Examining the Impacts of CO2 Concentration and Genetic Compatibility on Perennial Ryegrass—Epichloë festucae var lolii Interactions
Abstract
:1. Introduction
“Endophytic fungi, especially asexual, systemic endophytes in grasses, are generally viewed as plant mutualists, mainly through the action of mycotoxins, such as alkaloids in infected grasses, which protect the host plant from herbivores. Most of the evidence for the defensive mutualism concept is derived from studies of agronomic grass cultivars, which may be atypical of many endophyte-host interactions. I argue that endophytes in native plants, even asexual, seed-borne ones, rarely act as defensive mutualists. In contrast to domesticated grasses where infection frequencies of highly toxic plants often approach 100%, natural grass populations are usually mosaics of uninfected and infected plants. The latter, however, usually vary enormously in alkaloid levels, from none to levels that may affect herbivores. This variation may result from diverse endophyte and host genotypic combinations that are maintained by changing selective pressures, such as competition, herbivory and abiotic factors....”
“Ryegrass Staggers is the name given to a condition of tetanic muscle spasm that develops under certain conditions in grazing sheep, cattle, or horses. In most cases, the pastures on which animals become affected have contained a considerable proportion of perennial ryegrass, and this has given rise to the name, though there is no direct proof that ryegrass is the cause; at least one out-break has occurred on short-rotation ryegrass.”
- Do different strains of E. festucae var. lolii produce similar fungal concentrations in a genetically diverse host plant background?
- Do different strains of the fungus differentially moderate the impacts of elevated CO2 on the growth and seed production of perennial ryegrass?
- Are the metabolomes of the host plant–fungal strain combinations different from each other and how are they altered by elevated CO2?
- Are the proteomes of the host plant–fungal strain combinations different from each other and how are they altered by elevated CO2?
- Does an integrated analysis of the proteome and metabolome data yield different insights than those gained from considering the proteome and metabolome separately?
- Is there any evidence of host plant–fungal strain genetic incompatibility?
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Maternal Family Establishment
2.2. Chamber Experiment and Harvested Plant Tissue
2.3. Metabolomic Sample Preparation
2.4. Proteomic Sample Preparation
2.5. Mass Spectrometry
2.6. Omics Data Analysis
2.7. Endophyte Quantification
3. Results
“This measures the amount of information supplied by the test against the tested hypothesis (or model): Rounded off, the s-value s shows the number of heads in a row one would need to see when tossing a coin to get the same amount of information against the tosses being “fair” (independent with “heads” probability of ) instead of being loaded for heads. For example, if , this represents bits of information against the hypothesis (like getting 5 heads in a trial of “fairness” with 5 coin tosses); and if , this represents only bits of information against the hypothesis (like getting 2 heads in a trial of “fairness” with only 2 coin tosses).”([86], p. 12)
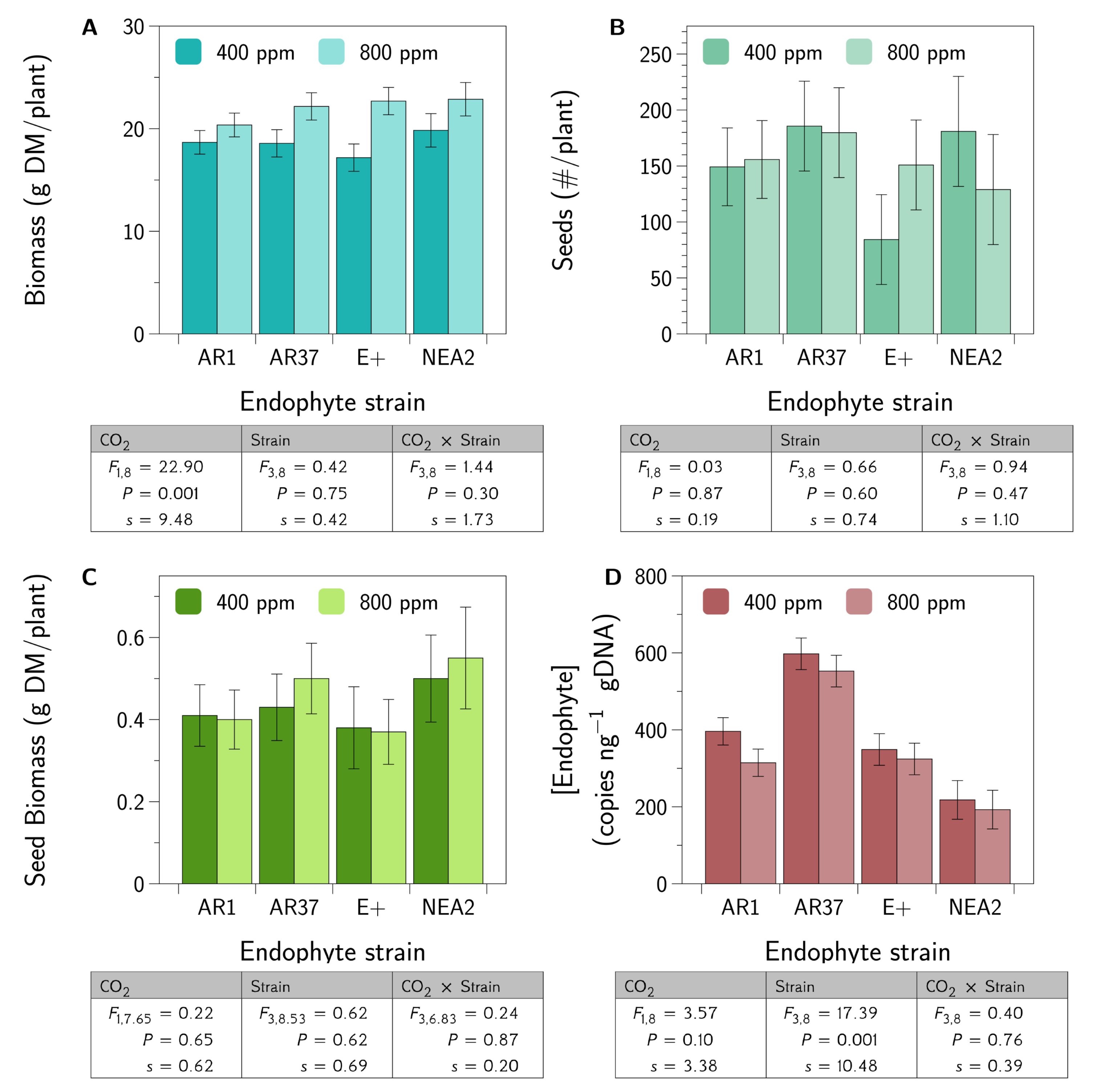
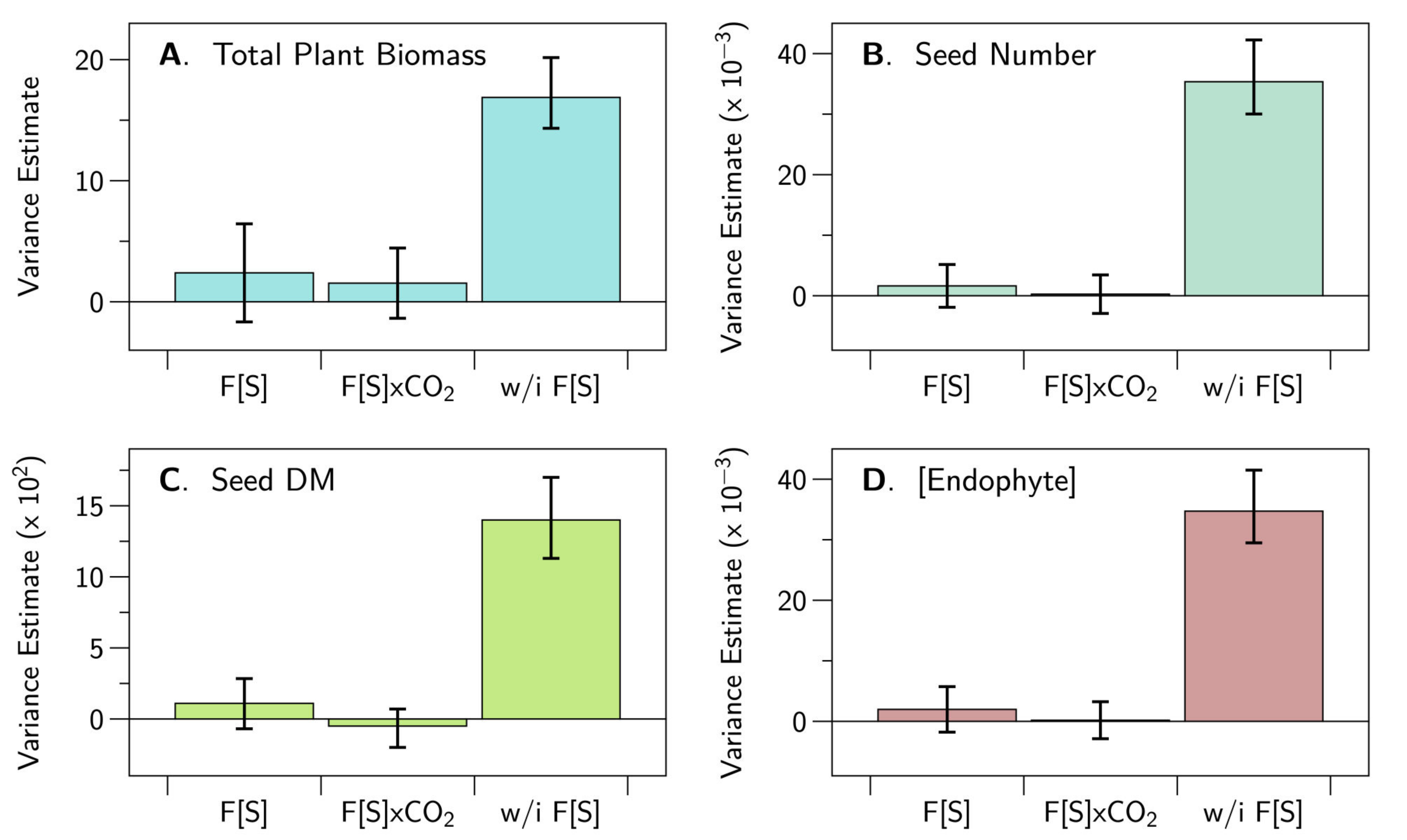
3.1. Plant and Fungal Growth Responses
3.2. Multi-OMICs Workflow
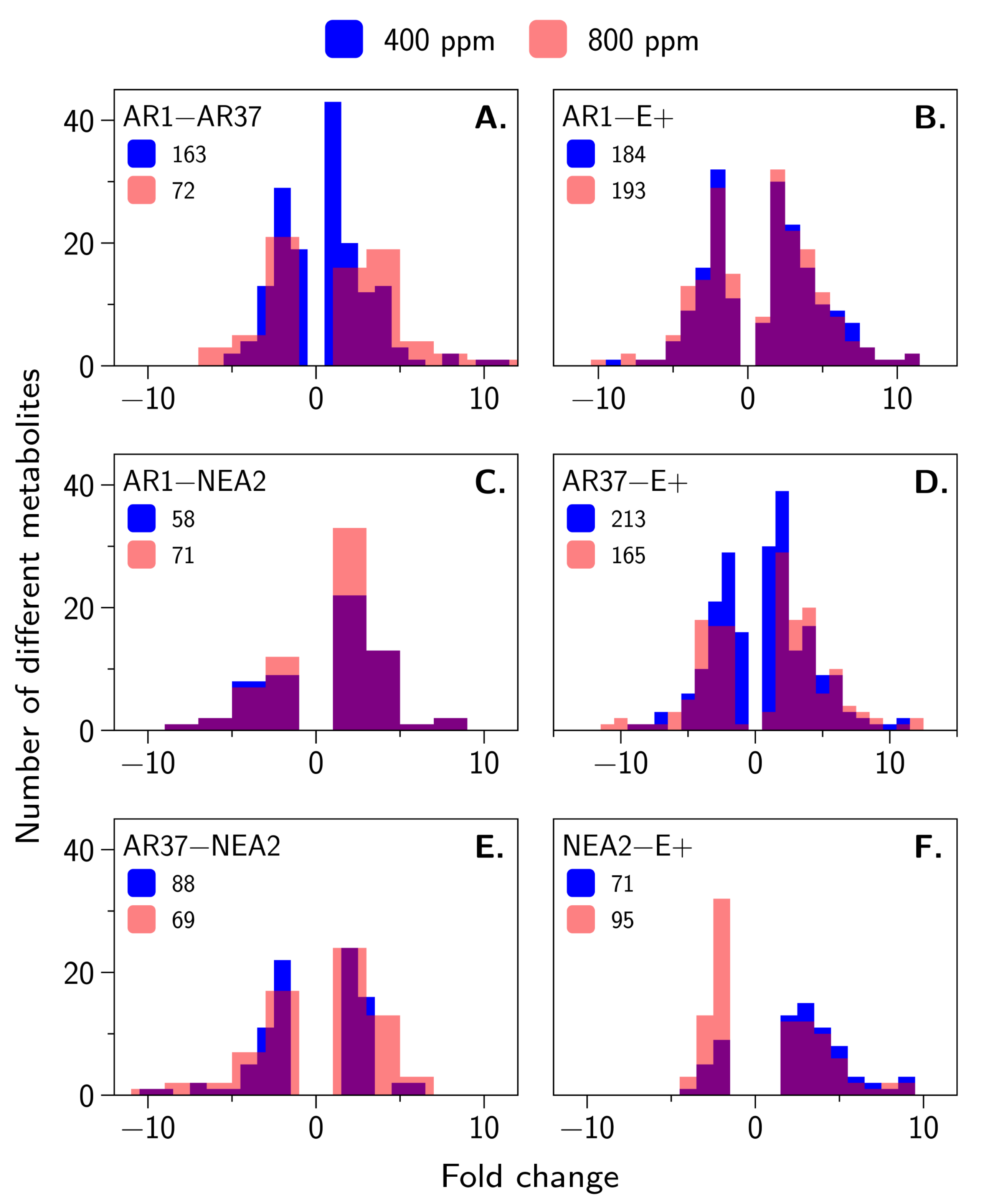
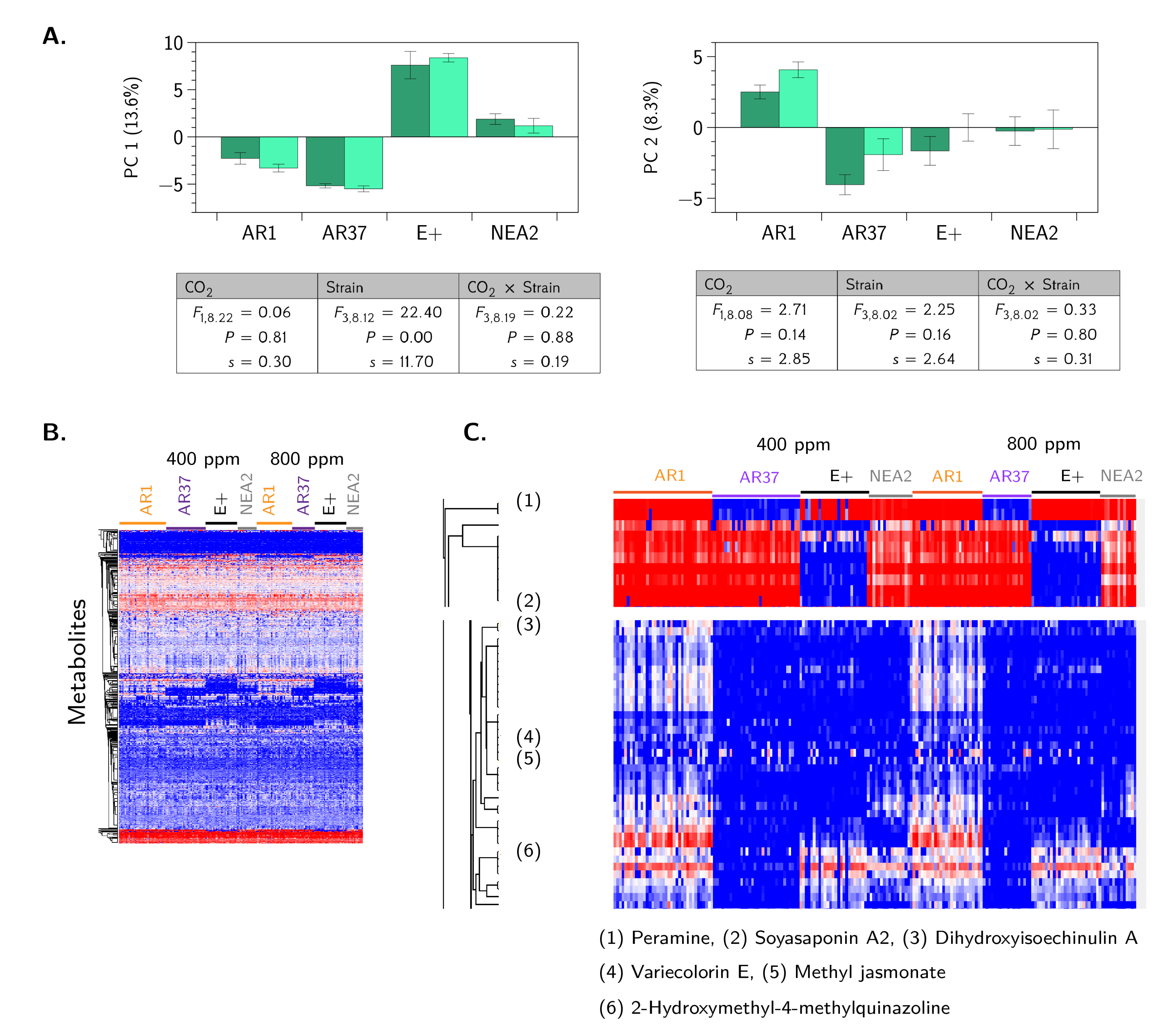
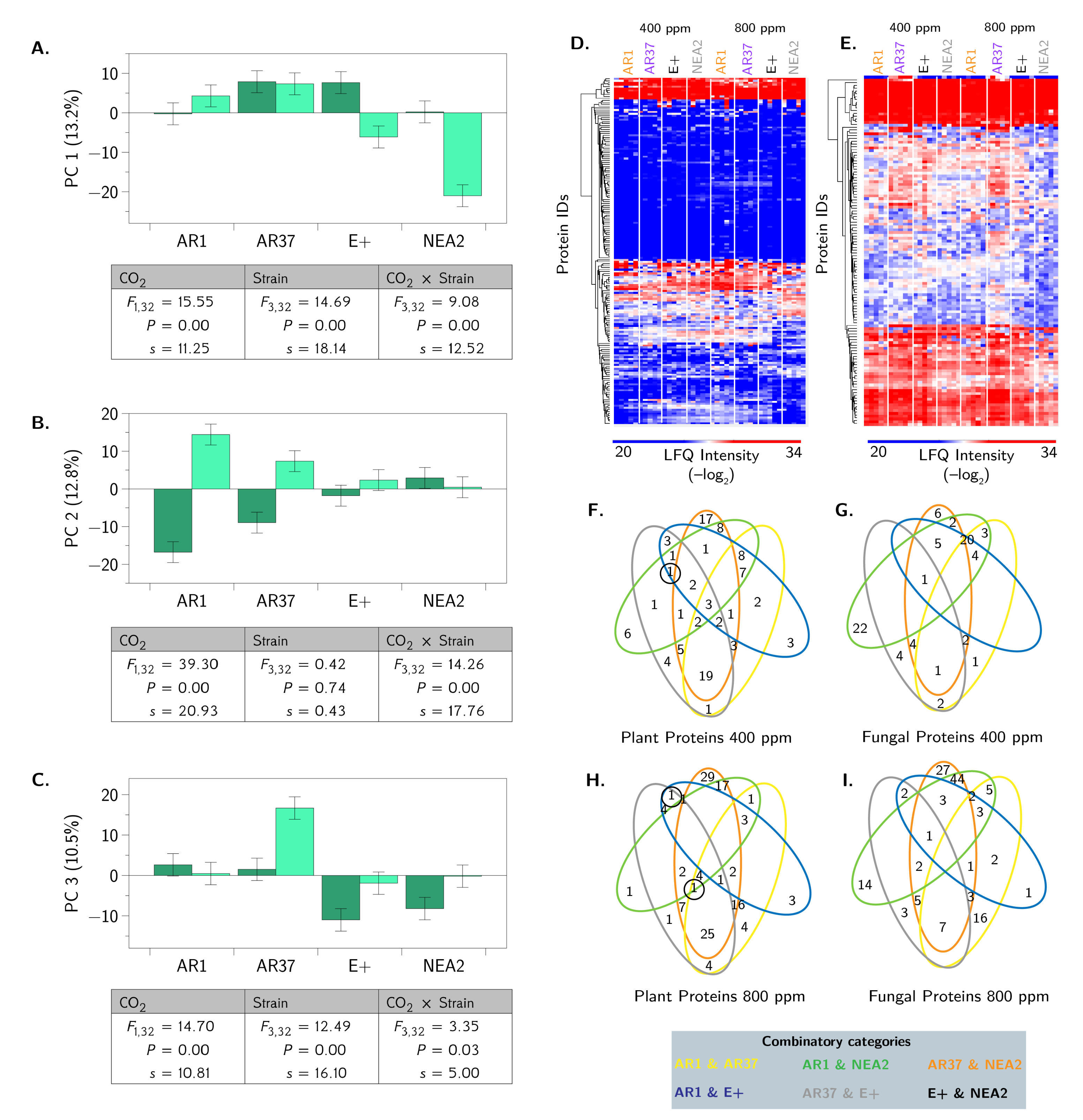
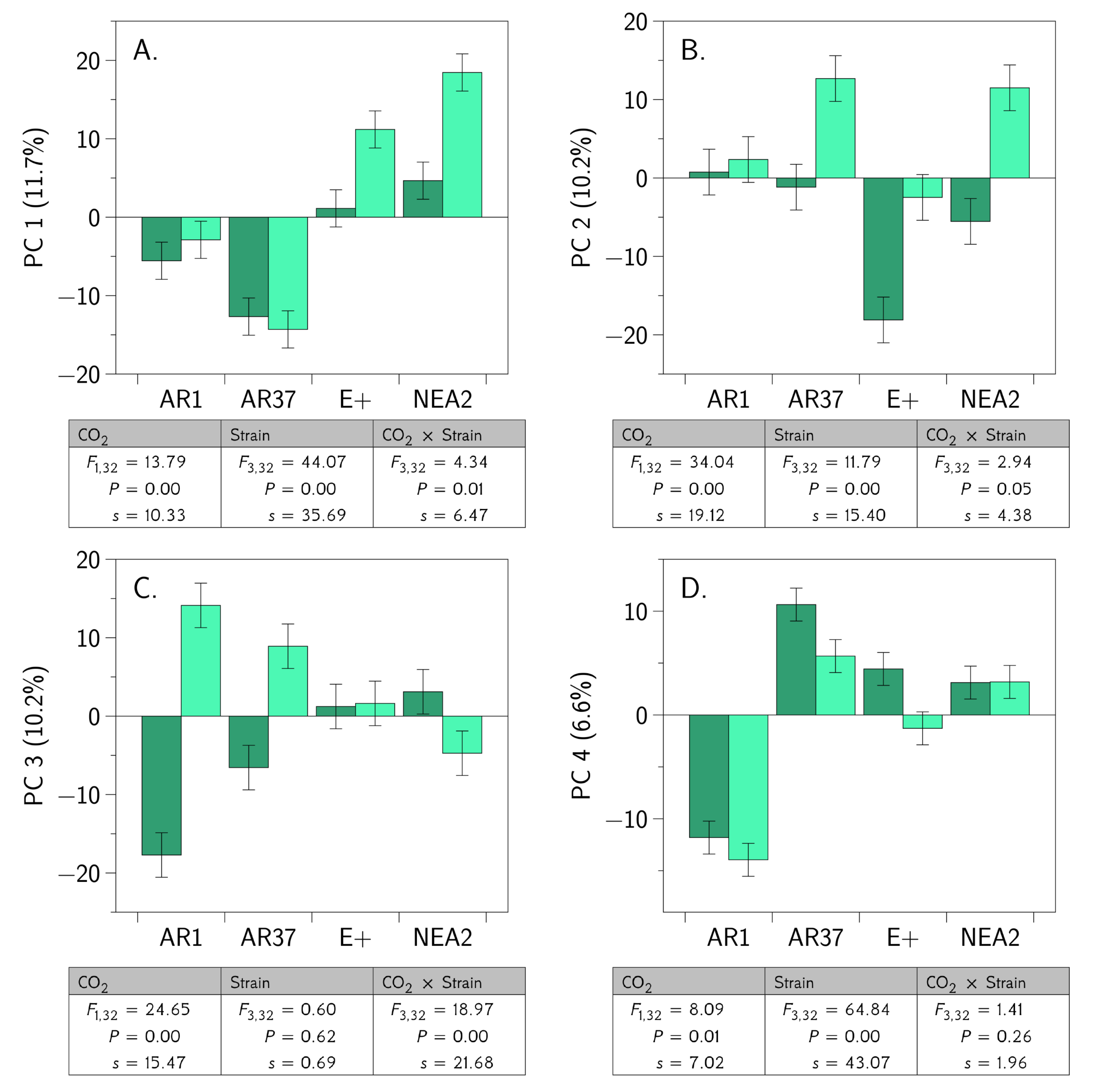
3.3. Metabolic Profiling Defines Endophyte-Specific Responses
3.4. Proteomic Profiling Reveals Fungal Strain by CO2 Interactions
3.5. Integrated OMICS Reveals Important Additonal Metabolites and Proteins
4. Discussion
4.1. Do Different Strains of Epichloë festucae var. lolii Produce Similar Fungal Concentrations in a Genetically Diverse Host Plant Background?
4.2. Do Different Strains of the Fungus Differentially Moderate the Impacts of Elevated CO2 on the Growth and Seed Production of Perennial Ryegrass?
4.3. Are the Metabolomes of the Host Plant–Fungal Strain Combinations Different From Each Other and How Are They Altered by Elevated CO2?
4.4. Are the Proteomes of the Host Plant–Fungal Strain Combinations Different From Each Other and How Are They Altered by Elevated CO2?
4.5. Does an Integrated Analysis of the Proteome and Metabolome Data Yield Different Insights Than Those Gained From Considering the Proteome and Metabolome Separately?
4.6. Is There Any Evidence of Host Plant–Fungal Strain Genetic Incompatibility?
5. Conclusions and Future Directions
- Do different strains of E. festucae var. lolii produce similar fungal concentrations in a genetically diverse host plant background?No, in our experiment AR37 produced greater concentrations of the endophyte than did any of the other strains (Figure 1).
- Do different strains of the fungus differentially moderate the impacts of elevated CO2 on the growth and seed production of perennial ryegrass?No, we did not find evidence that endophyte strains interact with CO2 to influence plant growth or seed production (Figure 1).
- Are the metabolomes of the host plant–fungal strain combinations different from each other and how are they altered by elevated CO2?
- Are the proteomes of the host plant–fungal strain combinations different from each other and how are they altered by elevated CO2?
- Does an integrated analysis of the proteome and metabolome data yield different insights than those gained from considering the proteome and metabolome separately?
- Is there any evidence of host plant–fungal strain genetic incompatibility?No, we found no evidence of genetic incompatibility for the degree of genetic diversity we were able to create in this experiment (Figure 2).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Metabolomics Results
| 1st Principal Component | |||
|---|---|---|---|
| Compound Name | Loading | ||
| 404.20 | 1.26 | unidentified #121 | 0.92 |
