Outcomes of Antifungal Prophylaxis in High-Risk Haematological Patients (AML under Intensive Chemotherapy): The SAPHIR Prospective Multicentre Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Ethics
2.2. Study Design and Patients
2.3. Data Source and Statistical Analysis
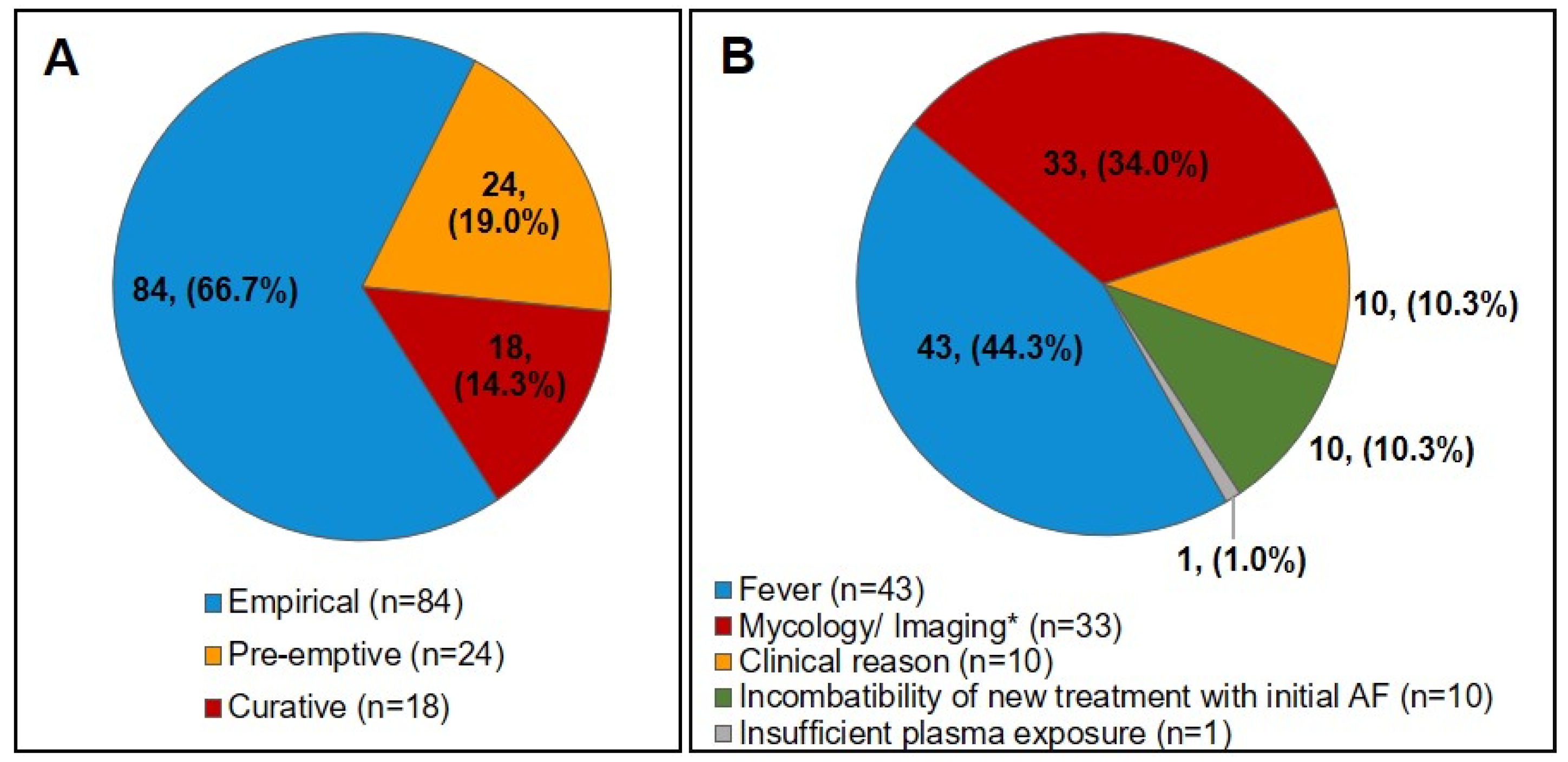
3. Results
3.1. Baseline Demographics, Medical History and Medical Condition
3.2. Initial AFP Treatment and Modifications during the Progression of the Disease
3.3. End of AFP Treatment
3.4. Patient Status 15 Days after the End of AFP Treatment and Deaths
3.5. Analyses of Significantly Different Parameters between the Subgroups
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michallet, M.; Ito, J.I. Approaches to the management of invasive fungal infections in hematologic malignancy and hematopoietic cell transplantation. J. Clin. Oncol. 2009, 27, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.T.; et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef] [Green Version]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Martino, B.; Specchia, G.; Pastore, D.; Stanzani, M.; Cattaneo, C.; Fanci, R.; et al. Invasive aspergillosis in patients with acute myeloid leukemia: A SEIFEM-2008 registry study. Haematologica 2010, 95, 644–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurosawa, M.; Yonezumi, M.; Hashino, S.; Tanaka, J.; Nishio, M.; Kaneda, M.; Ota, S.; Koda, K.; Suzuki, N.; Yoshida, M.; et al. Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int. J. Hematol. 2012, 96, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Auberger, J.; Lass-Florl, C.; Ulmer, H.; Nogler-Semenitz, E.; Clausen, J.; Gunsilius, E.; Einsele, H.; Gastl, G.; Nachbaur, D. Significant alterations in the epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int. J. Hematol. 2008, 88, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Pautas, C.; Maury, S.; Vekhoff, A.; Farhat, H.; Suarez, F.; Dhedin, N.; Isnard, F.; Ades, L.; Kuhnowski, F.; et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: A randomized, controlled trial. Clin. Infect. Dis. 2009, 48, 1042–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn-Ast, C.; Glasmacher, A.; Muckter, S.; Schmitz, A.; Kraemer, A.; Marklein, G.; Brossart, P.; von Lilienfeld-Toal, M. Overall survival and fungal infection-related mortality in patients with invasive fungal infection and neutropenia after myelosuppressive chemotherapy in a tertiary care centre from 1995 to 2006. J. Antimicrob. Chemother. 2010, 65, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Hammond, S.P.; Marty, F.M.; Bryar, J.M.; DeAngelo, D.J.; Baden, L.R. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am. J. Hematol. 2010, 85, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Caillot, D.; Cordonnier, C.; Auvrignon, A.; Thiebaut, A.; Brethon, B.; Michallet, M.; Mahlaoui, N.; Bertrand, Y.; Preziosi, P.; et al. Indications and outcomes of antifungal therapy in French patients with haematological conditions or recipients of haematopoietic stem cell transplantation. J. Antimicrob. Chemother. 