Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil, Plant and AMF Inoculum
2.2. Experimental Set-up and Procedure
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Root Colonization
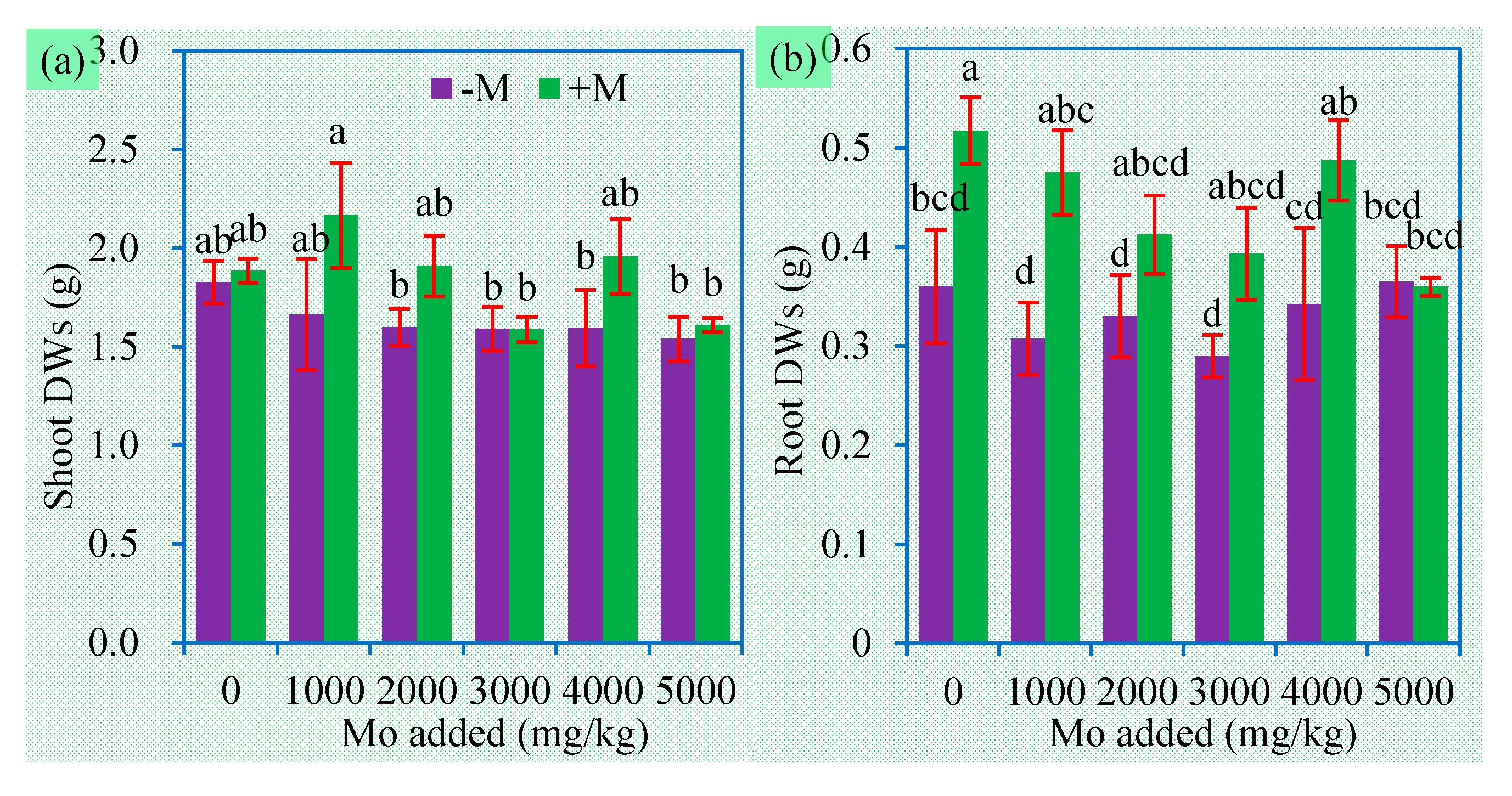
3.2. Plant Biomass
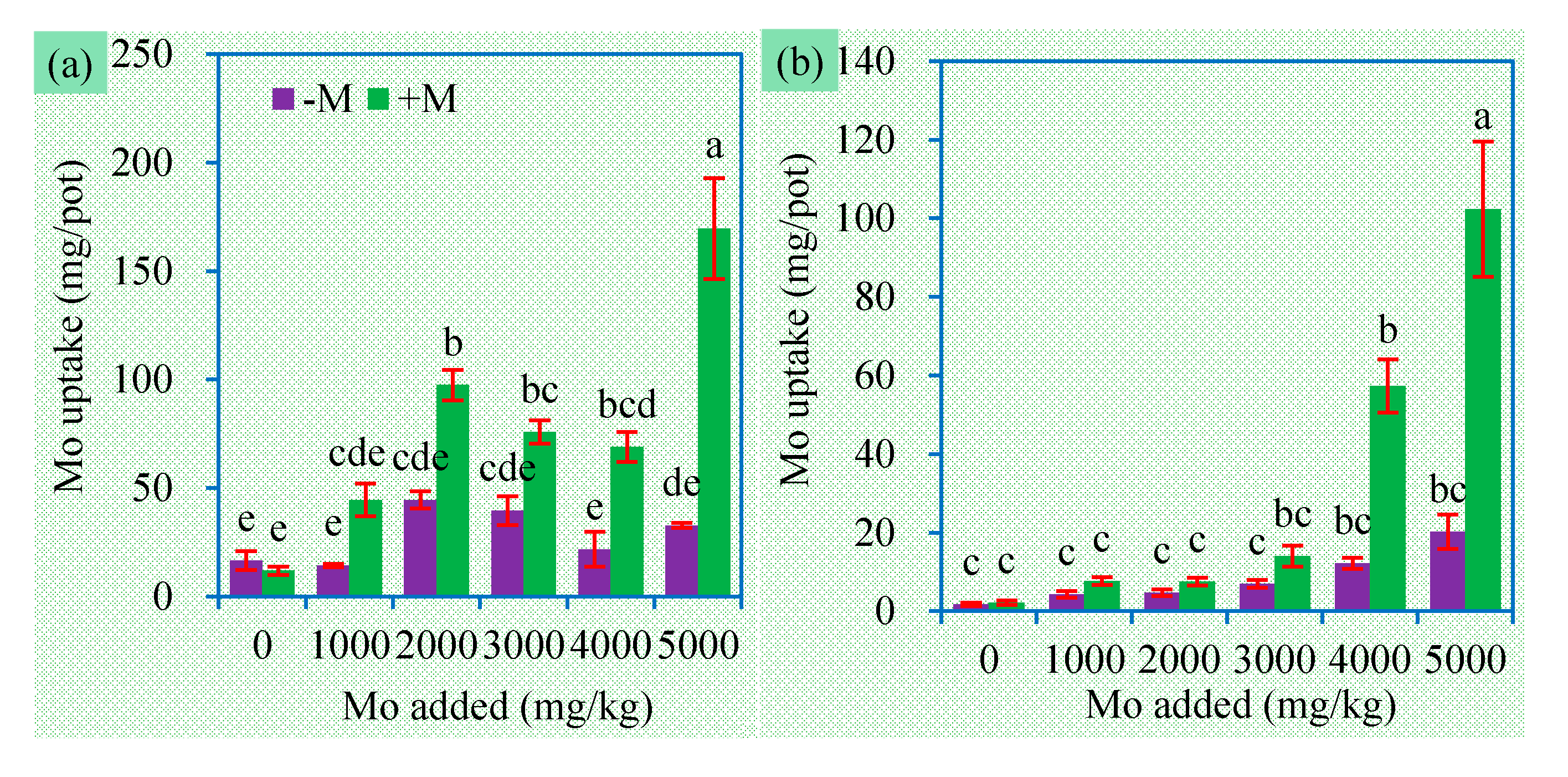
3.3. Mo Concentrations and Uptake in Plant Tissues
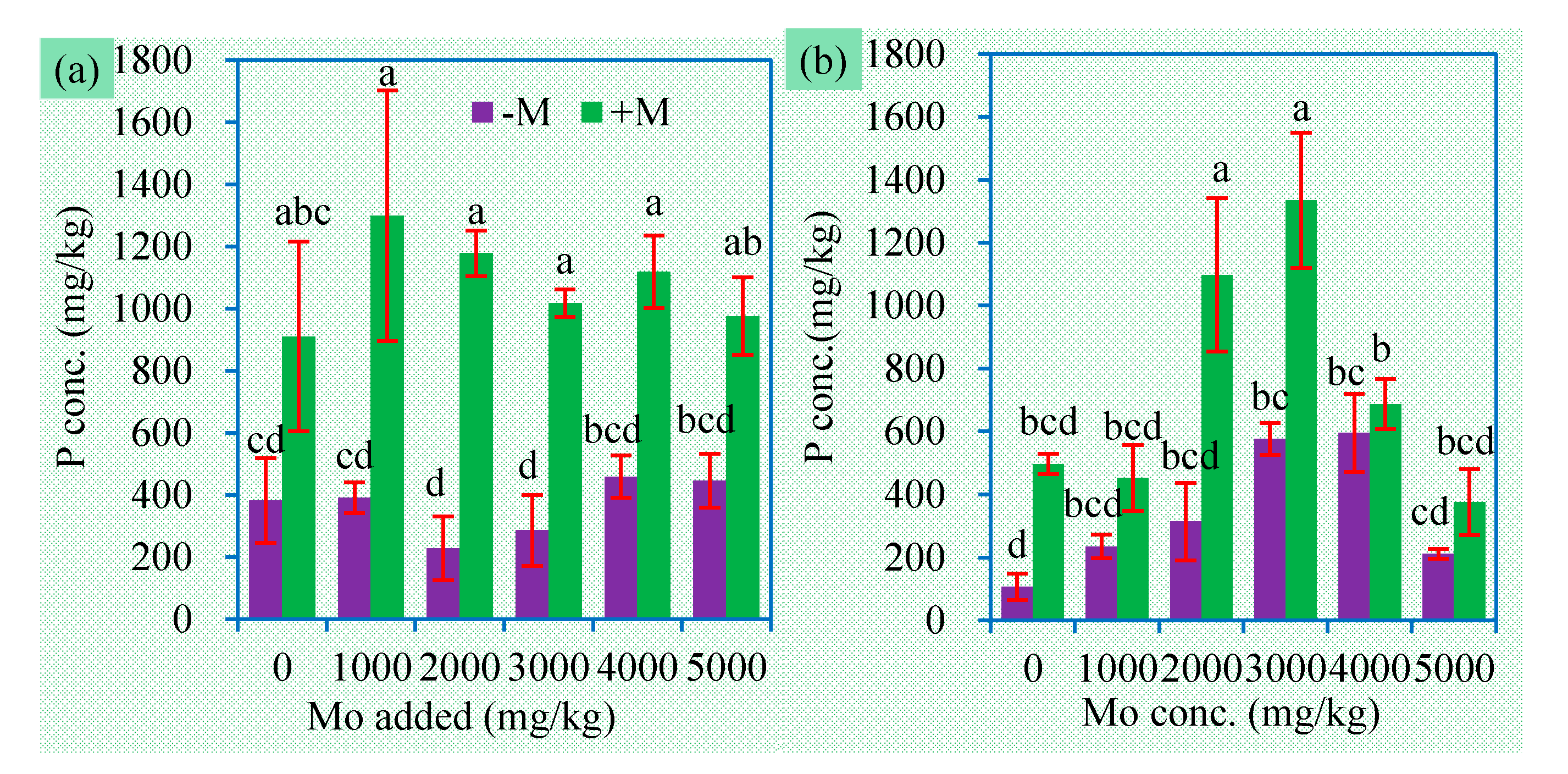
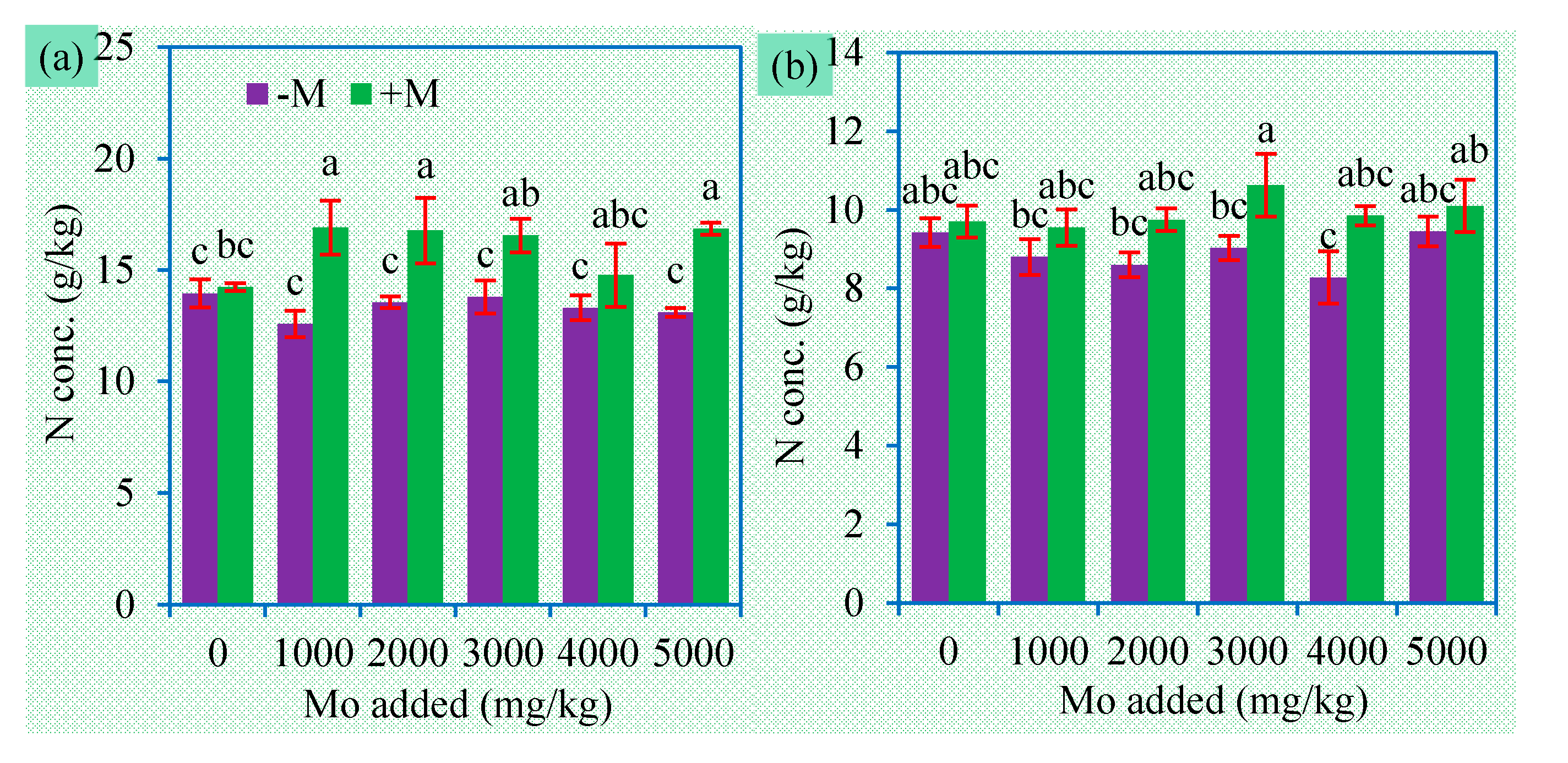
3.4. Concentrations of P, N, and S in Plants
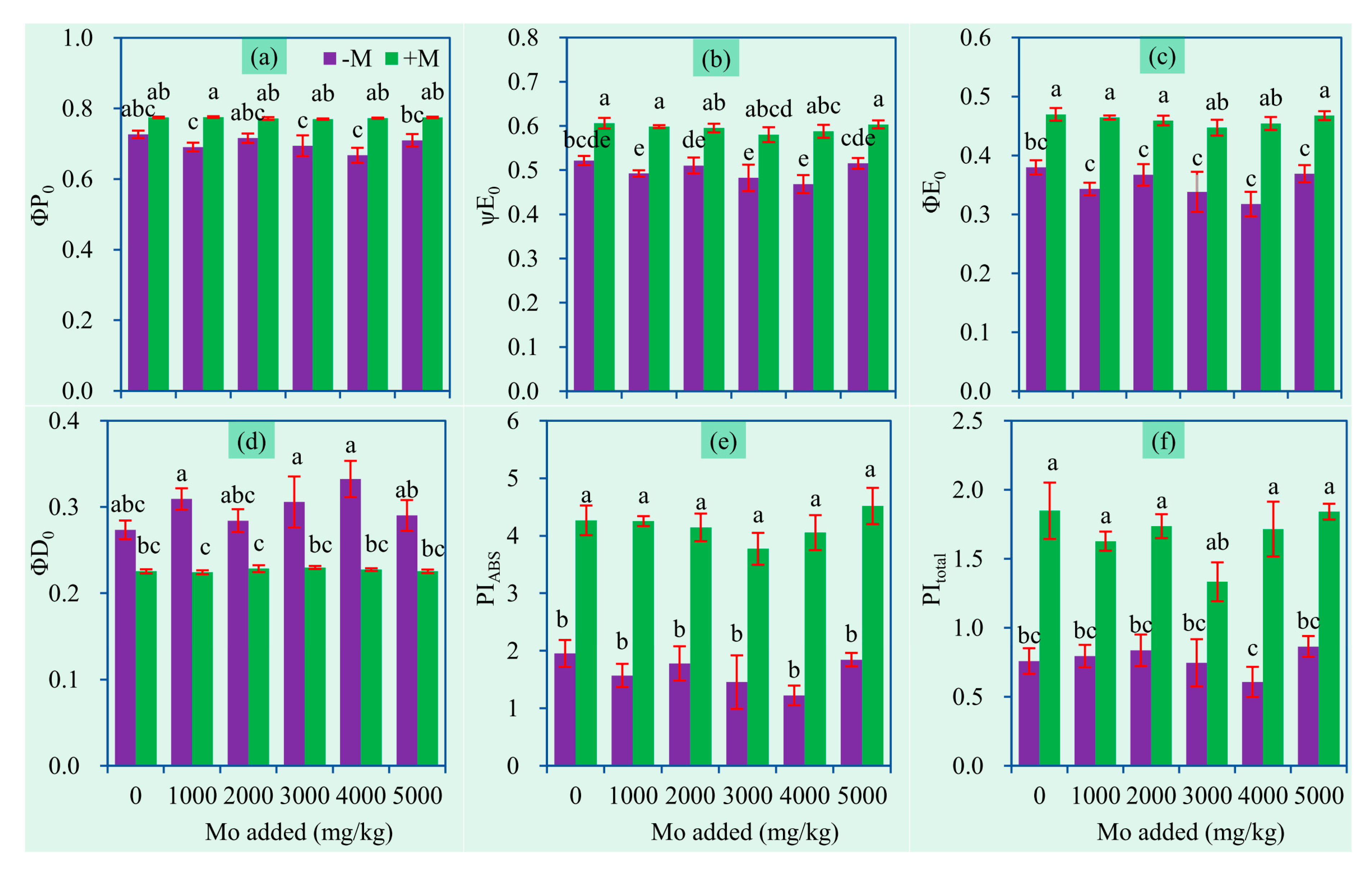
3.5. Chlorophyll Fluorescence Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, M.S.; Wei, C.; Fan, G.; Deng, Z.G.; Wang, S.F.; Wu, J. Molybdenum recovery from oxygen pressure water leaching residue of Ni–Mo ore. Rare Met. 2013, 32, 208–212. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Lucas, R.E. Micronutrients for Vegetables and Field Crops. In Cooperative Extension Service; Michigan State University: East Lansing, MI, USA, 1967. [Google Scholar]
- Gupta, U.C.; Lipsett, J. Molybdenum in soils, plants, and animals. Adv. Agron. 1981, 34, 73–115. [Google Scholar]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals; Springer: New York, NY, USA, 2001. [Google Scholar]
- Warington, K. The influence of iron supply on toxic effects of manganese, molybdenum and vanadium on soybean, peas and flax. Ann. Appl. Biol. 1954, 41, 1–22. [Google Scholar] [CrossRef]
- Ferguson, W.S.; Lewis, A.H.; Watson, S.J. The teart pastures of Somerset: I. The cause and cure of teartness. J. Agric. Sci. 1943, 33, 44–51. [Google Scholar] [CrossRef]
