Fungal Pigments and Their Roles Associated with Human Health
Abstract
:1. Introduction
2. Types of Fungal Pigments and Their Relevance to Human Health
2.1. Carotenoids
2.2. Melanin
2.3. Polyketides
2.4. Azaphilones
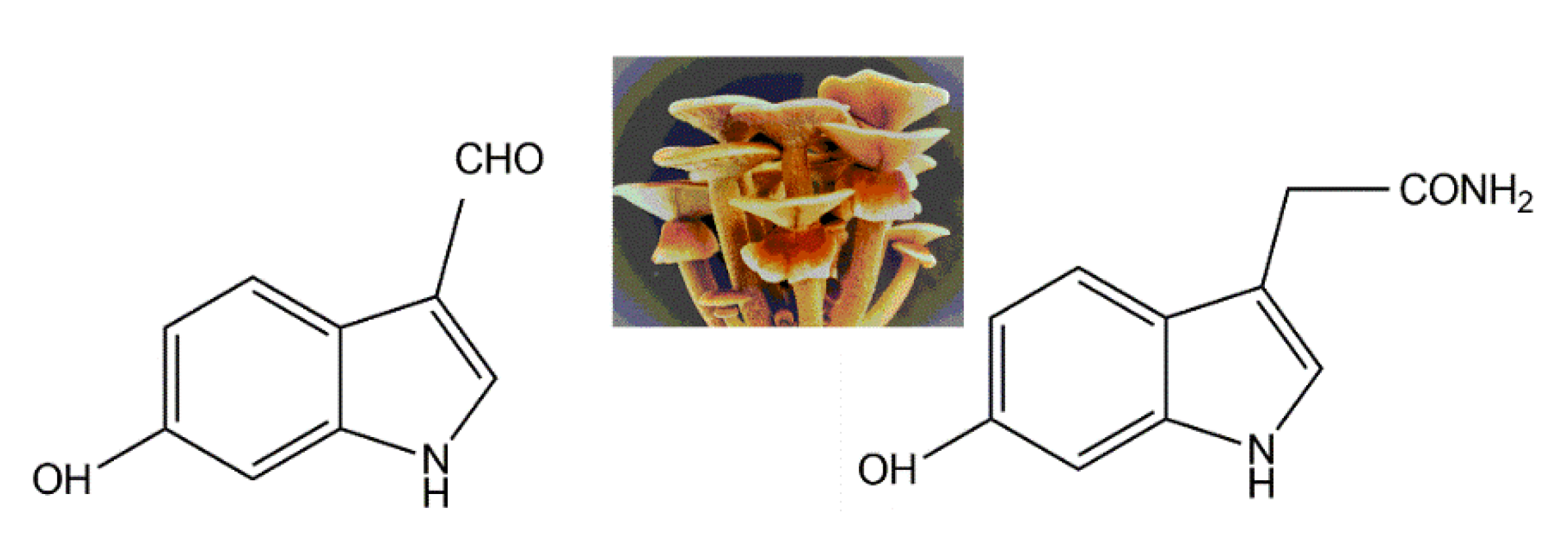
2.5. Other Fungal Pigments
3. Biosynthesis of Fungal Pigments
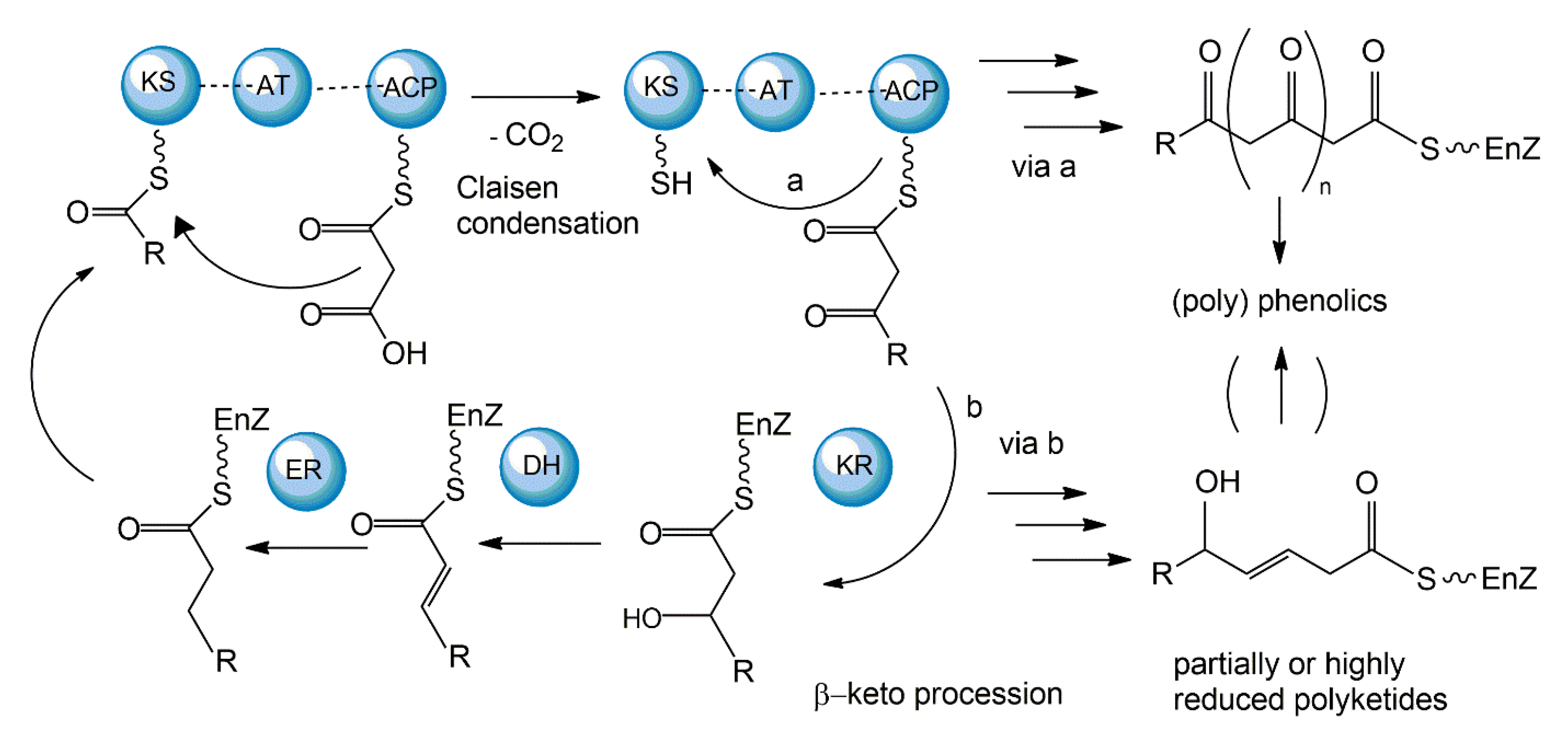
3.1. Polyketide Synthetic Pathways
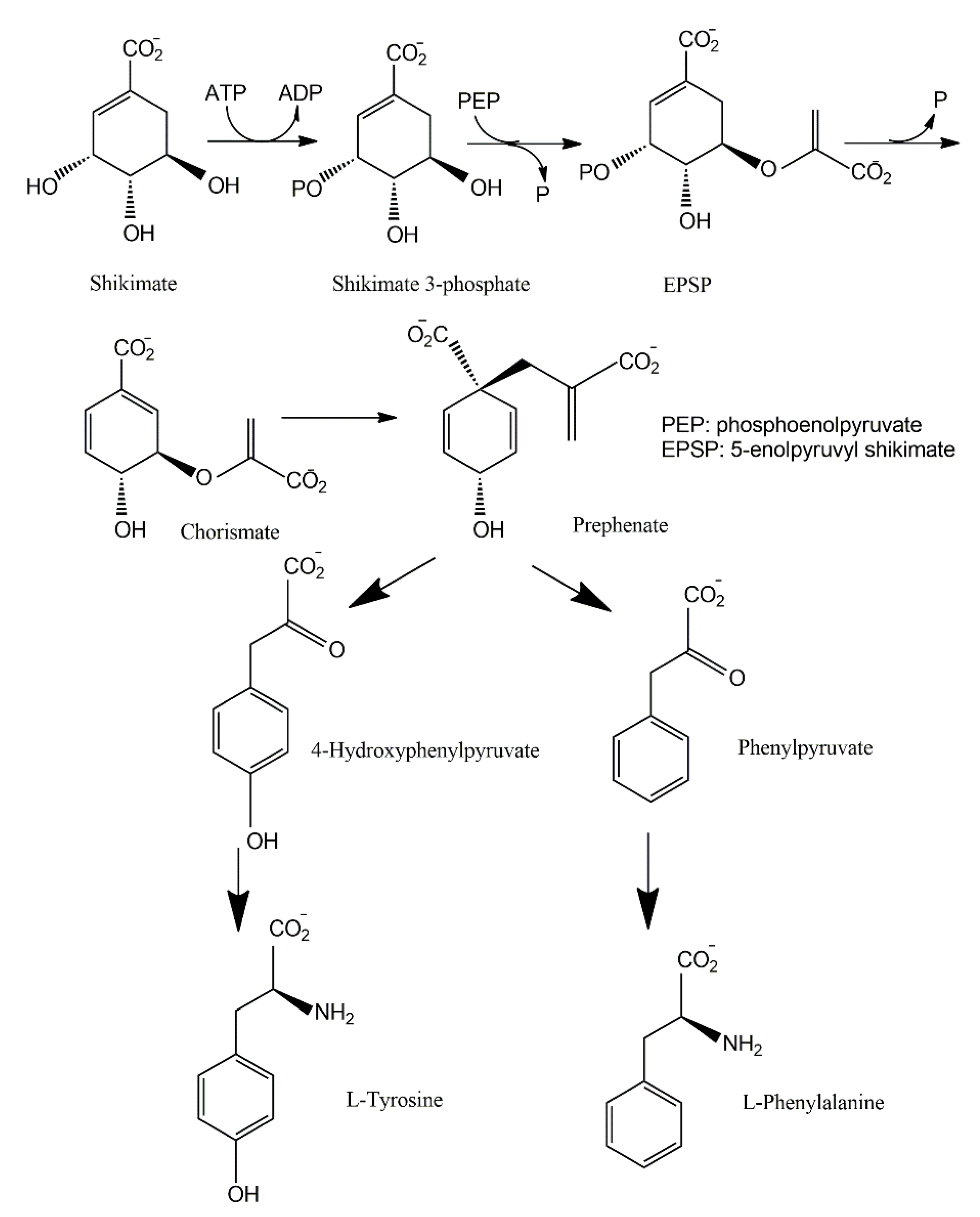
3.2. Shikimate Pathways
3.3. Terpenoid Synthetic Pathways
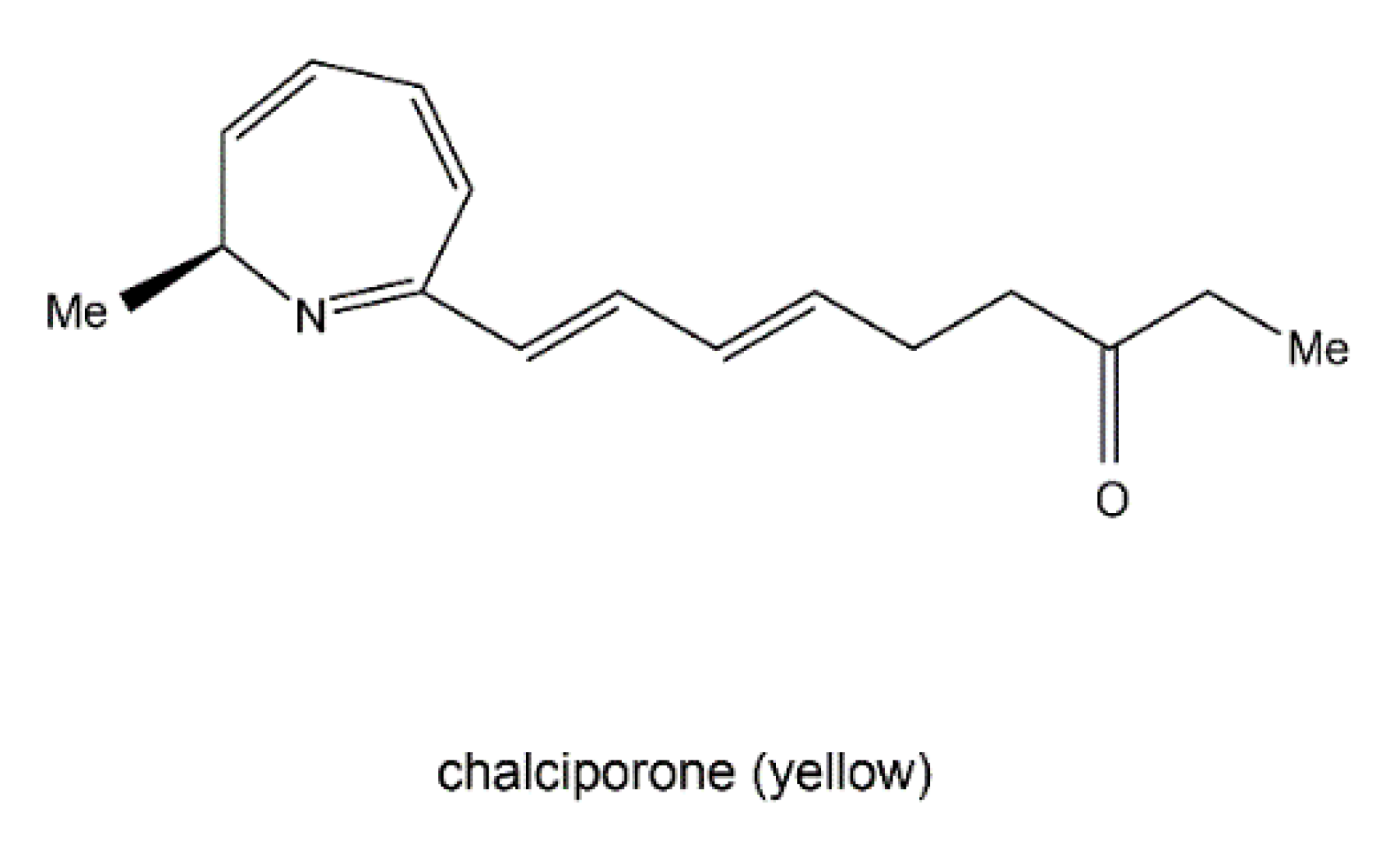
3.4. Nitrogen-Containing Metabolite Pathways
4. Interaction of Fungal Pigments with the Host Immune System
5. Medical Relevance of Fungal Pigments
5.1. Medical Roles of Melanins
5.2. Medical Roles of Other Pigments
5.2.1. Anti-Tumor Activities
5.2.2. Anti-Biofilm Activity
5.2.3. Photosensitizers
5.2.4. Cholesterol-Lowering and/or Anti-Atherosclerotic Agents
5.2.5. Promising Anti-Alzheimer Agents
5.2.6. Anti-Inflammatory Activity
5.2.7. Antimicrobial Activities
5.2.8. Others
6. Structure–Activity Relationship (SAR) Studies of Fungal Pigments
7. Conclusions and Further Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demain, A.L.; Vandamme, E.J.; Collins, J.; BuchhoIz, K. History of industrial biotechnology. In Industrial Biotechnology: Microorganisms; Wittmann, C., Liao, J.C., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 3–84. [Google Scholar]
- Boral, H.; Metinb, B.; Döğenc, A.; Seyedmousavi, S.; Ilkit, M. Overview of selected virulence attributes in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum and Exophiala dermatitidis. Fungal Genet. Biol. 2018, 111, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Poorniammal, R. Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898. [Google Scholar] [CrossRef] [Green Version]
- Méndez, A.; Pérez, C.; Monta˜éz, J.C.; Martínez, G.; Aguilar, C.N. Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J. Zhejiang Univ. Sci. B (Biomed. Biotechnol.) 2011, 12, 961–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akilandeswari1, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Celestino, J.; De Carvalho, L.E.; Da Paz Lima, M.; Lima, A.M.; Ogusku, M.M.; De Souza, J.V.B. Bioprospecting of amazon soil fungi with the potential for pigment production. Process Biochem. 2014, 49, 569–575. [Google Scholar] [CrossRef]
- Dufosse, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishanka, G.A. Microorganisms and microalgae as source of pigments for use: A scientific oddity or an industrial reality? Trends. Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Lee, D.; Jang, E.-H.; Lee, M.; Kim, S.-W.; Lee, Y.; Lee, K.-T.; Bahn, Y.-S. Unraveling melanin biosynthesis and signaling networks in Cryptococcus neoformans. mBio 2019, 10, e02267-19. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.-K.; Cary, J.W.; Lebar, M.D. Biosynthesis of conidial and sclerotial pigments in Aspergillus species. Appl. Microbiol. Biotech. 2020, 104, 2277–2286. [Google Scholar] [CrossRef]
- Brilhantea, R.S.N.; da Rochab, M.G.; de Oliveiraa, J.S.; Pereira-Neto, W.A.; de Melo Guedes, G.M.; de Aguiar Cordeiro, R.; Sidrim, J.J.; Rocha, M.F.; Castelo, D.D. Cryptococcus neoformans/Cryptococcus gattii species complex melanized by epinephrine: Increased yeast survival after amphotericin B exposure. Microb. Pathog. 2020, 143, 104123. [Google Scholar] [CrossRef]
- Santos, L.A.; Grisolia, J.C.; Burger, E.; de Araujo Paula, F.B.; Dias, A.L.T.; Malaquias, L.C.C. Virulence factors of Paracoccidioides brasiliensis as therapeutic targets: A review. Antonie Leeuwenhoek Int. J. Gen. 2020, 113, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Avalos, J.; Díaz-Sánchez, V.; García-Martínez, J.; Castrillo, M.; Ruger-Herreros, M.; Limón, M.C. Carotenoids. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Martín, J.F., García-Estrada, C., Zeilinger, S., Eds.; Springer: New York, NY, USA, 2014; pp. 149–185. [Google Scholar]
- Barredo, J.L.; García-Estrada, C.; Kosalkova, K.; Barreiro, C. Biosynthesis of astaxanthin as a main carotenoid in the heterobasidiomycetous yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avalos, J.; Limón, M.C. Biological roles of fungal carotenoids. Curr. Genet. 2015, 61, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Mata-Gómez, L.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Dufosse, L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006, 44, 313–321. [Google Scholar]
- Panesar, R.; Kaur, S.; Panesar, P.S. Production of microbial pigments utilizing agro-industrial waste: A review. Curr. Opin. Food. Sci. 2015, 1, 70–76. [Google Scholar] [CrossRef]
- Prado-Cabrero, A.; Scherzinger, D.; Avalos, J.; Al-Babili, S. Retinal biosynthesis in fungi: Characterization of the carotenoid oxygenase CarX from Fusarium fujikuroi. Eukaryot. Cell 2007, 6, 650–657. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Yuan, Q.-P.; Zhu, Y. Improved production of lycopene and β-carotene by Blakeslea trispora with oxygen-vectors. Process Biochem. 2007, 42, 289–293. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montaneza, J.C.; Aguilar-González, M.A.; Martínez-Ávila, C.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhodotorula glutinis as source of pigments and metabolites for food industry. Food Biosci. 2014, 5, 64–72. [Google Scholar] [CrossRef]
- Phaff, H.; Miller, M.; Yoneyama, M.; Soneda, M. A comparative study of the yeast florae associated with trees on the Japanese Islands and on the west coast of North America. In Proceedings of the 4th International Fermentation Symposium: Fermentation Technology Today, Kyoto, Japan, 19–25 March 1972; pp. 759–774. [Google Scholar]
- Andrewes, A.G.; Starr, M.P. (3R,3’R)-Astaxanthin from the yeast Phaffia rhodozyma. Phytochemistry 1976, 15, 1009–1011. [Google Scholar] [CrossRef]
- Rodriguez-Saiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2010, 8, 555–571. [Google Scholar] [CrossRef]
- Mathewaroth, M.M. Carotenoids in Erythropoietic protoporphyria and other photosensitivity diseases. Ann. N. Y. Acad. Sci. 1993, 691, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, G. Carotenoids of biotechnological importance. In Biotechnology of Isoprenoids, Advances in Biochemical Engineering/Biotechnology; Schrader, J., Bohlmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 148, pp. 449–467. [Google Scholar]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef]
- Wheeler, M.H.; Bell, A.A. Melanins and their importance in pathogenic fungi. Curr. Top. Med. Mycol. 1988, 2, 338–387. [Google Scholar] [PubMed]
- Perez-Cuesta, U.; Aparicio-Fernandez, L.; Guruceaga, X.; Martin-Souto, L.; Abad-Diaz-de-Cerio, A.; Antoran, A.; Buldain, I.; Hernando, F.L.; Ramirez-Garcia, A.; Rementeria, A. Melanin and pyomelanin in Aspergillus fumigatus: From its genetics to host interaction. Int. Microbiol. 2020, 23, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Heinekamp, T.; Thywißen, A.; Macheleidt, J.; Keller, S.; Valiante, V.; Brakhage, A.A. Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2012, 3, 440. [Google Scholar] [CrossRef] [Green Version]
