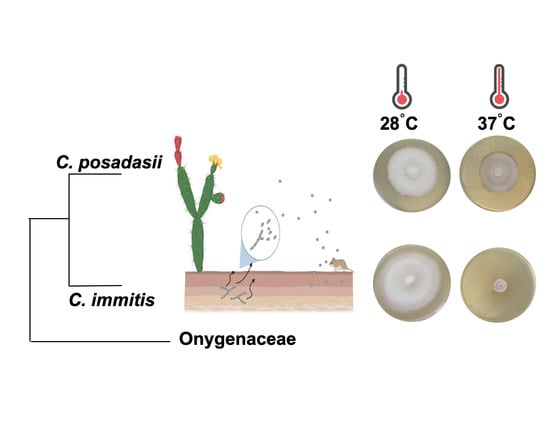
Differential Thermotolerance Adaptation between Species of Coccidioides
Abstract
:1. Introduction
2. Materials and Methods
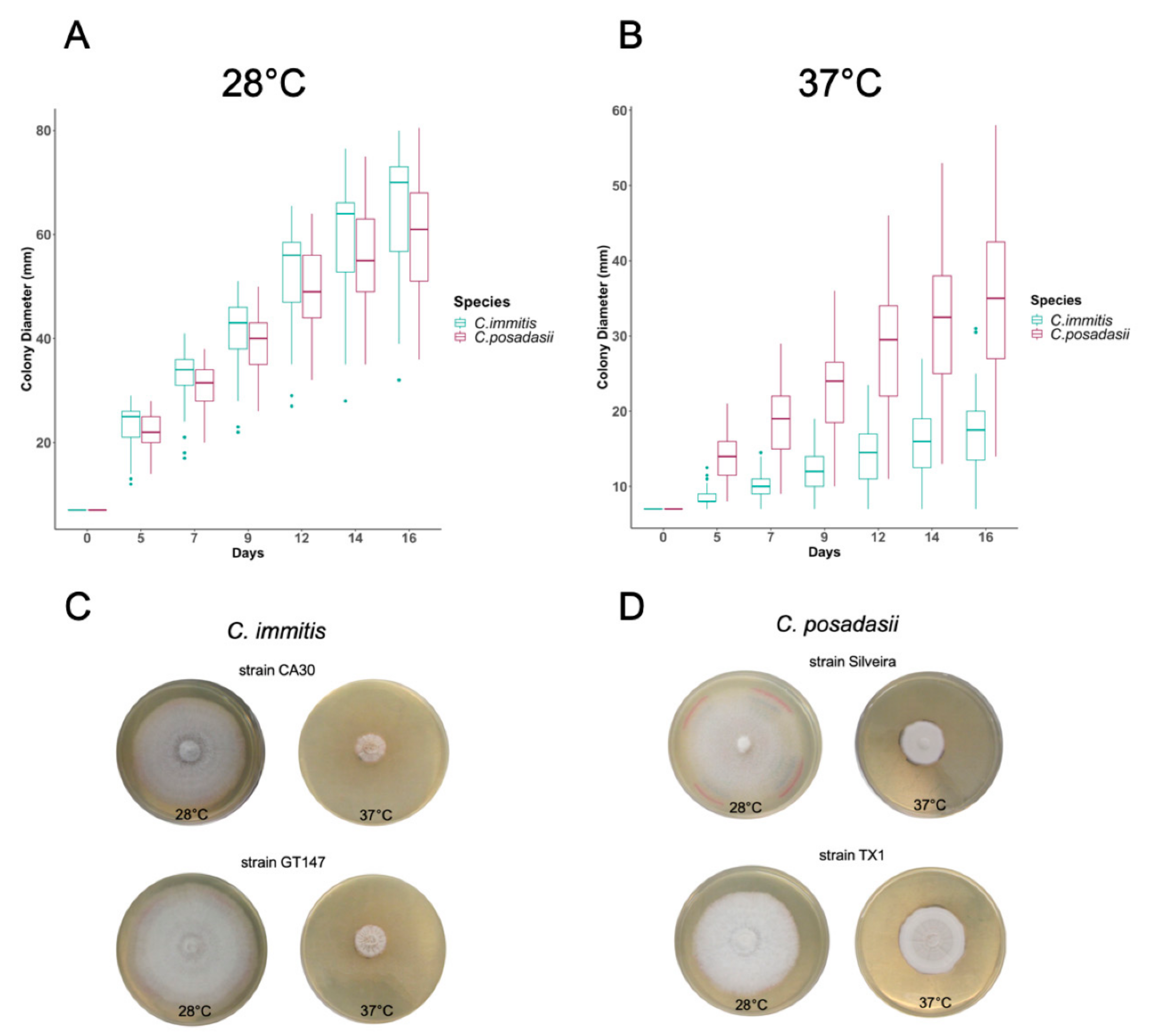
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cox, R.A.; Magee, D.M. Coccidioidomycosis: Host Response and Vaccine Development. Clin. Microbiol. Rev. 2004, 17, 804–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, E.R.G.; Bowers, J.R.; Barker, B.M. Dust Devil: The Life and Times of the Fungus That Causes Valley Fever. PLoS Pathog. 2015, 11, e1004762. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Catanzaro, A.; Johnson, R.H.; Stevens, D.A.; Williams, P.L. Practice Guidelines for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2000, 30, 658–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, M.B.; Jackson, B.R.; Benedict, R.; McCotter, O.; Benedict, K. Preliminary Estimates of Annual Burden of Coccidioidomycosis in the United States, 2010–2014. In Coccidioidomycosis Study Group 61st Annual Meeting in collaboration with the 7th International Coccidioidomycosis Symposium; Group CS, Ed.; Coccidioidomycosis Study Group: Palo Alto, CA, USA, 2017; p. 32. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal Wkly. Rep. 2013, 62, 217–221. [Google Scholar]
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and Phenotypic Description of Coccidioides posadasii sp. nov., Previously Recognized as the Non-California Population of Coccidioides immitis. Mycologia 2002, 94, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelthaler, D.M.; Roe, C.C.; Hepp, C.M.; Teixeira, M.; Driebe, E.M.; Schupp, J.M.; Gade, L.; Waddell, V.; Komatsu, K.; Arathoon, E.; et al. Local Population Structure and Patterns of Western Hemisphere Dispersal for Coccidioides spp., the Fungal Cause of Valley Fever. mBio 2016, 7, e00550-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, M.M.; Barker, B.M. Use of Population Genetics to Assess the Ecology, Evolution, and Population Structure of Coccidioides. Emerg. Infect. Dis. 2016, 22, 1022–1030. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, C.S.; Mattox, K.; Turissini, D.A.; Teixeira, M.M.; Barker, B.M.; Matute, D.R. Gene exchange between two divergent species of the fungal human pathogen, Coccidioides. Evolution 2019, 73, 42–58. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.M.; Alvarado, P.; Roe, C.C.; Thompson, G.R., 3rd; Patane, J.S.L.; Sahl, J.W.; Keim, P.; Galgiani, J.N.; Litvintseva, A.P.; Matute, D.R.; et al. Population Structure and Genetic Diversity among Isolates of Coccidioides posadasii in Venezuela and Surrounding Regions. mBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Neafsey, D.E.; Barker, B.M.; Sharpton, T.J.; Stajich, J.E.; Park, D.J.; Whiston, E.; Hung, C.-Y.; Mcmahan, C.; White, J.; Sykes, S.; et al. Population genomic sequencing of Coccidioides fungi reveals recent hybridization and transposon control. Genome Res. 2010, 20, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Mead, H.L.; Teixeira, M.D.M.; Galgiani, J.N.; Barker, B.M. Characterizing in vitro spherule morphogenesis of multiple strains of both species of Coccidioides. Med. Mycol. 2019, 57, 478–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, E.R.G.; David, V.R.; Doyle, A.L.; Rajabi, K.; Kiefer, J.A.; Pirrotte, P.; Barker, B.M. Differences in Host Innate Responses among Coccidioides Isolates in a Murine Model of Pulmonary Coccidioidomycosis. Eukaryot. Cell 2015, 14, 1043–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, L.; Smith, C.E. The comparison of four strains of Coccidioides immitis with diverse histories. Mycopathol. Mycol. Appl. 1957, 8, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.J.; Friedman, L.; Pappagianis, D.; Smith, C.E. Survival of Coccidioides immitis Under Controlled Conditions of Temperature and Humidity. Am. J. Public Health Nations Health 1956, 46, 1317–1324. [Google Scholar]
