Fungal Endophytic Community Associated with Guarana (Paullinia cupana Var. Sorbilis): Diversity Driver by Genotypes in the Centre of Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Fungal Endophyte Isolation
2.3. DNA Extraction, Amplification and Sequencing
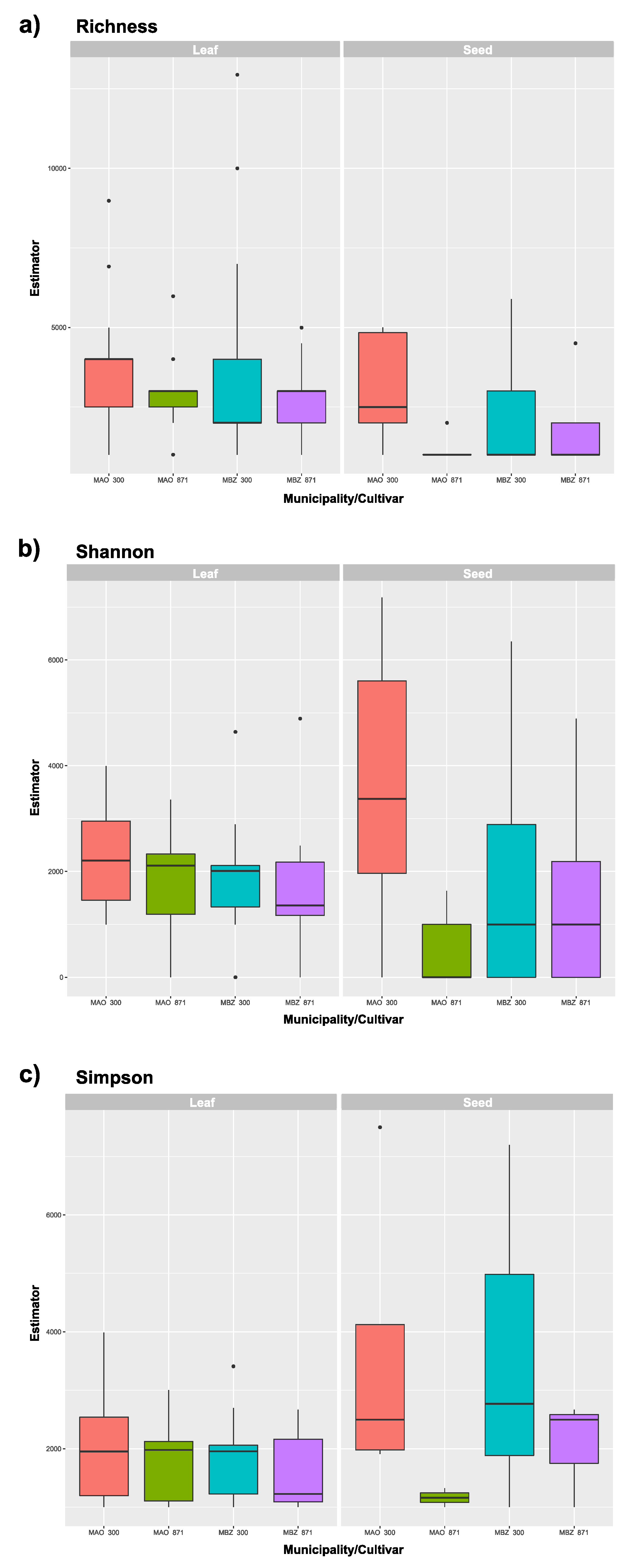
2.4. Fungal Diversity Analysis
3. Results and Discussion
3.1. Culture Medium
3.2. Taxonomic Composition
3.3. Composition of the Endophytic Microbiota of Genotypes and Municipalities
3.4. Diversity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atroch, A.L.; Nascimento Filho, F.J. Guarana—Paullinia cupana Kunth var. sorbilis (Mart.) Ducke. In Exotic Fruits; Rodrigues, S., Silva, E.O., Brito, E.S., Rodrigues, S., Silva, E.O., Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 225–236. [Google Scholar] [CrossRef]
- Babu, K.M.; Church, R.J.; Lewander, W. Energy drinks: The new eye-opener for adolescents. Clin. Pediatr. Emerg. Med. 2008, 9, 35–42. [Google Scholar] [CrossRef]
- Lorenz, S.D.S. Sateré-Mawé: Os Filhos do Guaraná; Centro de Trabalho Indigenista: São Paulo, Brazil, 1992. [Google Scholar]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef]
- Smith, N.; Atroch, A.L. Guaraná’s journey from regional tonic to aphrodisiac and global energy drink. Evid. Based Complement. Alt. Med. eCAM 2010, 7, 279–282. [Google Scholar] [CrossRef]
- Pomportes, L.; Davranche, K.; Brisswalter, I.; Hays, A.; Brisswalter, J.; Pomportes, L.; Davranche, K.; Brisswalter, I.; Hays, A.; Brisswalter, J. Heart rate variability and cognitive function following a multi-vitamin and mineral supplementation with added guarana (Paullinia cupana). Nutrients 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Bittencourt, L.d.S.; Zeidán-Chuliá, F.; Yatsu, F.K.J.; Schnorr, C.E.; Moresco, K.S.; Kolling, E.A.; Gelain, D.P.; Bassani, V.L.; Moreira, J.C.F. Guarana (Paullinia cupana Mart.) prevents β-amyloid aggregation, generation of advanced glycation-end products (AGEs), and acrolein-induced cytotoxicity on human neuronal-like cells. Phytother. Res. 2014, 28, 1615–1624. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; de Paula Oliveira, R. Guarana (Paullinia cupana) extract protects Caenorhabditis elegans models for Alzheimer disease and Huntington disease through activation of antioxidant and protein degradation pathways. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Barreto, G.; Galeano, P.; Romero, J.I.; Holubiec, M.I.; Badorrey, M.S.; Capani, F.; Giraldez Alvarez, L.D. Paullinia cupana Mart. var. sorbilis protects human dopaminergic neuroblastoma SH-SY5Y cell line against rotenone-induced cytotoxicity. Hum. Exp. Toxicol. 2011, 30, 1382–1391. [Google Scholar] [CrossRef]
- Ruchel, J.B.; Rezer, J.F.P.; Thorstenberg, M.L.; dos Santos, C.B.; Cabral, F.L.; Lopes, S.T.A.; da Silva, C.B.; Machado, A.K.; da Cruz, I.B.M.; Schetinger, M.R.C.; et al. Hypercholesterolemia and ecto-enzymes of purinergic system: Effects of Paullinia cupana. Phytother. Res. 2016, 30, 49–57. [Google Scholar] [CrossRef]
- Bortolin, R.C.; Vargas, A.R.; Ramos, V.D.M.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; da Boit Martinello, K.; Silveira, A.K.; Gomes, H.M.; Rabelo, T.K.; et al. Guarana supplementation attenuated obesity, insulin resistance, and adipokines dysregulation induced by a standardized human western diet via brown adipose tissue activation. Phytother. Res. 2019, 33, 1394–1403. [Google Scholar] [CrossRef]