- Morris-Jones, R.; Gomez, B.L.; Diez, S.; Uran, M.; Morris-Jones, S.D.; Casadevall, A.; Nosanchuk, J.D.; Hamilton, A.J. Synthesis of melanin pigment by Candida albicans in vitro and during infection. Infect. Immun. 2005, 73, 6147–6150. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.A.; Gomez, B.L.; Mora-Montes, H.M.; Mackenzie, K.S.; Munro, C.A.; Brown, A.J.; Gow, N.A.; Kibbler, C.C.; Odds, F.C. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot. Cell 2010, 9, 1329–1342. [Google Scholar] [CrossRef] [Green Version]
- Solano, F. Melanins: Skin pigments and much More—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef] [Green Version]
- Weijn, A.; van den Berg-Somhorst, D.B.P.M.; Slootweg, J.C.; Vincken, J.-P.; Gruppen, H.; Wichers, H.J.; Mes, J.J. Main phenolic compounds of the melanin biosynthesis pathway in bruising-tolerant and bruising-sensitive button mushroom (Agaricus bisporus) strains. J. Agric. Food Chem. 2013, 61, 8224–8231. [Google Scholar] [CrossRef] [PubMed]
- Belozerskaya, T.A.; Gessler, N.N.; Averýanov, A.A. Melanin pigments of fungi. In Fungal Metabolites. Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–29. [Google Scholar]
- Pacelli, C.; Cassaro, A.; Maturilli, A.; Timperio, A.M.; Gevi, F.; Cavalazzi, B.; Stefan, M.; Ghica, D.; Onofri, S. Multidisciplinary characterization of melanin pigments from the black fungus Cryomyces antarcticus. Appl. Microbiol. Biotechnol. 2020, 104, 6385–6395. [Google Scholar] [CrossRef] [PubMed]
- Ambrico, M. Special issue: Melanin, a long lasting history bridging natural pigments and organic bioelectronics. Polym. Int. 2016, 65, 1249–1250. [Google Scholar] [CrossRef] [Green Version]
- Kogej, T.; Wheeler, M.H.; Lanišnik RiŽner, T.; Gunde-Cimerman, N. Evidence for 1,8-dihydroxynaphthalene melanin in three halophilic black yeasts grown under saline and non-saline conditions. FEMS Microbiol. Lett. 2004, 232, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U.; Risvad, J.C.F. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale microbial cell factories. Microb. Cell Fact. 2009, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mapari, S.A.S.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants. Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef]
- Hobson, D.K.; Wales, D.S. Green dyes. J. Stud. Dyn. Chang. 1998, 114, 42–44. [Google Scholar] [CrossRef]
- Durán, N.; Teixeira, M.F.S.; Conti, R.D.; Esposito, E. Ecological-friendly pigments from fungi. Crit. Rev. Food Sci. Nutr. 2002, 42, 53–66. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Rana, K.L.; Yadav, N.; Singh, B.; Chauhan, V.S.; Rastegari, A.A.; Hesham, A.E.; Gupta, V.K. Metabolic engineering to synthetic biology of secondary metabolites production. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–320. [Google Scholar]
- Venil, C.K.; Velmurugan, P.; Devi, P.R.; Dufossé, L.; Ravi, A.V. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Gesslera, N.N.; Egorovaa, A.S.; Belozerskaya, T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013, 49, 85–99. [Google Scholar] [CrossRef]
- Augustin, N.; Nuthakki, V.K.; Abdullaha, M.; Hassan, Q.P.; Gandhi, S.G.; Bharate, S.B. Discovery of helminthosporin, an anthraquinone isolated from Rumex abyssinicus Jacq as a dual cholinesterase inhibitor. ACS Omega 2020, 5, 1616–1624. [Google Scholar] [CrossRef] [Green Version]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufossé, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Nielsen, K.F.; Larsen, T.O.; Frisvad, J.C.; Meyer, A.S.; Thrane, U. Exploring fungal biodiversity for the production of water-soluble pigments as potential natural food colorants. Curr. Opin. Biotechnol. 2005, 16, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, T.; Tao, H.; Lu, Z.; Fang, Y.; Gu, Q.; Zhu, W. Two new cytotoxic quinone type compounds from the halotolerant fungus Aspergillus variecolor. J. Antibiot. 2007, 60, 603–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.L.; Li, D.Y.; Xie, L.R.; Wu, X.; Hua, H.M.; Li, Z.L. Two new compounds from a marine-derived fungus Penicillium oxalicum. Nat. Prod. Res. 2014, 28, 290–293. [Google Scholar] [CrossRef]
- Li, S.-W.; Yang, T.-C.; Lai, C.-C.; Huang, S.-H.; Liao, J.-M.; Wan, L.; Lin, Y.-J.; Lin, C.-W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharm. 2014, 738, 125–132. [Google Scholar] [CrossRef]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV1, antioxidant and antifouling phenolic compounds from the deep sea derived fungus Aspergillus versicolor SCSIO41502. Bioorg. Med. Chem. Lett. 2017, 15, 787–791. [Google Scholar] [CrossRef]
- Miliani, C.; Romani, A.; Favaro, G. Acidichromic effects in 1,2-di- and 1,2,4-tri-hydroxyanthraquinones. A spectrophotometric and fluorimetric study. J. Phys. Org. Chem. 2000, 13, 141–150. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Havel, L.; Kizek, R. Noteworthy secondary metabolites naphthoquinones—Occurrence, pharmacological properties and analysis. Curr. Pharm. Anal. 2009, 5, 47–68. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Dufossé, L.; Caro, Y. Putative metabolic pathway for the bioproduction of bikaverin and intermediates thereof in the wild Fusarium oxysporum LCP531 strain. AMB Express 2019, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Burns, A.M.; Liu, M.X.; Faeth, S.H.; Gunatilaka, A.A. Search for cell mobility and angiogenesis inhibitors with potential anticancer activity: Beauvericin and other constituents of two endophytic strains of Fusarium oxysporum. J. Nat. Prod. 2007, 70, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.W.; Kim, H.Y.; Choi, G.J.; Lim, H.K.; Jang, K.S.; Lee, S.O.; Lee, S.; Sung, N.D.; Kim, J.C. Bikaverin and fusaric acid from Fusarium oxysporum show antioomycete activity against Phytophtora infestans. J. Appl Microbiol. 2008, 104, 692–698. [Google Scholar] [CrossRef]
- Limón, M.C.; Rodríguez-Ortiz, R.; Avalos, J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nirmaladevi, D.; Venkataramana, M.; Chandranayaka, S.; Ramesha, A.; Jameel, N.M.; Srinivas, C. Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar] [CrossRef]
- Sturdikova, M.; Slugen, D.; Lesova, K.; Rosenberg, M. Microbial production of coloured azaphiolone metabolites. Chem. Listy 2000, 94, 105–110. [Google Scholar]
- Zhu, J.; Nicholas, P.; Grigoriadis, N.P.; Lee, J.P.; Porco, J.A. Synthesis of the azaphilones using copper-mediated enantioselective oxidative dearomatization. J. Am. Chem. Soc. 2005, 127, 9342–9343. [Google Scholar] [CrossRef]
- Dong, J.; Zhou, Y.; Li, R.; Zhou, W.; Li, L.; Zhu, Y.; Huang, R.; Zhang, K. New nematicidal azaphilonesfrom the aquatic fungus Pseudohalonectria adversaria YMF1.01019. FEMS Microbiol. Lett. 2006, 264, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef]