- Friedman, L.; Smith, C.E.; Roessler, W.G.; Berman, R. The virulence and infectivity of twenty-seven strains of Coccidioides immitis. Am. J. Epidemiol. 1956, 64, 198–210. [Google Scholar] [CrossRef]
- Friedman, L.; Smith, C.E.; Gordon, L.E. The Assay of Virulence of Coccidioides in White Mice. J. Infect. Dis. 1955, 97, 311–316. [Google Scholar] [CrossRef]
- Huppert, M.; Walker, L.J. The selective and differential effects of cycloheximide on many strains of Coccidioides immitis. Am. J. Clin. Pathol. 1958, 29, 291–295. [Google Scholar] [CrossRef]
- Egeberg, R.O.; Elconin, A.E.; Egeberg, M.C. Effect of Salinity and Temperature on Coccidioides Immitis and Three Antagonistic Soil Saprophytes. J. Bacteriol. 1964, 88, 473–476. [Google Scholar] [CrossRef] [Green Version]
- Egeberg, R.O.; Ely, A.F. Coccidioides immitis in the soil of the southern San Joaquin Valley. Am. J. Med. Sci. 1956, 231, 151–154. [Google Scholar] [CrossRef]
- Elconin, A.F.; Egeberg, R.O.; Egeberg, M.C. Significance of soil salinity on the ecology of Coccidioides immitis. J. Bacteriol. 1964, 87, 500–503. [Google Scholar] [CrossRef] [Green Version]
- Emmons, C.W.; Ashburn, L.L. The Isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from Wild Rodents. Their Relationship to Coccidioidomycosis. Public Health Rep. 1942, 57, 1715. [Google Scholar] [CrossRef]
- Emmons, C.W. Isolation of Coccidioides from Soil and Rodents. Public Health Rep. 1942, 57, 109. [Google Scholar] [CrossRef]
- Maddy, K.T. The geographic distribution of Coccidioides immitis and possible ecologic implications. Ariz. Med. 1958, 15, 178–188. [Google Scholar] [PubMed]
- Maddy KT, C.H. Establishment of Coccidiodies immitis in Negative Soil Following Burial of Infected Animal Tissues. In Proceedings of the Second Symposium on Coccidioidomycosis, Phoenix, AZ, USA, 8–10 December 1965; pp. 309–312. [Google Scholar]
- Maddy, K.T. Observations on Coccidioides immitis found growing naturally in soil. Ariz. Med. 1965, 22, 281–288. [Google Scholar] [PubMed]
- Gorris, M.E.; Treseder, K.K.; Zender, C.; Randerson, J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. GeoHealth 2019, 3, 308–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, B.M.; Tabor, J.A.; Shubitz, L.F.; Perrill, R.; Orbach, M.J. Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. Fungal Ecol. 2012, 5, 163–176. [Google Scholar] [CrossRef]
- Barker, B.M.; Litvintseva, A.P.; Riquelme, M.; Vargas-Gastelum, L. Coccidioides ecology and genomics. Med. Mycol. 2019, 57 (Suppl. 1), S21–S29. [Google Scholar] [CrossRef]
- Luna-Isaac, J.A.; Muñiz-Salazar, R.; Baptista-Rosas, R.C.; Enríquez-Paredes, L.M.; Castañón-Olivares, L.R.; Contreras-Pérez, C.; Bazán-Mora, E.; González, G.M.; González-Martínez, M.R. Genetic analysis of the endemic fungal pathogens Coccidioides posadasii and Coccidioides immitis in Mexico. Med. Mycol. 2014, 52, 156–166. [Google Scholar] [CrossRef]
- McGinnis, M.R.; Smith, M.B.; Hinson, E. Use of the Coccidioides posadasii Deltachs5 strain for quality control in the ACCUPROBE culture identification test for Coccidioides immitis. J. Clin. Microbiol. 2006, 44, 4250–4251. [Google Scholar] [CrossRef] [Green Version]