2012, 67, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Azie, N.; Neofytos, D.; Pfaller, M.; Meier-Kriesche, H.U.; Quan, S.P.; Horn, D. The PATH (Prospective Antifungal Therapy) Alliance(R) registry and invasive fungal infections: Update 2012. Diagn. Microbiol. Infect. Dis. 2012, 73, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar] [PubMed]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and sites of involvement of invasive fungal infections in patients with haematological malignancies: A 20-year autopsy study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girmenia, C.; Aversa, F.; Busca, A.; Candoni, A.; Cesaro, S.; Luppi, M.; Pagano, L.; Rossi, G.; Venditti, A.; Nosari, A.M.; et al. A hematology consensus agreement on antifungal strategies for neutropenic patients with hematological malignancies and stem cell transplant recipients. Gruppo Italiano Malattie Ematologiche dell’Adulto, Gruppo Italiano Trapianto di Midollo Osseo, Associazione Italiana Ematologia ed Oncologia Pediatrica, Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer and Sorveglianza Epidemiologica delle Infezioni Fungine nelle Emopatie Maligne. Hematol. Oncol. 2013, 31, 117–126. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Heinz, W.J.; Hertenstein, B.; Horst, H.A.; Requadt, C.; Wagner, T.; Cornely, O.A.; Loffler, J.; Ruhnke, M.; IDEA Study Investigators. Immediate versus deferred empirical antifungal (IDEA) therapy in high-risk patients with febrile neutropenia: A randomized, double-blind, placebo-controlled, multicenter study. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 679–689. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Peman, J.; Bernhardt, H.; Klingspor, L.; Kibbler, C.C.; Faure, O.; Biraghi, E.; Canton, E.; Zimmermann, K.; Seaton, S.; et al. Epidemiology of candidaemia in Europe: Results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 317–322. [Google Scholar] [CrossRef]
- Rijnders, B.J.; Cornelissen, J.J.; Slobbe, L.; Becker, M.J.; Doorduijn, J.K.; Hop, W.C.; Ruijgrok, E.J.; Lowenberg, B.; Vulto, A.; Lugtenburg, P.J.; et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: A randomized, placebo-controlled trial. Clin. Infect. Dis. 2008, 46, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Michallet, M.; Gangneux, J.P.; Lafuma, A.; Herbrecht, R.; Ribaud, P.; Caillot, D.; Dupont, B.; Moreau, P.; Berger, P.; O’Sullivan, A.K. Cost effectiveness of posaconazole in the prophylaxis of invasive fungal infections in acute leukaemia patients for the French healthcare system. J. Med. Econ. 2011, 14, 28–35. [Google Scholar] [CrossRef]
- Gangneux, J.P.; El Cheikh, J.; Herbrecht, R.; Yakoub-Agha, I.; Quiniou, J.B.; Caillot, D.; Michallet, M. Systemic Antifungal Prophylaxis in Patients Hospitalized in Hematology Units in France: The AFHEM Cross-Sectional Observational Study. Infect. Dis. Ther. 2018, 7, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Maertens, J.; Marchetti, O.; Herbrecht, R.; Cornely, O.A.; Fluckiger, U.; Frere, P.; Gachot, B.; Heinz, W.J.; Lass-Florl, C.; Ribaud, P.; et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: Summary of the ECIL 3—2009 update. Bone Marrow Transplant. 2011, 46, 709–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellinghoff, S.C.; Panse, J.; Alakel, N.; Behre, G.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Kiehl, M.; Koldehoff, M.; Krause, S.W.; et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann. Hematol. 2018, 97, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [Green Version]
