Siderophore Scaffold as Carrier for Antifungal Peptides in Therapy of Aspergillus fumigatus Infections
Abstract
:1. Introduction
2. Materials and Methods
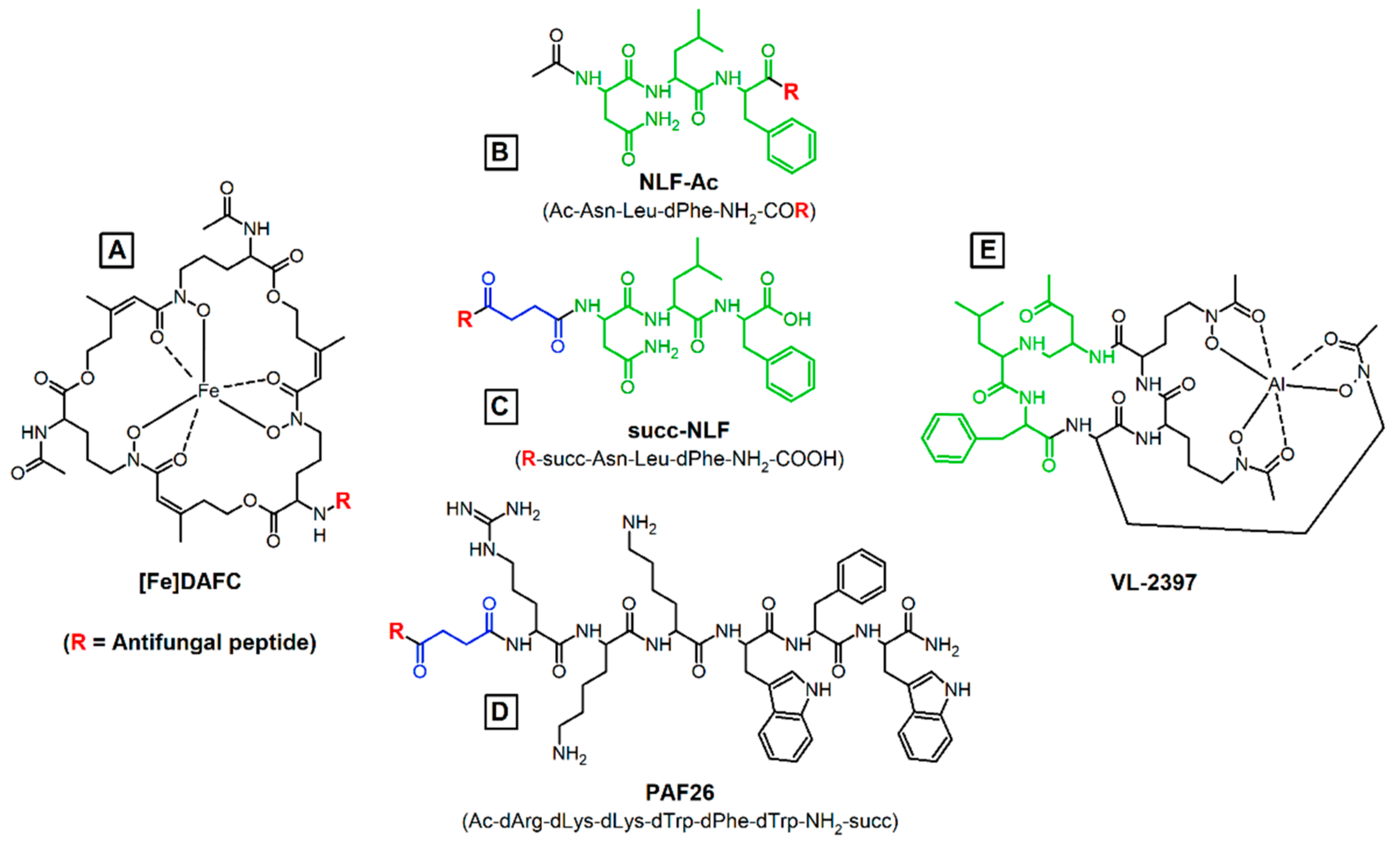
2.1. Synthesis of Antifungal Conjugates
2.2. Radiolabelling
2.3. In Vitro Characterization
2.3.1. Distribution Coefficient
2.3.2. Protein Binding
2.3.3. Serum Stability
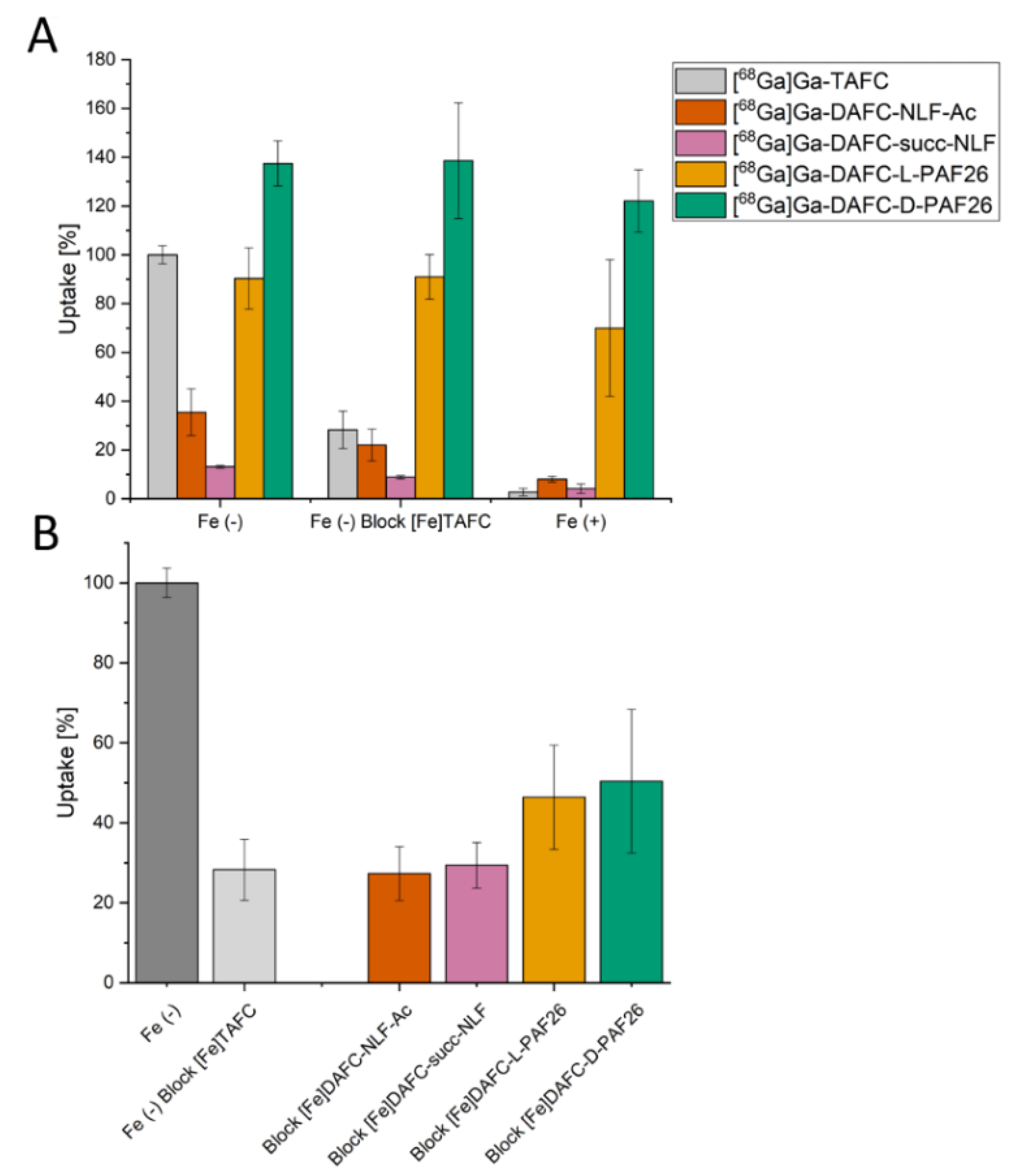
2.3.4. Uptake and Competition Assay
2.3.5. Growth Promotion Assay
2.3.6. Minimal Inhibitory Concentration (MIC) Assay
2.4. In Vivo Characterization
In Vivo Stability and Ex Vivo Biodistribution
3. Results
3.1. Synthesis and Radiolabelling
3.2. In Vitro Characterization
3.2.1. LogD, Protein Binding and Serum Stability
3.2.2. Uptake and Competition Assay
3.2.3. Growth Promotion Assay
3.2.4. Minimal Inhibitory Concentration (MIC)
3.3. In Vivo Experiments
In Vivo Stability and Biodistribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, H. Iron–A Key Nexus in the Virulence of Aspergillus fumigatus. Front. Microbiol. 2012, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrettl, M.; Bignell, E.; Kragl, C.; Sabiha, Y.; Loss, O.; Eisendle, M.; Wallner, A.; Arst, H.N.; Haynes, K.; Haas, H. Distinct roles for intra-and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007, 3, 1195–1207. [Google Scholar] [CrossRef]
- Raymond-Bouchard, I.; Carroll, C.S.; Nesbitt, J.R.; Henry, K.A.; Pinto, L.J.; Moinzadeh, M.; Scott, J.K.; Moore, M.M. Structural Requirements for the Activity of the MirB Ferrisiderophore Transporter of Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C.; et al. Hybrid Imaging of Aspergillus fumigatus Pulmonary Infection with Fluorescent, 68Ga-Labelled Siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Haas, H.; Laverman, P.; Schrettl, M.; Franssen, G.M.; Blatzer, M.; Decristoforo, C. 68Ga-Triacetylfusarinine C and 68Ga-Ferrioxamine E for Aspergillus Infection Imaging: Uptake Specificity in Various Microorganisms. Mol. Imaging Biol. 2014, 16, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Pfister, J.; Lichius, A.; Summer, D.; Haas, H.; Kanagasundaram, T.; Kopka, K.; Decristoforo, C. Live-cell imaging with Aspergillus fumigatus—Specific fluorescent siderophore conjugates. Sci. Rep. 2020, 10, 15519. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Parente-Rocha, J.A.; Bailão, A.M.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L.; Pereira, M. Antifungal Resistance, Metabolic Routes as Drug Targets, and New Antifungal Agents: An Overview about Endemic Dimorphic Fungi. Mediat. Inflamm. 2017, 2017, 9870679. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- López-García, B.; Pérez-Payá, E.; Marcos, J.F. Identification of Novel Hexapeptides Bioactive against Phytopathogenic Fungi through Screening of a Synthetic Peptide Combinatorial Library. Appl. Environ. Microbiol. 2002, 68, 2453–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Fernandez, A.; Avlonitis, N.; Vande Velde, G.; Bradley, M.; Read, N.D.; Vendrell, M. Searching for the Optimal Fluorophore to Label Antimicrobial Peptides. ACS Comb. Sci. 2016, 18, 689–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendive-Tapia, L.; Zhao, C.; Akram, A.R.; Preciado, S.; Albericio, F.; Lee, M.; Serrels, A.; Kielland, N.; Read, N.D.; Lavilla, R.; et al. Spacer-free BODIPY fluorogens in antimicrobial peptides for direct imaging of fungal infection in human tissue. Nat. Commun. 2016, 7, 10940. [Google Scholar] [CrossRef]
- Muñoz, A.; Gandía, M.; Harries, E.; Carmona, L.; Read, N.D.; Marcos, J.F. Understanding the mechanism of action of cell-penetrating antifungal peptides using the rationally designed hexapeptide PAF26 as a model. Fungal Biol. Rev. 2013, 26, 146–155. [Google Scholar] [CrossRef]
- Muñoz, A.; Harries, E.; Contreras-Valenzuela, A.; Carmona, L.; Read, N.D.; Marcos, J.F. Two Functional Motifs Define the Interaction, Internalization and Toxicity of the Cell-Penetrating Antifungal Peptide PAF26 on Fungal Cells. PLoS ONE 2013, 8, e54813. [Google Scholar]