| 1049.53 | 5.09 | unidentified #278 | 0.89 |
| 893.44 | 4.45 | unidentified #269 | 0.89 |
| 236.13 | 4.71 | unidentified #34 | 0.88 |
| 477.26 | 4.56 | ✓ VM 54159 | 0.87 |
| 480.89 | 5.15 | unidentified #153 | 0.87 |
| 481.55 | 5.15 | unidentified #155 | 0.86 |
| 1078.52 | 4.98 | unidentified #280 | 0.86 |
| 1148.60 | 5.15 | unidentified #289 | 0.84 |
| 121.05 | 5.15 | ✓ unidentified #3 | 0.83 |
| 514.28 | 5.16 | Cucurbitacin I | 0.82 |
| 992.47 | 5.15 | unidentified #275 | 0.82 |
| 891.46 | 5.12 | unidentified #268 | 0.82 |
| 1106.56 | 5.16 | ✓ Soyasaponin A2 | 0.81 |
| 368.85 | 5.17 | ✓ unidentified #91 | 0.80 |
| 1122.55 | 4.45 | ✓ unidentified #283 | 0.80 |
| 374.18 | 4.45 | unidentified #96 | 0.80 |
| 860.42 | 5.17 | unidentified #263 | 0.79 |
| 1120.57 | 5.16 | unidentified #282 | 0.79 |
| 373.85 | 5.17 | unidentified #95 | 0.79 |
| 2st Principal Component | |||
| Compound Name | Loading | ||
| 212.08 | 5.14 | Benzyl benzoate | 0.79 |
| 374.85 | 4.44 | unidentified #98 | 0.78 |
| 256.16 | 4.75 | Chanoclavine-I | 0.77 |
| 1498.71 | 5.15 | unidentified #301 | 0.77 |
| 645.33 | 5.15 | unidentified #214 | 0.74 |
| 480.56 | 5.15 | unidentified #152 | 0.74 |
| 514.31 | 1.22 | unidentified #164 | 0.74 |
| 1458.69 | 5.15 | unidentified #300 | 0.73 |
| 878.37 | 5.16 | unidentified #266 | 0.70 |
| 2nd Principal Component | |||
| Compound Name | |||
| 364.14 | 7.51 | unidentified #86 | −0.70 |
| 348.11 | 5.75 | unidentified #75 | −0.71 |
| 332.12 | 5.32 | unidentified #69 | −0.72 |
| 316.08 | 5.31 | unidentified #61 | −0.73 |
| 362.09 | 4.70 | unidentified #84 | −0.73 |
| 396.13 | 6.05 | unidentified #115 | −0.73 |
| 378.12 | 6.04 | Macfadienoside | −0.75 |
| 346.13 | 7.50 | 3-Methoxyxanthocillin X dimethyl ether | −0.75 |
| 558.18 | 5.02 | unidentified #182 | −0.78 |
| 315.21 | 5.29 | unidentified #310 | −0.81 |
| 396.13 | 6.05 | unidentified #115 | −0.81 |
| 142.06 | 6.02 | unidentified #6 | −0.82 |
| 558.18 | 5.08 | unidentified #181 | −0.85 |
| 334.13 | 8.61 | unidentified #71 | −0.86 |
| 302.07 | 5.30 | unidentified #55 | −0.86 |
Appendix B. Detailed Proteomics Results
| PCA Loadings | |||
|---|---|---|---|
| Protein | PC1 | PC2 | PC3 |
| ✓ probable methionine synthase | 0.85 | ||
| 6-phosphogluconate dehydrogenase, decarboxylating | 0.84 | ||
| FAD dependent oxidoreductase | 0.80 | ||
| ✓-actin | 0.78 | ||
| hypothetical protein | 0.78 | ||
| heat shock protein | 0.75 | ||
| probable nucleoside-diphosphate kinase | 0.75 | ||
| ✓ related to sporulation-specific gene SPS2 | 0.75 | ||
| † 7-cephem-methoxylase P8 chain related protein | 0.75 | ||
| Iso_dh domain-containing protein | 0.74 | ||
| 60S ribosomal protein L13 | 0.73 | ||
| † Saccharopine dehydrogenase | 0.72 | ||
| unnamed protein product | −0.71 | ||
| peptidyl-prolyl cis-trans isomerase CYP20-1 isoform X2 | −0.71 | ||
| GTP-binding protein | −0.72 | ||
| dipeptidase | −0.72 | ||
| proteasome subunit type-7-B | −0.72 | ||
| citrate synthase | −0.73 | ||
| protein TPR2 | −0.74 | ||
| † transmembrane 9 superfamily member | −0.75 | ||
| glucose-6-phosphate isomerase | −0.75 | ||
| predicted protein | −0.76 | ||
| zinc protease | −0.77 | ||
| guanosine nucleotide diphosphate dissociation inhibitor | −0.80 | ||
| ✓ unnamed protein product / MPN domain-containing protein | 0.86 | ||
| ✓† plant SNARE 13 | 0.86 | ||
| ✓ pyruvate kinase, cytosolic isozyme | 0.86 | ||
| ✓† endoglucanase | 0.86 | ||
| chromatin assembly factor 1 subunit A isoform X1 | 0.84 | ||
| unnamed protein product / Importin N-terminal domain-containing protein | 0.83 | ||
| vesicle-associated protein | 0.81 | ||
| † N-acetyl-D-glucosamine kinase | 0.80 | ||
| ✓ GTP-binding protein SAR1A | 0.80 | ||
| uncharacterized protein | 0.80 | ||
| predicted protein | 0.79 | ||
| ATP-dependent Clp protease proteolytic subunit | 0.78 | ||
| ✓-adaptin-like protein | 0.75 | ||
| elongation factor 1- | 0.75 | ||
| † cytosolic acetyl-CoA carboxylase 2 | 0.75 | ||
| unnamed protein product / SRP54 domain-containing protein | 0.75 | ||
| uncharacterized protein | 0.74 | ||
| ✓† histidine–tRNA ligase | 0.73 | ||
| peroxisomal acyl-coenzyme A oxidase | 0.73 | ||
| putative 6-phosphogluconolactonase 4 | 0.73 | ||
| † chloroplast protoporphyrinogen IX oxidase | 0.72 | ||
| 40S ribosomal protein S20 | 0.72 | ||
| † 60S ribosomal protein L10a | 0.71 | ||
| hypothetical protein | 0.71 | ||
| ✓† ubiquitin carboxyl-terminal hydrolase | 0.70 | ||
| ✓ Ras family protein | 0.79 | ||
| ✓ probable glutathione peroxidase | 0.77 | ||
| ✓ 14-3-3E | 0.77 | ||
| putative glycerophosphoryl diester phosphodiesterase | 0.75 | ||
| fructose-bisphosphate aldolase | 0.75 | ||
| ran-binding protein | 0.74 | ||
| ✓ vacuolar proton-inorganic pyrophosphatase | 0.74 | ||
| hypothetical protein | 0.73 | ||
| protein CROWDED NUCLEI | 0.71 | ||
| probable ribosomal protein | 0.71 | ||
| polyketide synthase | −0.73 | ||
| † 60S ribosomal protein L32-1 | −0.73 | ||
| oxygen-dependent coproporphyrinogen-III oxidase | −0.81 | ||
| uncharacterized protein | −0.81 | ||
| LFQ (log2; 800 vs. 400 ppm) | |||||
|---|---|---|---|---|---|
| Gene Identifier | Protein Name | AR1 | AR37 | E+ | NEA2 |
| GOBP: Metabolism | |||||
| E2368|EfP3.074780.mRNA-1 | dihydrolipoamide acetyltransferase | 1.91 | |||
| E2368|EfP3.082070.mRNA-1 | probable -glucosidase 1 precursor | 1.73 | |||
| E2368|EfP3.025570.mRNA-1 | † saccharopine dehydrogenase | −2.16 | |||
| E2368|EfP3.029340.mRNA-1 | LysM domain-containing protein | −1.61 | |||
| E2368|EfP3.034550.mRNA-1 | probable H+-transporting ATPase | 1.94 | |||
| E2368|EfP3.040190.mRNA-1 | glycoside hydrolase family 10 protein | 2.57 | −2.24 | ||
| E2368|EfP3.046900.mRNA-1 | glyceraldehyde-3-phosphate dehydrogenase | −1.60 | |||
| E2368|EfP3.005420.mRNA-1 | glycoside hydrolase family 3 protein | −2.46 | |||
| E2368|EfP3.059150.mRNA-1 | inorganic pyrophosphatase | −2.25 | |||
| E2368|EfP3.015680.mRNA-1 | -mannosidase | −1.68 | |||
| E2368|EfP3.019300.mRNA-1 | adenosylhomocysteinase | −2.39 | |||
| E2368|EfP3.019390.mRNA-1 | probable trehalase precursor | −2.86 | |||
| E2368|EfP3.027680.mRNA-1 | Acyl-CoA-binding protein | −2.97 | −2.08 | ||