- Lima, N.D.S.; Teixeira, L.; Gambero, A.; Ribeiro, M.L. Guarana (Paullinia cupana) stimulates mitochondrial biogenesis in mice fed high-fat diet. Nutrients 2018, 10, 165. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Ko, Y.-F.; Tseng, S.-F.; Lai, H.-C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Santana, Á.L.; Macedo, G.A. Health and technological aspects of methylxanthines and polyphenols from guarana: A review. J. Funct. Foods 2018, 47, 457–468. [Google Scholar] [CrossRef]
- Basile, A.; Ferrara, L.; Pezzo, D.M.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol. 2005, 102, 32–36. [Google Scholar] [CrossRef]
- Hamerski, L.; Vieira Somner, G.; Tamaio, N. Paullinia cupana Kunth (Sapindaceae): A review of its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plants Res. 2013, 7, 2221–2229. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Montero-Rodríguez, A.F.; Aguilar-Alonso, P.; Vera-López, O.; Lazcano-Hernández, M.; Morales-Medina, J.C.; Navarro-Cruz, A.R. Antioxidant properties of amazonian fruits: A mini review of in vivo and in vitro studies. Oxid. Med. Cell. Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Cadoná, F.C.; Rosa, J.L.; Schneider, T.; Cubillos-Rojas, M.; Sánchez-Tena, S.; Azzolin, V.F.; Assmann, C.E.; Machado, A.K.; Ribeiro, E.E.; da Cruz, I.B.M. Guaraná, a highly caffeinated food, presents in vitro antitumor activity in colorectal and breast cancer cell lines by inhibiting AKT/mTOR/S6K and MAPKs pathways. Nutr. Cancer 2017, 69, 800–810. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Ferreira, E.D.F.; Paula, D.M.N.; Klein, T.; Mello, D.J.C.P. Paullinia cupana: A multipurpose plant—A review. Braz. J. Pharmacogn. 2018, 29, 77–110. [Google Scholar] [CrossRef]
- Antonelli-Ushirobira, T.M.; Kaneshima, E.N.; Gabriel, M.; Audi, E.A.; Marques, L.C.; Mello, J.C.P. Acute and subchronic toxicological evaluation of the semipurified extract of seeds of guaraná (Paullinia cupana) in rodents. Food Chem. Toxicol. 2010, 48, 1817–1820. [Google Scholar] [CrossRef]
- Marx, F. Analysis of guarana seeds II. Studies on the composition of the tannin fraction. Z. Lebensm. Unters. Forsch. 1990, 190, 429–431. [Google Scholar] [CrossRef]
- Pinto, C.E.D.L.; Atroch, A.L.; Fajardo, J.D.V.; Nascimento Filho, D.F.J. Seleção de clones de guaranazeiro para adaptabilidade e estabilidade no estado do Amazonas. Rev. Ciênc. Agrár. 2018, 61, 1–7. [Google Scholar] [CrossRef]
- Market Data Forecast. Guarana Seed Extract Market by Form (Powder and Liquid) by Distribution (Health Stores, Drug Stores, Online Retailing and Other Channels) by Application (Pharmaceuticals, Dietary Supplements, Cosmetics, Food and Beverages, and Others), and by Region—Global Industry Analysis, Size, Share, Growth, Trends, And Forecast To 2024; Custom Market Research Services: Albany, NY, USA, 2019; Volume 145. [Google Scholar]
- IBGE. Levantamento sistemático da produção agrícola. In Intituto Brasileiro de Geografia e Estatistica; IBGE: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- IBGE. Levantamento sistemático da produção agrícola: Pesquisa mensal de previsão e acompanhamento das safras agrícolas no ano civil. In Intituto Brasileiro de Geografia e Estatistica; IBGE: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Clima Maués: Temperatura, Tempo e Dados Climatológicos Maués—Climate-Data.org. Available online: https://pt.climate-data.org/america-do-sul/brasil/amazonas/maues-879673/ (accessed on 27 May 2020).