- Breda, G.L.; Tuon, F.F.; Meis, J.F.; Herkert, P.F.; Hagen, F.; de Oliveira, L.Z.; Dias, V.C.; da Cunha, C.A.; Queiroz-Telles, F. Breakthrough candidemia after the introduction of broad spectrum antifungal agents: A 5-year retrospective study. Med. Mycol. 2018, 56, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.R.; DiPippo, A.J.; Bose, P.; Kontoyiannis, D.P. Breakthrough Fungal Infections in Patients With Leukemia Receiving Isavuconazole. Clin. Infect. Dis. 2018, 67, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Auberger, J.; Lass-Florl, C.; Aigner, M.; Clausen, J.; Gastl, G.; Nachbaur, D. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: Real-life data from a single-centre institutional retrospective observational study. J. Antimicrob. Chemother. 2012, 67, 2268–2273. [Google Scholar] [CrossRef] [Green Version]
- Cornely, O.A.; Hoenigl, M.; Lass-Florl, C.; Chen, S.C.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., 3rd; Mycoses Study Group Education; Research Consortium (MSG-ERC); the European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Bougnoux, M.E.; Hennequin, C.; Godet, C.; Chandenier, J.; Denning, D.W.; Dupont, B. An estimation of burden of serious fungal infections in France. J. Mycol. Med. 2016, 26, 385–390. [Google Scholar] [CrossRef]
- Lortholary, O.; Gangneux, J.P.; Sitbon, K.; Lebeau, B.; de Monbrison, F.; Le Strat, Y.; Coignard, B.; Dromer, F.; Bretagne, S.; French Mycosis Study Group. Epidemiological trends in invasive aspergillosis in France: The SAIF network (2005–2007). Clin. Microbiol. Infect. 2011, 17, 1882–1889. [Google Scholar] [CrossRef] [Green Version]
- Maertens, J.A.; Girmenia, C.; Bruggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.A.; Cornely, O.A.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef]
- Vehreschild, J.J.; Ruping, M.J.; Wisplinghoff, H.; Farowski, F.; Steinbach, A.; Sims, R.; Stollorz, A.; Kreuzer, K.A.; Hallek, M.; Bangard, C.; et al. Clinical effectiveness of posaconazole prophylaxis in patients with acute myelogenous leukaemia (AML): A 6 year experience of the Cologne AML cohort. J. Antimicrob. Chemother. 2010, 65, 1466–1471. [Google Scholar] [CrossRef] [Green Version]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Florl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Chin, A.; Pergam, S.A.; Fredricks, D.N.; Hoofnagle, A.N.; Baker, K.K.; Jain, R. Evaluation of Posaconazole Serum Concentrations from Delayed-Release Tablets in Patients at High Risk for Fungal Infections. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Kraft, W.K.; Chang, P.S.; van Iersel, M.L.; Waskin, H.; Krishna, G.; Kersemaekers, W.M. Posaconazole tablet pharmacokinetics: Lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob. Agents Chemother. 2014, 58, 4020–4025. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, A.J.; Lipton, J.H.; Vesole, D.H.; Chandrasekar, P.; Langston, A.; Tarantolo, S.R.; Greinix, H.; Morais de Azevedo, W.; Reddy, V.; Boparai, N.; et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 2007, 356, 335–347. [Google Scholar] [CrossRef]