| E2368|EfP3.043990.mRNA-2 | probable pyruvate decarboxylase | −1.66 | |||
| GOBP: Biosynthetic & Catabolic processes | |||||
| E2368|EfP3.011820.mRNA-1 | cobalamin-independent methionine synthase | −2.15 | |||
| E2368|EfP3.002240.mRNA-1 | argininosuccinate lyase | 1.53 | |||
| E2368|EfP3.032860.mRNA-1 | 3-isopropylmalate dehydrogenase | 1.91 | −1.97 | ||
| E2368|EfP3.064110.mRNA-2 | probable phosphogluconate dehydrogenase | −1.90 | |||
| GOBP: Translation & Transcription | |||||
| E2368|EfP3.056000.mRNA-1 | 40S ribosomal protein S15 | −2.15 | |||
| E2368|EfP3.066450.mRNA-1 | probable ribosomal protein L12 | 6.22 | 3.71 | ||
| E2368|EfP3.011650.mRNA-1 | 40S ribosomal protein S0 | −2.04 | |||
| E2368|EfP3.046770.mRNA-1 | 40S ribosomal protein S7 | −2.60 | |||
| E2368|EfP3.020500.mRNA-1 | ribonuclease HI large subunit | −2.89 | −4.47 | ||
| E2368|EfP3.026100.mRNA-1 | Histone H2B | −2.20 | |||
| GOBP: Uncharacterized | |||||
| E2368|EfP3.004630.mRNA-1 | † 7-cephem-methoxylase P8 chain related protein | 3.81 | -4.95 | ||
| E2368|EfP3.014290.mRNA-1 | endosomal peripheral membrane protein | 1.83 | |||
| E2368|EfP3.053290.mRNA-1 | uncharacterized protein | −2.29 | |||
| E2368|EfP3.057600.mRNA-1 | hypothetical protein | −2.44 | |||
| E2368|EfP3.080080.mRNA-1 | WD repeat protein | −3.04 | |||
| GOBP: Protein folding | |||||
| E2368|EfP3.079510.mRNA-1 | 40 kDa peptidyl-prolyl cis-trans isomerase | −5.12 | |||
| E2368|EfP3.059210.mRNA-1 | calreticulin | −1.86 | |||
| GOBP: Pathogenesis | |||||
| E2368|EfP3.079280.mRNA-1 | Vesicle-associated membrane protein | −1.80 | |||
| GOBP:: Aerobic respiration | |||||
| E2368|EfP3.007970.mRNA-1 | cytochrome b-c1 complex subunit 2 | 2.14 | −1.79 | ||
| GOBP: Genome maintenance | |||||
| E2368|EfP3.031010.mRNA-1 | ATP citrate lyase | 2.05 | −1.90 | ||
| GOBP: DNA binding | |||||
| E2368|EfP3.059770.mRNA-1 | cold-shock DNA-binding domain-containing protein | −3.00 | |||
| LFQ (log2; 800 vs. 400 ppm) | |||||
|---|---|---|---|---|---|
| Gene Identifier | Protein Name | AR1 | AR37 | E+ | NEA2 |
| GOBP: Translation & RNA processing | |||||
| ref0006279-exonerate_est2genome-gene-0.0-mRNA-1 | sm-like protein | 2.69 | |||
| ref0046235-exonerate_est2genome-gene-0.1-mRNA-1 | 30S ribosomal protein 3 | −1.95 | |||
| ref0003115-exonerate_est2genome-gene-0.3-mRNA-1 | ✓† histidine–tRNA ligase | 1.38 | |||
| ref0012853-exonerate_est2genome-gene-1.5-mRNA-1 | RGG repeats nuclear RNA binding protein A-like | 2.12 | |||
| ref0029525-exonerate_est2genome-gene-0.5-mRNA-1 | 50S ribosomal protein L31 | 3.30 | |||
| ref0002751-exonerate_est2genome-gene-0.0-mRNA-1 | eukaryotic translation initiation factor 4G | 1.90 | |||
| ref0037446-exonerate_est2genome-gene-0.1-mRNA-1 | † 60S ribosomal protein L10a | 4.72 | |||
| ref0039514-exonerate_est2genome-gene-0.1-mRNA-1 | valine–tRNA ligase | 1.26 | −1.76 | ||
| ref0005003-exonerate_est2genome-gene-0.0-mRNA-3 | serine/arginine-rich-splicing factor SR34 isoform | 2.33 | |||
| ref0004691-exonerate_est2genome-gene-0.6-mRNA-1 | DEAD-box ATP-dependent RNA helicase 3 | 1.58 | |||
| ref0026558-exonerate_est2genome-gene-0.0-mRNA-2 | eukaryotic translation initiation factor 6-2 | −2.04 | |||
| ref0005830-exonerate_est2genome-gene-0.0-mRNA-1 | small nuclear ribonucleoprotein SmD1a | 1.63 | −1.98 | ||
| ref0031460-exonerate_est2genome-gene-0.1-mRNA-1 | splicing factor 3B subunit 1 | 1.71 | −1.73 | ||
| ref0002750-exonerate_est2genome-gene-0.0-mRNA-1 | 50S ribosomal protein L29 | −2.82 | −2.59 | ||
| ref0027372-exonerate_est2genome-gene-0.0-mRNA-1 | † 60S ribosomal protein L32-1 | −2.37 | |||
| ref0024485-exonerate_est2genome-gene-0.5-mRNA-1 | DEAD-box ATP-dependent RNA helicase 20 | −1.71 | |||
| ref0020192-exonerate_est2genome-gene-0.1-mRNA-1 | nardilysin-like | −1.85 | |||
| ref0047393-exonerate_est2genome-gene-0.0-mRNA-1 | translation initiation factor IF3-4 | 1.92 | |||
| ref0005329-exonerate_est2genome-gene-0.0-mRNA-2 | ✓ glycine–tRNA ligase | 1.54 | |||
| GOBP: Biosynthetic & catabolic processes | |||||
| ref0029850-exonerate_est2genome-gene-0.0-mRNA-1 | † chloroplast protoporphyrinogen IX oxidase 1 | 1.56 | |||
| ref0045266-exonerate_est2genome-gene-0.0-mRNA-1 | Cytochrome P450 | 3.02 | 2.73 | −2.45 | |
| ref0014716-exonerate_est2genome-gene-0.1-mRNA-1 | glutamyl-tRNA(Gln) amidotransferase subunit C | 2.04 | |||
| ref0040294-exonerate_est2genome-gene-0.0-mRNA-2 | lipoamide acyltransferase | 2.40 | −2.67 | ||
| ref0010297-exonerate_est2genome-gene-0.2-mRNA-1 | glutamate–glyoxylate aminotransferase 2 isoform | 1.60 | |||
| ref0029399-exonerate_est2genome-gene-0.0-mRNA-1 | trehalose-6-phosphate synthase | 2.32 | |||
| ref0008372-exonerate_est2genome-gene-0.1-mRNA-1 | phospholipase A1-II 7 | 2.15 | |||
| ref0020040-exonerate_est2genome-gene-0.1-mRNA-1 | -aminolevulinic acid dehydratase | 2.62 | −2.22 | ||
| ref0041371-exonerate_est2genome-gene-0.0-mRNA-1 | 12-oxophytodienoate reductase 11 | 2.25 | |||
| ref0026877-exonerate_est2genome-gene-0.0-mRNA-1 | linoleate 9S-lipoxygenase 3 | 3.43 | |||
| ref0042665-exonerate_est2genome-gene-0.2-mRNA-1 | endoglucanase 24 | 3.13 | 1.98 | ||
| ref0023177-exonerate_est2genome-gene-1.6-mRNA-1 | pyruvate dehydrogenase E1 component subunit -3 | 1.71 | |||
| ref0046445-exonerate_est2genome-gene-0.3-mRNA-1 | chloroplast stem-loop binding protein of 41 kDa b | 1.69 | −1.61 | ||
| ref0025738-exonerate_est2genome-gene-0.0-mRNA-1 | putative monogalactosyldiacylglycerol synthase 1 | 2.03 | 2.12 | ||
| ref0013554-exonerate_est2genome-gene-0.5-mRNA-1 | aldehyde oxidase 2 | −1.42 | |||
| ref0032452-exonerate_est2genome-gene-0.2-mRNA-1 | protein ECERIFERUM 26-like | −2.13 | |||
| ref0000037-exonerate_est2genome-gene-0.2-mRNA-1 | alcohol dehydrogenase 4 | −1.34 | |||
| ref0043257-exonerate_est2genome-gene-0.0-mRNA-1 | cinnamyl alcohol dehydrogenase | 2.02 | |||
| GOBP: Transport | |||||
| ref0016004-exonerate_est2genome-gene-0.3-mRNA-1 | ✓† Plant SNARE 13 | 2.87 | 1.62 | ||
| ref0007462-exonerate_est2genome-gene-0.1-mRNA-2 | cation-chloride cotransporter 1-like isoform X2 | −1.84 | |||
| ref0029020-exonerate_est2genome-gene-0.0-mRNA-1 | -soluble NSF attachment protein | 1.73 | |||