- Albuquerque, F.C. Antracnose do Guaraná; 1-37: Boletim Técnico; IAN: Belém, Brazil, 1960. [Google Scholar]
- Araújo, J.C.A.; Pereira, J.C.R.; Gasparotto, L.; Arruda, D.M.R. O Complexo Superbrotamento do Guaranazeiro e Seu Controle; Embrapa Amazônia Central: Manaus, Brazil, 2006. [Google Scholar]
- Gonçalves, J.R.C. Notas Sobre as Doenças e Pragas do Guaraná no Estado do Amazonas; IPEAN: Belém, Brazil, 1967. [Google Scholar]
- Tavares, A.M.; Atroch, A.L.; Nascimento Filho, D.F.J.; Pereira, J.C.R.; Araújo, D.J.C.A.; Moares, L.A.C.; Santos, L.P.; Garcia, M.V.B.; Arruda, D.M.R.; Sousa, N.R.; et al. Cultura do Guaranazeiro no Amazonas, 4th ed.; Embrapa Amazônia Ocidental: Manaus, Brazil, 2005. [Google Scholar]
- Araújo, J.C.A.; Pereira, J.C.R.; Gasparotto, L.; Arruda, D.M.R.; Moreira, A. Antracnose do Guaranazeiro e Seu Controle; Embrapa Amazônia Ocidental: Manaus, Brazil, 2007; pp. 3–6. [Google Scholar]
- Araújo, J.C.A.; Pereira, J.C.R.; Gasparotto, L.; Arruda, D.M.R.; Nascimento Filho, D.F.J.; Moreira, A. Avaliação de fungicidas no controle da antracnose do guaranazeiro. In Proceedings of the Embrapa Amazônia Ocidental-Artigo em Anais de Congresso (ALICE), Manaus, Brazil, 31 October 2007. [Google Scholar]
- Baedke, J.; Fábregas-Tejeda, A.; Delgado, A.N. The holobiont concept before Margulis. J. Exp. Zoology Part B Mol. Dev. Evol. 2020, 334, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Margulis, L.; Fester, R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; MIT Press: Cambridge, MA, USA, 1991; p. 470. [Google Scholar]
- Zilber-Rosenberg, I.; Rosenberg, E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol. Rev. 2008, 32, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Toju, H.; Guimarães, P.R.; Olesen, J.M.; Thompson, J.N. Assembly of complex plant-fungus networks. Nat. Commun. 2014, 5, 5273. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2016, 90, 645–655. [Google Scholar] [CrossRef]
- Raghavendra, A.K.H.; Newcombe, G. The contribution of foliar endophytes to quantitative resistance to Melampsora rust. New Phytol. 2013, 197, 909–918. [Google Scholar] [CrossRef]
- Ahlholm, J.U.; Helander, M.; Henriksson, J.; Metzler, M.; Saikkonen, K. Environmental conditions and host genotype direct genetic diversity of Venturia ditricha, a fungal endophyte of birch trees. Evolution 2002, 56, 1566–1573. [Google Scholar] [CrossRef]
- Wäli, P.R.; Helander, M.; Nissinen, O.; Saikkonen, K. Susceptibility of endophyte-infected grasses to winter pathogens (snow molds). Can. J. Bot. 2006, 84, 1043–1051. [Google Scholar] [CrossRef]
- Azevedo Silva, F.; Liotti, R.G.; Boleti, A.P.D.A.; Reis, É.D.M.; Passos, M.B.S.; dos Santos, E.L.; Sampaio, O.M.; Januário, A.H.; Branco, C.L.B.; Silva, D.G.F.; et al. Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS ONE 2018, 13, e0195874. [Google Scholar] [CrossRef]
- Elias, L.M.; Fortkamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, V.Q.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Braz. J. Microbiol. 2018, 49, 840–847. [Google Scholar] [CrossRef]