- Litvintseva, A.P.; Marsden-Haug, N.; Hurst, S.; Hill, H.; Gade, L.; Driebe, E.M.; Ralston, C.; Roe, C.; Barker, B.M.; Goldoft, M.; et al. Valley Fever: Finding New Places for an Old Disease: Coccidioides immitis Found in Washington State Soil Associated with Recent Human Infection. Clin. Infect. Dis. 2014, 60, e1–e3. [Google Scholar] [CrossRef] [Green Version]
- Bowers, J.R.; Parise, K.L.; Kelley, E.J.; Lemmer, D.; Schupp, J.M.; Driebe, E.M.; Engelthaler, D.M.; Keim, P.; Barker, B.M. Direct detection of Coccidioides from Arizona soils using CocciENV, a highly sensitive and specific real-time PCR assay. Med. Mycol. 2019, 57, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheff, K.; York, E.; Driebe, E.; Barker, B.M.; Rounsley, S.; Waddell, V.; Beckstrom-Sternberg, S.; Beckstrom-Sternberg, J.S.; Keim, P.; Engelthaler, D. Development of a rapid, cost-effective TaqMan Real-Time PCR Assay for identification and differentiation of Coccidioides immitis and Coccidioides posadasii. Med. Mycol. 2009, 48, 1–4. [Google Scholar] [CrossRef]
- Hamm, P.S.; Hutchison, M.I.; Leonard, P.; Melman, S.; Natvig, D.O. First Analysis of Human Coccidioides Isolates from New Mexico and the Southwest Four Corners Region: Implications for the Distributions of C. posadasii and C. immitis and Human Groups at Risk. J. Fungi 2019, 5, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catanzaro, A.; Drutz, D.J. Pulmonary Coccidioidomycosis. Coccidioidomycosis 1980, 32, 147–161. [Google Scholar]
- Odio, C.D.; Marciano, B.E.; Galgiani, J.N.; Holland, S.M. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg. Infect. Dis. 2017, 23, 308–311. [Google Scholar] [CrossRef]
- Galgiani, J. How does genetics influence valley fever? Research underway now to answer this question. Southwest J. Pulm. Crit. Care 2014, 9, 230–237. [Google Scholar] [CrossRef]
- Shubitz, L.F.; Powell, A.D.; Butkiewicz, C.D.; Lewis, M.L.; Trinh, H.T.; Frelinger, A.J.; Orbach, M.J.; Galgiani, J.N. A Chronic Murine Disease Model of Coccidioidomycosis Using Coccidioides posadasii, Strain 1038. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Davis, K.M. All Yersinia Are Not Created Equal: Phenotypic Adaptation to Distinct Niches Within Mammalian Tissues. Front. Cell. Infect. Microbiol. 2018, 8, 261. [Google Scholar] [CrossRef]
- Phan, H.T.; Rybak, K.; Furuki, E.; Breen, S.; Solomon, P.S.; Oliver, R.P.; Tan, K.-C. Differential effector gene expression underpins epistasis in a plant fungal disease. Plant J. 2016, 87, 343–354. [Google Scholar] [CrossRef]
- Abdelsamed, H.; Peters, J.; Byrne, G.I. Genetic variation in Chlamydia trachomatis and their hosts: Impact on disease severity and tissue tropism. Future Microbiol. 2013, 8, 1129–1146. [Google Scholar] [CrossRef] [Green Version]
- Freguja, R.; Gianesin, K.; Zanchetta, M.; De Rossi, A. Cross-talk between virus and host innate immunity in pediatric HIV-1 infection and disease progression. New Microbiol. 2012, 35, 249–257. [Google Scholar] [PubMed]
- Gauthier, G.M. Fungal Dimorphism and Virulence: Molecular Mechanisms for Temperature Adaptation, Immune Evasion, and In Vivo Survival. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sil, A.; Andrianopoulos, A. Thermally Dimorphic Human Fungal Pathogens—Polyphyletic Pathogens with a Convergent Pathogenicity Trait. Cold Spring Harb. Perspect. Med. 2015, 5, a019794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perini, L.; Mogrovejo, D.C.; Tomazin, R.; Gostinčar, C.; Brill, F.H.H.; Gunde-Cimerman, N. Phenotypes Associated with Pathogenicity: Their Expression in Arctic Fungal Isolates. Microorganisms 2019, 7, 600. [Google Scholar] [CrossRef] [Green Version]