| ref0006742-exonerate_est2genome-gene-0.0-mRNA-2 | plasma membrane ATPase 1 | −1.73 | |||
| ref0036493-exonerate_est2genome-gene-0.3-mRNA-1 | exportin-2 | 2.70 | |||
| ref0009047-exonerate_est2genome-gene-0.0-mRNA-2 | ✓† vesicle-associated protein 1-3-like | 2.47 | 2.09 | ||
| ref0017192-exonerate_est2genome-gene-0.0-mRNA-1 | vacuolar targeting receptor bp-80 | 1.64 | |||
| ref0004589-exonerate_est2genome-gene-0.0-mRNA-1 | ABC transporter F family member 1-like | 1.87 | |||
| ref0032029-exonerate_est2genome-gene-0.1-mRNA-3 | † transmembrane 9 superfamily member 12-like | 2.36 | |||
| ref0006339-exonerate_est2genome-gene-0.4-mRNA-1 | importin subunit -1-like | 1.43 | |||
| GOBP: Defense response | |||||
| ref0030923-exonerate_est2genome-gene-0.2-mRNA-1 | aspartyl protease family protein 1 | 2.16 | |||
| ref0035348-exonerate_est2genome-gene-0.1-mRNA-1 | primary amine oxidase 1 | 1.77 | |||
| ref0043342-exonerate_est2genome-gene-1.4-mRNA-1 | AIG2-like protein D | 1.63 | |||
| ref0014914-exonerate_est2genome-gene-0.0-mRNA-1 | tryptophan synthase chain 2 | 2.35 | |||
| ref0036720-exonerate_est2genome-gene-0.2-mRNA-2 | endo-1,3(4)--glucanase 2 | 1.61 | |||
| ref0029599-exonerate_est2genome-gene-0.3-mRNA-1 | peroxidase 1-like | 1.85 | |||
| ref0009434-exonerate_est2genome-gene-0.0-mRNA-1 | peroxidase 43-like | 1.57 | |||
| ref0032529-exonerate_est2genome-gene-0.0-mRNA-1 | peroxidase 47-like | 1.97 | |||
| ref0038358-exonerate_est2genome-gene-0.3-mRNA-1 | pathogen-related protein 10-3 | 2.63 | |||
| ref0000436-exonerate_est2genome-gene-0.1-mRNA-1 | protein DJ-1 homolog A | 1.92 | |||
| ref0042726-exonerate_est2genome-gene-0.0-mRNA-1 | ferritin-1 | −3.07 | |||
| ref0046713-exonerate_est2genome-gene-0.1-mRNA-1 | Glucan endo-1,3--glucosidase 4 | −1.76 | |||
| ref0041974-exonerate_est2genome-gene-0.0-mRNA-1 | nonspecific lipid transfer protein-like 1 | −3.04 | |||
| ref0022536-exonerate_est2genome-gene-0.1-mRNA-1 | metacaspase 3 | −1.73 | |||
| GOBP: Uncharacterized | |||||
| ref0011040-exonerate_est2genome-gene-0.0-mRNA-1 | uncharacterized protein | 2.05 | |||
| ref0007943-exonerate_est2genome-gene-0.4-mRNA-3 | hypothetical protein | −2.04 | |||
| ref0026121-exonerate_est2genome-gene-0.3-mRNA-1 | hypothetical protein | 1.99 | −3.23 | ||
| ref0036333-exonerate_est2genome-gene-0.0-mRNA-1 | unnamed protein product | 2.65 | −2.53 | ||
| ref0025567-exonerate_est2genome-gene-0.1-mRNA-1 | ✓ hypothetical protein | 1.54 | |||
| ref0034929-exonerate_est2genome-gene-0.0-mRNA-1 | uncharacterized protein | 2.05 | |||
| ref0040401-processedgene-0.3-mRNA-1 | hypothetical protein | 1.96 | |||
| ref0038111-exonerate_est2genome-gene-0.0-mRNA-1 | unnamed protein product | −1.77 | |||
| ref0018438-exonerate_est2genome-gene-0.0-mRNA-2 | large proline-rich protein bag6-B isoform | −2.03 | |||
| ref0044732-processed-gene-0.3-mRNA-1 | predicted protein | −2.01 | |||
| GOBP: Metabolism | |||||
| ref0036350-exonerate_est2genome-gene-0.2-mRNA-1 | aldo-keto reductase family 4 member C10 | 1.81 | −1.98 | ||
| ref0046846-exonerate_est2genome-gene-0.2-mRNA-1 | putative aldo-keto reductase 2 | 2.74 | |||
| ref0036721-exonerate_est2genome-gene-0.0-mRNA-2 | sphingosine-1-phosphate lyase | 2.98 | |||
| ref0037951-exonerate_est2genome-gene-0.0-mRNA-1 | Glu1 protein | −2.67 | |||
| ref0021220-exonerate_est2genome-gene-0.2-mRNA-1 | methylcrotonoyl-CoA carboxylase subunit | 1.59 | |||
| ref0015207-exonerate_est2genome-gene-0.0-mRNA-1 | -L-arabinofuranosidase 1-like | 1.56 | −2.84 | ||
| ref0042878-exonerate_est2genome-gene-0.0-mRNA-2 | † acyl-coenzyme A oxidase 2 | 2.34 | |||
| ref0040981-exonerate_est2genome-gene-0.1-mRNA-1 | UDP-N-acetylglucosamine diphosphorylase 1 | −2.71 | |||
| GOBP: Cell cycle & development | |||||
| ref0006993-exonerate_est2genome-gene-0.0-mRNA-1 | dynamin-related protein 1E | 1.94 | |||
| ref0040384-exonerate_est2genome-gene-0.2-mRNA-1 | myosin-17-like | 1.92 | |||
| ref0013885-snap-gene-0.15-mRNA-1 | early nodulin-like protein 1 | 2.01 | 2.83 | ||
| ref0032994-exonerate_est2genome-gene-0.0-mRNA-2 | † chromatin assembly factor 1 subunit A isoform | 2.50 | |||
| ref0042157-exonerate_est2genome-gene-0.4-mRNA-1 | probable cellulose synthase A catalytic subunit 8 | 3.07 | |||
| ref0033306-exonerate_est2genome-gene-0.0-mRNA-1 | NADH dehydrogenase [ubiquinone] 1 subcomplex subunit | 2.03 | |||
| ref0034289-exonerate_est2genome-gene-0.1-mRNA-1 | † cytosolic acetyl-CoA carboxylase 2 | 2.04 | |||
| ref0003264-exonerate_est2genome-gene-0.0-mRNA-2 | protein RCC2 | −1.75 | |||
| ref0025755-exonerate_est2genome-gene-0.0-mRNA-1 | Protein phosphatase 1 regulatory subunit | −2.03 | |||
| GOBP: Proteolysis, protein ubiquitination & protein folding | |||||
| ref0044837-exonerate_est2genome-gene-0.2-mRNA-1 | ATP-dependent zinc metalloprotease | 1.40 | 1.68 | ||
| ref0019120-exonerate_est2genome-gene-0.0-mRNA-3 | ✓ ubiquitin carboxyl-terminal hydrolase 13 | 2.33 | −1.72 | ||
| ref0026335-exonerate_est2genome-gene-0.1-mRNA-1 | ubiquitin conjugation factor | 2.57 | −2.66 | ||
| ref0025653-exonerate_est2genome-gene-1.2-mRNA-1 | proteasome subunit type-5 | −1.54 | |||
| ref0043339-exonerate_est2genome-gene-1.2-mRNA-1 | dnaJ protein P58IPK homolog B isoform X1 | 2.05 | |||
| ref0035568-exonerate_est2genome-gene-0.2-mRNA-1 | proteasome subunit type-3 | −1.56 | |||
| GOBP: Signal transduction | |||||
| ref0037043-exonerate_est2genome-gene-0.0-mRNA-1 | † N-acetyl-D-glucosamine kinase | 3.38 | 2.48 | −1.68 | |
| ref0012852-exonerate_est2genome-gene-0.1-mRNA-1 | nicalin | 1.20 | |||
| ref0030401-exonerate_est2genome-gene-0.0-mRNA-1 | signal recognition particle 54 kDa protein | 1.86 | |||
| ref0013500-exonerate_est2genome-gene-0.0-mRNA-1 | signal recognition particle subunit SRP72 | 2.53 | |||
Appendix C. Detailed Integrated OMICs Results
| PCA Loadings | ||||
|---|---|---|---|---|
| Identity | PC1 | PC2 | PC3 | PC4 |
| related to sporulation-specific gene SPS2 (Table A2) | −0.85 | |||
| Superoxide dismutase [Cu-Zn] | −0.84 | |||
| probable methionine synthase (Table A2) | −0.79 | |||
| unidentified protein | −0.79 | |||
| -actin (Table A2) | −0.78 | |||