- Sia, D.E.; Marcon, J.; Luvizotto, D.; Quecine, M.; Tsui, S.; Pereira, J.; Pizzirani-Kleiner, A.; Azevedo, J. Endophytic fungi from the Amazonian plant Paullinia cupana and from Olea europaea isolated using cassava as an alternative starch media source. Springer Plus 2013, 2, 579. [Google Scholar] [CrossRef]
- Souza, D.A.Q.L.; Souza, D.A.D.L.; Astolfi Filho, S.; Pinheiro, M.L.B.; Sarquis, M.I.D.M.; Pereira, J.O. Atividade antimicrobiana de fungos endofíticos isolados de plantas tóxicas da Amazônia: Palicourea longiflora (aubl.) Rich e Strychnos cogens Bentham. Acta Amazon. 2004, 34, 185–195. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 3rd ed.; Burgess Publishing Company: Minneapolis, MI, USA, 1972; p. 241. [Google Scholar]
- Seifert, K.; Morgan-Jones, G.; Gams, W.; Kendrick, B. The Genera of Hyphomycetes; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2011; p. 997. [Google Scholar]
- Pitt, J.I. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press: London, UK, 1979. [Google Scholar]
- Sutton, B.C. The Coelomycetes. Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycol. Inst.: Surrey, UK, 1980; Volume 696. [Google Scholar] [CrossRef]
- Takashio, M. Single-spore and single-cell cultures of fungi. Two new methods particularly useful in the isolation of fungal spores and cells. Ann. Microbiol. 1974, 125A, 45–56. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocls: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Inoculum: London, UK, 1990; pp. 315–322. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Singh, D.K.; Sharma, V.K.; Kumar, J.; Mishra, A.; Verma, S.K.; Sieber, T.N.; Kharwar, R.N. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn.f.: Spatiotemporal and tissue type effects. Sci. Rep. 2017, 7, 3745. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-M.; Zhou, Y.-Q.; Zhou, X.-L.; Xia, X.-H.; Wei, Y.; He, L.-L.; Tang, H.-Z.; Yu, L.-Y. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 2018, 8, 5929. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol. 2000, 147, 617–630. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.D.; Hyde, K.D. Taxonomic placement of sterile morphotypes of endophytic fungi from Pinus tabulaeformis (Pinaceae) in northeast China based on rDNA sequences. Fungal Divers. 2005, 20, 235–260. [Google Scholar]
- Sun, X.; Guo, L.D. Endophytic fungal diversity: Review of traditional and molecular techniques. Mycology 2012, 3, 65–76. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Crous, P.W. Removing chaos from confusion: Assigning names to common human and animal pathogens in Neocosmospora. Persoonia 2018, 41, 109–129. [Google Scholar] [CrossRef]
- Diogo, H.C.; Sarpieri, A.; Pires, M.C. Preservação de fungos em água destilada. Anais Bras. Dermatol. 2005, 80, 591–594. [Google Scholar] [CrossRef]
- Burdsall, H.H.; Dorworth, E.B. Preserving cultures of wood-decaying Basidiomycotina using sterile distilled water in cryovials. Mycologia 2007, 86, 275. [Google Scholar] [CrossRef]
- Okafor, N. Modern Industrial Microbiolohy and Biotechnology; Science Publishers: Enfield, NH, USA, 2007. [Google Scholar]
- Sarma, P.; Dkhar, M.S.; Kayang, H.; Kumar, M.; Dubey, N.K.; Raghuwanshi, R. Diversity of endophytic fungi associated with the medicinally important aromatic plant Gaultheria fragrantissima. Stud. Fungi 2018, 3, 309–320. [Google Scholar] [CrossRef]