- Han, Z.; Wan, D.; Tian, H.; He, W.; Wang, Z.; Liu, Q. Pollution assessment of heavy metals in soils and plants around a molybdenum mine in central China. Pol. J. Environ. Stud. 2019, 28, 123–133. [Google Scholar] [CrossRef]
- McBride, M.; Cherney, J. Molybdenum, sulfur, and other trace elements in farm soils and forages after sewage sludge application. Commun. Soil Sci. Plant Anal. 2004, 35, 517–535. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Cong, Q.; Qu, J. Assessment of heavy metal pollution in orchard soil and its affections to fruit quality around molybdenum mining area. Sci. Technol. Eng. 2010, 10, 5831–5834. [Google Scholar]
- Qu, J.; Yuan, X.; Wang, L.L.; Wang, N. Analysis and assessment on the pollution condition of heavy metals in the soil of molybdenum mine. Environ. Prot. Sci. 2007, 33, 36–38. [Google Scholar]
- Jia, T.; Jia, Y.Y.; Yu, S.T.; Qu, Y.M.; Jiang, Z.L.; Chen, Y.H.; Wang, G. Pollution of molybdenum and heavy metals of the soils and rice near a molybdenum mining site in eastern Fujian. Environ. Monit. China 2015, 31, 45–49. [Google Scholar]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Wang, F.; Adams, C.A.; Yang, W.; Sun, Y.; Shi, Z. Benefits of arbuscular mycorrhizal fungi in reducing organic contaminant residues in crops: Implications for cleaner agricultural production. Crit. Rev. Environ. Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Cabral, L.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant. Nutrition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zhuang, D.; Jiang, D.; Liu, L.; Huang, Y. Assessment of bioenergy potential on marginal land in China. Renew. Sustain. Energy Rev. 2011, 15, 1050–1056. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Shi, Z. Arbuscular mycorrhiza enhances biomass production and salt tolerance of sweet sorghum. Microorganisms 2019, 7, 289. [Google Scholar] [CrossRef] [Green Version]