- Rodriguez-Veiga, R.; Montesinos, P.; Boluda, B.; Lorenzo, I.; Martinez-Cuadron, D.; Salavert, M.; Peman, J.; Calvillo, P.; Cano, I.; Acuna, E.; et al. Incidence and outcome of invasive fungal disease after front-line intensive chemotherapy in patients with acute myeloid leukemia: Impact of antifungal prophylaxis. Ann. Hematol. 2019, 98, 2081–2088. [Google Scholar] [CrossRef]
- Tormo, M.; Perez-Martinez, A.; Calabuig, M.; Hernandez-Boluda, J.C.; Amat, P.; Navarro, D.; Solano, C. Primary prophylaxis of invasive fungal infections with posaconazole or itraconazole in patients with acute myeloid leukaemia or high-risk myelodysplastic syndromes undergoing intensive cytotoxic chemotherapy: A real-world comparison. Mycoses 2018, 61, 206–212. [Google Scholar] [CrossRef]
- Hebart, H.; Klingspor, L.; Klingebiel, T.; Loeffler, J.; Tollemar, J.; Ljungman, P.; Wandt, H.; Schaefer-Eckart, K.; Dornbusch, H.J.; Meisner, C.; et al. A prospective randomized controlled trial comparing PCR-based and empirical treatment with liposomal amphotericin B in patients after allo-SCT. Bone Marrow Transplant. 2009, 43, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Bodey, G.P.; McCredie, K.B.; Keating, M.J.; Freireich, E.J. Treatment of acute leukemia in protected environment units. Cancer 1979, 44, 431–436. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Adjide, C.C.; Bernard, L.; Botterel, F.; Carel, A.; Castel, O.; Derouin, F.; Hoarau, G.; Labussiere, H.; Lafaurie, M.; et al. Quantitative assessment of fungal risk in the case of construction works in healthcare establishments: Proposed indicators for the determination of the impact of management precautions on the risk of fungal infection. J. Mycol. Med. 2012, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Switch to a Non-Prophylactic Treatment | |||
|---|---|---|---|---|
| No (S1) | Yes (S2) | Total | p-Value | |
| Age (years) | n = 278 | n = 126 | n = 404 | 0.273 |
| Mean (SD) | 55.9 (14.8) | 57.6 (12.2) | 56.4 (14.0) | |
| Median | 59.5 | 60.5 | 60.0 | |
| Range | 19.0–88.0 | 21.0–78.0 | 19.0–88.0 | |
| Gender | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.340 |
| Male | 138 (49.6) | 69 (54.8) | 207 (51.2) | |
| Medical and treatment history | ||||
| Time since AML diagnosis (days) | n = 263 | n = 116 | n = 379 | 0.823 |
| Mean (SD) | 66.0 (152) | 62.3 (227) | 64.9 (178) | |
| Median | 10.0 | 7.5 | 9.0 | |
| Range | 1.0–1256 | 1.0–1737 | 1.0–1737 | |
| Cytogenetics/molecular biology of prognosis | n = 274 (%) | n = 125 (%) | n = 399 (%) | 0.001 |
| Favourable | 87 (31.8) | 22 (17.6) | 109 (27.3) | |
| Intermediate | 108 (39.4) | 54 (43.2) | 162 (40.6) | |
| Unfavourable | 59 (21.5) | 45 (36.0) | 104 (26.1) | |
| Do not know | 20 (7.3) | 4 (3.2) | 24 (6.0) | |
| AML onset | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.004 |
| Newly diagnosed | 177 (63.7) | 88 (69.8) | 265 (65.6) | |
| Relapsed | 20 (7.2) | 8 (6.4) | 28 (6.9) | |
| Refractory | 2 (0.7) | 2 (1.6) | 4 (1.0) | |