- Selim, K.A.; Waill, A.E.; Ahmed, M.T.; Ahmed, A.E.-B.; Tahany, M.A.-R.; Ahmed, I.E.-D.; Eman, F.A. Antiviral and antioxidant potential of fungal endophytes of Egyptian medicinal plants. Fermentation 2018, 4, 49. [Google Scholar] [CrossRef]
- Mahmoud, A.G.Y.; Zaher, E.H.F. Why nuclear ribosomal Internal Transcribed Spacer (ITS) has been selected as the DNA barcode for fungi? Adv. Gen. Eng. 2015, 4, 1–2. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Alfenas, A.C.; Mafia, R.G. Métodos em Fitopatologia, 2nd ed.; Editora UFV: Viçosa, Brazil, 2016. [Google Scholar]
- Carroll, G. Fungal endophytes in stems and leaves: From latent pathogen to mutualistic symbiont. Ecology 1988, 69, 2–9. [Google Scholar] [CrossRef]
- Rajamanikyam, M.; Vadlapudi, V.; amanchy, R.; Upadhyayula, S.M.; Rajamanikyam, M.; Vadlapudi, V.; amanchy, R.; Upadhyayula, S.M. Endophytic fungi as novel resources of natural therapeutics. Braz. Arch. Biol. Technol. 2017, 60. [Google Scholar] [CrossRef]
- Martin, R.; Gazis, R.; Skaltsas, D.; Chaverri, P.; Hibbett, D. Unexpected diversity of basidiomycetous endophytes in sapwood and leaves of Hevea. Mycologia 2015, 107, 284–297. [Google Scholar] [CrossRef]
- Figueiredo, Á.; Silva, A.C.e. Atividade in vitro de extratos de Pycnoporus sanguineus e Lentinus crinitus sobre o fitopatógeno Fusarium sp. Acta Amazon. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Iqbal, M.; Dubey, M.; Gudmundsson, M.; Viketoft, M.; Jensen, D.F.; Karlsson, M. Comparative evolutionary histories of fungal proteases reveal gene gains in the mycoparasitic and nematode-parasitic fungus Clonostachys rosea. BMC Evol. Biol. 2018, 18, 171. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Huang, X.; Zhang, K.Q. Purification and characterization of an extracellular serine protease from Clonostachys rosea and its potential as a pathogenic factor. Process Biochem. 2006, 41, 925–929. [Google Scholar] [CrossRef]
- Zou, C.G.; Xu, Y.F.; Liu, W.J.; Zhou, W.; Tao, N.; Tu, H.H.; Huang, X.W.; Yang, J.K.; Zhang, K.Q. Expression of a serine protease gene prCIs up-regulated by oxidative stress in the fungus Clonostachys rosea: Implications for fungal survival. PLoS ONE 2010, 5, e13386. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.I.S.; Valente, A.M.M.P.; Kavamura, V.N.; Vilela, E.S.D.; Faull, J.L. Mycoparasitic nature of Bionectria sp. strain 6.21. J. Plant Protect. Res. 2014, 54, 327–333. [Google Scholar] [CrossRef]
- Salamone, A.L.; Gundersen, B.; Inglis, D.A. Clonostachys rosea, a potential biological control agent for Rhizoctonia solani AG-3 causing black scurf on potato. Biocontr. Sci. Technol. 2018, 28, 895–900. [Google Scholar] [CrossRef]
- Vivas, J.M.S.; da Silveira, S.F.; dos Santos, P.H.D.; Carvalho, B.M.; Poltronieri, T.P.D.S.; Jorge, T.S.; Santos, J.S.; Kurosawa, R.D.N.F.; de Moraes, R. Antagonism of fungi with biocontrol potential of papaya black spot caused by Asperisporium caricae. Austr. J. Crop Sci. 2018, 12, 827–833. [Google Scholar] [CrossRef]
- Hassan, M.M.; Daffalla, H.M.; Modwi, H.I.; Osman, M.G.; Ahmed, I.I.; Gani, M.E.A.; El, A.; Babiker, G.E. Effects of fungal strains on seeds germination of millet and Striga hermonthica. Univ. J. Agricult. Res. 2013, 2, 83–88. [Google Scholar] [CrossRef]
- Hung, R.; Lee Rutgers, S. Applications of Aspergillus in plant growth promotion. New Future Dev. Microbial Biotechnol. Bioeng. 2016, 223–227. [Google Scholar] [CrossRef]