- Regassa, T.H.; Wortmann, C.S. Sweet sorghum as a bioenergy crop: Literature review. Biomass Bioenergy 2014, 64, 348–355. [Google Scholar] [CrossRef]
- Houx, J.H.; Roberts, C.A.; Fritschi, F.B. Evaluation of sweet sorghum bagasse as an alternative livestock feed. Crop. Sci. 2013, 53, 1784–1790. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Wang, F.; Li, K.; Yuan, W.; Liu, J. Arbuscular mycorrhizal inoculation increases molybdenum accumulation but decreases molybdenum toxicity in maize plants grown in polluted soil. RSC Adv. 2018, 8, 37069–37076. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.C.; Yang, X.L.; Xie, G.H. Establishing sustainable sweet sorghum-based cropping systems for forage and bioenergy feedstock in North China Plain. Field Crops Res. 2018, 227, 144–154. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Xiong, T.; Yao, S.; Liu, C.; Yin, Y.N.; Li, H.C.; Li, N.S. A real filed phytoremediation of multi-metals contaminated soils by selected hybrid sweet sorghum with high biomass and high accumulation ability. Chemosphere 2019, 237, 124536. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, F.; Zhang, C.; Yang, Z. Exploitation of phosphorus patches with different phosphorus enrichment by three arbuscular mycorrhizal fungi. J. Plant Nutr. 2011, 34, 1096–1106. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Force, L.; Critchley, C.; van Rensen, J.J. New fluorescence parameters for monitoring photosynthesis in plants. Photosynth. Res. 2003, 78, 17. [Google Scholar] [CrossRef]