- Williams-Rhaesa, A.M.; Awuku, N.K.; Lipscomb, G.L.; Poole, F.L.; Rubinstein, G.M.; Conway, J.M.; Kelly, R.M.; Adams, M.W.W. Native xylose-inducible promoter expands the genetic tools for the biomass-degrading, extremely thermophilic bacterium Caldicellulosiruptor bescii. Extremophiles 2018, 22, 629–638. [Google Scholar] [CrossRef]
- Besten, H.M.D.; Wells-Bennik, M.H.; Zwietering, M.H. Natural Diversity in Heat Resistance of Bacteria and Bacterial Spores: Impact on Food Safety and Quality. Annu. Rev. Food Sci. Technol. 2018, 9, 383–410. [Google Scholar] [CrossRef]
- Barzkar, N.; Homaei, A.; Hemmati, R.; Patel, S. Thermostable marine microbial proteases for industrial applications: Scopes and risks. Extremophiles 2018, 22, 335–346. [Google Scholar] [CrossRef]
- Choi, D.-H.; Park, E.-H.; Kim, M.-D. Isolation of thermotolerant yeast Pichia kudriavzevii from nuruk. Food Sci. Biotechnol. 2017, 26, 1357–1362. [Google Scholar] [CrossRef]
- Kamthan, A.; Kamthan, M.; Datta, A. Expression of C-5 sterol desaturase from an edible mushroom in fisson yeast enhances its ethanol and thermotolerance. PLoS ONE 2017, 12, e0173381. [Google Scholar] [CrossRef]
- Matsushita, K.; Azuma, Y.; Kosaka, T.; Yakushi, T.; Hoshida, H.; Akada, R.; Yamada, M. Genomic analyses of thermotolerant microorganisms used for high-temperature fermentations. Biosci. Biotechnol. Biochem. 2015, 80, 655–668. [Google Scholar] [CrossRef] [Green Version]
- Reidy, M.; Sharma, R.; Shastry, S.; Roberts, B.-L.; Albino-Flores, I.; Wickner, S.; Masison, D.C. Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines. PLoS Genet. 2014, 10, e1004720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhabhra, R.; Askew, D.S. Thermotolerance and virulence of Aspergillus fumigatus: Role of the fungal nucleolus. Med. Mycol. 2005, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Robert, V.A.; Casadevall, A. Vertebrate Endothermy Restricts Most Fungi as Potential Pathogens. J. Infect. Dis. 2009, 200, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Steenbergen, J.N.; Nosanchuk, J.D. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi—The Cryptococcus neoformans paradigm. Curr. Opin. Microbiol. 2003, 6, 332–337. [Google Scholar] [CrossRef]
- Denham, S.T.; Wambaugh, M.A.; Brown, J.C.S. How Environmental Fungi Cause a Range of Clinical Outcomes in Susceptible Hosts. J. Mol. Biol. 2019, 431, 2982–3009. [Google Scholar] [CrossRef]
- Reyes-Montes, M.D.R.; Pérez-Huitrón, M.A.; Ocaña-Monroy, J.L.; Frías-De-León, M.G.; Martínez-Herrera, E.; Arenas, R.; Duarte-Escalante, E. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infect. Dis. 2016, 16, 550. [Google Scholar]
- Shubitz, L.F. Comparative Aspects of Coccidioidomycosis in Animals and Humans. Ann. N. Y. Acad. Sci. 2007, 1111, 395–403. [Google Scholar] [CrossRef]
- Eulalio, K.D.; De Macedo, R.L.; Cavalcanti, M.D.A.S.; Martins, L.M.S.; Lazéra, M.D.S.; Wanke, B. Coccidioides immitis isolated from armadillos (Dasypus novemcinctus) in the state of Piauí, northeast Brazil. Mycopathologia 2001, 149, 57–61. [Google Scholar]