| related to gluconate 5-dehydrogenase | −0.77 | |||
| unidentified protein | −0.76 | |||
| uncharacterized proetin | −0.75 | |||
| ; ; unidentified #19 | −0.70 | |||
| Epichloë fesctucae var. lolii concentration (Figure 1) | −0.62 | |||
| ; ; Soyasaponin A2 (Table A1) | 0.68 | |||
| ; ; unidentified #283 (Table A1) | 0.69 | |||
| ; ; unidentified #91 (Table A1) | 0.75 | |||
| phospho-2-dehydro-3-deoxyheptonate aldolase 2, chloroplastic | 0.75 | |||
| 40S ribosomal protein S5-1 | 0.76 | |||
| 26S proteasome non-ATPase regulatory subunit 1 homolog | 0.76 | |||
| uncharacterized proetin | 0.77 | |||
| uncharacterized proetin | 0.79 | |||
| 26S protease regulatory subunit 8 homolog A-like | 0.80 | |||
| GDP-mannose 3,5-epimerase 2 | 0.86 | |||
| ; ; unidentified #65 | −0.72 | |||
| ; ; VM54159 (Table A1) | −0.69 | |||
| ; ; unidentified #3 (Table A1) | −0.65 | |||
| DNAJ-like protein | 0.76 | |||
| RanBD1 domain-containing protein | 0.77 | |||
| probable glutathione peroxidase 4 (Table A2) | 0.77 | |||
| vacuolar proton-inorganic pyrophosphatase (Table A2) | 0.78 | |||
| cytosolic copper zinc superoxide dismutase | 0.79 | |||
| putative ADP-ribosylation factor | 0.81 | |||
| 14-3-3E (Table A2) | 0.81 | |||
| Ras family protein (Table A2) | 0.87 | |||
| U-box domain-containing protein | 0.75 | |||
| uncharacterized protein | 0.77 | |||
| glycine–tRNA ligase, chloroplastic/mitochondrial 2-like (Table A4) | 0.78 | |||
| aldo_ket_red domain-containing protein | 0.79 | |||
| histidine–tRNA ligase, cytoplasmic (Table A2 and Table A4) | 0.79 | |||
| protoporphyrinogen oxidase | 0.79 | |||
| uncharacterized protein | 0.80 | |||
| uncharacterized protein (Table A2 and Table A4) | 0.80 | |||
| acetyltransferase component of pyruvate dehydrogenase complex | 0.80 | |||
| importin N-terminal domain-containing protein | 0.81 | |||
| -adaptin-like protein (Table A2) | 0.82 | |||
| MI domain-containing protein | 0.82 | |||
| ubiquitin carboxyl-terminal hydrolase 13 (Table A2 and Table A4) | 0.82 | |||
| pyruvate kinase, cytosolic isozyme (Table A2) | 0.83 | |||
| MPN domain-containing protein (Table A2) | 0.84 | |||
| GTP-binding protein SAR1A (Table A2) | 0.86 | |||
| vesicle-associated protein 1-3-like (Table A4) | 0.86 | |||
| predicted protein | 0.87 | |||
| uncharacterized protein | 0.89 | |||
| UBA domain-containing protein | 0.91 | |||
| endoglucanase (Table A2) | 0.92 | |||
| plant SNARE 13 (Table A2 and Table A4) | 0.93 | |||
| hypothetical protein IFM46972_10396 | −0.81 | |||
| expansin B2 | −0.77 | |||
| ; ; unidentified #249 | −0.74 | |||
| ; ; unidentified #254 | −0.70 | |||
| uncharacterized protein | 0.77 | |||
References
- Gibson, D.J.; Newman, J.A. Grasslands and Climate Change; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Beddows, A. Lolium Perenne L. J. Ecol. 1967, 55, 567–587. [Google Scholar] [CrossRef]
- McEvoy, M.; O’Donovan, M.; Shalloo, L. Development and application of an economic ranking index for perennial ryegrass cultivars. J. Dairy Sci. 2011, 94, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Thorogood, D.; Skøt, L. Directed use of wild perennial ryegrass germplasm in turfgrass improvement programmes. Czech J. Genet. Plant Breed. 2003, 39, 147–157. [Google Scholar]
- Schapendonk, A.; Dijkstra, P.; Groenwold, J.; Pot, C.; Van de Geijn, S. Carbon balance and water use efficiency of frequently cut Lolium perenne L. swards at elevated carbon dioxide. Glob. Chang. Biol. 1997, 3, 207–216. [Google Scholar] [CrossRef]
- Laing, W.A.; Greer, D.H.; Campbell, B.D. Strong responses of growth and photosynthesis of five C3 pasture species to elevated CO2 at low temperatures. Funct. Plant Biol. 2002, 29, 1089–1096. [Google Scholar] [CrossRef]
- Ainsworth, E.; Davey, P.; Hymus, G.; Osborne, C.; Rogers, A.; Blum, H.; Nösberger, J.; Long, S.P. Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant Cell Environ. 2003, 26, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Farfan-Vignolo, E.R.; Asard, H. Effect of elevated CO2 and temperature on the oxidative stress response to drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 2012, 59, 55–62. [Google Scholar] [CrossRef]
- Newton, P.; Clark, H.; Bell, C.; Glasgow, E. Interaction of soil moisture and elevated CO2 on the above-ground growth rate, root length density and gas exchange of turves from temperate pasture. J. Exp. Bot. 1996, 47, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Nijs, I.; Impens, I. Effects of elevated CO2 concentration and climate-warming on photosynthesis during winter in Lolium perenne. J. Exp. Bot. 1996, 47, 915–924. [Google Scholar] [CrossRef]
- Ferris, R.; Nijs, I.; Behaeghe, T.; Impens, I. Contrasting CO2 and temperature effects on leaf growth of perennial ryegrass in spring and summer. J. Exp. Bot. 1996, 47, 1033–1043. [Google Scholar] [CrossRef] [Green Version]
- Ryle, G.; Powell, C.; Tewson, V. Effect of elevated CO2 on the photosynthesis, respiration and growth of perennial ryegrass. J. Exp. Bot. 1992, 43, 811–818. [Google Scholar] [CrossRef]
- Schapendonk, A.; Dijkstra, P.; Groenwold, J.; Pot, C.; Van de Geijn, S. Implications of elevated carbon dioxide concentration on Lolium perenne L. swards. Growth analyses and carbon balance. Asp. Appl. Biol. 1996, 45, 31–40. [Google Scholar]
- Nijs, I.; Impens, I.; Behaeghe, T. Leaf and canopy responses of Lolium perenne to long-term elevated atmospheric carbon-dioxide concentration. Planta 1989, 177, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Suter, D.; Nösberger, J.; Lüscher, A. Response of perennial ryegrass to free-air CO2 enrichment (FACE) is related to the dynamics of sward structure during regrowth. Crop Sci. 2001, 41, 810–817. [Google Scholar] [CrossRef]
- Brinkhoff, R.; Porter, M.; Hovenden, M.J. Elevated CO2 causes large changes to morphology of perennial ryegrass (Lolium perenne). Crop Pasture Sci. 2019, 70, 555–565. [Google Scholar] [CrossRef]
- Sæbø, A.; Mortensen, L.M. Growth and regrowth of Phleum pratense, Lolium perenne, Trifolium repens and Trifolium pratense at normal and elevated CO2 concentration. Agric. Ecosyst. Environ. 1995, 55, 29–35. [Google Scholar] [CrossRef]