- Trouvelot, E.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche et méthodes d’estimation ayant une signification fonctionnelle. In The Mycorrhizae: Physiology and Genetic; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Lee, E.-H.; Ka, K.-H.; Eom, A.-H. Community structures of arbuscular mycorrhizal fungi in soils and plant roots inhabiting abandoned mines of Korea. Mycobiology 2016, 44, 277–282. [Google Scholar] [CrossRef] [Green Version]
- González-Guerrero, M.; Escudero, V.; Saéz, Á.; Tejada-Jiménez, M. Transition metal transport in plants and associated endosymbionts: Arbuscular mycorrhizal fungi and rhizobia. Front. Plant Sci. 2016, 7, 1088. [Google Scholar] [CrossRef] [Green Version]
- Campo, R.J.; Araujo, R.S.; Hungria, M. Molybdenum-enriched soybean seeds enhance N accumulation, seed yield, and seed protein content in Brazil. Field Crops Res. 2009, 110, 219–224. [Google Scholar] [CrossRef]
- Mcgrath, S.P.; Micó, C.; Zhao, F.J.; Zhao, F.J.; Stroud, J.L.; Zhang, H.; Fozard, S. Predicting molybdenum toxicity to higher plants: Estimation of toxicity threshold values. Environ. Pollut. 2010, 158, 3085–3094. [Google Scholar] [CrossRef]
- Kluge, R. Molybdenum toxicity in plants. In Proceedings of Mengen-und Spurenelemente Arbeitstagung, Leipzig; Leipzig, 1983; Anke, M., Groppel, B., Gürtler, H., Grün, M., Lombeck, I., Schneider, H.J., Eds.; Institut für Pflanzenernährung: Leipzig, Germany, 1983; pp. 10–17. [Google Scholar]