- Nakamura, I.; Yoshimura, S.; Masaki, T.; Takase, S.; Ohsumi, K.; Hashimoto, M.; Furukawa, S.; Fujie, A. ASP2397: A novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. (Tokyo) 2017, 70, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Dietl, A.M.; Misslinger, M.; Aguiar, M.M.; Ivashov, V.; Teis, D.; Pfister, J.; Decristoforo, C.; Hermann, M.; Sullivan, S.M.; Smith, L.R.; et al. The siderophore transporter Sit1 determines susceptibility to the antifungal VL-2397. Antimicrob. Agents Chemother. 2019, 63, e00807-19. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Pfister, J.; Misslinger, M.; Decristoforo, C.; Haas, H. Siderophore—Based Molecular Imaging of Fungal and Bacterial Infections—Current Status and Future Perspectives. J. Fungi 2020, 6, 73. [Google Scholar] [CrossRef]
- Turner, J.H. Recent advances in theranostics and challenges for the future. Br. J. Radiol. 2018, 91, 20170893. [Google Scholar] [CrossRef]
- Abou, D.; Benabdallah, N.; Jiang, W.; Peng, L.; Zhang, H.; Villmer, A.; Longtine, M.S.; Thorek, D.L.J. Prostate Cancer Theranostics—An Overview. Front. Oncol. 2020, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, E.D.G.; Versleijen-Jonkers, Y.M.H.; Heskamp, S.; Van Herpen, C.M.L.; Oyen, W.J.G.; Van der Graaf, W.T.A.; Boerman, O.C. Theranostic applications of antibodies in oncology. Mol. Oncol. 2014, 8, 799–812. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.; Summer, D.; Rangger, C.; Petrik, M.; Von Guggenberg, E.; Minazzi, P.; Giovenzana, G.B.; Aloj, L.; Decristoforo, C. Influence of a novel, versatile bifunctional chelator on theranostic properties of a minigastrin analogue. EJNMMI Res. 2015, 5, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaeopookum, P.; Summer, D.; Pfister, J.; Orasch, T.; Lechner, B.E.; Petrik, M.; Novy, Z.; Matuszczak, B.; Rangger, C.; Haas, H.; et al. Modifying the Siderophore Triacetylfusarinine C for Molecular Imaging of Fungal Infection. Mol. Imaging Biol. 2019, 21, 1097–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontecorvo, G.; Roper, J.A.; Chemmons, L.M.; Macdonald, K.D.; Bufton, A.W.J. The Genetics of Aspergillus nidulans. Adv. Genet. 