Abstract
Aspergillus species are known to cause damage to food crops and are associated with opportunistic infections in humans. In the United States, significant losses have been reported in peanut production due to contamination caused by the Aspergillus species. This study evaluated the antifungal effect and anti-aflatoxin activity of selected plant-based essential oils (EOs) against Aspergillus flavus in contaminated peanuts, Tifguard, runner type variety. All fifteen essential oils, tested by the poisoned food technique, inhibited the growth of A. flavus at concentrations ranging between 125 and 4000 ppm. The most effective oils with total clearance of the A. flavus on agar were clove (500 ppm), thyme (1000 ppm), lemongrass, and cinnamon (2000 ppm) EOs. The gas chromatography-mass spectrometry (GC-MS) analysis of clove EO revealed eugenol (83.25%) as a major bioactive constituent. An electron microscopy study revealed that clove EO at 500 ppm caused noticeable morphological and ultrastructural alterations of the somatic and reproductive structures. Using both the ammonia vapor (AV) and coconut milk agar (CMA) methods, we not only detected the presence of an aflatoxigenic form of A. flavus in our contaminated peanuts, but we also observed that aflatoxin production was inhibited by clove EO at concentrations between 500 and 2000 ppm. In addition, we established a correlation between the concentration of clove EO and AFB1 production by reverse-phase high-performance liquid chromatography (HPLC). We demonstrate in our study that clove oil could be a promising natural fungicide for an effective bio-control, non-toxic bio-preservative, and an eco-friendly alternative to synthetic additives against A. flavus in Georgia peanuts.
1. Introduction
Contamination of food commodities by toxigenic fungi and the presence of mycotoxins during pre-harvest and post-harvest has attracted the attention of scientific, political, and economic organizations. Mycotoxins are toxins produced naturally by several types of molds [1]. Among the group of mycotoxins (aflatoxins B1, B2, G1, and G2) produced by Aspergillus flavus and Aspergillus parasiticus, aflatoxin B1 (AFB1) has been classified as a class I human carcinogen by the International Agency for Research on Cancer and is reportedly the most toxic [2,3]. The aflatoxins produced by these two species are known to affect crops such as peanut, maize, yams, cassava, and cereals, recognized as basic staple diets globally, particularly in Africa [4]. It is estimated that 25% or more of global food crops are destroyed annually due to aflatoxins [5]. These economic losses are suffered at a global level with significant public health consequences, and it is estimated that losses due to aflatoxin contamination in the corn industry are between USD 52.1 million and USD 1.68 billion annually [6].
More specifically, AFB1 production is a serious problem in peanut-growing countries where the crops are produced under rain-fed conditions [7]. In the United States (US), the impact of aflatoxins could cost the peanut industry up to USD 58 million annually [8], making it an expensive problem for the agricultural industry. The US remains one of the largest producers of peanuts in the world, with more than USD 2 billion at the retail level and a farm value of over one billion dollars [9]. Lawley [10] reported that A. flavus is the most common fungus contaminating peanuts by producing carcinogenic aflatoxins, which destroy peanut shells before they are harvested. These bacteria also produce aflatoxins, which are both highly toxic and carcinogenic, thereby threatening humans and livestock. Pitt et al. [11] reported systemic infection of peanuts by A. flavus in soil and contaminated seeds, and Achar et al. [12] demonstrated, for the first time using electron microscopy, the seed-borne nature of A. flavus in Georgia peanuts and the establishment of the mycelium in seed tissues.
Despite the recognized impact and consequences of aflatoxin-producing fungi in peanut production, the measures in place to address this global issue remain limited [12,13]. Current strategies to control this fungus rely heavily on synthetic fungicides or preservatives belonging to the aromatic hydrocarbons, benzimidazoles [14]. Extensive use of these substances might produce several side effects, such as carcinogenicity, teratogenicity, and toxicity to consumers, as well as increased risk of high-level toxic residues in food products [15]. Chemical methods require sophisticated equipment and expensive chemicals or reagents [16].