- Adriano, D. Trace Elements in the Terrestrial Environment; Springer-Verlag: New York, NY, USA, 1986; pp. 329–361. [Google Scholar]
- Gupta, U.C. Deficient, sufficient, and toxic concentrations of molybdenum in crops. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: New York, NY, USA, 1997; pp. 150–159. [Google Scholar]
- Clark, R.; Zeto, S. Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 2000, 23, 867–902. [Google Scholar] [CrossRef]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Relationships among phosphorus, molybdenum and free-living nitrogen fixation in tropical rain forests: Results from observational and experimental analyses. Biogeochemistry 2013, 114, 135–147. [Google Scholar] [CrossRef]
- Reddy, K.J.; Munn, L.C.; Wang, L. Chemistry and mineralogy of molybdenum in soils. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: New York, NY, USA, 1997; pp. 4–22. [Google Scholar]
- Wang, F.Y.; Lin, X.G.; Yin, R. Inoculation with arbuscular mycorrhizal fungus Acaulospora mellea decreases Cu phytoextraction by maize from Cu-contaminated soil. Pedobiologia 2007, 51, 99–109. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, L.; Shi, Z.Y.; Li, Y.J.; Song, Z.M. Effects of AM inoculation and organic amendment, alone or in combination, on growth, P nutrition, and heavy-metal uptake of tobacco in Pb-Cd-contaminated soil. J. Plant Growth Regul. 2012, 31, 549–559. [Google Scholar] [CrossRef]