- Nakanishi, K. Studies in microbial and insect natural products chemistry. J. Nat. Med. 2006, 60, 2–20. [Google Scholar] [CrossRef]
- Gill, M. Pigments of fungi (Macromycetes). Nat. Prod. Rep. 2003, 20, 615–639. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Meyer, A.S.; Thrane, U. Colorimetric characterization for comparative analysis of fungal pigments and natural food colorants. J. Agric. Food Chem. 2006, 54, 7027–7035. [Google Scholar] [CrossRef] [PubMed]
- Jou, P.C.; Ho, B.Y.; Hsu, Y.W.; Pan, T.M. The effect of Monascus secondary polyketide metabolites, monascin and ankaflavin, on adipogenesis and lipolysis activity in 3T3-L1. J. Agric. Food Chem. 2010, 58, 12703–12709. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Kung, Y.H.; Wu, C.L.; Hsu, Y.W.; Pan, T.M. Monascin and ankaflavin act as novel hypolipidemic and high-density lipoprotein cholesterol-raising agents in red mold dioscorea. J. Agric. Food Chem. 2010, 59, 8199–8207. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Wen, J.-Y.; Hsu, Y.-W.; Pan, T.-M. Monascus-fermented yellow pigments monascin and ankaflavin showed antiobesity effect via the suppression of differentiation and lipogenesis in obese rats fed a high-fat diet. J. Agric. Food Chem. 2013, 61, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Hung, Y.-P.; Hsu, Y.-W.; Pan, T.-M. Monascin and ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages. J. Agric. Food Chem. 2013, 61, 143–150. [Google Scholar] [CrossRef]
- Cheng, C.-F.; Pan, T.-M. Monascus-fermented red mold dioscorea protects mice against alcohol-induced liver injury, whereas its metabolites ankaflavin and monascin regulate ethanol-induced peroxisome proliferator-activated receptor-γ and sterol regulatory element-binding transcription factor-1 expression in HepG2 cells. J. Sci. Food Agric. 2018, 98, 1889–1898. [Google Scholar]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Iyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, G.J.; Jang, K.S.; Lim, H.K.; Kim, H.T.; Cho, K.W.; Kim, J.-C. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol. Lett. 2005, 252, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Takahashi, M.; Natori, S.; Kawai, K.; Yamazaki, M.; Takeuchi, M.; Takido, M. Azaphilones inhibit tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mice. Oncology 1994, 51, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H.; Matsushima, C.; Tabata, N.; Namatame, I.; Tanaka, H.; Bamberger, M.J.; Arai, H.; Fukazawa, M.; Inoue, K.; Omura, S. Structure-specific inhibition of cholesteryl ester transfer protein by azaphilones. J. Antibiot. 1999, 52, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chidananda, C.; Rao, L.J.M.; Sattur, A.P. Sclerotiorin, from Penicillium frequentans, a potent inhibitor of aldose reductase. Biotechnol. Lett. 2006, 28, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Pairet, L.; Wrigley, S.K.; Chetland, I.; Reynolds, I.E.; Hayes, M.A.; Holloway, J.; Ainsworth, A.M.; Katzer, W.; Cheng, X.-M.; Hupe, D.J.; et al. Azaphilones with endothelin receptor binding activity produced by Penicillium sclerotiorum: Taxonomy, fermentation, isolation, structure elucidation and biological activity. J. Antibiot. 1995, 48, 913–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, E.M.F.; de Castro, M.C.M.; Takahashi, J.A. Antimicrobial properties of sclerotionrin, isochromophilone VI and pencolide, metabolites from a Brazilian cerrado isolate of Penicillum sclerotiorum van beyma. Braz. J. Microbiol. 2007, 38, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Giridharan, P.; Verekar, S.A.; Khanna, A.; Mishra, P.D.; Deshmukh, S.K. Anticancer activity of sclerotiorin, isolated from an endophytic fungus Cephalotheca faveolata Yaguchi, Nishim & Udagawa. Indian J. Exp. Biol. 2012, 50, 464–468. [Google Scholar] [PubMed]
- Arunpanichlert, J.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Tewtrakul, S.; Rungjindamai, N.; Sakayaroj, J. Azaphilone and isocoumarin derivatives from the endophytic fungus Penicillium sclerotiorum PSU-A13. Chem. Pharm. Bull. 2010, 58, 1033–1036. [Google Scholar] [CrossRef] [Green Version]
- Salo, O.; Guzmán-Chávez, F.; Ries, M.I.; Lankhorst, P.P.; Bovenberg, R.A.L.; Vreeken, R.J.; Driessen, A.J.M. Identification of a polyketide synthase involved in sorbicillin biosynthesis by Penicillium chrysogenum. Appl. Environ. Microbiol. 2016, 82, 3971–3978. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Chu, J. Characterization of two polyketide synthases involved in sorbicillinoid biosynthesis by Acremonium chrysogenum using the CRISPR/Cas9 system. Appl. Biochem. Biotechnol. 2019, 188, 1134–1144. [Google Scholar] [CrossRef]
- Pastre, R.; Marinho, A.M.R.; Rodrigues-Filho, E.; Souza, A.Q.L.; Pereira, J.O. Diversity of polyketides produced by Penicillium species isolated from Melia azedarach and Murraya paniculata. Quim. Nova 2007, 30, 1867–1871. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.F.S.; Martins, M.S.; Da Silva, J.C.; Kirsch, L.S.; Fernandes, O.C.C.; Carneiro, A.L.B.; Da Conti, R.; Durán, N. Amazonian biodiversity: Pigments from Aspergillus and Penicillium–characterizations, antibacterial activities and their toxicities. Curr. Trends Biotechnol. Pharm. 2012, 6, 300–311. [Google Scholar]
- Velíšek, J.; Davídek, J.; Cejpek, K. Biosynthesis of food constituents: Natural pigments. Part 1—A review. Czech J. Food Sci. 2007, 25, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Velíšek, J.; Cejpek, K. Pigments of higher fungi: A review. Czech J. Food Sci. 2011, 29, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Fujii, I.; Watanabe, A.; Sankawa, U.; Ebizuka, Y. Identification of claisen cyclase domain in fungal polyketide synthase WA, a naphthopyrone synthase of Aspergillus nidulans. Chem. Biol. 2001, 8, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Wohlert, S.E.; Wendt-Pienkowski, E.; Bao, W.L.; Hutchinson, C.R. Production of aromatic minimal polyketides by the daunorubicin polyketide synthase genes reveals the incompatibility of the heterologous DpsY and JadI cyclases. J. Nat. Prod. 2001, 64, 1077–1080. [Google Scholar] [CrossRef]
- Schümann, J.; Hertweck, C. Advances in cloning, functional analysis and heterologous expression of fungal polyketide synthase genes. J. Biotechnol. 2006, 124, 690–703. [Google Scholar] [CrossRef]
- Hajjaj, H.; Klaébé, A.; Goma, G.; Blanc, P.J.; Barbier, E.; Francois, J. Medium-chain fatty acids Affect citrinin production in the filamentous fungus Monascus ruber. Appl. Envriron. Microbiol. 2000, 66, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Velíšek, J.; Davídek, J.; Cejpek, K. Biosynthesis of food constituents: Natural pigments. Part 2—A review. Czech J. Food Sci. 2008, 26, 73–98. [Google Scholar] [CrossRef] [Green Version]
- Jensen, R.A.; Pierson, D.A. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature 1975, 254, 667–671. [Google Scholar] [CrossRef]
- Musso, H. The pigments of fly agaric, Amanita muscaria. Tetrahedron 1979, 35, 2843–2853. [Google Scholar] [CrossRef]