The WHO recommends an integrated approach to control and prevent aflatoxin-affected crops at different stages of production (pre and post-harvest), policies and regulation on the levels of aflatoxin allowed, targeted farming practices, as well as seeking ways to remove the contamination [1]. A recent development in the field of biological management of aflatoxin in pre and post-harvested crops includes the successful application of competitive nontoxigenic strains of A. flavus and A. parasiticus [17]. The introduction of nontoxigenic strains of Aspergillus spp. in field studies of peanut and cotton has led to a significant reduction in aflatoxin contamination [17,18,19].
In addition, there is an increased interest in sourcing safer alternate natural products instead of synthetic chemical fungicides to combat Aspergillus spp. in the food chain. The use of essential oils with antifungal and anti-aflatoxigenic activity, which are environmentally friendly, generally regarded as safe (GRAS), and do not pose health risks, are currently being explored or used as biocontrol agents against this fungus. Several studies have reported antifungal properties of essential oils, with evidence of historical and long-term use of essential oils in human, health, and food settings for the prevention and management of fungal infections [14,16,20,21,22,23].
We investigated the antifungal activities of plant-based essential oils against A. flavus in Georgia peanuts. The peanut variety used in this study is the Tifguard, runner-type, which is highly recommended for peanut farmers by the United States Department of Agriculture [24]. It is the first peanut variety known to be resistant to two difficult pathogens; the peanut root-knot nematode (Meloidogyne arenaria (Neal) Chitwood race 1) and the tomato spotted wilt tospovirus [25]. In addition, we investigated the mode of action of selected essential oils on the morphological and ultrastructural changes in A. flavus and their impact on aflatoxin (AFB1) production. Hence, this study moves towards a novel, sustainable, eco-friendly solution to a serious problem of fungal contamination of peanuts and AFB1 production by the A. flavus in Georgia, USA and other peanut farming communities worldwide.
2. Materials and Methods
2.1. Isolation of A. flavus from Peanuts
Peanut seeds, variety Tifguard, runner-type, courtesy of Agricultural Research Service (ARS), Tifton, Georgia, US, were incubated on moist filter paper for seven days. A. flavus was isolated from a contaminated peanut and were directly plated onto potato dextrose agar (PDA) medium (Fisher Scientific, Waltham, MA, USA), and the plates were incubated with alternate periods of 12 h light and 12 h darkness for seven days. In addition, isolates from the contaminated peanut were compared to standard strains of A. flavus (ATCC 11498) from the American Type Culture Collection. The fungal colonies were observed under a light microscope (Leica, M13595, Leica Microsystems, Wetzlar, Germany) and were identified based on their macro and morphological characteristics such as the color of the colony, conidial heads, vesicle, phialides and conidia, using fungal keys and manuals [7,10,26,27,28,29]. Standard spore suspension of A. flavus was freshly prepared by the suspension of a loop full of spores from a 5-day-old pure culture plate in 5 mL of sterile water. The cell concentration of 10−6/mL in photo calorimeter was adjusted by diluting further with sterile water so that the optical density (OD) of the suspension was 0.01 at 460 nm. Yeast extract sucrose (YES) agar was used as a medium for aflatoxin production [30]. The isolated A. flavus was inoculated to YES agar medium, and the plates were sealed and incubated at a temperature of 27 °C in a CO2 incubator (Fisher Scientific, Isotemp, Waltham, MA, USA) for 10–15 days. After incubation, the plates were observed under ultraviolet light (UV) (Spectroline CC-80, Fisher Scientific, Waltham, MA, USA) to detect the presence of aflatoxin production. If the mold fluoresced under UV light, it was considered aflatoxin positive and confirmed as an aflatoxigenic form of A. flavus.
2.2. Selection of Plant-Based Essential Oils
Essentials oils (EOs) reported having antifungal properties against a myriad of Aspergillus spp. were selected. Fifteen essential oils were utilized in this study as follows: cedarwood (Cedrus atlantica), cumin (Cuminum cyminum), citronella (Cymbopogon winterianus), black pepper (Piper nigrum), cardamom (Elettaria cardamomum), cinnamon (Cinnamomum verum), ginger (Zingiber officinale), lemongrass (Cymbopogon citratus), orange (Citrus sinensis), spearmint (Mentha spicata), thyme (Thymus vulgaris), clove (Syzygium aromaticum), eucalyptus (Eucalyptus globulus), lavender (Lavandula dentata) and peppermint (Mentha piperita). All essential oils were purchased from Fisher Scientific and Sigma Aldrich, St. Louis, MO, USA. The essential oils were emulsified with 0.5% Tween 20 (v/v) stock concentrations of 10,000 ppm for further use.