- Schenk, U.; Manderscheid, R.; Hugen, J.; Weigel, H. Effects of CO2 enrichment and intraspecific competition on biomass partitioning, nitrogen content and microbial biomass carbon in soil of perennial ryegrass and white clover. J. Exp. Bot. 1995, 46, 987–993. [Google Scholar] [CrossRef]
- Clark, H.; Newton, P.; Barker, D. Physiological and morphological responses to elevated CO2 and a soil moisture deficit of temperate pasture species growing in an established plant community. J. Exp. Bot. 1999, 50, 233–242. [Google Scholar] [CrossRef]
- Clark, H.; Newton, P.; Bell, C.; Glasgow, E. The influence of elevated CO2 and simulated seasonal changes in temperature on tissue turnover in pasture turves dominated by perennial ryegrass (Lolium perenne) and white clover (Trifolium repens). J. Appl. Ecol. 1995, 34, 128–136. [Google Scholar] [CrossRef]
- Daepp, M.; Nösberger, J.; Lüscher, A. Nitrogen fertilization and developmental stage alter the response of Lolium perenne to elevated CO2. New Phytol. 2001, 150, 347–358. [Google Scholar] [CrossRef]
- Beechey-Gradwell, Z.; Cooney, L.; Winichayakul, S.; Andrews, M.; Hea, S.Y.; Crowther, T.; Roberts, N. Storing carbon in leaf lipid sinks enhances perennial ryegrass carbon capture especially under high N and elevated CO2. J. Exp. Bot. 2019, 71, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Tang, S.; Wang, R.; Ju, X.; Ding, Y.; Tu, S.; Smith, D.L. Effects of elevated CO2 on growth, photosynthesis, elemental composition, antioxidant level, and phytochelatin concentration in Lolium mutiforum and Lolium perenne under Cd stress. J. Hazard. Mater. 2010, 180, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.W.; Marshall, C.; Williams, G.; Blum, H.; Harmens, H.; Jones, D.; Farrar, J. The fate of photosynthetically-fixed carbon in Lolium perenne grassland as modified by elevated CO2 and sward management. New Phytol. 2007, 173, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.G.; Rasmussen, S.; Newton, P.C.; Parsons, A.J.; Newman, J.A. Near-term impacts of elevated CO2, nitrogen and fungal endophyte-infection on Lolium perenne L. growth, chemical composition and alkaloid production. Plant Cell Environ. 2005, 28, 1345–1354. [Google Scholar] [CrossRef]
- Bazot, S.; Ulff, L.; Blum, H.; Nguyen, C.; Robin, C. Effects of elevated CO2 concentration on rhizodeposition from Lolium perenne grown on soil exposed to 9 years of CO2 enrichment. Soil Biol. Biochem. 2006, 38, 729–736. [Google Scholar] [CrossRef]
- Gorissen, A.; Van Ginkel, J.; Van de Beek, H. Carbon allocation in mature grass (Lolium perenne) under elevated CO2 at two soil nitrogen levels. In Studies in Environmental Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 65, pp. 735–740. [Google Scholar]
- Hardacre, A.; Laing, W.; Christeller, J. The response of simulated swards of perennial ryegrass and white clover to enriched atmospheric CO2: Interaction with nitrogen and photosynthetic photon flux density. N. Z. J. Agric. Res. 1986, 29, 567–573. [Google Scholar] [CrossRef]
- Daepp, M.; Suter, D.; Almeida, J.P.; Isopp, H.; Hartwig, U.A.; Frehner, M.; Blum, H.; Nösberger, J.; Lüscher, A. Yield response of Lolium perenne swards to free air CO2 enrichment increased over six years in a high N input system on fertile soil. Glob. Chang. Biol. 2000, 6, 805–816. [Google Scholar] [CrossRef]
- Schenk, U.; Jäger, H.J.; Weigel, H.J. The response of perennial ryegrass/white clover mini-swards to elevated atmospheric CO2 concentrations: Effects on yield and fodder quality. Grass Forage Sci. 1997, 52, 232–241. [Google Scholar] [CrossRef]
- Clark, H.; Newton, P.; Bell, C.; Glasgow, E. Dry matter yield, leaf growth and population dynamics in Lolium perenne/Trifolium repens-dominated pasture turves exposed to two levels of elevated CO2. J. Appl. Ecol. 1997, 34, 304–316. [Google Scholar] [CrossRef]
- Suter, D.; Frehner, M.; Fischer, B.U.; Nösberger, J.; Lüscher, A. Elevated CO2 increases carbon allocation to the roots of Lolium perenne under free-air CO2 enrichment but not in a controlled environment. New Phytol. 2002, 154, 65–75. [Google Scholar] [CrossRef]
- Van Ginkel, J.; Gorissen, A.; Van Veen, J. Carbon and nitrogen allocation in Lolium perenne in response to elevated atmospheric CO2 with emphasis on soil carbon dynamics. Plant Soil 1997, 188, 299–308. [Google Scholar] [CrossRef]
- Soussana, J.; Casella, E.; Loiseau, P. Long-term effects of CO2 enrichment and temperature increase on a temperate grass sward. Plant Soil 1996, 182, 101–114. [Google Scholar] [CrossRef]
- Loiseau, P.; Soussana, J. Effects of elevated CO2, temperature and N fertilization on nitrogen fluxes in a temperate grassland ecosystem. Glob. Chang. Biol. 2000, 6, 953–965. [Google Scholar] [CrossRef]
- Leuchtmann, A.; Bacon, C.W.; Schardl, C.L.; White, J.F., Jr.; Tadych, M. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 2014, 106, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L. The Epichloë, Symbionts of the Grass Subfamily Poöideae. Ann. Mo. Bot. Gard. 2010, 97, 646–665. [Google Scholar] [CrossRef]
- Newman, J.A.; Gillis, S.; Hager, H.A. Costs, Benefits, Parasitism and Mutualisn: A Note on the Use and Abuse of the ‘Mutualism–Parasitism Continuum’ Concept for Epichloë Fungi. Philos. Theory Pract. Biol. 2020. in review. [Google Scholar]
- Faeth, S.H. Are endophytic fungi defensive plant mutualists? Oikos 2002, 98, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1. [Google Scholar] [CrossRef] [Green Version]
- Hesse, U.; Schöberlein, W.; Wittenmayer, L.; Förster, K.; Warnstorff, K.; Diepenbrock, W.; Merbach, W. Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass Forage Sci. 2003, 58, 407–415. [Google Scholar] [CrossRef]
- Hesse, U.; Schöberlein, W.; Wittenmayer, L.; Förster, K.; Warnstorff, K.; Diepenbrock, W.; Merbach, W. Influence of water supply and endophyte infection (Neotyphodium spp.) on vegetative and reproductive growth of two Lolium perenne L. genotypes. Eur. J. Agron. 2005, 22, 45–54. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Malinowski, D.; Belesky, D.; Hill, N.; Baligar, V.; Fedders, J. Influence of phosphorus on the growth and ergot alkaloid content of Neotyphodium coenophialum-infected tall fescue (Festuca arundinacea Schreb.). Plant Soil 1998, 198, 53–61. [Google Scholar] [CrossRef]