| In consolidation | 40 (14.4) | 4 (3.2) | 44 (10.9) | |
| Secondary | 39 (14.0) | 24 (19.0) | 63 (15.6) | |
| Previous chemotherapy | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.001 |
| No | 190 (68.3) | 106 (84.1) | 296 (73.3) | |
| Chemotherapy at baseline | ||||
| Time since start of ongoing chemotherapy (days) | n = 277 | n = 125 | n = 402 | 0.001 |
| Mean (SD) | −1.6 (4.7) | 0.03 (3.9) | −1.0 (4.5) | |
| Median | 1.0 | 1.0 | 1.0 | |
| Range | −16.0–16.0 | −13.0–10.0 | −55.0–29.0 | |
| Ongoing chemotherapy | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.003 |
| Consolidation | 40 (14.4) | 4 (3.2) | 44 (10.9) | |
| Induction | 216 (77.7) | 112 (88.9) | 328 (81.2) | |
| Relapse | 22 (7.9) | 10 (7.9) | 32 (7.9) | |
| Characteristics | Switch to a Non-Prophylactic Treatment | |||
|---|---|---|---|---|
| No (S1) | Yes (S2) | Total | p-Value | |
| Risk factors | ||||
| Neutropenia | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.344 |
| Yes | 211 (75.9) | 101 (80.2) | 312 (77.2) | |
| Advanced age | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.857 |
| Yes | 93 (33.5) | 41 (32.5) | 134 (33.2) | |
| Monocytopenia | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.116 |
| Yes | 46 (16.5) | 18 (14.3) | 64 (15.8) | |
| Absence of air filtration by high efficiency particulate air (HEP)filter | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.046 |
| Yes | 17 (6.1) | 15 (11.9) | 32 (7.9) | |
| Baseline medical conditions | ||||
| Time to neutropenia start (days) | n = 206 | n = 100 | n = 306 | 0.314 |
| Mean (SD) | 9.8 (25.7) | 13.4 (36.5) | 11.0 (29.7) | |
| Median | 4.0 | 4.5 | 4.0 | |
| Range | −10.0–289 | −5.0–305 | −10.0–305 | |
| Medical condition potentially influencing AFP absorption | n = 277 (%) | n = 126 (%) | n = 403 (%) | 0.303 |
| Yes | 143 (51.6) | 72 (57.1) | 215 (53.3) | |
| Gastric cytoprotectants and/ or PPI | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.537 |
| Yes | 132 (47.5) | 64 (50.8) | 196 (48.5) | |
| Patient can eat | n = 278 (%) | n = 126 (%) | n = 404 (%) | 1.000 |
| Yes | 266 (95.7) | 121 (96.0) | 387 (95.8) | |
| No, oral medication intake possible | 11 (4.0) | 5 (4.0) | 16 (4.0) | |
| No, oral medication intake impossible | 1 (0.4) | - | 1 (0.3) | |
| If able to eat, patient receives food: | n = 265 (%) | n = 121 (%) | n = 386 (%) | 0.274 |
| Sterile | 115 (43.4) | 49 (40.5) | 164 (42.5) | |
| Protected | 132 (49.8) | 68 (56.2) | 200 (51.8) | |
| Normal | 18 (6.8) | 4 (3.3) | 22 (5.7) | |
| Method of digestive decontamination | n = 276 (%) | n = 126 (%) | n = 402 (%) | 0.068 |
| Yes | 107 (38.8) | 37 (29.4) | 144 (35.8) | |
| Type of digestive decontamination | n = 107 (%) | n = 37 (%) | n = 144 (%) | 0.513 |
| Antibacterial | 67 (62.6) | 26 (70.3) | 93 (64.6) | |
| Antifungal | 2 (1.9) | 1 (2.7) | 3 (2.1) | |
| Antibacterial + Antifungal | 38 (35.5) | 10 (27.0) | 48 (33.3) | |
| Hospitalisation conditions | n = 276 (%) | n = 126 (%) | n = 402 (%) | 0.070 |
| Conventional area | 22 (8.0) | 4 (3.2) | 26 (6.5) | |
| Sterile/isolated area | 254 (92.0) | 122 (96.8) | 376 (93.5) | |
| If sterile/isolated area | n = 254 (%) | n = 122 (%) | n = 376 (%) | 0.110 |