1953, 141–238. [Google Scholar]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N.; Haynes, K.; Haas, H. Siderophore Biosynthesis But Not Reductive Iron Assimilation Is Essential for Aspergillus fumigatus Virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef] [Green Version]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Huber, A.; Hajdu, D.; Bratschun-Khan, D.; Gáspári, Z.; Varbanov, M.; Philippot, S.; Fizil, Á.; Czajlik, A.; Kele, Z.; Sonderegger, C.; et al. New Antimicrobial Potential and Structural Properties of PAFB: A Cationic, Cysteine-Rich Protein from Penicillium chrysogenum Q176. Sci. Rep. 2018, 8, 1751. [Google Scholar] [CrossRef]
- Ocak, M.; Helbok, A.; Rangger, C.; Peitl, P.K.; Nock, B.A.; Morelli, G.; Eek, A.; Sosabowski, J.K.; Breeman, W.A.P.; Reubi, J.C.; et al. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1426–1435. [Google Scholar] [CrossRef] [Green Version]
- Petrik, M.; Franssen, G.M.; Haas, H.; Laverman, P.; Hörtnagl, C.; Schrettl, M.; Helbok, A.; Lass-Flörl, C.; Decristoforo, C. Preclinical evaluation of two 68Ga-siderophores as potential radiopharmaceuticals for Aspergillus fumigatus infection imaging. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1175–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Goldenberg, D.M.; Becker, W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: Present status, future prospects and limitations. Eur. J. Nucl. Med. Mol. Imaging 1998, 25, 201–212. [Google Scholar] [CrossRef]
- Petrik, M.; Haas, H.; Dobrozemsky, G.; Lass-Florl, C.; Helbok, A.; Blatzer, M.; Dietrich, H.; Decristoforo, C. 68Ga-Siderophores for PET Imaging of Invasive Pulmonary Aspergillosis: Proof of Principle. J. Nucl. Med. 2010, 51, 639–645. [Google Scholar] [CrossRef] [Green Version]