- Von Ardenne, R.; Döpp, H.; Musso, H.; Steiglich, W. Über das vorkommen von muscaflavin bei hygrocyben (Agaricales) und seine dihydroazepin-strukture. Z. Nat. C 1974, 29, 637–639. [Google Scholar]
- Hallsworth, J.E.; Magan, N. Culture age, temperature, and pH affect the polyol and trehalose contents of fungal propagules. Appl. Environ. Microbiol. 1996, 62, 2435–2442. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Sharma, K.; Sharma, A.; Garg, A.; Kumar, S.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultraviolet irradiation. Pharmacogn. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Ghazaei, C. Molecular insights into pathogenesis and infection with Aspergillus fumigatus. Malays. J. Med. Sci. 2017, 24, 10–20. [Google Scholar] [CrossRef]
- Marcos, C.M.; de Oliveira, H.C.; de Melo, W.D.; da Silva, J.D.; Assato, P.A.; Scorzoni, L.; Rossi, S.A.; de Paula e Silva, A.C.; Mendes-Giannini, M.J.; Almeida, A.M. Anti-immune strategies of pathogenic fungi. Front. Cell. Infect. Microbiol. 2016, 6, 142. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, A.R.; Lamb, H.K.; Moore, J.D.; Charles, I.G.; Roberts, C.F. The pre-chorismate (shikimate) and quinate pathways in filamentous fungi: Theoretical and practical aspects. J. Gen. Microbiol. 1993, 139, 2891–2899. [Google Scholar] [CrossRef] [Green Version]
- Kὅgl, F.; Becker, H.; de Voss, G.; Wirth, E.L. Untersuchungen über Pilzfarbstoffe. VII. Die Synthese des Atromentins. Zur Kenntnis der Atromentinsäure. Ann. Chem. 1928, 465, 243. [Google Scholar] [CrossRef]
- Liu, J. Natural terphenyls: Developments since 1877. Chem. Rev. 2006, 106, 2209–2223. [Google Scholar] [CrossRef]
- Dewick, P.M. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 1994, 11, 173–203. [Google Scholar] [CrossRef]
- Tauber, J.P.; Gallegos-Monterrosa, R.; Kovacs, A.T.; Shelest, E.; Hoffmeister, D. Dissimilar pigment regulation in Serpula lacrymans and Paxillus involutus during inter-kingdom interactions. Microbiology 2018, 164, 65–77. [Google Scholar] [CrossRef]
- Sullivan, G.; Garrett, R.D.; Lenehan, R.F. Occurrence of atromentin and thelephoric acid in cultures of Clitocybe subilludens. J. Pharm. Sci. 1971, 60, 1727. [Google Scholar] [CrossRef] [PubMed]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: Handbook; Birkhauser: Basel, Switzerland, 2004. [Google Scholar]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids; Birkhäuser: Basel, Switzerland, 1998; Volume 1–2. [Google Scholar]
- Spiteller, P.; Hamprecht, D.; Steglich, W. Biosynthesis of the 2H-azepine alkaloid chalciporone. J. Am. Chem. Soc. 2001, 123, 4837–4838. [Google Scholar] [CrossRef] [PubMed]
- Eggert, C. Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus. Microbiol. Res. 1997, 152, 315–318. [Google Scholar] [CrossRef]
- Kim, W.-G.; Lee, I.-K.; Kim, J.-P.; Ryoo, I.-J.; Koshino, H.; Yoo, I.-D. New indole derivatives with free radical scavenging activity from Agrocybe cylindracea. J. Nat. Prod. 1997, 60, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Koshcheenko, K.A.; Baklashova, T.G.; Kozlovskii, A.G.; Arinbasarov, M.U.; Skryabin, G.K. Hydroxylation of indolyl-3-acetic acid by the fungus Aspergillus niger IBFM-F-12. Prikl. Biokhim. Mikrobiol. 1977, 13, 248–254. [Google Scholar]
- Ringø, E.; Olsen, R.E.; Mayhew, T.M.; Myklebust, R. Electron microscopy of the intestinal microflora of fish. Aquaculture 2003, 227, 395–415. [Google Scholar] [CrossRef]
- Bogusławska-Wąs, E.; Dłubała, A.; Laskowska, M. The role of Rhodotorula mucilaginosa in selected biological process of wild fish. Fish Physiol. Biochem. 2019, 45, 511–521. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rodríguez, L. Carotenoids in evolutionary ecology: Re-evaluating the antioxidant role. BioEssays 2009, 31, 1116–1126. [Google Scholar] [CrossRef]
- Garbe, A.; Buck, J.; Hämmerling, U. Retinoids are important cofactors in T cell activation. J. Exp. Med. 1992, 176, 109–117. [Google Scholar] [CrossRef]
- Geissmann, F.; Revy, P.; Brousse, N.; Lepelletier, Y.; Folli, C.; Durandy, A.; Chambon, P.; Dy, M. Retinoids regulate survival and antigen presentation by immature dendritic cells. J. Exp. Med. 2003, 198, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Cornet, S.; Biard, C.; Moret, Y. Is there a role for antioxidant carotenoids in limiting self-harming immune response in invertebrates? Biol. Lett. 2007, 3, 284–288. [Google Scholar] [CrossRef] [Green Version]
- El-Sheekh, M.M.; Mahmoud, Y.A.; Abo-Shady, A.M.; Hamza, W. Efficacy of Rhodotorula glutinis and Spirulina platensis carotenoids in immunopotentiation of mice infected with Candida albicans SC5314 and Pseudomonas aureginosa 35. Folia Microbiol. 2010, 55, 61–67. [Google Scholar] [CrossRef]
- Pais, P.; Costa, C.; Cavalheiro, M.; Romão, D.; Teixeira, M.C. Transcriptional control of drug resistance, virulence and immune system evasion in pathogenic fungi: A cross-species comparison. Front. Cell. Infect. Microbiol. 2016, 6, 131. [Google Scholar] [CrossRef] [Green Version]
- Bayry, J.; Beaussart, A.; Dufrêne, Y.F.; Sharma, M.; Bansal, K.; Kniemeyer, O.; Aimanianda, V.; Brakhage, A.A.; Kaveri, S.V.; Kwon-Chung, K.J.; et al. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response. Infect. Immun. 2014, 82, 3141–3153. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Canadien, V.; Lam, G.Y.; Steinberg, B.E.; Dinauer, M.C.; Magalhaes, M.A.O.; Glogauer, M.; Grinstein, S.; Brumell, J.H. Activation of antibacterial autophagy by NADPH oxidases. Proc. Natl. Acad. Sci. USA 2009, 106, 6226–6231. [Google Scholar] [CrossRef] [Green Version]
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.C.; Kontoyiannis, D.P.; et al. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Chamilos, G.; Akoumianaki, T.; Kyrmizi, I.; Brakhage, A.; Beauvais, A.; Latge, J.-P. Melanin targets LC3-associated phagocytosis (LAP): A novel pathogenetic mechanism in fungal disease. Autophagy 2016, 12, 888–889. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [Green Version]
- Sarna, T.; Plonka, P.M. Biophysical studies of melanin. In Paramagnetic, Ion-Exchange, and Redox Properties of Melanin Pigments and Their Photoreactivity; Eaton, S.R., Eaton, G.R., Berliner, L.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 125–146. [Google Scholar]
- Pacelli, C.; Bryan, R.A.; Onofri, S.; Selbmann, L.; Shuryak, I.; Dadachova, E. Melanin is effective in protecting fast and slow growing fungi from various types of ionizing radiation. Environ. Microbiol. 2017, 19, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Cordero, R.; Bryan, R.; Nosanchuk, J.; Dadachova, E. Melanin, radiation, and energy transduction in fungi. In The Fungal Kingdom; Heitman, J., Howlett, B., Crous, P., Stukenbrock, E., James, T., Gow, N., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 509–514. [Google Scholar]
