emmonsia sp.) from HIV-infected South African patients. J. Clin. Microbiol. 2017, 55, 1812–1820. [Google Scholar] [CrossRef]
- Gast, K.B.; van der Hoeven, A.; de Boer, M.G.J.; van Esser, J.W.J.; Kuijper, E.J.; Verweij, J.J.; van Keulen, P.H.J.; van der Beek, M.T. Two cases of Emergomyces pasteurianus infection in immunocompromised patients in the Netherlands. Med. Mycol. Case Rep. 2019, 24, 5–8. [Google Scholar] [CrossRef]
- Schwartz, I.S.; McLoud, J.D.; Berman, D.; Botha, A.; Lerm, B.; Colebunders, R.; Levetin, E.; Kenyon, C. Molecular detection of airborne Emergomyces africanus, a thermally dimorphic fungal pathogen, in Cape Town, South Africa. PLoS Negl. Trop. Dis. 2018, 12, e0006174. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Lerm, B.; Hoving, J.C.; Kenyon, C.; Horsnell, W.G.; Basson, W.J.; Otieno-Odhiambo, P.; Govender, N.P.; Colebunders, R.; Botha, A. Emergomyces africanus in soil, South Africa. Emerg. Infect. Dis. 2018, 24, 377–380. [Google Scholar] [CrossRef]
- Rooms, I.; Mugisha, P.; Hadaschik, E.; Esser, S.; Rath, P.-M.; Haase, G.; Wilmes, D.; McCormick-Smith, I.; Rickerts, V. Disseminated emergomycosis in a person with HIV infection from Uganda: Molecular identification of Emergomyces pasteurianus or a close relative from a pathology block. Emerg. Infect. Dis. 2019, 25. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Sources of Blastomycosis. Available online: https://www.cdc.gov/fungal/diseases/blastomycosis/causes.html (accessed on 25 March 2019).
- McCullough, M.J.; DiSalvo, A.F.; Clemons, K.V.; Park, P.; Stevens, D.A. Molecular Epidemiology of Blastomyces dermatitidis. Clin. Infect. Dis. 2000, 30, 328–335. [Google Scholar] [CrossRef][Green Version]
- Meece, J.K.; Anderson, J.L.; Fisher, M.C.; Henk, D.A.; Sloss, B.L.; Reed, K.D. Population genetic structure of clinical and environmental isolates of Blastomyces dermatitidis, based on 27 polymorphic microsatellite markers. Appl. Environ. Microbiol. 2011, 77, 5123–5131. [Google Scholar] [CrossRef]
- Brown, E.M.; McTaggart, L.R.; Zhang, S.X.; Low, D.E.; Stevens, D.A.; Richardson, S.E. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS ONE 2013, 8, e59237. [Google Scholar] [CrossRef]
- Dalcin, D.; Ahmed, S.Z. Blastomycosis in northwestern Ontario, 2004 to 2014. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 259–262. [Google Scholar] [CrossRef]
- Dalcin, D.; Rothstein, A.; Spinato, J.; Escott, N.; Kus, J.V. Blastomyces gilchristii as cause of fatal acute respiratory distress syndrome. Emerg. Infect. Dis. 2016, 22, 306–308. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Wiederhold, N.P.; Hanson, K.E.; Patterson, T.F.; Sigler, L. Blastomyces helicus, a new dimorphic fungus causing fatal pulmonary and systemic disease in humans and animals in Western Canada and the United States. Clin. Infect. Dis. 2019, 68, 188–195. [Google Scholar] [CrossRef]
- Frean, A.; Carman, W.; Crewe-Brown, H.; Culligan, G.; Young, C. Blastomyces dermatitidis infections in the RSA. S. Afr. Med. J. 1989, 76, 13–16. [Google Scholar]
- Sepúlveda, V.E.; Márquez, R.; Turissini, D.A.; Goldman, W.E.; Matute, D.R. Genome Sequences Reveal Cryptic Speciation in the Human Pathogen Histoplasma capsulatum. mBio 2017, 8, e01339-17. [Google Scholar] [CrossRef]
- Durkin, M.M.; Connolly, P.A.; Karimi, K.; Wheat, E.; Schnizlein-Bick, C.; Allen, S.D.; Alves, K.; Tewari, R.P.; Keath, E. Pathogenic Differences between North American and Latin American Strains of Histoplasma capsulatum var. capsulatum in Experimentally Infected Mice. J. Clin. Microbiol. 2004, 42, 4370–4373. [Google Scholar] [CrossRef]
- Dubois, A.; Janssens, P.; Brutsaert, P.; Vanbreuseghem, R. A case of African histoplasmosis; with a mycological note on Histoplasma duboisii n.sp. Ann. Soc. Belg. Med. Trop. 1952, 32, 569–584. [Google Scholar]
- Pakasa, N.; Biber, A.; Nsiangana, S.; Imposo, D.; Sumaili, E.; Muhindo, H.; Buitrago, M.J.; Barshack, I.; Schwartz, E. African Histoplasmosis in HIV-Negative Patients, Kimpese, Democratic Republic of the Congo. Emerg. Infect. Dis. 2018, 24, 2068–2070. [Google Scholar] [CrossRef]
- Gauthier, G.M. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog. 2015, 11, e1004608. [Google Scholar] [CrossRef]