2.3. Antifungal Activity Assay of Essential Oils
The in vitro antifungal activities of each of the essential oils (EO) from above, at different concentrations, were evaluated by the poisoned food technique [9]. Known volumes of EO and a commercial fungicide, prothioconazole (positive control), were incorporated into the potato dextrose agar (PDA) medium along with 0.5% of Tween 20 (v/v), which acts as an emulsifying agent to get the required concentrations of 125–4000 ppm. Further, the plates consisting of agar medium mixed with 0.5% of Tween 20 (v/v) without any essential oil was considered as blank control. A 5–10 µL conidial suspension from a 4–6-day-old A. flavus culture was inoculated in the center of the agar plates by using a capillary tube. The plates were incubated at 28 ± 2 °C, with an alternating period of 12 h of dark and light for 7 days until the mycelial growth in the control plates reached the edge of the plates. The efficacy of each EO as an antifungal agent was evaluated by measuring fungal colony diameter using a centimeter scale. Percentage inhibition of the radial growth with different oils compared to control was calculated using the following formula: Percentage mycelial inhibition (%) = [(dc − dt)/dc] × 100, where dc is the mean colony diameter for the control sets and dt is the mean colony diameter for the treatment sets.
2.4. Gas Chromatographic-Mass Spectrometry of Clove Oil
Based on the antifungal activity of all EOs from above, clove oil was selected and analyzed for its major composition using GC-MS (Shimadzu QP 2010 Plus, Tokyo, Japan), fitted with a flame ionization detector (FID) and Japan capillary column (0.32 mm i.d., length: 30 m, film thickness 0.25 µm). Injector temperature and ion source temperature were maintained at 280 and 230 °C, respectively. The oil sample (0.2 µL) was injected into the column with a split ratio of 80:1. The temperature program comprised 60 °C for 2 min, raised to 250 °C for 5 min at 10 °C/min and 280 °C for 15 min at 10 °C/min. The composition (%) was estimated with peak normalization and assuming its equal detector response for each run. The range of mass acquisition was 40–650 m/z. The peaks were detected by comparing the individual mass spectra with the reference database at the National Institute of Standards and Technology (NIST12 or NIST62) and Wiley 229 mass spectrometry libraries.
2.5. Transmission Electron Microscopy
Sporulating A. flavus mycelia treated with 500 ppm of clove EO were observed using transmission electron microscopy (TEM). Untreated mycelia served as the control. All samples were infiltrated with 1:1 then 1:2 ratios of ethanol to resin in a vacuum from four hours to overnight, then two changes of 100% resin under vacuum four hours to overnight. The samples were then fixed and allowed to evacuate overnight before being placed into an oven to polymerize for three to four days. The samples were trimmed and thin sectioned (~70 to 80 nm) using a diamond knife and RMC PT-XL Ultramicrotome (RMC Corporation, Tucson, AZ, USA). The sections were post stained with 7.5% uranyl acetate and Reynolds’s lead citrate. TEM micrographs of the samples were taken using JEM-1210 TEM instrument (JEOL USA Inc., Peabody, MA, USA) and operated at 90 kV. Ultrastructural alterations of the somatic and reproductive structures of treated and untreated samples were compared to assess the effect of clove EO against A. flavus.
2.6. Scanning Electron Microscopy
Sporulating A. flavus treated mycelia, with 500 ppm of clove EO, were also used for scanning electron microscopy (SEM) using standard chemical fixation and critical point drying methods. Untreated mycelia served as the control. Samples were fixed with 2.5% glutaraldehyde solution overnight at 4 °C. Thereafter, the samples were washed with 0.1 M sodium phosphate buffer solution (pH 7.2) three times for 20 min each. Following this, the samples were dehydrated in ascending ethanol series ending in three changes of 100% dry ethanol for about 45 min. Samples were dried in liquid carbon dioxide and were mounted on a silver stub and gold-covered by cathodic spraying (Polaron gold). The morphology of the fungus was observed using Topcon DS-130F Field Emission SEM/STEM (Topcon Technologies, Inc., Paramus, NJ, USA) and operated at 20 kV. Morphological alterations in the somatic and reproductive structures of treated and untreated samples were compared to assess the effect of clove EO against A. flavus.