- Selbmann, L.; Pacelli, C.; Zucconi, L.; Dadachova, E.; Moeller, R.; de Vera, J.; Onofri, S. Resistance of an Antarctic cryptoendolithic black fungus to radiation gives new insights of astrobiological relevance. Fungal Biol. 2018, 122, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E.; Bryan, R.A.; Howell, R.C.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.D.; Casadevall, A. The radioprotective properties of fungal melanin are a function of its chemical composition, stable radical presence and spatial arrangement. Pigment. Cell Melanoma Res. 2008, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Khajo, A.; Bryan, R.A.; Friedman, M.; Burger, R.M.; Levitsky, Y.; Casadevall, A.; Magliozzo, R.S.; Dadachova, E. Protection of melanized Cryptococcus neoformans from lethal dose gamma irradiation involves changes in melanin’s chemical structure and paramagnetism. PLoS ONE 2011, 6, e25092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef]
- Géry, A.; Dubreule, C.; André, V.; Rioult, J.-P.; Bouchart, V.; Heutte, N.; de Pécoulas, P.E.; Krivomaz, T.; Garon, D. Chaga (Inonotus obliquus), a future potential medicinal fungus in oncology? A chemical study and a comparison of the cytotoxicity against human lung adenocarcinoma cells (A549) and human bronchial epithelial cells (BEAS-2B). Integr. Cancer Ther. 2018, 17, 832–843. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Hyun, C.K. Insulin-sensitizing and beneficial lipid metabolic effects of the water-soluble melanin complex extracted from Inonotus obliquus. Phytother. Res. 2014, 28, 1320–1328. [Google Scholar] [CrossRef]
- Youn, M.J.; Kim, J.K.; Park, S.Y.; Kim, Y.; Kim, S.J.; Lee, J.S.; Park, R. Chaga mushroom (Inonotus obliquus) induces G0/G1 arrest and apoptosis in human hepatoma HepG2 cells. World J. Gastroenterol. 2008, 14, 511–517. [Google Scholar] [CrossRef]
- Youn, M.J.; Kim, J.K.; Park, S.Y.; Kim, Y.; Park, C.; Kim, E.S.; Park, R. Potential anticancer properties of the water extract of Inonotus obliquus by induction of apoptosis in melanoma B16-F10 cells. J. Ethnopharmacol. 2009, 121, 221–228. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Y.; Zheng, X.; Liu, Y.; Pan, S.; Dai, Y.; Liu, F. Production of antioxidant and antitumor metabolites by submerged cultures of Inonotus obliquus cocultured with Phellinus punctatus. Appl. Microbiol. Biotechnol. 2011, 89, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Babitskaya, V.G.; Shcherba, V.V.; Ikonnikova, N.V. Melanin Complex of the Fungus Inonotus obliquus. Appl. Biochem. Microbiol. 2000, 36, 377–381. [Google Scholar] [CrossRef]
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 81, 6469–6477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taborda, C.P.; da Silva, M.B.; Nosanchuk, J.D.; Travassos, L.R. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: A minireview. Mycopathologia 2008, 165, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Wei, L.; Guo, T.; Tan, W. Detection of DOPA-melanin in the dimorphic fungal pathogen Penicillium marneffei and its effect on macrophage phagocytosis in vitro. PLoS ONE 2014, 9, e92610. [Google Scholar] [CrossRef]
- Cunha, M.M.; Franzen, A.J.; Seabra, S.H.; Herbst, M.H.; Vugman, N.V.; Borba, L.P.; de Souza, W.; Rozental, S. Melanin in Fonsecaea pedrosoi: A trap for oxidative radicals. BMC Microbiol. 2010, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Romero-Martinez, R.; Wheeler, M.; Guerrero-Plata, A.; Rico, G.; Torres-Guerrero, H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 2000, 68, 3696–3703. [Google Scholar] [CrossRef] [Green Version]
- Rambach, G.; Blum, G.; Latge, J.P.; Fontaine, T.; Heinekamp, T.; Hagleitner, M.; Jeckstrom, H.; Weigel, G.; Wurtinger, P.; Pfaller, K.; et al. Identification of Aspergillus fumigatus surface components that mediate interaction of conidia and hyphae with human platelets. J. Infect. Dis. 2015, 212, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, L.; Xi, L.; Huang, H.; Hu, Y.; Li, X.; Huang, X.; Lu, S.; Sun, J. Melanin in a meristematic mutant of Fonsecaea monophora inhibits the production of nitric oxide and Th1 cytokines of murine macrophages. Mycopathologia 2013, 175, 515–522. [Google Scholar] [CrossRef]
- Shi, M.; Sun, J.; Lu, S.; Qin, J.; Xi, L.; Zhang, J. Transcriptional profiling of macrophages infected with Fonsecaea monophora. Mycoses 2019, 62, 374–383. [Google Scholar] [CrossRef]
- Hsu, W.H.; Pan, T.M. Monascus purpureus-fermented products and oral cancer: A review. Appl. Microbiol. Biotechnol. 2012, 93, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Z. A new classification system of anticancer drugs—Based on cell biological mechanisms. Med. Hypotheses 2006, 66, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.Y.; Wu, Y.M.; Hsu, Y.W.; Hsu, L.C.; Kuo, Y.H.; Chang, K.J.; Pan, T.M. Effects of Monascus-fermented rice extract on malignant cell-associated neovascularization and intravasation determined using the chicken embryo chorioallantoic membrane model. Integr. Cancer Ther. 2010, 9, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Effects of red mold dioscorea on oral carcinogenesis in DMBA-induced hamster animal model. Food Chem. Toxicol. 2011, 49, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Yasukawa, K.; Ukiya, M.; Kiyota, A.; Sakamoto, N.; Suzuki, T.; Tanabe, N.; Nishino, H. Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J. Agric. Food Chem. 2005, 53, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Su, N.W.; Lin, Y.L.; Lee, M.H.; Ho, C.Y. Ankaflavin from Monascus-fermented red rice exhibits selective cytotoxic effect and induces cell death on Hep G2 cells. J. Agric. Food Chem. 2005, 53, 1949–1954. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, L.C.; Liang, Y.H.; Kuo, Y.H.; Pan, T.M. Monaphilones A–C, three new antiproliferative azaphilone derivatives from Monascus purpureus NTU 568. J. Agric. Food Chem. 2010, 58, 8211–8216. [Google Scholar] [CrossRef]
- Zheng, Y.; Xin, Y.; Shi, X.; Guo, Y. Anti-cancer effect of rubropunctatin against human gastric carcinoma cells BGC-823. Appl. Microbiol. Biotechnol. 2010, 88, 1169–1177. [Google Scholar] [CrossRef]
- Knecht, A.; Cramer, B.; Humpf, H.U. New Monascus metabolites: Structure elucidation and toxicological properties studied with immortalized human kidney epithelial cells. Mol. Nutr. Food Res. 2006, 50, 314–321. [Google Scholar] [CrossRef]
- Jo, D.; Choe, D.; Nam, K.; Shin, C.S. Biological evaluation of novel derivatives of the orange pigments from Monascus sp. as inhibitors of melanogenesis. Biotechnol. Lett. 2014, 36, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-W.; Fang, W.-H.; Chen, Y.-L.; Wu, M.-D.; Yuan, G.-F.; Ho, S.-Y.; Wang, Y.-J. Monascuspiloin enhances the radiation sensitivity of human prostate cancer cells by stimulating endoplasmic reticulum stress and inducing autophagy. PLoS ONE 2012, 7, e40462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, S.K.; Mishra, P.D.; Kulkarni-Almeida, A.; Verekar, S.; Sahoo, M.R.; Periyasamy, G.; Goswami, H.; Khanna, A.; Balakrishnan, A.; Vishwakarma, R. Anti-inflammatory and anticancer activity of ergoflavin isolated from an endophytic fungus. Chem. Biodivers. 2009, 6, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, M.M.; Clardy, J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 2001, 64, 1006–1009. [Google Scholar] [CrossRef]