| Portable air treatment | 53 (20.9) | 17 (13.9) | 70 (18.6) | |
| Laminar air flow | 50 (19.7) | 33 (27.0) | 83 (22.1) | |
| HEPA filter | 95 (37.4) | 52 (42.6) | 147 (39.1) | |
| Laminar air flow and HEPA filter | 56 (22.0) | 20 (16.4) | 76 (20.2) | |
| Characteristics | Switch to a Non-Prophylactic Treatment | |||
|---|---|---|---|---|
| No (S1) | Yes (S2) | Total | p-Value | |
| Reason(s) for AFP prescription | n = 276 (%) | n = 126 (%) | n = 402 (%) | 0.784 |
| Hospital specific protocol | 268 (97.1) | 121 (96.0) | 389 (96.8) | |
| Link to medical history | 3 (1.1) | 1 (0.8) | 4 (1.0) | |
| Both | 5 (1.8) | 4 (3.2) | 9 (2.2) | |
| Loading dose | n = 279 (%) | n = 124 (%) | n = 403 (%) | 0.032 |
| Yes | 205 (73.5) | 78 (62.9) | 283 (70.2) | |
| AFP modification | n = 278 (%) | n = 126 (%) | n = 404 (%) | 0.369 |
| Yes | 25 (9.0) | 8 (6.3) | 33 (8.2) | |
| Time to first modification (days) | n = 25 | n = 6 | n = 31 | 0.915 |
| Mean (SD) | 17.5 (25.5) | 16.3 (17.8) | 17.3 (23.9) | |
| Median | 12.0 | 10.0 | 11.0 | |
| Range | 0.0–108 | 2.0–51 | 0.0–108 | |
| Characteristics | Switch to a Non-Prophylactic Treatment | |||
|---|---|---|---|---|
| No (S1) | Yes (S2) | Total | p-Value | |
| Global PPC measurements (mg/L) | ||||
| Global PPC of all measurements taken | n = 172 | n = 105 | n = 277 | 0.027 |
| Mean (SD) | 1.1 (0.7) | 1.3 (1.1) | 1.2 (0.9) | |
| Median | 0.9 | 1.0 | 1.0 | |
| IQR | 0.6–1.3 | 0.6–1.8 | 0.6–1.6 | |
| Duration to 1st PPC per patient (days) | n = 83 | n = 56 | n = 139 | 0.148 |
| Mean (SD) | 9.9 (6.5) | 8.1 (8.1) | 9.1 (7.2) | |
| Median | 7.0 | 6.0 | 7.0 | |
| Range | 3.0–33 | 0.0–48 | 0.0–48 | |
| First PPC per patient | n = 83 | n = 56 | n = 139 | 0.420 |
| Mean (SD) | 1.0 (0.6) | 1.1 (0.8) | 1.1 (0.7) | |
| Median | 0.9 | 1.0 | 1.0 | |
| IQR | 0.6–1.3 | 0.5–1.6 | 0.6–1.3 | |
| Patients with PPC < 0.7 mg/L | n = 83 (%) | n = 56 (%) | n = 139 (%) | 0.735 |
| 23 (27.7) | 17 (30.4) | 40 (28.8) | ||
| AFP modification during the study | ||||
| Patient changes AFP | No (n = 124) | Yes (n = 15) | Total (n = 139) | p-value |
| PPC (mg/L) of all measurements taken | n = 252 | n = 25 | n = 277 | 0.189 |
| Mean (SD) | 1.2 (0.9) | 0.9 (0.6) | 1.2 (0.9) | |
| Median | 1.0 | 0.8 | 1.0 | |
| IQR | 0.6–1.6 | 0.5–1.2 | 0.6–1.6 | |
| Characteristics | Switch to a Non-Prophylactic Treatment | |||
| No (S1) | Yes (S2) | Total | p-value | |
| PPC measurements at end of AFP (mg/L) | ||||
| PPC at end of AFP | n = 20 | n = 30 | n = 50 | 0.586 |
| Mean (SD) | 1.5 (0.9) | 1.3 (1.0) | 1.4 (1.0) | |
| Median | 1.2 | 1.1 | 1.1 | |
| IQR | 0.7–2.0 | 0.5–2.0 | 0.7–2.0 | |
| Patients with PPC < 0.7 mg/L | n = 20 (%) | n = 30 (%) | n = 50 (%) | 0.430 |
| Yes | 4 (20.0) | 9 (30.0) | 13 (26.0) | |
| PPC in patients with potential AFP absorption issues | n = 14 | n = 23 | n = 37 | 0.373 |
| Mean (SD) | 1.5 (1.0) | 1.2 (1.0) | 1.3 (1.0) | |
| Median | 1.2 | 0.8 | 1.0 | |
| IQR | 0.7–2.0 | 0.4–1.7 | 0.6–1.9 | |
| Patients with PPC < 0.7 mg/L | n = 14 (%) | n = 23 (%) | n = 37 (%) | 0.477 |
| Yes | 3 (21.4) | 8 (34.8) | 11 (29.7) | |
| Characteristics | Switch to a Non-Prophylactic Treatment | |||
|---|---|---|---|---|
| No (S1) | Yes (S2) | Total | p-Value | |