2.7. Detection of Aflatoxin by Qualitative Methods
2.7.1. Ammonia Vapor Method
ATCC 11498 is a toxigenic strain of A. flavus. The ammonia vapor (AV) method was used to confirm the aflatoxigenic form of A. flavus in contaminated peanuts, variety Tifguard, following protocol of Saito, et al. [31]. In addition, we determined the effect of clove EO on mycelial growth at different concentrations of the oil. Briefly, yeast extract sucrose (YES) agar plates were prepared by supplementing different concentrations of clove EO: 500, 1000, 1500, and 2000 ppm, respectively. YES plates without EO were treated as control. Ten microliters of the fungal spore suspension were inoculated at the center of the YES plates and were incubated at 25 ± 2 °C for five days. Following incubation, each dish was inverted, and approximately 200 µL of ammonium hydroxide solution (25%) was placed on the inside of the lid. The YES plates containing fungal mycelium and spores with different concentrations of clove EO were then inverted on the plates containing the ammonium hydroxide. EO-treated and untreated plates with active colonies were observed hourly for color change. A plum-red color change on the undersides of the plated colonies was an indication of aflatoxin producing strain of A. flavus isolate. No color change was categorized as a non-toxin producing strain. Mycelial growth was also monitored for plates impregnated with different concentrations of clove EO.
2.7.2. Coconut Milk Agar Method
The coconut milk agar (CMA) method was used to further confirm the presence of aflatoxin in the aflatoxigenic form of A. flavus in contaminated peanuts, variety Tifguard, following the protocol of Davis et al. [32]. In addition, we determined the effect of clove EO on mycelial growth at different concentrations of the oil. CMA plates were prepared by supplementing with different concentrations of clove oil: 500, 1000, 1500, and 2000 ppm, respectively. CMA plates without oil were treated as control. Ten microliters of the fungal spore suspension were inoculated onto the solidified agar plates and were incubated at 25 ± 2 °C for five days. Following incubation, plates were exposed to 460 nm UV light and observed for fluorescence.
2.7.3. Quantification of Aflatoxin by High-Performance Liquid Chromatography
Following incubation of clove EO-treated and untreated mycelia and spores of A. flavus on YES medium at 25 ± 2 °C for five days, extraction and purification of AFB1 were performed using High Performance Liquid Chromatography (HPLC; Waters 2790 HPLC-Photodiode Array UV detector, Milford, MA, USA) in accordance with standard protocols, using methanol as the solvent. Thirty grams of treated (500–2500 ppm) and untreated samples were collected from YES plates and homogenized with 30 mL of HPLC grade methanol. The mixture was vortexed for 5 min, followed by extraction and centrifugation. Extracts of 3 replicates were collected into a rotary evaporator flask, following which methanol was eliminated by evaporation under reduced pressure. The purified AFB1 from the treated samples was placed under a UV light along with AFB1 standard (Sigma Aldrich, St. Louis, MO, USA) that caused aflatoxin to fluoresce. Purified samples were stored at 4 °C in the dark for a couple of days prior to HPLC injection. A Waters 2790 Separations Module reversed- phase HPLC equipped with a photodiode array detector 2996 set between 200 and 500 nm was employed to capture the AFB1 spectrum in the sample. A Lunar C18 separation column, 100 mm by 4.6 mm internal diameter, with 5 µm packing material was used. A methanol/ultrapure water (60:40) mobile phase at a flow rate of 0.5 mL/min was used according to Vosough et al. [33] with minor modifications. The AFB1 standard equation was based on a four-point calibration curve and yielding the equation Y = 16976X + 8060.1, with a regression coefficient of R2 = 0.9957. The peak areas obtained from the fungal extracts for the control and clove EO treated samples were used in the regression equation to calculate the concentrations of AFB1.