- Lohrenz, S.; Minion, J.; Pandey, M.; Karunakaran, K. Blastomycosis in Southern Saskatchewan 2000–2015: Unique presentations and disease characteristics. Med. Mycol. 2018, 56, 787–795. [Google Scholar] [CrossRef]
- McDonald, R.; Dufort, E.; Jackson, B.R.; Tobin, E.H.; Newman, A.; Benedict, K.; Blog, D. Blastomycosis cases occurring outside of regions with known endemicity—New York, 2007–2017. MMWR Morb. Mortal. Wkly. Rep 2018, 67, 1077–1078. [Google Scholar] [CrossRef]
- Nett, R.J.; Skillman, D.; Riek, L.; Davis, B.; Blue, S.R.; Sundberg, E.E.; Merriman, J.R.; Hahn, C.G.; Park, B.J. Histoplasmosis in Idaho and Montana, USA, 2012–2013. Emerg. Infect. Dis. 2015, 21, 1071–1072. [Google Scholar] [CrossRef]
- Anderson, H.; Honish, L.; Taylor, G.; Johnson, M.; Tovstiuk, C.; Fanning, A.; Tyrrell, G.; Rennie, R.; Jaipaul, J.; Sand, C.; et al. Histoplasmosis cluster, golf course, Canada. Emerg. Infect. Dis. 2006, 12, 163–165. [Google Scholar] [CrossRef]
- Litvintseva, A.P.; Marsden-Haug, N.; Hurst, S.; Hill, H.; Gade, L.; Driebe, E.M.; Ralston, C.; Roe, C.; Barker, B.M.; Goldoft, M.; et al. Valley Fever: Finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin. Infect. Dis. 2015, 60, e1–e3. [Google Scholar] [CrossRef]
- Maiga, A.W.; Deppen, S.; Scaffidi, B.K.; Baddley, J.; Aldrich, M.C.; Dittus, R.S.; Grogan, E.L. Mapping Histoplasma capsulatum exposure, United States. Emerg. Infect. Dis. 2018, 24, 1835–1839. [Google Scholar] [CrossRef]
- Marimon, R.; Cano, J.; Gene, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol 2007, 45, 3198–3206. [Google Scholar] [CrossRef]
- Marimon, R.; Gené, J.; Cano, J.; Guarro, J. Sporothrix luriei: A rare fungus from clinical origin. Med. Mycol. 2008, 46, 621–625. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Téllez-Martínez, D.; de Roberto Andrade, C.; Portuondo, D.L.; Jellmayer, J.A.; Polesi, M.C.; Carlos, I.Z. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells responses than Sporothrix schenckii sensu stricto in mice. Fungal Biol. 2018, 122, 1163–1170. [Google Scholar] [CrossRef]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Marine, M.; Genis, J.; Cano, J.; Guarro, J. Different virulence levels of the species of Sporothrix in a murine model. Clin. Microbiol. Infect. 2009, 15, 651–655. [Google Scholar] [CrossRef]
- Montenegro, H.; Rodrigues, A.M.; Dias, M.A.G.; da Silva, E.A.; Bernardi, F.; de Camargo, Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: An emerging animal infection in São Paulo, Brazil. BMC Vet. Res. 2014, 10, 269. [Google Scholar] [CrossRef]
- Schubach, A.; de Barros, M.B.L.; Wanke, B. Epidemic sporotrichosis. Curr. Opin. Infect. Dis. 2008, 21, 129. [Google Scholar] [CrossRef]
- de Barros, M.B.L.; Schubach, T.M.P.; Gutierrez Galhardo, M.C.; de Schubach, A.O.; Monteiro, P.C.F.; Reis, R.S.; Zancopé-Oliveira, R.M.; Lazéra, M.; dos, S.; Cuzzi-Maya, T.; et al. Sporotrichosis: An emergent zoonosis in Rio de Janeiro. Mem. Inst. Oswaldo Cruz 2001, 96, 777–779. [Google Scholar] [CrossRef]
- Sanchotene, K.O.; Madrid, I.M.; Klafke, G.B.; Bergamashi, M.; Terra, P.P.D.; Rodrigues, A.M.; de Camargo, Z.P.; Xavier, M.O. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses 2015, 58, 652–658. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Oliveira, M.M.E.; Freitas, D.F.S.; do Valle, A.C.F.; Zancopé-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl. Trop. Dis. 2014, 8, e3094. [Google Scholar] [CrossRef]
- Brandolt, T.M.; Madrid, I.M.; Poester, V.R.; Sanchotene, K.O.; Basso, R.P.; Klafke, G.B.; de Rodrigues, M.L.; Xavier, M.O. Human sporotrichosis: A zoonotic outbreak in southern Brazil, 2012–2017. Med. Mycol. 2018, 57, 527–533. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedman, D.Z.P.; Schwartz, I.S. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. J. Fungi 2019, 5, 67. https://doi.org/10.3390/jof5030067
Friedman DZP, Schwartz IS. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. Journal of Fungi. 2019; 5(3):67. https://doi.org/10.3390/jof5030067
Chicago/Turabian StyleFriedman, Daniel Z.P., and Ilan S. Schwartz. 2019. "Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens" Journal of Fungi 5, no. 3: 67. https://doi.org/10.3390/jof5030067
APA StyleFriedman, D. Z. P., & Schwartz, I. S. (2019). Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. Journal of Fungi, 5(3), 67. https://doi.org/10.3390/jof5030067