| Duration of prophylaxis period (days) | n = 277 | n = 126 | n = 403 | 0.017 |
| Mean (SD) | 26.8 (33.4) | 18.6 (28.1) | 24.2 (32.1) | |
| Median | 22.0 | 13.0 | 19.0 | |
| Range | 1.0–375 | 1.0–209 | 1.0–375 | |
| Medical conditions influencing AFP absorption | ||||
| Medical condition potentially influencing AFP absorption | n = 277 (%) | n = 126 (%) | n = 403 (%) | 0.008 |
| Yes | 164 (59.2) | 92 (73.0) | 256 (63.5) | |
| Gastric cytoprotectants and/or PPI | n = 277 (%) | n = 126 (%) | n = 403 (%) | 0.386 |
| Yes | 141 (50.9) | 70 (55.6) | 211 (52.4) | |
| Patient can eat | n = 275 (%) | n = 125 (%) | n = 401 (%) | 0.001 |
| Yes | 250 (90.9) | 97 (77.0) | 347 (86.5) | |
| No, oral medication intake possible | 16 (5.8) | 19 (15.1) | 35 (8.7) | |
| No, oral medication intake impossible | 9 (3.3) | 10 (7.9) | 19 (4.7) | |
| If able to eat, patient receives food: | n = 249 (%) | n = 97 (%) | n = 346 (%) | <0.001 |
| Sterile | 69 (27.7) | 33 (34.0) | 102 (29.5) | |
| Protected | 76 (30.5) | 60 (61.9) | 136 (39.3) | |
| Normal | 104 (41.8) | 4 (4.1) | 108 (31.2) | |
| Method of digestive decontamination used | n = 275 (%) | n = 126 (%) | n = 401 (%) | 0.070 |
| Yes | 56 (20.4) | 36 (28.6) | 92 (22.9) | |
| Type of digestive decontamination | ||||
| Antibacterial | 50 (89.3) | 33 (91.7) | 83 (90.2) | |
| Antifungal | 13 (23.2) | 4 (11.1) | 17 (18.5) | |
| Change of hospitalisation conditions | n = 275 (%) | n = 126 (%) | n = 401 (%) | <0.001 |
| No | 166 (60.4) | 115 (91.3) | 281 (70.1) | |
| Patient no longer hospitalised | 89 (32.4) | 7 (5.6) | 96 (23.9) | |
| Yes | 20 (7.3) | 4 (3.2) | 24 (6.0) | |
| Characteristics | Switch to a Non-Prophylactic Treatment | |||
|---|---|---|---|---|
| No (S1) | Yes (S2) | Total | p-Value | |
| Signs/symptoms of infection at day 15: | n = 268 (%) | n = 119 (%) | n = 387 (%) | <0.001 |
| Yes | 2 (0.7) | 20 (16.8) | 22 (5.7) | |
| Change of hospitalisation conditions | n = 268 (%) | n = 119 (%) | n = 387 (%) | <0.001 |
| No | 76 (28.4) | 95 (79.8) | 171 (44.2) | |
| Yes, patient no longer hospitalised | 167 (62.3) | 20 (16.8) | 187 (48.3) | |
| Yes | 25 (9.3) | 4 (3.4) | 29 (7.5) | |
| Profound neutropenia periods during the entire follow up period | ||||
| Neutropenia periods, n = number of patients | n = 249 | n = 121 | n = 370 | 0.021 |
| Mean (SD) | 1.0 (0.3) | 1.2 (0.8) | 1.1 (0.5) | |
| Median | 1.0 | 1.0 | 1.0 | |
| Range | 1.0–5.0 | 1.0–6.0 | 1.0–6.0 | |
| Duration (days), n = number of neutropenia periods | n = 254 | n = 139 | n = 393 | 0.388 |
| Mean (SD) | 22.7 (16.7) | 24.2 (16.6) | 23.3 (16.6) | |
| Median | 21.0 | 22.0 | 22.0 | |
| Range | 2.0–172 | 1.0–162 | 1.0–172 | |
| Deaths | ||||
| Time between inclusion and death (days) | n = 11 | n = 9 | n = 20 | 0.634 |
| Mean (SD) | 62.6 (56.1) | 51.4 (44.8) | 57.6 (50.3) | |
| Median | 30.0 | 19.0 | 28.5 | |
| Range | 15.0–184 | 11.0–122 | 11.0–184 | |
| Signs/symptoms of infection: | n = 11 (%) | n = 9 (%) | n = 20 (%) | 0.160 |
| Yes | 2 (18.2) | 5 (55.6) | 7 (35.0) | |
| Death linked to IFD | n = 11 (%) | n = 9 (%) | n = 20 (%) | 0.074 |
| Yes | - | 3 (33.3) | 3 (15.0) | |
| Baseline and AFP initiation | |||