- Pereira, F.T.; Oliveira, D.J.B.; Muniz, P.H.P.C.; Peixoto, G.H.S.; Guimarães, R.R.; Carvalho, D.D.C.; Pereira, F.T.; Oliveira, D.J.B.; Muniz, P.H.P.C.; Peixoto, G.H.S.; et al. Growth promotion and productivity of lettuce using Trichoderma spp. commercial strains. Horticult. Bras. 2019, 37, 69–74. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kang, S.-M.; Kim, J.-G.; Lee, I.-J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Xia, C.; Li, N.; Zhang, X.; Feng, Y.; Christensen, M.J.; Nan, Z. An Epichloë endophyte improves photosynthetic ability and dry matter production of its host Achnatherum inebrians infected by Blumeria graminis under various soil water conditions. Fungal Ecol. 2016, 22, 26–34. [Google Scholar] [CrossRef]
- Zavala-Gonzalez, E.A.; Rodríguez-Cazorla, E.; Escudero, N.; Aranda-Martinez, A.; Martínez-Laborda, A.; Ramírez-Lepe, M.; Vera, A.; Lopez-Llorca, V.L. Arabidopsis thaliana root colonization by the nematophagous fungus Pochonia chlamydosporia is modulated by jasmonate signaling and leads to accelerated flowering and improved yield. New Phytol. 2017, 213, 351–364. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Naraghi, L.; Heydari, A.; Rezaee, S.; Razavi, M. Biocontrol agent Talaromyces flavus stimulates the growth of cotton and potato. J. Plant Growth Regul. 2012, 31, 471–477. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. The plant growth-promoting fungus Penicillium simplicissimum GP17-2 induces resistance in Arabidopsis thaliana by activation of multiple defense signals. Plant Cell Physiol. 2007, 48, 1724–1736. [Google Scholar] [CrossRef]
- Hamayun, M.; Afzal Khan, S.; Ahmad, N.; Tang, D.-S.; Kang, S.-M.; Na, C.-I.; Sohn, E.-Y.; Hwang, Y.-H.; Shin, D.-H.; Lee, B.-H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Kim, H.; Nam, Y.-J.; Oh, Y.; Jeong, M.-J.; Choi, H.-R.; You, Y.-H.; Choo, Y.-S.; Lee, I.-J.; Shin, J.-H.; et al. Diversity and plant growth promoting capacity of endophytic fungi associated with halophytic plants from the west coast of Korea. Mycobiology 2015, 43, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Chithra, S.; Jasim, B.; Mathew, J.; Radhakrishnan, E.K. Endophytic Phomopsis sp. colonization in Oryza sativa was found to result in plant growth promotion and piperine production. Physiol. Plant. 2017, 160, 437–446. [Google Scholar] [CrossRef]
- Berlanas, C.; Berbegal, M.; Elena, G.; Laidani, M.; Cibriain, J.F.; Sagües, A.; Gramaje, D. The fungal and bacterial rhizosphere microbiome associated with grapevine rootstock genotypes in mature and young vineyards. Front. Microbiol. 2019, 10, 1142. [Google Scholar] [CrossRef]
- Toju, H.; Okayasu, K.; Notaguchi, M. Leaf-associated microbiomes of grafted tomato plants. Sci. Rep. 2019, 9, 1787. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.D.; Crous, P.W.; Ades, P.K.; Hyde, K.D.; Taylor, P.W.J. Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biol. Rev. 2017, 31, 155–168. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. Dissecting endophytic lifestyle along the parasitism/mutualism continuum in Arabidopsis. Curr. Opin. Microbiol. 2016, 32, 103–112. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nat. Genet. 2012, 44, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Rhoden, S.A.; Mota, T.R.; Azevedo, J.L.; Pamphile, J.A.; de Souza, C.G.M.; Polizeli, M.D.L.T.D.M.; Bracht, A.; Peralta, R.M. Endophytic fungi: Expanding the arsenal of industrial enzyme producers. J. Ind. Microbiol. Biotechnol. 2014, 41, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K.; Zabalgogeazcoa, I.; Vázquez de Aldana, B.R.; Boyom, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S. Afr. J. Bot. 2017, 109, 146–153. [Google Scholar] [CrossRef]