- Swatek, F.E.; Plunkett, O.A. Experimental Infections of Wild Rodents and Animals other than Mammals. In Proceedings of the First Symposium on Coccidiodomycosis; Ajello, L., Ed.; University of Arizona Press: Phoenix, AZ, USA, 1957; pp. 161–167. [Google Scholar]
- Burt, A.; Carter, D.A.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl. Acad. Sci. USA 1996, 93, 770–773. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Australia, 2020. [Google Scholar]
- Canty, A.R.; Ripley, B.D. Boot: Bootstrap R (S-Plus) Functions, R package version 1.3-24; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Application; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1997. [Google Scholar]
- Tiwari, S.; Thakur, R.; Shankar, J. Role of Heat-Shock Proteins in Cellular Function and in the Biology of Fungi. Biotechnol. Res. Int. 2015, 2015, 132635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.J.P.; Leach, M.D.; Nicholls, S. The relevance of heat shock regulation in fungal pathogens of humans. Virulence 2010, 1, 330–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viriyakosol, S.; Singhania, A.; Fierer, J.; Goldberg, J.; Kirkland, T.N.; Woelk, C.H. Gene expression in human fungal pathogen Coccidioides immitis changes as arthroconidia differentiate into spherules and mature. BMC Microbiol. 2013, 13, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiston, E.; Wise, H.Z.; Sharpton, T.J.; Jui, G.; Cole, G.T.; Taylor, J.W. Comparative Transcriptomics of the Saprobic and Parasitic Growth Phases in Coccidioides spp. PLoS ONE 2012, 7, e41034. [Google Scholar] [CrossRef] [Green Version]
- Mead, H.L.; Roe, C.C.; Keppler, E.A.H.; Van Dyke, M.C.C.; Laux, K.L.; Funke, A.; Miller, K.J.; Bean, H.D.; Sahl, J.W.; Barker, B.M. Defining Critical Genes During Spherule Remodeling and Endospore Development in the Fungal Pathogen, Coccidioides posadasii. Front. Genet. 2020, 11, 483. [Google Scholar] [CrossRef]
- Narra, H.P.; Shubitz, L.F.; Mandel, M.A.; Trinh, H.T.; Griffin, K.; Buntzman, A.S.; Frelinger, J.A.; Galgiani, J.N.; Orbach, M.J. A Coccidioides posadasii CPS1 Deletion Mutant Is Avirulent and Protects Mice from Lethal Infection. Infect. Immun. 2016, 84, 3007–3016. [Google Scholar] [CrossRef] [Green Version]
- Gorovits, R.; Propheta, O.; Kolot, M.; Dombradi, V.; Yarden, O. A Mutation within the Catalytic Domain of COT1 Kinase Confers Changes in the Presence of Two COT1 Isoforms and in Ser/Thr Protein Kinase and Phosphatase Activities in Neurospora crassa. Fungal Genet. Biol. 1999, 27, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Yarden, O.; Plamann, M.; Ebbole, D.J.; Yanofsky, C. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 1992, 11, 2159–2166. [Google Scholar] [CrossRef]
- Li, X.C.; Peris, D.; Hittinger, C.T.; Sia, E.A.; Fay, J.C. Mitochondria-encoded genes contribute to evolution of heat and cold tolerance in yeast. Sci. Adv. 2019, 5, eaav1848. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.d.M.; Lang, B.F.; Daniel, R.; Matute, D.R.; Stajich, J.E.; Barker, B. Structural characterization and evolutionary analyses of the Coccidioides immitis and Coccidioides posadasii mitochondrial genomes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Fisher, M.C.; Koenig, G.L.; White, T.J.; San-Blas, G.; Negroni, R.; Alvarez, I.G.; Wanke, B.; Taylor, J.W. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc. Natl. Acad. Sci. USA 2001, 10, 4558–4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.J.; Sigel, K.; Vaz, V.; Komatsu, K.; McRill, C.; Phelan, M.; Colman, T.; Comrie, A.C.; Warnock, D.W.; Galgiani, J.N.; et al. An Epidemic of Coccidioidomycosis in Arizona Associated with Climatic Changes, 1998–2001. J. Infect. Dis. 2005, 191, 1981–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolivras, K.N.; Comrie, A.C. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int. J. Biometeorol. 2003, 47, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Gorris, M.E.; Cat, L.A.; Zender, C.; Treseder, K.K.; Randerson, J.T. Coccidioidomycosis Dynamics in Relation to Climate in the Southwestern United States. GeoHealth 2018, 2, 6–24. [Google Scholar] [CrossRef]
- Coopersmith, E.J.; Bell, J.E.; Benedict, K.; Shriber, J.; McCotter, O.; Cosh, M.H. Relating coccidioidomycosis (valley fever) incidence to soil moisture conditions. GeoHealth 2017, 1, 51–63. [Google Scholar] [CrossRef]
- Comrie, A.C. Climate Factors Influencing Coccidioidomycosis Seasonality and Outbreaks. Environ. Health Perspect. 2005, 113, 688–692. [Google Scholar] [CrossRef]
- Maddy, K.T.; Coccozza, J. The Probable Geographic Distribution of Coccidioides Immitis in Mexico. Bol. Oficina Sanit. Panam. 1964, 57, 44–54. [Google Scholar]
- NOAA. National Centers for Environmental information. In Climate at a Glance: County Mapping; NOAA: Asheville, NC, USA, 2020. [Google Scholar]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the industrial revolution. Elife 2020, 9. [Google Scholar] [CrossRef]
- Sampaio, E.P.; Hsu, A.P.; Pechacek, J.; Bax, H.I.; Dias, D.L.; Paulson, M.L.; Chandrasekaran, P.; Rosen, L.B.; Carvalho, D.S.; Ding, L.; et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J. Allergy Clin. Immunol. 2013, 131, 1624–1634. [Google Scholar] [CrossRef]
- González, Á.; Hung, C.-Y.; Cole, G.T. Coccidioides releases a soluble factor that suppresses nitric oxide production by murine primary macrophages. Microb. Pathog. 2011, 50, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.Y.; Zhang, H.; Castro-Lopez, N.; Ostroff, G.R.; Khoshlenar, P.; Abraham, A.; Cole, G.T.; Negron, A.; Forsthuber, T.; Peng, T.; et al. Glucan-Chitin Particles Enhance Th17 Response and Improve Protective Efficacy of a Multivalent Antigen (rCpa1) against Pulmonary Coccidioides posadasii Infection. Infect. Immun. 2018, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkland, T.N.; Fierer, J. Coccidioides immitis and posadasii; A review of their biology, genomics, pathogenesis, and host immunity. Virulence 2018, 9, 1426–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viriyakosol, S.; Kapoor, M.; Okamoto, S.; Covel, J.; Soltow, Q.A.; Trzoss, M.; Shaw, K.J.; Fierer, J. APX001 and Other Gwt1 Inhibitor Prodrugs Are Effective in Experimental Coccidioides immitis Pneumonia. Antimicrob. Agents Chemother. 2018, 63, e01715-18. [Google Scholar] [CrossRef] [Green Version]
| ID | Species | NCBI Accession | Geographic Origin a | Source | Testing Institution b |
|---|---|---|---|---|---|
| CA22 | C. immitis | NA | California | University of Texas Health Science Center (UTHSC) | NAU |
| 500 | C. posadasii | NA | Soil, Tucson, AZ | University of Arizona (UA) | UA |
| IL1 | C. posadasii | NA | Illinois | UTHSC | NAU |
| CA23 | C. immitis | NA | California | UTHSC | NAU |
| HS-I-000718 | C. posadasii | NA | Arizona | Flagstaff Medical Center (FMC) | NAU |
| GT164 | C. posadasii | NA | Texas | University of California Davis (UCD) | NAU |
| GT163 | C. immitis | NA | California | UCD | NAU |
| HS-I-000588 | C. posadasii | NA | Arizona | FMC | NAU |
| CA28 | C. immitis | NA | California | UTHSC | NAU |
| TX4 | C. posadasii | NA | Texas | UTHSC | NAU |