2.8. Statistical Analysis
Data analysis was performed using statistical software SPSS for windows version 10.0.1 to calculate the means, standard errors, and standard deviations. One-way analysis of variance (ANOVA) was applied to the data to determine differences between treatments with significance levels set at p = 0.05. To check substantial differences between the levels of the mean factor, Tukey’s multiple comparison tests were applied to determine the levels of significance at 5% significance was applied.
3. Results
3.1. Isolation of A. flavus from Peanuts
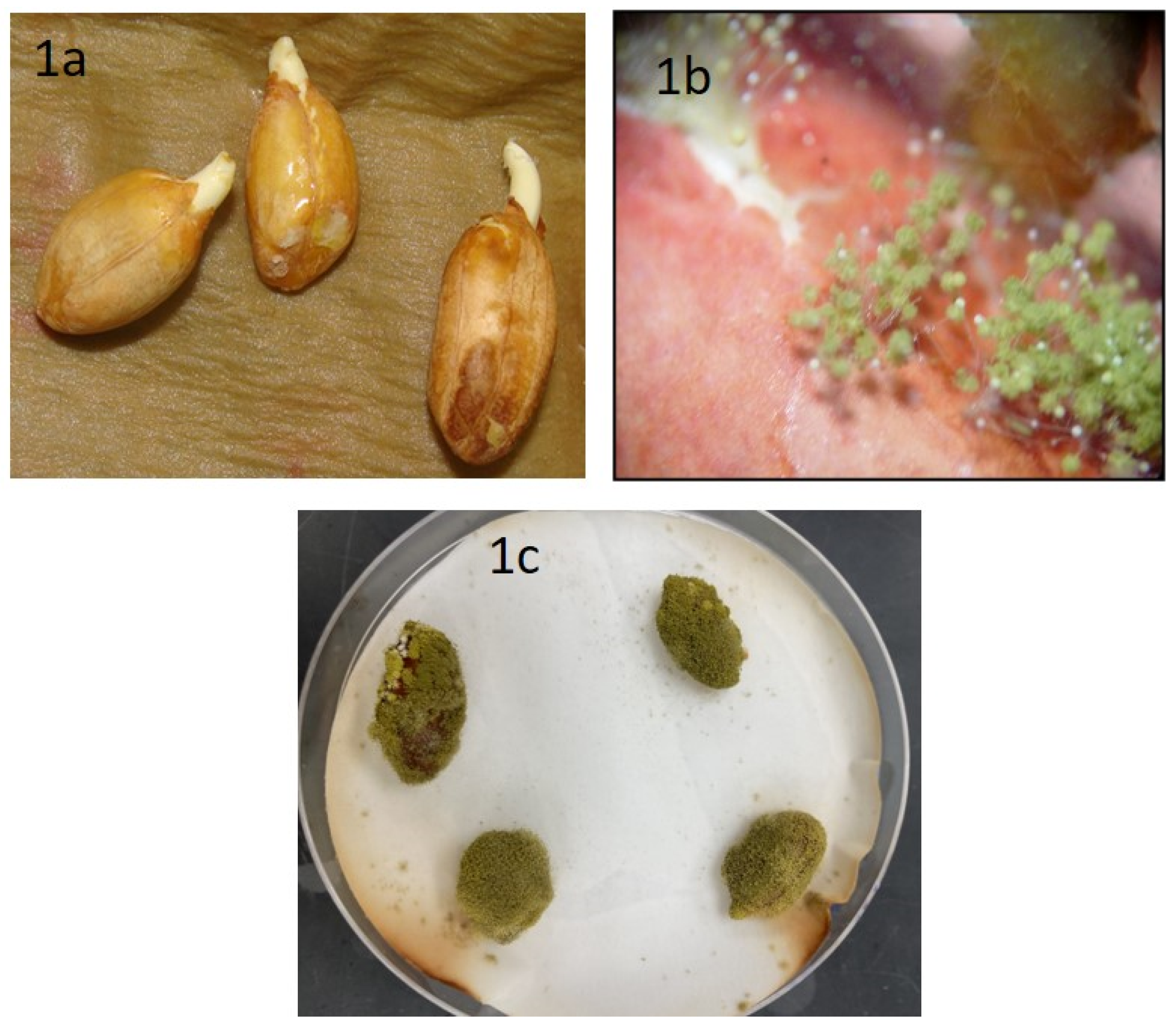
After seven days of incubation, we observed healthy peanut seeds, variety Tifguard, germinating in a few plates (Figure 1a). Under light microscopic examination, these seeds showed the presence of both mycelia and spores of A. flavus (Figure 1b) on the cracked seed coat. After 10 days, seed germination was arrested, seed coats and radicles were completely covered with a mesh of mycelia and spores (Figure 1c). After 10–15 days of incubation, the A. flavus isolates on YES media fluoresced under UV light compared to the non-toxigenic forms.