- Eken, C.; Demirci, E. First report of Colletotrichum truncatum on alfalfa in Turkey. Plant Dis. 2007, 84, 100. [Google Scholar] [CrossRef]
- Yang, H.C.; Haudenshield, J.S.; Hartman, G.L. First report of Colletotrichum chlorophyti causing soybean anthracnose. Plant Dis. 2012, 96, 1699. [Google Scholar] [CrossRef]
- Górzyńska, K.; Węgrzyn, E.; Sandecki, R.; Lembicz, M. Endophytic fungi and latent pathogens in the sedge Carex secalina (Cyperaceae), a critically endangered species in Europe. Plant Protect. Sci. 2019, 55, 102–108. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; McKenzie, E.H.C.; Hyde, K.D.; Photita, W.; Lumyong, S.; Lumyong, P.; Hyde, M.E.H.C. Are some endophytes of Musa acuminata latent pathogens? Fungal Divers. 2004, 16, 131–140. [Google Scholar]
- Sessa, L.; Abreo, E.; Lupo, S. Diversity of fungal latent pathogens and true endophytes associated with fruit trees in Uruguay. J. Phytopathol. 2018, 166, 633–647. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Soumya, P.R.; Rukshana Begum, S.; Tamil Selvi, K.S. Endophytic fungi as latent pathogens in Eichhornia crassipes (Mart.) Solms. Int. J. Adv. Sci. Res. Manag. 2018, 3, 140–146. [Google Scholar]
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar] [CrossRef]
- Verhoeff, K. Latent infections by fungi. Ann. Rev. Phytopathol. 1974, 12, 99–110. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.A.; Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.R. Variabilidade Genética e Estimativas de Parâmetros Genéticos em Germoplasma de Guaranazeiro; Embrapa Amazônia Ocidental: Maues, Brazil, 2004. [Google Scholar]
- Freeman, S.; Rodriguez, R.J. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 1993. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2018. [Google Scholar] [CrossRef]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Lombard, L.; Wingfield, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 31–49. [Google Scholar] [CrossRef]
- Unterseher, M.; Gazis, R.; Chaverri, P.; Guarniz, C.F.G.; Tenorio, D.H.Z. Endophytic fungi from Peruvian highland and lowland habitats form distinctive and host plant-specific assemblages. Biodivers. Conserv. 2013, 22, 999–1016. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Nokso-Koivisto, J.; Hottola, J.; Rajala, T.; Pennanen, T.; Ali-Kovero, H.; Miettinen, O.; Oinonen, P.; Auvinen, P.; Paulin, L.; et al. Identifying wood-inhabiting fungi with 454 sequencing—What is the probability that BLAST gives the correct species? Fungal Ecol. 2010, 3, 274–283. [Google Scholar] [CrossRef]
- Tedersoo, L.; Nilsson, R.H.; Abarenkov, K.; Jairus, T.; Sadam, A.; Saar, I.; Bahram, M.; Bechem, E.; Chuyong, G.; Kõljalg, U. 454 Pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol. 2010, 188, 291–301. [Google Scholar] [CrossRef]
- Unterseher, M.; Schnittler, M. Dilution-to-extinction cultivation of leaf-inhabiting endophytic fungi in beech (Fagus sylvatica L.)—Different cultivation techniques influence fungal biodiversity assessment. Mycol. Res. 2009, 113, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yao, Y.-F. Endophytic fungal communities associated with vascular plants in the high Arctic zone are highly diverse and host-plant specific. PLoS ONE 2015, 10, e0130051. [Google Scholar] [CrossRef] [PubMed]