| HS-I-000235 | C. posadasii | NA | Arizona | FMC | NAU |
| TX1 | C. posadasii | NA | Texas | UTHSC | NAU |
| HS-I-000778 | C. posadasii | NA | Arizona | FMC | NAU |
| GT147 | C. immitis | NA | California | UCD | NAU |
| HS-I-000234 | C. posadasii | NA | Texas | FMC | NAU |
| CA30 | C. immitis | NA | California | UTHSC | NAU |
| HS-I-000547 | C. posadasii | NA | Arizona | FMC | NAU |
| HS-I-000233 | C. posadasii | NA | Arizona | FMC | NAU |
| GT166 | C. posadasii | NA | Texas | UCD | NAU |
| CA24 | C. immitis | NA | California | UTHSC | NAU |
| CA29 | C. immitis | NA | California | UTHSC | NAU |
| M211 | C. posadasii | NA | Central Mexico | Unidad de Micologia, UNAM | NAU |
| GT158 | C. posadasii | NA | Arizona | UCD | NAU |
| CA15 | C. immitis | NA | California | UTHSC | NAU |
| CA27 | C. immitis | NA | California | UTHSC | NAU |
| TX3 | C. posadasii | NA | Texas | UTHSC | NAU |
| CA20 | C. immitis | NA | California | UTHSC | NAU |
| RS | C. immitis | AAEC00000000.3 | California | Common Laboratory Strain | NAU |
| Silveira | C. posadasii | ABAI00000000.2 | California | Common Laboratory Strain | NAU |
| RMSCC2378 | C. posadasii | NA | Argentina | R. Negroni | UA |
| RMSCC2377 | C. posadasii | NA | Argentina | R. Negroni | UA |
| RMSCC2379 | C. posadasii | NA | Argentina | R. Negroni | UA |
| RMSCC3698 | C. immitis | NA | Barstow, California | Naval Hospital | UA |
| RMSCC3490 c | C. posadasii | SRR3468073 | Coahuila, Mexico | I. Gutierrez | UA |
| RMSCC3505 | C. immitis | NA | Coahuila, Mexico | I. Gutierrez | UA |
| RMSCC3506 c | C. posadasii | SRR3468053 | Coahuila, Mexico | I. Gutierrez | UA |
| RMSCC3472 | C. posadasii | NA | Michoacán, Mexico | I. Gutierrez | UA |
| RMSCC3474 | C. immitis | NA | Michoacán, Mexico | I. Gutierrez | UA |
| RMSCC3475 | C. immitis | NA | Michoacán, Mexico | I. Gutierrez | UA |
| RMSCC3476 c | C. immitis | SRR3468020 | Michoacán, Mexico | I. Gutierrez | UA |
| RMSCC3478 | C. posadasii | NA | Michoacán, Mexico | I. Gutierrez | UA |
| RMSCC3479 c | C. immitis | SRR3468018 | Michoacán, Mexico | I. Gutierrez | UA |
| RMSCC3377 | C. immitis | NA | Monterey, California | UCD | UA |
| RMSCC2343 c | C. posadasii | SRR3468064 | Nuevo Leon, Mexico | R. Diaz | UA |
| RMSCC2346 c | C. posadasii | SRR3468065 | Nuevo Leon, Mexico | R. Diaz | UA |
| RMSCC3738 | C. posadasii | NA | Piaui, Brazil | B. Wanke | UA |
| RMSCC3740 | C. posadasii | NA | Piaui, Brazil | B. Wanke | UA |
| RMSCC2127 | C. posadasii | NA | Texas | UTHSC | UA |
| RMSCC2133 | C. posadasii | GCA_000150185.1 | Texas | UTHSC | UA |
| RMSCC2234 | C. posadasii | NA | Texas | UTHSC | UA |
| RMSCC2102 | C. immitis | NA | San Diego, California | University of California San Diego (UCSD) Medical Center | UA |
| RMSCC2394 | C. immitis | GCA_000149895.1 | San Diego, California | UCSD Medical Center | UA |
| RMSCC2395 | C. immitis | NA | San Diego, California | UCSD Medical Center | UA |
| RMSCC3693 | C. immitis | NA | San Diego, California | Naval Hospital | UA |
| RMSCC3703 | C. immitis | GCA_000150085.1 | San Diego, California | UCSD Medical Center | UA |
| RMSCC3705 | C. immitis | NA | San Diego, California | UCSD Medical Center | UA |
| RMSCC3706 c | C. immitis | SRR3468019 | San Diego, California | UCSD Medical Center | UA |
| RMSCC2006 | C. immitis | NA | San Joaquin Valley | Kern County Public Health (KCPH) | UA |
| RMSCC2009 c | C. immitis | SRR3468015 | San Joaquin Valley | KCPH | UA |
| RMSCC2010 | C. immitis | NA | San Joaquin Valley | KCPH | UA and NAU |