- Li, X.; Sheng, Y.T.; Zhang, Y.-M.; Qin, J.-C. Cytotoxic azaphilone alkaloids from Chaetomium globosum TY1. Bioorg. Med. Chem. Lett. 2013, 23, 2945–2947. [Google Scholar] [CrossRef]
- Cao, S.; McMillin, D.W.; Tamayo, G.; Delmore, J.; Mitsiades, C.S.; Clardy, J. Inhibition of tumor cells interacting with stromal cells by xanthones isolated from a Costa Rican Penicillium sp. J. Nat. Prod. 2012, 75, 793–797. [Google Scholar] [CrossRef] [Green Version]
- Yasuhide, M.; Yamada, T.; Numata, A.; Tanaka, R. Chaetomugilins, new selectively cytotoxic metabolites, produced by a marine fish-derived Chaetomium species. J. Antibiot. 2008, 61, 615–622. [Google Scholar] [CrossRef]
- Sakurai, M.; Kohno, J.; Yamamoto, K.; Okuda, T.; Nishio, M.; Kawano, K.; Ohnuki, T. TMC-256A1 and C1, New inhibitors of IL-4 signal transduction produced by Aspergillus niger var niger TC 1629. J. Antibiot. 2002, 55, 685–692. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.B.; Xiao, Z.E.; Feng, X.J.; Huang, C.-H.; Zhu, X.; Ju, J.-H.; Li, M.-F.; Lin, Y.-C.; Liu, L.; She, Z.-G. Cytotoxic naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis (GX1-5E). Helv. Chim. Acta 2011, 94, 1732–1740. [Google Scholar] [CrossRef]
- Myobatake, Y.; Takeuchi, T.; Kuramochi, K.; Kuriyama, I.; Ishido, T.; Hirano, K.; Sugawara, F.; Yoshida, H.; Mizushina, Y. Pinophilins A and B, inhibitors of mammalian A-, B-, and Y-family DNA polymerases and human cancer cell proliferation. J. Nat. Prod. 2012, 75, 135–141. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A-F, cytotoxic chloroazaphilones from the marine mangrove endophytic fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ge, X.; Mudassir, S.; Zhou, L.; Yu, G.; Che, Q.; Zhang, G.; Peng, J.; Gu, Q.; Zhu, T.; et al. New Glutamine-containing azaphilone alkaloids from deep-sea-derived fungus Chaetomium globosum HDN151398. Mar. Drugs 2019, 17, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Li, Q.; Li, J.; Shao, C.; Zhang, J.; Zhang, Y.; Liu, X.; Lin, Y.; Liu, C.; She, Z. Two new derivatives of griseofulvin from the mangrove endophytic Fungus Nigrospora sp. (Strain No.1403) from Kandelia candel (L.) druce. Planta Med. 2011, 77, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, J.T.; Amagata, T.; Amagata, A.; Valeriote, F.A.; Mooberry, S.L.; Crews, P. Expanding the strategies in natural product studies of marine-derived fungi: A chemical investigation of Penicillium obtained from deep water sediment. J. Nat. Prod. 2004, 67, 362–367. [Google Scholar] [CrossRef]
- Stierle, A.A.; Stierle, D.B.; Girtsman, T.; Mou, T.C.; Antczak, C.; Djaballah, H. Azaphilones from an acid mine extremophile strain of a Pleurostomophora sp. J. Nat. Prod. 2015, 78, 2917–2923. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Sperlich, J.; Mádi, A.; Kurtá, T.; Dai, H.; Teusch, N.; Guo, Z.-Y.; Zou, K.; Liu, Z.; Proksch, P. Azaphilone derivatives from the fungus Coniella fragariae inhibit NF-κB activation and reduce tumor cell migration. J. Nat. Prod. 2018, 81, 2493–2500. [Google Scholar] [CrossRef]
- Kim, K.H.; Noh, H.J.; Choi, S.U.; Park, K.M.; Seok, S.-J.; Lee, K.R. Lactarane sesquiterpenoids from Lactarius subvellereus and their cytotoxicity. Bioorg. Med. Chem. Lett. 2010, 20, 5385–5388. [Google Scholar] [CrossRef]
- Song, J.; Manir, M.M.; Moon, S.-S. Cytotoxic grifolin derivatives isolated from the wild mushroom Boletus pseudocalopus (Basidiomycetes). Chem. Biodivers. 2009, 6, 1435–1440. [Google Scholar] [CrossRef]
- Yang, X.-L.; Qin, C.; Wang, F.; Dong, Z.-J.; Liu, J.-K. A New meroterpenoid pigment from the Basidiomycete Albatrellus confluens. Chem. Biodivers. 2008, 5, 484–489. [Google Scholar] [CrossRef]
- Yaqoob, A.; Li, W.M.; Liu, V.; Wang, C.; Mackedenski, S.; Tackaberry, L.E.; Massicotte, H.B.; Egger, K.N.; Reimer, K.; Lee, C.H. Grifolin, neogrifolin and confluentin from the terricolous polypore Albatrellus flettii suppress KRAS expression in human colon cancer cells. PLoS ONE 2020, 15, e0231948. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Chen, X.H.; Jiang, M.; Dong, M.S. In vitro antibiofilm activity of the melanin from Auricularia auricula, an edible jelly mushroom. Ann. Microbiol. 2012, 62, 1523–1530. [Google Scholar]
- Zhu, H.; He, C.-C.; Chu, Q.-H. Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett. Appl. Microbiol. 2011, 52, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum sensing inhibitors: A bargain of effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulrooey, C.A.; O’Brien, E.M.; Morgan, B.J.; Kozlowski, M.C. Perylenequinones: Isolation, synthesis, and biological activity. Eur. J. Org. Chem. 2012, 21, 3887–3904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, J.; Wu, D. Novel therapeutically and diagnostic applications of hypocrellins and hypericins. Photochem Photobiol. 1995, 61, 529–539. [Google Scholar]
- Ma, Y.J.; Zheng, L.P.; Wang, J.W. Inducing perylenequinone production from a bambusicolous fungus Shiraia sp. S9 through co-culture with a fruiting body-associated bacterium Pseudomonas fulva SB1. Microb. Cell Fact. 2019, 18, 121. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Morgan, B.J.; Mulrooney, C.A.; Carroll, P.J.; Kozlowski, M.C. Perylene-quinone natural products: Total synthesis of hypocrellin A. J. Org. Chem. 2010, 75, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Rissanen, T.; Voutilainen, S.; Nyyssonen, K.; Salonen, J.T. Lycopene, atherosclerosis, and coronary heart disease. Exp. Biol. Med. 2002, 227, 900–907. [Google Scholar] [CrossRef]
- Hernández-Almanza, A.; Montanez, J.; Martínez, G.; Aguilar-Jimenez, A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Lycopene: Progress in microbial production. Trends Food Sci. Technol. 2016, 56, 142–148. [Google Scholar] [CrossRef]
- Paranjape, S.R.; Riley, A.P.; Somoza, A.D.; Oakley, C.E.; Wang, C.C.C.; Prisinzano, T.E.; Oakley, B.R.; Gamblin, T.C. Azaphilones inhibit Tau aggregation and dissolve Tau aggregates in vitro. ACS Chem. Neurosci. 2015, 6, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Jansen, N.; Ohlendorf, B.; Arlette Erhard, A.; Bruhn, T.; Bringmann, G.; Imhoff, J.F. Helicusin E, isochromophilone X and isochromophilone XI: New chloroazaphilones produced by the fungus Bartalinia robillardoides strain LF550. Mar. Drugs 2013, 11, 800–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 48, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Hsu, L.-C.; Hsu, Y.-W.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. Anti-tumor and anti-inflammatory properties of ankaflavin and monaphilone A from Monascus purpureus NTU 568. J. Agric. Food Chem. 2011, 59, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fang, L.-Z.; Liu, F.-L.; Pang, X.-J.; Qin, H.-L.; Zhao, T.; Xu, L.-L.; Yang, D.-F.; Yang, X.-L. New prenylxanthones, polyketide hemiterpenoid pigments from the endophytic fungus Emericella sp. XL029 and their anti-agricultural pathogenic fungal and antibacterial activities. RSC Adv. 2017, 7, 31115. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Shen, N.; Chen, Z.-Q.; Zhang, F.-M.; Chen, Y. Penicilones A−D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs. 2018, 16, 61. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shang, X.-Y.; Wang, S.-J.; Mo, S.-Y.; Li, S.; Yang, Y.-C.; Ye, F.; Shi, J.-G.; He, L. Structures, biogenesis, and biological activities of pyrano[4,3-c]isochromen-4-one derivatives from the fungus Phellinus igniarius. J. Nat. Prod. 2007, 70, 296–299. [Google Scholar] [CrossRef]