- Kovács, B.; Puskás-Preszner, A.; Huzsvai, L.; Lévai, L.; Bódi, É. Effect of molybdenum treatment on molybdenum concentration and nitrate reduction in maize seedlings. Plant Physiol. Biochem. 2015, 96, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Hu, C.; Tan, Q.; Qin, S.; Sun, X. Subcellular distribution of molybdenum, ultrastructural and antioxidative responses in soybean seedlings under excess molybdenum stress. Plant Physiol. Biochem. 2017, 123, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Selim, H.M. Molybdenum-phosphate retention and transport in soils. Geoderma 2017, 308, 60–68. [Google Scholar] [CrossRef]
- Barshad, I. Factors affecting the molybdenum content of pasture plants: II. Effect of soluble phosphates, available nitrogen, and soluble sulfates. Soil Sci. 1951, 71, 387–398. [Google Scholar] [CrossRef]
- Heuwinkel, H.; Kirkby, E.A.; Lebot, J.; Marschner, H. Phosphorus deficiency enhances molybdenum uptake by tomato plants. J. Plant Nutr. 1992, 15, 549–568. [Google Scholar] [CrossRef]
- Srivastava, P. Biochemical significance of molybdenum in crop plants. In Molybdenum in Agriculture; Gupta, U.C., Ed.; Cambridge University Press: New York, NY, USA, 1997; pp. 47–70. [Google Scholar]
- Yu, M.; Hu, C.; Sun, X.; Wang, Y.H. Influences of Mo on nitrate reductase, glutamine synthetase and nitrogen accumulation and utilization in Mo-efficient and Mo-inefficient winter wheat cultivars. J. Integr. Agric. 2010, 9, 355–361. [Google Scholar] [CrossRef]
- Allen, J.W.; Shachar-Hill, Y. Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol. 2009, 149, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Shende, S.; Strasser, R. JIP test for fast fluorescence transients as a rapid and sensitive technique in assessing the effectiveness of arbuscular mycorrhizal fungi in Zea mays: Analysis of chlorophyll a fluorescence. Plant Biosyst. 2008, 142, 191–198. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D. Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 2011, 346, 189–199. [Google Scholar] [CrossRef]