- Müller, C.B.; Krauss, J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005, 8, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.E.; Ryan, G.D.; Gibert, A.; Helander, M.; Mirlohi, A.; Sabzalian, M.R. Epichloë fungal endophytes for grassland ecosystems. In Sustainable Agriculture Reviews; Prashar, P., Shah, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 233–305. [Google Scholar]
- Cunningham, I.J.; Hartley, W.J. Ryegrass staggers. N. Z. Vet. J. 1959, 7, 1–7. [Google Scholar] [CrossRef]
- Gallagher, R.; Campbell, A.; Hawkes, A.; Holland, P.; McGaveston, D.; Pansier, E.; Harvey, I. Ryegrass staggers: The presence of lolitrem neurotoxins in perennial ryegrass seed. N. Z. Vet. J. 1982, 30, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Prestidge, R. Causes and control of perennial ryegrass staggers in New Zealand. Agric. Ecosyst. Environ. 1993, 44, 283–300. [Google Scholar] [CrossRef]
- Fletcher, L.; Easton, H. The evaluation of use of endophytes for pasture improvement. In Neotyphodium/Grass Interactions; Bacon, C., Hill, N., Eds.; Plenum Press: New York, NY, USA, 1997; pp. 209–228. [Google Scholar]
- Easton, H.S. Grasses and Neotyphodium endophytes: Co-adaptation and adaptive breeding. Euphytica 2007, 154, 295–306. [Google Scholar] [CrossRef]
- Fletcher, L.; Finch, S.; Sutherland, B.; deNicolo, G.; Mace, W.; van Koten, C.; Hume, D. The occurrence of ryegrass staggers and heat stress in sheep grazing ryegrass-endophyte associations with diverse alkaloid profiles. N. Z. Vet. J. 2017, 65, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.; Rasmussen, S.; Parsons, A.; Newman, J. The effects of carbohydrate supply and host genetic background on Epichloë endophyte and alkaloid concentrations in perennial ryegrass. Fungal Ecol. 2015, 18, 115–125. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R.; et al. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Newman, J.A. Metabolomics analysis of the Lolium perenne–Neotyphodium lolii symbiosis: More than just alkaloids? Phytochem. Rev. 2009, 8, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.; Newman, J. Festuca arundinacea Schreber (F. elatior L. ssp. arundinacea (Schreber) Hackel). J. Ecol. 2001, 89, 304–324. [Google Scholar]
- Hunt, M.G.; Newman, J.A. Reduced herbivore resistance from a novel grass–endophyte association. J. Appl. Ecol. 2005, 42, 762–769. [Google Scholar] [CrossRef]
- Hager, H.; Newman, J. Methodology I: Detecting and predicting grassland change. In Grasslands and Climate Change; Gibson, D.J., Newman, J.A., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 19–39. [Google Scholar]
- Cheplick, G.P.; Faeth, S.H. Ecology and Evolution of the Grass-Endophyte Symbiosis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marks, S.; Clay, K. Effects of CO2 enrichment, nutrient addition, and fungal endophyte-infection on the growth of two grasses. Oecologia 1990, 84, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Marks, S.; Lincoln, D.E. Antiherbivore defense mutualism under elevated carbon dioxide levels: A fungal endophyte and grass. Environ. Entomol. 1996, 25, 618–623. [Google Scholar] [CrossRef]
- Newman, J.; Abner, M.; Dado, R.; Gibson, D.; Brookings, A.; Parsons, A. Effects of elevated CO2, nitrogen and fungal endophyte-infection on tall fescue: Growth, photosynthesis, chemical composition and digestibility. Glob. Chang. Biol. 2003, 9, 425–437. [Google Scholar] [CrossRef]
- Ryan, G.D.; Rasmussen, S.; Xue, H.; Parsons, A.J.; Newman, J.A. Metabolite analysis of the effects of elevated CO2 and nitrogen fertilization on the association between tall fescue (Schedonorus arundinaceus) and its fungal symbiont Neotyphodium coenophialum. Plant Cell Environ. 2014, 37, 204–212. [Google Scholar] [CrossRef]
- Ryan, G.D.; Shukla, K.; Rasmussen, S.; Shelp, B.J.; Newman, J.A. Phloem phytochemistry and aphid responses to elevated CO2, nitrogen fertilization and endophyte infection. Agric. For. Entomol. 2014, 16, 273–283. [Google Scholar] [CrossRef]
- Brosi, G.B.; McCulley, R.L.; Bush, L.P.; Nelson, J.A.; Classen, A.T.; Norby, R.J. Effects of multiple climate change factors on the tall fescue–fungal endophyte symbiosis: Infection frequency and tissue chemistry. New Phytol. 2011, 189, 797–805. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Uppal, K.; Tran, V.T.; Jones, D.P.; Filipov, N.M. Metabolomics of fescue toxicosis in grazing beef steers. Food Chem. Toxicol. 2017, 105, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mote, R.S.; Filipov, N.M. Use of Integrative Interactomics for Improvement of Farm Animal Health and Welfare: An Example with Fescue Toxicosis. Toxins 2020, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; McCulley, R.L.; McNear, D.H., Jr. Tall fescue cultivar and fungal endophyte combinations influence plant growth and root exudate composition. Front. Plant Sci. 2015, 6, 183. [Google Scholar] [CrossRef] [Green Version]
- Wakelin, S.; Harrison, S.; Mander, C.; Dignam, B.; Rasmussen, S.; Monk, S.; Fraser, K.; O’Callaghan, M. Impacts of endophyte infection of ryegrass on rhizosphere metabolome and microbial community. Crop Pasture Sci. 2015, 66, 1049–1057. [Google Scholar] [CrossRef]
- Green, K.A.; Berry, D.; Feussner, K.; Eaton, C.J.; Ram, A.; Mesarich, C.H.; Solomon, P.; Feussner, I.; Scott, B. Lolium perenne apoplast metabolomics for identification of novel metabolites produced by the symbiotic fungus Epichloë festucae. New Phytol. 2020, 227, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Parsons, A.J.; Jones, C.S. Metabolomics of forage plants: A review. Ann. Bot. 2012, 110, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.; Bassett, S.; Cao, M.; Christensen, M.; Gaborit, C.; Johnson, L.; Koulman, A.; Rasmussen, S.; Voisey, C.; Bryan, G. A multidisciplinary approach to dissect the molecular basis of the Neotyphodium lolii/ryegrass symbiosis. Adv. Pasture Plant Breed. Grassl. Res. Pract. Ser. 2006, 12, 107–114. [Google Scholar]