- Kostrzewa, T.; Styszko, J.; Gorska-Ponikowska, M.; Sledzinski, T.; Kuban-Jan-kowska, A. Inhibitors of protein tyrosine phosphatase PTP1B with anticancer potential. Anticancer Res. 2019, 39, 3379–3384. [Google Scholar] [CrossRef]
- Pan, B.-Q.; Xie, Z.-H.; Hao, J.-J.; Zhang, Y.; Xu, X.; Cai, Y.; Wang, M.-R. PTP1B up-regulates EGFR expression by dephosphorylating MYH9 at Y1408 to promote cell migration and invasion in esophageal squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2020, 522, 53–60. [Google Scholar] [CrossRef]
- Sibero, M.T.; Triningsih, D.W.; Radjasa, O.K.; Sabdono, A.; Trianto, A. Evaluation of antimicrobial activity and identification of yellow pigmented marine sponge associated fungi from Teluk Awur, Jepara, Central Java. Indones. J. Biotechnol. 2016, 21, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.A.; Sivanandhan, G.; Thakare, D.B. Effect of physical and chemical parameters on the production of red exopigment from Penicillium purpurogenum isolated from spoilt onion and study of its antimicrobial activity. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 599–609. [Google Scholar]
- Vendruscolo, F.; Tosin, I.; Giachini, D.J.; Schmidell, W.; Ninow, J.L. Antimicrobial activity of Monascus pigments produced in submerged fermentation. J. Food Process. Preserv. 2014, 38, 1860–1865. [Google Scholar] [CrossRef]
- Mani, V.M.; Priya, M.S.; Dhaylini, S.; Preethi, K. Antioxidant and antimicrobial evaluation of bioactive pigment from Fusarium sp. isolated from stressed environment. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 1147–1158. [Google Scholar]
- Saravanan, D.; Radhakrishnan, M. Antimicrobial activity of pigments produced by fungi from Western Ghats. J. Chem. Pharm. Res. 2016, 8, 634–638. [Google Scholar]
- Yolmeh, M.; Hamedi, H.; Khomeiri, M. Antimicrobial activity of pigments extracted from Rhodotorula glutinis against some bacteria and fungi. Zahedan J. Res. Med Sci. 2016, 18, e4954. [Google Scholar] [CrossRef] [Green Version]
- Miao, F.-P.; Li, X.-D.; Liu, X.-H.; Cichewicz, R.H.; Ji, N.-Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs 2012, 10, 131–139. [Google Scholar] [CrossRef]
- Seddon, J.M.; Ajani, A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994, 272, 1413–1420. [Google Scholar] [CrossRef]
- Panthama, N.; Kanokmedhakul, S.; Kanokmedhakul, K.; Soytong, K. Cytotoxic and antimalarial azaphilones from Chaetomium longirostre. J. Nat. Prod. 2011, 74, 2395–2399. [Google Scholar] [CrossRef]
- Martínková, L.; Patáková-Juzlová, P.; Krên, V.; Kucêrová, Z.; Havlícêk, V.; Olsŏ ovský, P.; Hovorka, O.; Ríhová, B.; Veselý, D.; Veselá, D.; et al. Biological activities of oligoketide pigments of Monascus purpureus. Food Addit. Contam. 1999, 16, 15–24. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Takara, H.; Inokoshi, J.; Tanaka, H.; Masuma, R.; Omura, S. New brominated and halogen-less derivatives and structure-activity relationship of azaphilones inhibiting gp120-CD4 binding. J. Antibiot. 1998, 51, 1004–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quang, D.N.; Harinantenaina, L.; Nishizawa, T.; Hashimoto, T.; Kohchi, C.; Soma, G.; Asakawa, Y. Inhibition of nitric oxide production in RAW 264.7 cells by azaphilones from Xylariaceous fungi. Biol. Pharm. Bull. 2006, 29, 34–37. [Google Scholar] [CrossRef] [Green Version]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; George, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Chisté, R.C.; Mercadante, A.Z. Development of a novel micro-assay for evaluation of peroxyl radical scavenger capacity: Application to carotenoids and structure–activity relationship. Food Chem. 2012, 135, 2103–2111. [Google Scholar] [CrossRef] [Green Version]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551. [Google Scholar] [CrossRef]
- Miyashita, K. Function of Marine Carotenoids. In Food Factors for Health Promotion; Yoshikawa, T., Ed.; Forum Nutr; Karger AG: Basel, Switzerland, 2009; Volume 61, pp. 136–146. [Google Scholar]
- Endo, A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J. Antibiot. 1979, 32, 852–854. [Google Scholar] [CrossRef] [Green Version]
- Yamada, R.; Yoshie, T.; Wakai, S.; Asai-Nakashima, N.; Okazaki, F.; Ogino, C.; Hisada, H.; Tsutsumi, H.; Hata, Y.; Kondo, A. Aspergillus oryzae-based cell factory for direct kojic acid production from cellulose. Microb. Cell Fact. 2014, 13, 71. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]







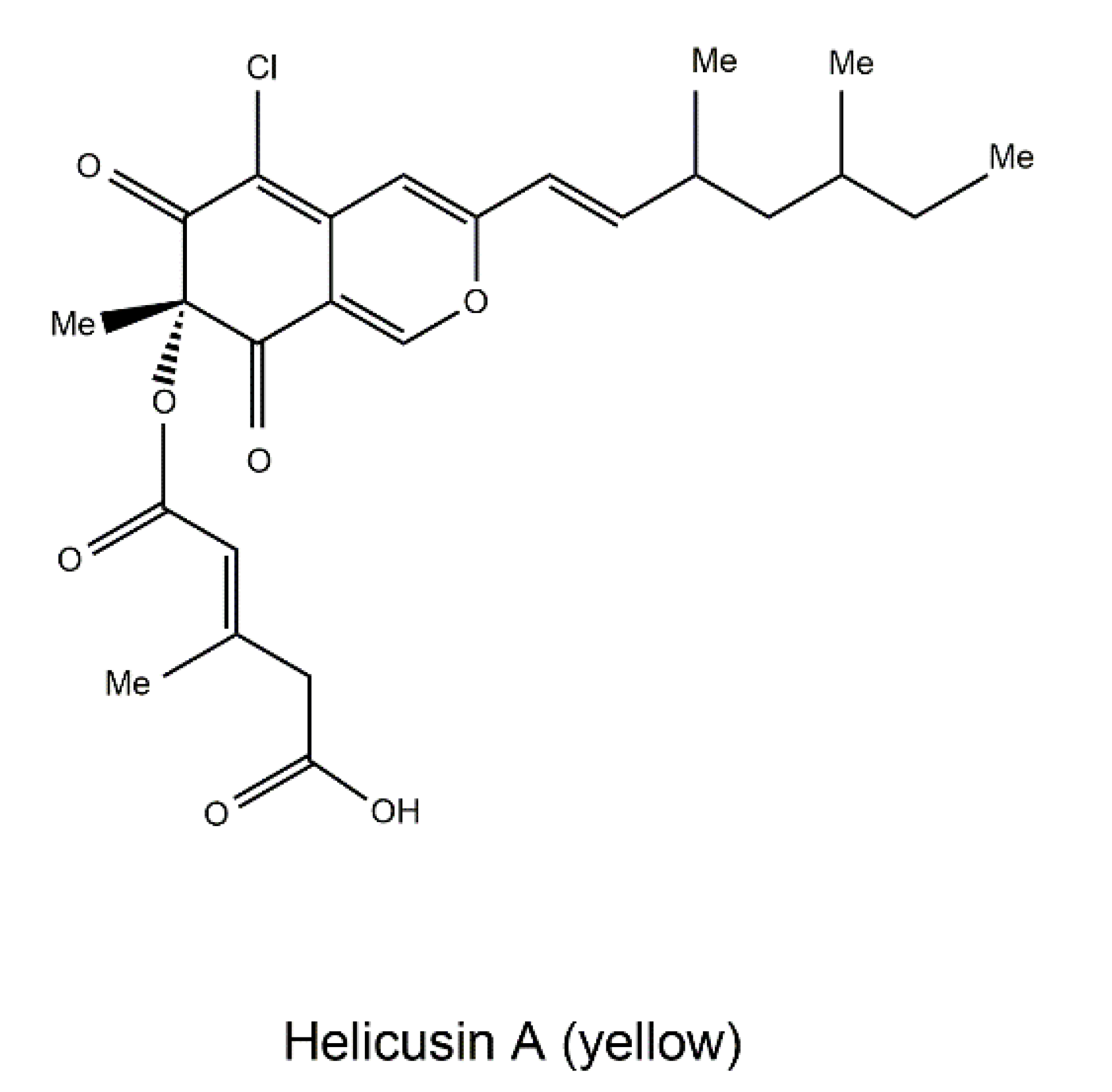
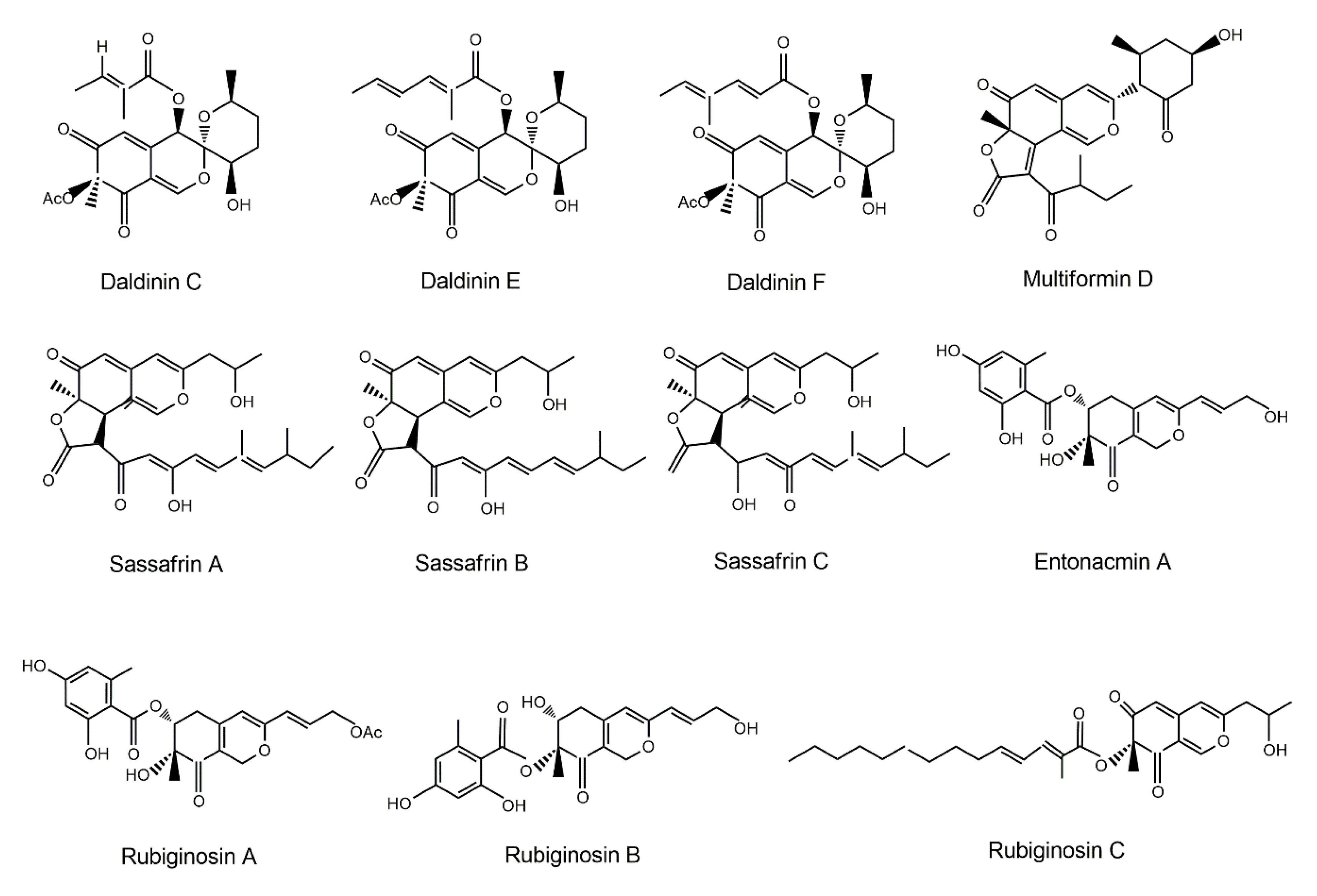
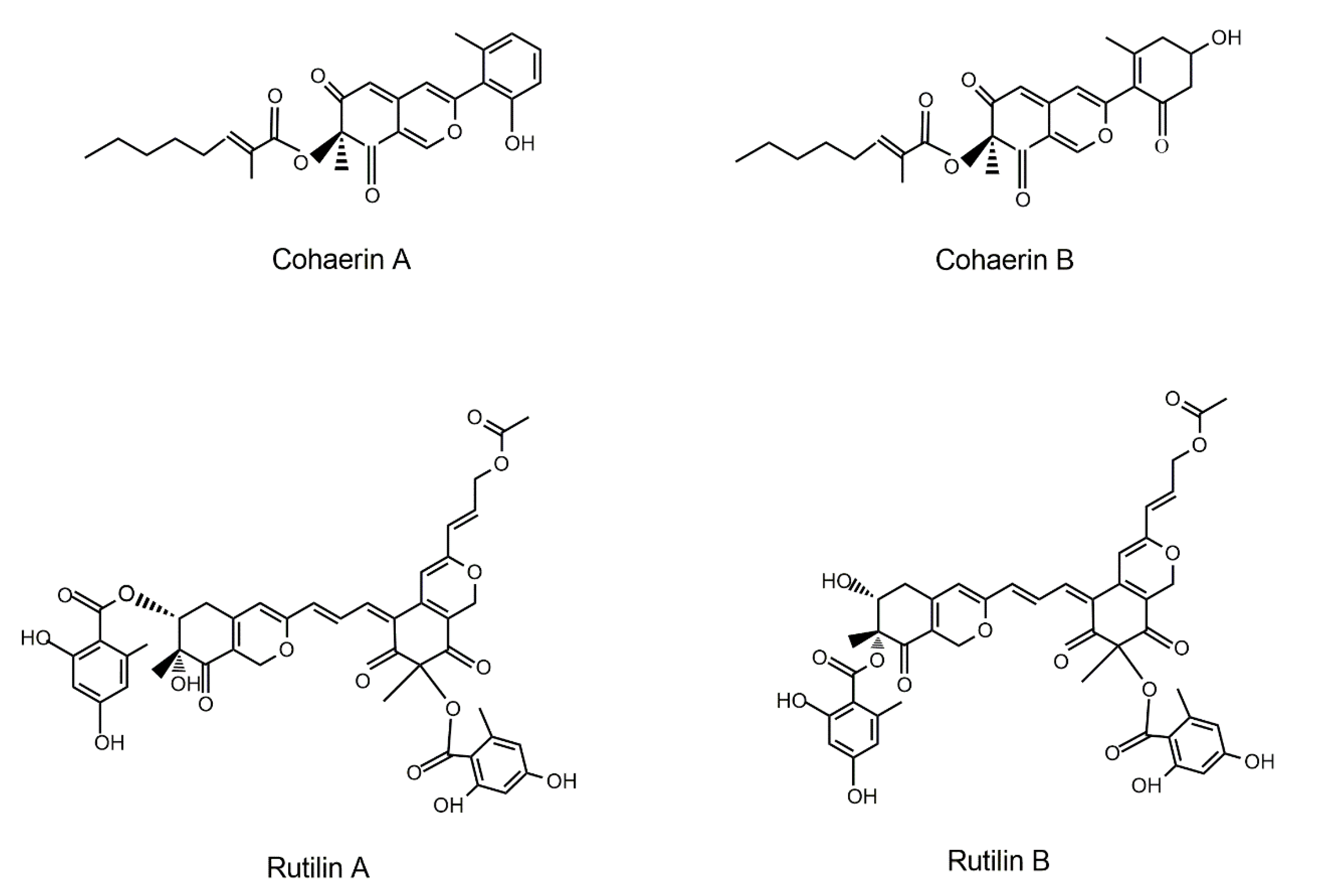
| Fungal Sources | Fungal Species/Strain | Isolated Compound | Chemical Nature | Tumor Model/Cell Lines/Target Enzyme | Activity/Active Concentration | References |
|---|---|---|---|---|---|---|
| Monascus-fermented red rice | Monascus pilosus | Monascin & ankaflavin rubropunctamine & monascorubramine | Azaphilones | Ames test and Peroxynitrite-and UVB-induced mouse skin carcinogenesis model | Accelerate the mutagen decomposition | Ho et al. [154] Hsu et al. [155] Akihisa et al. [156] |
| Monascus sp. | Ankaflavin | HepG2, A549 #/IC50 | 15 μg/mL | Su et al. [157] | ||
| Monascus purpureus | Monaphilone A Monoaphilone B | HEp-2, WiDr #/IC50 HEp-2, WiDr/IC50 | 72.1, 55.8 μM 77.6, 55.3 μM | Hsu et al. [158] | ||
| Rubropunctatin | BGC-823 #/IC50 and in vivo mouse model | 12.57 μM | Zheng et al. [159] | |||
| M. purpureus | Monascopyridine C & D | IHKE (kidney epithelial cell) CCK8 assay/EC50 | 20.7–43.2 μmol/L | Knecht et al. [160] | ||
| Monascus sp. | Glutamic acid derivative of Monascus orange pigments† (S)-(+)-1-amino-2-propanol derivative of the above orange pigments† | B16F10 (mouse melanoma cells) tyrosinase expression | 30% inhibition 35% inhibition | Jo et al. [161] | ||
| Monascus pilosus | Monascuspiloin | Monascin analog | PC-3 tumors of nude mice | 42.5% inhibition (in vivo) | Chiu et al. [162] | |
| Endophytic fungi | A fungus endophytic to Mimosops elengi | Ergoflavin | Xanthenes | ACHN (renal cell carcinoma), H460 (non-small-cell lung carcinoma), Panc1(pancreas), HCT116 (colon cancer), and Calu-1 (lung carcinoma) | 1.2, 4.0, 2.4, 8.0, 1.5μM/IC50 | Deshmukh et al. [163] |