- Rozpadek, P.; Wezowicz, K.; Stojakowska, A.; Malarz, J.; Surowka, E.; Sobczyk, L.; Anielska, T.; Wazny, R.; Miszalski, Z.; Turnau, K. Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 2014, 112, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Shamshiri, M.; Fattahi, M. Effects of arbuscular mycorrhizal fungi on photosystem II activity of three pistachio rootstocks under salt stress as probed by the OJIP-test. Russ. J. Plant Physiol. 2016, 63, 101–110. [Google Scholar] [CrossRef]
- Shi, Z.; Li, K.; Zhu, X.; Wang, F. The worldwide leaf economic spectrum traits are closely linked with mycorrhizal traits. Fungal Ecol. 2020, 43, 100877. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Zinc protects chloroplasts and associated photochemical functions in cadmium exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci. 2004, 166, 1321–1327. [Google Scholar] [CrossRef]
- Hale, K.L.; McGrath, S.P.; Lombi, E.; Stack, S.M.; Terry, N.; Pickering, I.J.; George, G.N.; Pilon-Smits, E.A.H. Molybdenum sequestration in Brassica species. A role for anthocyanins? Plant Physiol. 2001, 126, 1391–1402. [Google Scholar] [CrossRef] [Green Version]



| pH | Organic Matter | Total N | Total P | Total K | Total Mo | Total S |
|---|---|---|---|---|---|---|
| 7.32 | 7.6% | 13 g/kg | 337.3 mg/kg | 2.7 g/kg | 8.4 mg/kg | 147.6 mg/kg |
| Parameter | Definition |
|---|---|
| φPo | The maximum quantum efficiency of PSII |