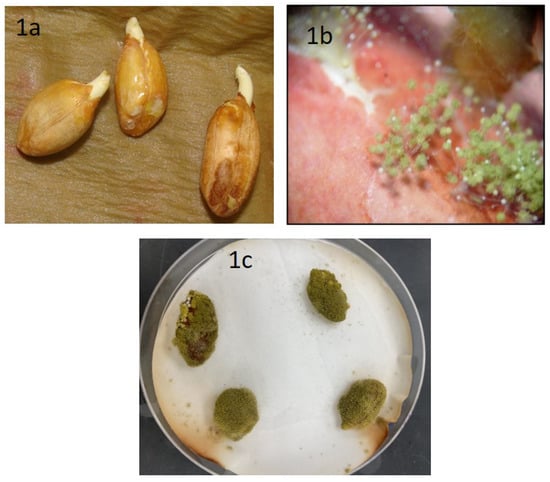
Figure 1.
Isolation and screening of A. flavus from peanut seeds, variety Tifguard. Image of (a) healthy peanuts, (b) fungus sporulation from infected peanuts under light microscopy examination, and (c) peanuts infected with A. flavus, incubated for 7 days in moist filter paper.
3.2. Antifungal Activity Assay of Essential Oils
Fifteen essential oils (EOs) from above were tested for antifungal activities against the mycelial growth of A. flavus in peanuts. All EOs investigated demonstrated different levels of growth inhibition at different concentrations (Table 1). Total inhibition of mycelial growth was observed in 8 of 15 essential oils at concentrations between 500 and 4000 ppm. The most effective EO in inhibiting mycelial growth, based on the antifungal inhibition assay, was clove, with total inhibition at 500 ppm and over 90% inhibition at 250 ppm. This was closely followed by thyme, with total inhibition at 1000 ppm and approximately 90% inhibition at 500 ppm. EOs of lemongrass and cinnamon totally inhibited mycelial growth at concentrations ≤2000 ppm and at least 90% inhibition at 1000 ppm. The commercial fungicide, prothioconazole (positive control) completely inhibited the A. flavus at all tested concentrations ranging from 125 to 4000 ppm. As the concentration of most of the essential oils increased, the inhibitory effect also increased. Tukey’s multiple comparison test (p = 0.05) showed that all EOs tested, at different concentrations, showed varying degrees of antifungal activity against mycelial growth. Of all the EOs tested, clove EO was the most effective antifungal agent against this fungus, while ginger EO was the least effective, irrespective of the concentration.

Table 1.
Inhibitory effect (%) of fifteen essential oils measured by the zone of clearance on the mycelial growth of A. flavus, isolated from peanuts, after 7 days of incubation.
3.3. Major Active Compounds of Clove Oil
Following the strength of the antifungal activities observed against A. flavus by clove EO from the diffusion assay, GC-MS was performed to investigate the major components of this oil. Our results show that the clove EO was predominantly composed of eugenol (83.25%), then E-caryophyllene (13.36%), followed by α-humulene (2.18%), which represented the marker compounds comprising approximately 99% of the whole oil (Table 2).

Table 2.
GC-MS analysis of clove essential oil showing major chemical components; RI: retention index literature comparison; the remaining compounds were in traces (<0.1%).