| Phomopsis longicolla, endophytic to Dicerandra frutescens | Dicerandrol A, Dicerandrol B, Dicerandrol C | Xanthenes | A549, HCT116 #/IC50 A549, HCT116 A549, HCT116 | 7.0, 7.0 μg/mL 1.8, 1.8 μg/mL 1.8, 7.0 μg/mL | Wagenaar & Clardy [164] | |
| Chaetomium globosum endophytic to Ginkgo biloba Penicillium sp. CR1642D endophytic to Costa Rican rainforest | Chaetomugilides A–C | Azaphilone alkaloids | HepG-2# | 1.7−3.4 μM/IC50 | Li et al. [165] | |
| Penexanthone A Penexanthone B Dicerandrols B | Xanthones | A panel of cancer cell lines (Myeloma, lymphoma, leukemia, breast, prostate), also showing enhanced effects regarding tumor-stromal interaction | 1−17 μM/IC50 IC50 of 1.2 μM (+stroma) vs. 2.4 μM (-stroma) in RPMI8226; 3.4 μM (+ stroma) vs 10.2 μM (-stroma) in H929 | Cao et al. [166] | ||
| Chaetomium globosum endophytic to marine fish Mugil cephalus | Chaetomugilin A Chaetomugilin C Chaetomugilin F | Azaphilone alkaloids | P388(murine), HL-60 (human) leukemia | 8.7, 7.3 μM/IC50 3.6, 2.7 μM/IC50 3.3, 1.3 μM/IC50 | Yasuhide et al. [167] | |
| Marine fungi | Aspergillus tubingensis GX1-5E | TMC 256 A1 | Naphtho-γ-pyrone | MCF-7 & MDA-MB-435 (breast carcinoma), Hep3B & Huh7 (hepatoma), SNB19 & U87 MG (glioblastoma) | 19.92−47.98 μM/IC50 | Sakurai et al. [168] Huang et al. [169] |
| Penicillium pinophilum Hedgcok | Pinophilin A Pinophilin B | Hydrogenated azaphilones | Mammalian DNA polymerases (pols)A, B, Y | 48.6–55.6 μM/IC50 | Myobatake et al. [170] | |
| Diaporthe sp. SCSIO 41011 | epi-isochromophilone II isochromophilone D | Chloroazaphilones | ACHN, 786-O, OS-RC-2 (three renal carcinoma) 786-O renal carcinoma | 4.4, 3.0, 3.9 μM/IC50 8.9 μM/IC50 | Luo et al. [171] | |
| Chaetomium globosum HDN151398 | N-glutarylchaetoviridin C Chaetomugilin A Chaetomugilin C | chloroazaphilones Azaphilone Alkaloids | MGC-803, HO8910 # HL-60, HCT-116 # HL-60, HO8910 | 6.6, 9.7 μM/IC50 6.4, 6.1 μM/IC50 6.6, 8.8 μM/IC50 | Sun et al. [172] | |
| Nigrospora sp. strain 1403 | Bostrycindeoxybostrycin | Anthraquinones | A549, HepG2 # A549, HepG2 | 2.64, 5.90 μg/mL 2.44, 4.41 μg/mL | Xia et al. [173] | |
| Penicillium sp. | (+)-formylanserinone B anserinones B | Pentaketides | MDA-MB-435 # | 2.90 μg/mL 3.60 μg/mL | Gautschi et al. [174] | |
| Fungi in special habitats | Pleurostomophora sp. from a copper mine of North America | Berkchaetoazaphilones A, C Berkchaetorubramine berkchaetoazaphilone B | Azaphilones | Caspase-1 MMP-3 ξ Y79 # LOX IMVI # | 150,25,50 μM/IC50 130,15, 45 μM/IC50 1.1 μM /IC50 10 μM/IC50 | Stierle et al. [175] |
| Coniella fragariae from goose dung | Coniellin A Coniellin A, D, E | Azaphilones | MDA-MB-231# | 4.4 μM /IC50 and suppress tumor migration by 98% at 10 μM | Yu et al. [176] | |
| Macrofungi (mushroom) | Lactarius subvellereus | Subvellerolactone B, Subvellerolactone D, Subvellerolactone E | Sesquiterpene hydroxylactones | A549, SK-MEL-2 #, HCT-15 A549, HCT-15 A549, HCT-15 | 26.5, 18.3, 14.2 μM/IC50 25.1,17.8μM/IC50 19.6, 28.7μM/IC50 | Kim et al. [177] |
| Boletus pseudocalopus | Grifolin derivatives 1–3 | Phenolic compounds | A549, B16F1 (mouse melanoma) | 5.0–9.0 μg/mL 3.5–7.3 μg/mL | Song et al. [178] | |
| Albatrellus confluens | Albatrellin | Meroterpenoid | HepG2 | 1.55 μg/mL | Yang et al. [179] | |
| Albatrellus flettii | Grifolin, neogrifolin, confluentin | Phenolic compounds | SW480 & HT29 (two colon cancer lines) | 35.4, 30.7μM/IC50 34.6, 30.1μM/IC50 33.5, 25.8μM/IC50 | Yaqoob et al. [180] |
| Fungal Sources | Fungal Species/Strain | Bioactive Component | Target Microbes 1 | Antimicrobial Assay 2 | Reference |
|---|---|---|---|---|---|
| Marine sponge-associated, Indonesia | Trichoderma parareesei | Yellow pigment | Salmonella typhi, Escherichia coli, multi-drug resistant strain | MIC: 1000 μg/mL (weak) | Sibero et al. [201] |
| Deep sea, West Pacific Ocean | Chaetomium sp. NA-S0-R1 | Chaetoviridide A, B | Vibrio rotiferianus, Vibrio vulnificus and MRSA (Staphylococcus aureus ATCC 43300 & CGMCC 1.12409) | MIC: 7.3–7.8μg/mL | Wang et al. [197] |
| Spoiled onion | Penicillium purpurogenum | Red exopigment | S. aureus, Salmonella typhi, E. coli, Corynebacterium diptheriae, Pseudomonas aeruginosa | Agar diffusion assay showing inhibition zone (diameter 1.5–2.3 cm) | Patil et al. [202] |
| Tropical Culture Collection André Tosello (Campinas, SP, Brazil). | Monascus ruber CCT 3802 | Orange pigments (monascorubrin, rubropunctatin) Red pigments (monascorubramine, rubropunctamine) | Foodborne bacterium S. aureus ATCC 25923, S. aureus ATCC 25923, E. coli ATCC 25922 | Radial diffusion assay showing inhibition zone (diameter 0.15 cm) Inhibition zone (diameter 0.35, 0.63 cm, respectively) | Vendruscolo et al. [203] |
| Stressed environment | Fusarium sp. | Reddish orange pigment | Klebsiella pnuemoniae, E. coli, Shigella sp. (bacteria) Aspergillus niger, Candida albicans (fungi) | Well diffusion assay showing inhibition zone (diameter 1.6–2.9 cm) | Mani et al. [204] |
| Western Ghats forest, India | Penicillium sp. MF5 | Yellow pigments | Bacillus subtilis | MIC: 12.5 μg/mL | Saravanan & Radhakrishnan [205] |
| Persian type culture collection (PTCC), Tehran, Iran | Rhodotorula glutinis PTCC 5256 | Carotenoid pigments | S. aureus, Bacillus cereus, Streptococcus pyogenes, E. coli, Salmonella enteritidis, Enterococcus faecalis, Listeria monocytogenes | Disk diffusion assay showing inhibition zone (diameter 0.9–1.1 cm) | Yolmeh et al. [206] |
| Endophyte on marine brown algae, eastern China | Aspergillus versicolor | Asperversin, brevianamide M | E. coli, S. aureus | Disk diffusion assay showing inhibition zone (diameter 2.0–2.2 cm) | Miao et al. [207] |
| Endophyte on leaves of Panax notoginseng | Emericella sp. XL029 | 14-hydroxyltajixanthone 14-hydroxyltajixanthone, its hydrate, chloride derivative as well as epitajixanthone hydrate | Fungus- Drechslera maydis, Rhizoctonia cerealis, Fusarium oxysporum and Physalospora piricola Effective against all tested bacteria (except for drug resistant Staphylococcus aureus) | MIC: 25 μg/mL MIC: 12.5–50 μg/mL | Wu et al. [195] |
| Mangrove rhizosphere soil | Penicillium janthinellum HK1-6 | Penicilones B−D | MRSA (S. aureus ATCC 43300, ATCC 33591) | MIC: 3.13–6.25 μg/mL | Chen et al. [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, L.; Xu, J. Fungal Pigments and Their Roles Associated with Human Health. J. Fungi 2020, 6, 280. https://doi.org/10.3390/jof6040280
Lin L, Xu J. Fungal Pigments and Their Roles Associated with Human Health. Journal of Fungi. 2020; 6(4):280. https://doi.org/10.3390/jof6040280
Chicago/Turabian StyleLin, Lan, and Jianping Xu. 2020. "Fungal Pigments and Their Roles Associated with Human Health" Journal of Fungi 6, no. 4: 280. https://doi.org/10.3390/jof6040280
APA StyleLin, L., & Xu, J. (2020). Fungal Pigments and Their Roles Associated with Human Health. Journal of Fungi, 6(4), 280. https://doi.org/10.3390/jof6040280