| ψEo | Efficiency/probability that an electron moves further than reduced QA (primary electron acceptor of PSII) |
| φEo | Quantum yield of electron transport |
| φDo | Quantum yield of energy dissipation |
| PIABS | Performance index (potential) for energy conservation from exciton to reduction of intersystem electron acceptors |
| PItotal | Performance index (potential) for energy conservation from exciton to reduction of PSI end acceptors |
| Parameter | AM Inoculation | Mo | AM × Mo |
|---|---|---|---|
| φPo | 89.14 ** | 1.31 ns | 1.22 ns |
| ψEo | 121.40 ** | 1.86 ns | 0.4 ns |
| φEo | 142.09 ** | 1.86 ns | 0.68 ns |
| φDo | 89.14 ** | 1.31 ns | 1.22 ns |
| PIABS | 272.70 ** | 1.58 ns | 0.36 ns |
| PItotal | 157.18 ** | 1.62 ns | 1.16 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Zhang, J.; Lu, S.; Li, Y.; Wang, F. Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-Contaminated Soil. J. Fungi 2020, 6, 44. https://doi.org/10.3390/jof6020044
Shi Z, Zhang J, Lu S, Li Y, Wang F. Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-Contaminated Soil. Journal of Fungi. 2020; 6(2):44. https://doi.org/10.3390/jof6020044
Chicago/Turabian StyleShi, Zhaoyong, Jiacheng Zhang, Shichuan Lu, Yang Li, and Fayuan Wang. 2020. "Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-Contaminated Soil" Journal of Fungi 6, no. 2: 44. https://doi.org/10.3390/jof6020044
APA StyleShi, Z., Zhang, J., Lu, S., Li, Y., & Wang, F. (2020). Arbuscular Mycorrhizal Fungi Improve the Performance of Sweet Sorghum Grown in a Mo-Contaminated Soil. Journal of Fungi, 6(2), 44. https://doi.org/10.3390/jof6020044