- Ganz, T. Iron in innate immunity: Starve the invaders. Curr. Opin. Immunol. 2009, 21, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| [68Ga]Ga-DAFC-NLF-Ac | [68Ga]Ga-DAFC-succ-NLF | [68Ga]Ga-DAFC-L-PAF26 | [68Ga]Ga-DAFC-D-PAF26 | ||
|---|---|---|---|---|---|
| Distribution coefficient n = 3 | Log D (pH 7.4) | −0.80 ± 0.08 | −2.84 ± 0.17 | −3.13 ± 0.02 | −3.35 ± 0.16 |
| Protein binding [%] n = 3 | 30 min | 1.6 ± 0.8 | 1.2 ± 1.1 | 10.5 ± 1.1 | 17.7 ± 4.1 |
| 60 min | 2.4 ± 1.9 | 1.3 ± 1.4 | 23.8 ± 1.4 | 22.3 ± 2.6 | |
| 120 min | 1.4 ± 1.1 | 0.7 ± 0.4 | 24.1 ± 2.2 | 20.3 ± 6.1 | |
| Serum stability n = 2 | 60 min | >99% | >99% | 56% | 99% |
| 120 min | >99% | >99% | 39% | 98% | |
| 240 min | >99% | >99% | 14% | 98% |
| Compound | MIC 24 h | MIC 48 h | ||
|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | |
| [Fe]DAFC-NLF-Ac | >16 | >12.5 | >16 | >12.5 |
| [Fe]DAFC-succ-NLF | >16 | >12.0 | >16 | >12.0 |
| HOOC-NLF-Ac | >16 | >36.8 | >16 | >36.8 |
| [Fe]DAFC-L-PAF26 | >16 | >8.4 | >16 | >8.4 |
| [Fe]DAFC-D-PAF26 | >16 | >8.4 | >16 | >8.4 |
| HOOC-L-PAF26-NH2 | >16 | >16.8 | >16 | >16.8 |
| H2N-D-PAF26-NH2 | >16 | >16.9 | >16 | >16.9 |
| H2N-D-PAF26-Ac | >16 | >16.1 | >16 | >16.1 |
| Compound | MIC 24 h | MIC 48 h | ||
|---|---|---|---|---|
| µg/mL | µM | µg/mL | µM | |
| [Fe]DAFC-D-PAF26 | >16 | >8.4 | >16 | >8.4 |
| H2N-D-PAF26-NH2 | 8 | 8.4 | 8 | 8.4 |
| H2N-D-PAF26-Ac | 16 | 16.1 | >16 | >16.1 |
| [68Ga]Ga-DAFC-NLF-Ac | [68Ga]Ga-DAFC-succ-NLF | [68Ga]Ga-DAFC-D-PAF26 | |
|---|---|---|---|
| Blood | 86.6% | >99% | >99% |
| Urine | 64.0% | 89.4% | 57.0% |
| Organ | [68Ga]Ga-DAFC-NLF-Ac | [68Ga]Ga-DAFC-succ-NLF | [68Ga]Ga-DAFC-D-PAF26 | [68Ga]Ga-TAFC * | ||||
|---|---|---|---|---|---|---|---|---|
| 45 min | 90 min | 45 min | 90 min | 45 min | 90 min | 30 min | 90 min | |
| Blood | 0.46 ± 0.10 | 0.13 ± 0.10 | 0.41 ± 0.14 | 0.05 ± 0.03 | 1.08 ± 0.12 | 0.40 ± 0.04 | 1.60 ± 0.37 | 0.06 ± 0.04 |
| Spleen | 0.35 ± 0.10 | 0.24 ± 0.07 | 0.23 ± 0.04 | 0.19 ± 0.03 | 2.21 ± 0.15 | 1.97 ± 0.21 | 0.38 ± 0.11 | 0.05 ± 0.02 |