- Christian, N.; Sullivan, C.; Visser, N.D.; Clay, K. Plant host and geographic location drive endophyte community composition in the face of perturbation. Microbial Ecol. 2016, 72, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol Res. 2008, 112, 331–344. [Google Scholar] [CrossRef]
- David, A.S.; Seabloom, E.W.; May, G. Plant host species and geographic distance affect the structure of aboveground fungal symbiont communities, and environmental filtering affects belowground communities in a coastal dune ecosystem. Microbial Ecol. 2016, 71, 912–926. [Google Scholar] [CrossRef]
- De Arruda, M.R.; Clério, J.; Pereira, R.; Moreira, A.; Geraldes Teixeira, W. Survival rate of guarana herbaceous cuttings in different substrates. Cienc. Agrotecnol. 2007, 31, 236–241. [Google Scholar]
- Garcia, T.B.; Nascimento Filho, D.F.J. O Cultivo do Guarana no Amazonas; Embrapa Amazônia Ocidental: Manaus, Brazil, 1999. [Google Scholar]
- Albertino, S.M.F.; Filho, F.J.D.N.; da Silva, J.F.; Atroch, A.L.; Galvão, A.K.D.L. Enraizamento de estacas de cultivares de guaranazeiro com adubação de plantas matrizes. Pesqui. Agropecu. Bras. 2012, 47, 1449–1454. [Google Scholar] [CrossRef]
- Plácido, C.G.; Moreira, A.; Moraes, L.A.C. Spacing and plant density in the yield components, nutritional status, and soil fertility of guarana varieties grown in humid tropical Amazon. Commun. Soil Sci. Plant Anal. 2015, 46, 1551–1565. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]



| Leaves | Seeds | |||||
|---|---|---|---|---|---|---|
| Cultivar | BRS300 | BRS871 | BRS300 | BRS871 | Total | |
| Local | Manaus | 1533 | 1947 | 19 | 11 | 3510 |
| Maués | 2721 | 1240 | 23 | 20 | 4004 | |
| Total | 4254 | 3187 | 42 | 31 | 7514 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.; Santos da Silva, B.N.; Amorim Ferreira e Ferreira, A.F.T.; Santos, C.; Lima, N.; Silva Bentes, J.L.d. Fungal Endophytic Community Associated with Guarana (Paullinia cupana Var. Sorbilis): Diversity Driver by Genotypes in the Centre of Origin. J. Fungi 2020, 6, 123. https://doi.org/10.3390/jof6030123
Santos C, Santos da Silva BN, Amorim Ferreira e Ferreira AFT, Santos C, Lima N, Silva Bentes JLd. Fungal Endophytic Community Associated with Guarana (Paullinia cupana Var. Sorbilis): Diversity Driver by Genotypes in the Centre of Origin. Journal of Fungi. 2020; 6(3):123. https://doi.org/10.3390/jof6030123
Chicago/Turabian StyleSantos, Carla, Blenda Naara Santos da Silva, Ana Francisca Tibúrcia Amorim Ferreira e Ferreira, Cledir Santos, Nelson Lima, and Jânia Lília da Silva Bentes. 2020. "Fungal Endophytic Community Associated with Guarana (Paullinia cupana Var. Sorbilis): Diversity Driver by Genotypes in the Centre of Origin" Journal of Fungi 6, no. 3: 123. https://doi.org/10.3390/jof6030123
APA StyleSantos, C., Santos da Silva, B. N., Amorim Ferreira e Ferreira, A. F. T., Santos, C., Lima, N., & Silva Bentes, J. L. d. (2020). Fungal Endophytic Community Associated with Guarana (Paullinia cupana Var. Sorbilis): Diversity Driver by Genotypes in the Centre of Origin. Journal of Fungi, 6(3), 123. https://doi.org/10.3390/jof6030123