- Bassett, S.A.; Bond, J.J.; Kwan, F.Y.; McCulloch, A.F.; Haynes, P.A.; Johnson, R.D.; Bryan, G.T.; Jordan, T.W. Proteomic analysis of a filamentous fungal endophyte using EST datasets. Proteomics 2009, 9, 2295–2300. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef] [Green Version]
- Nagabhyru, P.; Dinkins, R.D.; Schardl, C.L. Transcriptomics of Epichloë-grass symbioses in host vegetative and reproductive stages. Mol. Plant Microbe Interact. 2019, 32, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prudhomme, N.; Gianetto-Hill, C.; Pastora, R.; Cheung, W.-F.; Allen-Vercoe, E.; McLean, M.D.; Cossar, D.; Geddes-McAlister, J. Quantitative proteomic profiling of shake flask versus bioreactor growth reveals distinct responses of Agrobacterium tumefaciens for preparation in molecular pharming. Can. J. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Gaugaz, F.Z. Fast and sensitive total protein and Peptide assays for proteomic analysis. Anal. Chem. 2015, 87, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nat. Methods 2016, 13, 731. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [Green Version]
- Takach, J.E.; Mittal, S.; Swoboda, G.A.; Bright, S.K.; Trammell, M.A.; Hopkins, A.A.; Young, C.A. Genotypic and chemotypic diversity of I endophytes in tall fescue from Greece. Appl. Environ. Microbiol. 2012, 78, 5501–5510. [Google Scholar] [CrossRef] [Green Version]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a world beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar]
- Greenland, S. Valid p-values behave exactly as they should: Some misleading criticisms of p-values and their resolution with s-values. Am. Stat. 2019, 73, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Kalde, M.; Nühse, T.S.; Findlay, K.; Peck, S.C. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc. Natl. Acad. Sci. USA 2007, 104, 11850–11855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, K.; DebRoy, S.; Lee, Y.H.; Pumplin, N.; Jones, J.; He, S.Y. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science 2006, 313, 220–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, R.A.; De Sordi, L.; MacCallum, D.M.; Topal, H.; Eaton, R.; Bloor, J.W.; Robinson, G.K.; Levin, L.R.; Buck, J.; Wang, Y.; et al. CO2 acts as a signalling molecule in populations of the fungal pathogen Candida albicans. PLoS Pathog. 2010, 6, e1001193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Sun, D.; Wang, X.; Mao, S. An integrated metabolomic and proteomic study revealed the difference in metabolite and protein expression profiles in ruminal tissue from goats fed hay or high grain diets. Front. Physiol. 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clay, K. Effects of fungal endophytes on the seed and seedling biology of Lolium perenne and Festuca arundinacea. Oecologia 1987, 73, 358–362. [Google Scholar] [CrossRef] [PubMed]
- White, J., Jr.; Morgan-Jones, G.; Morrow, A. Taxonomy, life cycle, reproduction and detection of Acremonium endophytes. Agric. Ecosyst. Environ. 1993, 44, 13–37. [Google Scholar] [CrossRef]
- Lane, G.A.; Christensen, M.J.; Miles, C.O. Coevolution of fungal endophytes with grasses: The significance of secondary metabolites. In Microbial Endophytes; Bacon, C., White, J., Eds.; Marcel Dekker: New York, NY, USA, 2000; Volume 2000, pp. 341–388. [Google Scholar]
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337. [Google Scholar] [CrossRef] [Green Version]
- Dinkins, R.D.; Nagabhyru, P.; Young, C.A.; West, C.P.; Schardl, C.L. Transcriptome analysis and differential expression in tall fescue harboring different endophyte strains in response to water deficit. Plant Genome 2019, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]



| Strain | 400 ppm | 800 ppm | Measurements |
|---|---|---|---|
| AR1 | |||
| Family a | Biomass, qPCR, proteomics, | ||
| metabolomics, integrated OMICS | |||
| Family b | Biomass, qPCR, metabolomics | ||
| Family c | Biomass, qPCR, metabolomics | ||
| Family d | Biomass, qPCR, metabolomics | ||
| AR37 | |||
| Family e | Biomass, qPCR, proteomics, | ||
| metabolomics, integrated OMICS | |||
| Family f | Biomass, qPCR, metabolomics | ||
| Family g | Biomass, qPCR, metabolomics | ||
| E+ | |||
| Family h | Biomass, qPCR, proteomics, | ||
| metabolomics, integrated OMICS | |||
| Family i | Biomass, qPCR, metabolomics | ||
| Family j | Biomass, qPCR, metabolomics | ||
| NEA2 | |||
| Family k | Biomass, qPCR, proteomics, | ||
| metabolomics, integrated OMICS | |||
| Family l | Biomass, qPCR, metabolomics | ||
| Totals | 144 plants | 144 plants |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddes-McAlister, J.; Sukumaran, A.; Patchett, A.; Hager, H.A.; Dale, J.C.M.; Roloson, J.L.; Prudhomme, N.; Bolton, K.; Muselius, B.; Powers, J.; et al. Examining the Impacts of CO2 Concentration and Genetic Compatibility on Perennial Ryegrass—Epichloë festucae var lolii Interactions. J. Fungi 2020, 6, 360. https://doi.org/10.3390/jof6040360
Geddes-McAlister J, Sukumaran A, Patchett A, Hager HA, Dale JCM, Roloson JL, Prudhomme N, Bolton K, Muselius B, Powers J, et al. Examining the Impacts of CO2 Concentration and Genetic Compatibility on Perennial Ryegrass—Epichloë festucae var lolii Interactions. Journal of Fungi. 2020; 6(4):360. https://doi.org/10.3390/jof6040360
Chicago/Turabian StyleGeddes-McAlister, Jennifer, Arjun Sukumaran, Aurora Patchett, Heather A. Hager, Jenna C. M. Dale, Jennifer L. Roloson, Nicholas Prudhomme, Kim Bolton, Benjamin Muselius, Jacqueline Powers, and et al. 2020. "Examining the Impacts of CO2 Concentration and Genetic Compatibility on Perennial Ryegrass—Epichloë festucae var lolii Interactions" Journal of Fungi 6, no. 4: 360. https://doi.org/10.3390/jof6040360
APA StyleGeddes-McAlister, J., Sukumaran, A., Patchett, A., Hager, H. A., Dale, J. C. M., Roloson, J. L., Prudhomme, N., Bolton, K., Muselius, B., Powers, J., & Newman, J. A. (2020). Examining the Impacts of CO2 Concentration and Genetic Compatibility on Perennial Ryegrass—Epichloë festucae var lolii Interactions. Journal of Fungi, 6(4), 360. https://doi.org/10.3390/jof6040360