| Pancreas | 0.21 ± 0.06 | 0.10 ± 0.06 | 0.14 ± 0.05 | 0.06 ± 0.02 | 0.41 ± 0.02 | 0.26 ± 0.03 | 0.42 ± 0.16 | 0.04 ± 0.01 |
| Stomach | 0.69 ± 0.31 | 2.04 ± 0.74 | 0.19 ± 0.09 | 0.11 ± 0.01 | 0.45 ± 0.17 | 0.35 ± 0.03 | 0.77 ± 0.22 | 0.06 ± 0.02 |
| Intestine | 3.09 ± 0.03 | 4.85 ± 1.78 | 0.40 ± 0.10 | 0.36 ± 0.07 | 0.63 ± 0.02 | 0.56 ± 0.03 | 1.71 ± 10.04 | 1.03 ± 0.26 |
| Kidneys | 35.69 ± 1.73 | 39.88 ± 2.66 | 3.27 ± 0.61 | 2.93 ± 0.38 | 149.62 ± 17.99 | 158.18 ± 25.65 | 5.51 ± 1.22 | 1.27 ± 0.39 |
| Liver | 0.85 ± 0.51 | 0.44 ± 0.05 | 0.37 ± 0.07 | 0.30 ± 0.05 | 20.79 ± 0.58 | 23.00 ± 0.89 | 0.70 ± 0.04 | 0.15 ± 0.09 |
| Heart | 0.22 ± 0.02 | 0.12 ± 0.11 | 0.18 ± 0.09 | 0.05 ± 0.02 | 0.76 ± 0.02 | 0.60 ± 0.23 | 0.67 ± 0.16 | 0.03 ± 0.01 |
| Lung | 0.62 ± 0.01 | 0.32 ± 0.06 | 0.61 ± 0.14 | 0.21 ± 0.05 | 2.16 ± 0.19 | 1.31 ± 0.23 | 1.54 ± 0.27 | 0.10 ± 0.03 |
| Muscle | 0.18 ± 0.08 | 0.17 ± 0.07 | 0.08 ± 0.01 | 0.11 ± 0.14 | 0.42 ± 0.18 | 0.28 ± 0.10 | 0.52 ± 0.25 | 0.03 ± 0.01 |
| Femur | 0.16 ± 0.05 | 0.59 ± 0.50 | 1.76 ± 0.17 | 0.31 ± 0.13 | 1.47 ± 0.09 | 1.11 ± 0.16 | 0.51 ± 0.79 | 0.15 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfister, J.; Bata, R.; Hubmann, I.; Hörmann, A.A.; Gsaller, F.; Haas, H.; Decristoforo, C. Siderophore Scaffold as Carrier for Antifungal Peptides in Therapy of Aspergillus fumigatus Infections. J. Fungi 2020, 6, 367. https://doi.org/10.3390/jof6040367
Pfister J, Bata R, Hubmann I, Hörmann AA, Gsaller F, Haas H, Decristoforo C. Siderophore Scaffold as Carrier for Antifungal Peptides in Therapy of Aspergillus fumigatus Infections. Journal of Fungi. 2020; 6(4):367. https://doi.org/10.3390/jof6040367
Chicago/Turabian StylePfister, Joachim, Roland Bata, Isabella Hubmann, Anton Amadeus Hörmann, Fabio Gsaller, Hubertus Haas, and Clemens Decristoforo. 2020. "Siderophore Scaffold as Carrier for Antifungal Peptides in Therapy of Aspergillus fumigatus Infections" Journal of Fungi 6, no. 4: 367. https://doi.org/10.3390/jof6040367