|---|---|---|---|
| Factor | Modalities | Odds ratio | 95% CI |
| Cytogenetics/molecular biology of prognosis | Favourable versus Unknown | 1.411 | 0.426–4.676 |
| Intermediate versus Unknown | 2.669 | 0.844–8.444 | |
| Unfavourable versus. Unknown | 3.639 | 1.129–11.723 | |
| Previous chemotherapy | No versus Yes | 2.033 | 1.151–3.589 |
| Duration between start of 1st chemotherapy and inclusion | Increasing duration between ongoing chemotherapy and inclusion (in days) | 1.064 | 1.007–1.124 |
| Loading dose | No versus Yes | 1.811 | 1.116–2.938 |
| End of AFP treatment and 15-day follow-up | |||
| Factor | Modalities | Odds ratio | 95% CI |
| Patient alimentation at the end of AFP | Patient cannot eat, and oral medication intake is impossible versus Normal food | 5.031 | 0.907–27.905 |
| Patient cannot eat, but oral medication intake is possible versus Normal food | 4.983 | 1.155–21.504 | |
| Protected food versus Normal food | 4.741 | 1.392–16.147 | |
| Sterile food versus Normal food | 2.131 | 0.596–7.613 | |
| Change of hospitalisation conditions at the end of AFP | No versus Yes, the patient is no longer hospitalized | 5.452 | 1.851–16.060 |
| Yes versus Yes, the patient is no longer hospitalized | 1.515 | 0.166–13.828 | |
| Signs and symptoms of infection at day 15 | Yes versus No | 7.286 | 1.275–41.648 |
| Change of hospitalisation conditions at 15 days | No versus Yes, the patient is no longer hospitalized | 7.370 | 3.776–14.383 |
| Yes versus Yes, the patient is no longer hospitalized | 1.083 | 0.303–3.870 | |
| Number of neutropenia periods | Increasing number of neutropenia periods | 9.939 | 3.420–28.881 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangneux, J.-P.; Padoin, C.; Michallet, M.; Saillio, E.; Kumichel, A.; Peffault de La Tour, R.; Ceballos, P.; Gastinne, T.; Pigneux, A. Outcomes of Antifungal Prophylaxis in High-Risk Haematological Patients (AML under Intensive Chemotherapy): The SAPHIR Prospective Multicentre Study. J. Fungi 2020, 6, 281. https://doi.org/10.3390/jof6040281
Gangneux J-P, Padoin C, Michallet M, Saillio E, Kumichel A, Peffault de La Tour R, Ceballos P, Gastinne T, Pigneux A. Outcomes of Antifungal Prophylaxis in High-Risk Haematological Patients (AML under Intensive Chemotherapy): The SAPHIR Prospective Multicentre Study. Journal of Fungi. 2020; 6(4):281. https://doi.org/10.3390/jof6040281
Chicago/Turabian StyleGangneux, Jean-Pierre, Christophe Padoin, Mauricette Michallet, Emeline Saillio, Alexandra Kumichel, Régis Peffault de La Tour, Patrice Ceballos, Thomas Gastinne, and Arnaud Pigneux. 2020. "Outcomes of Antifungal Prophylaxis in High-Risk Haematological Patients (AML under Intensive Chemotherapy): The SAPHIR Prospective Multicentre Study" Journal of Fungi 6, no. 4: 281. https://doi.org/10.3390/jof6040281
APA StyleGangneux, J.-P., Padoin, C., Michallet, M., Saillio, E., Kumichel, A., Peffault de La Tour, R., Ceballos, P., Gastinne, T., & Pigneux, A. (2020). Outcomes of Antifungal Prophylaxis in High-Risk Haematological Patients (AML under Intensive Chemotherapy): The SAPHIR Prospective Multicentre Study. Journal of Fungi, 6(4), 281. https://doi.org/10.3390/jof6040281