| RMSCC2011 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2012 c | C. immitis | SRR3468016 | San Joaquin Valley | KCPH | UA |
| RMSCC2014 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2015 c | C. immitis | SRR3468027 | San Joaquin Valley | KCPH | UA |
| RMSCC2017 c | C. immitis | SRR3468038 | San Joaquin Valley | KCPH | UA |
| RMSCC2268 c | C. immitis | SRR3468049 | San Joaquin Valley | KCPH | UA |
| RMSCC2269 c | C. immitis | SRR3468060 | San Joaquin Valley | KCPH | UA |
| RMSCC2271 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2273 c | C. immitis | SRR3468071 | San Joaquin Valley | KCPH | UA |
| RMSCC2274 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2275 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2276 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2277 c | C. immitis | SRR3468079 | San Joaquin Valley | KCPH | UA |
| RMSCC2278 | C. immitis | NA | San Joaquin Valley | KCPH | UA |
| RMSCC2279 c | C. immitis | SRR3468080 | San Joaquin Valley | KCPH | UA |
| RMSCC2280 c | C. immitis | SRR3468081 | San Joaquin Valley | KCPH | UA |
| RMSCC2281 c | C. immitis | SRR3468017 | San Joaquin Valley | KCPH | UA |
| RMSCC3480 c | C. posadasii | SRR3468051 | Sonora, Mexico | I. Gutierrez | UA |
| RMSCC3487 c | C. posadasii | SRR3468052 | Sonora, Mexico | I. Gutierrez | UA |
| RMSCC3488 | C. posadasii | GCA_000150055.1 | Sonora, Mexico | I. Gutierrez | UA |
| RMSCC1040 | C. posadasii | NA | Tucson, Arizona | UA | UA |
| RMSCC1043 | C. posadasii | NA | Tucson, Arizona | UA | UA |
| RMSCC1044 | C. posadasii | NA | Tucson, Arizona | UA | UA |
| RMSCC1045 | C. posadasii | NA | Tucson, Arizona | UA | UA |
| RMSCC3796 | C. posadasii | NA | Venezuela | G. San-Blas |
| Colony Diameter at 28 °C | Colony Diameter at 37 °C | |||||
|---|---|---|---|---|---|---|
| Species | mm/Day | 95% CI | P a | mm/day | 95% CI | P a |
| C. immitis × Day | 3.73 | 3.53–3.92 | 0.072 | 0.64 | 0.51–0.78 | <0.001 |
| C. posadasii × Day | 3.47 | 0.55–0.02 | 1.82 | 0.98–1.38 | ||
| N b | 85 | 85 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mead, H.L.; Hamm, P.S.; Shaffer, I.N.; Teixeira, M.d.M.; Wendel, C.S.; Wiederhold, N.P.; Thompson, G.R., III; Muñiz-Salazar, R.; Castañón-Olivares, L.R.; Keim, P.; et al. Differential Thermotolerance Adaptation between Species of Coccidioides. J. Fungi 2020, 6, 366. https://doi.org/10.3390/jof6040366
Mead HL, Hamm PS, Shaffer IN, Teixeira MdM, Wendel CS, Wiederhold NP, Thompson GR III, Muñiz-Salazar R, Castañón-Olivares LR, Keim P, et al. Differential Thermotolerance Adaptation between Species of Coccidioides. Journal of Fungi. 2020; 6(4):366. https://doi.org/10.3390/jof6040366
Chicago/Turabian StyleMead, Heather L., Paris S. Hamm, Isaac N. Shaffer, Marcus de Melo Teixeira, Christopher S. Wendel, Nathan P. Wiederhold, George R. Thompson, III, Raquel Muñiz-Salazar, Laura Rosio Castañón-Olivares, Paul Keim, and et al. 2020. "Differential Thermotolerance Adaptation between Species of Coccidioides" Journal of Fungi 6, no. 4: 366. https://doi.org/10.3390/jof6040366
APA StyleMead, H. L., Hamm, P. S., Shaffer, I. N., Teixeira, M. d. M., Wendel, C. S., Wiederhold, N. P., Thompson, G. R., III, Muñiz-Salazar, R., Castañón-Olivares, L. R., Keim, P., Plude, C., Terriquez, J., Galgiani, J. N., Orbach, M. J., & Barker, B. M. (2020). Differential Thermotolerance Adaptation between Species of Coccidioides. Journal of Fungi, 6(4), 366. https://doi.org/10.3390/jof6040366