3.4. Effects of Clove Oil on Morphology and Ultrastructure of A. flavus Fungus
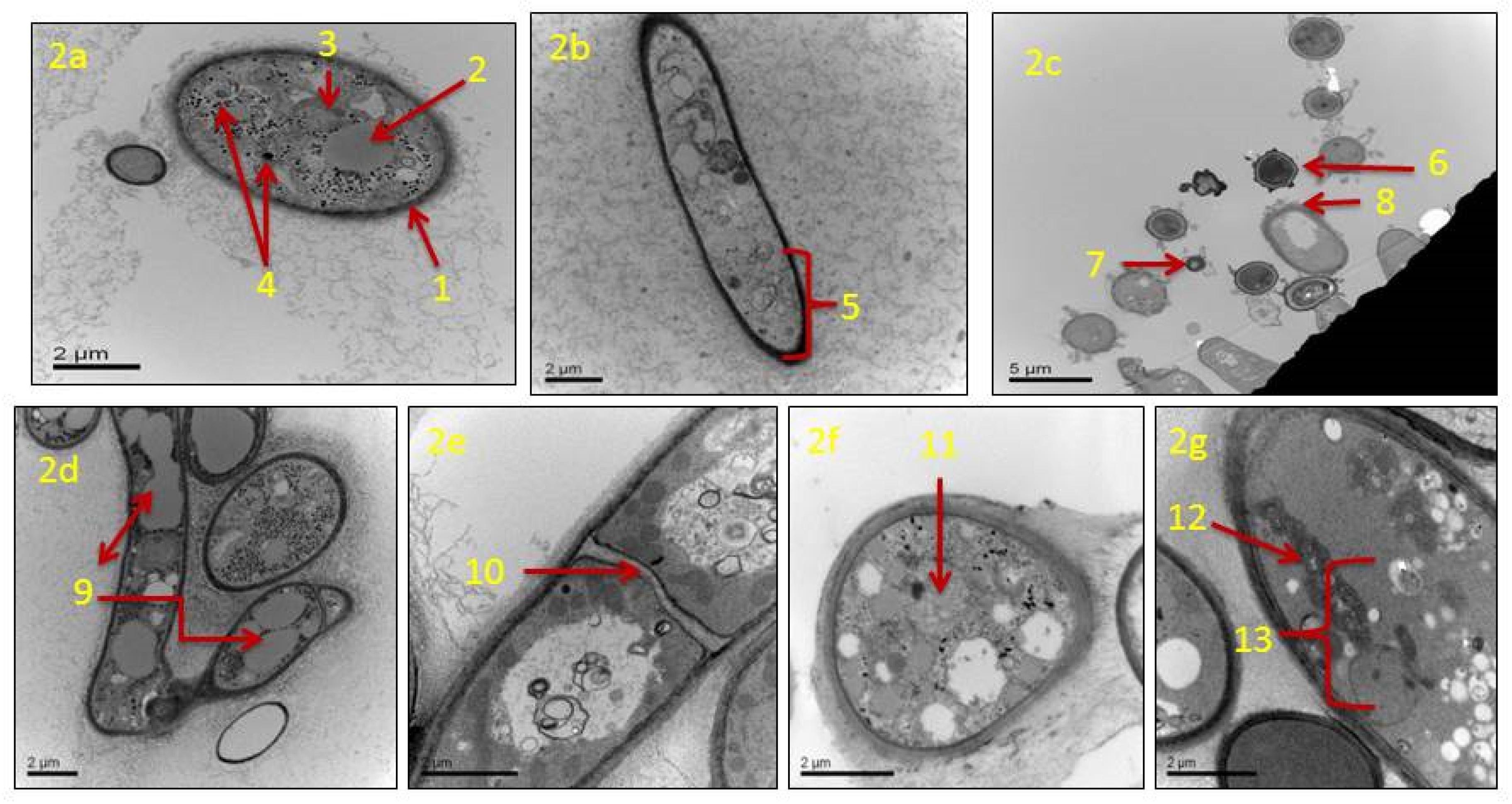
TEM analysis showed that in actively growing mycelia of A. flavus, untreated with clove EO, cellular components of conidia and hyphae remain unaltered. Untreated conidium showed a normal cell wall, and its cell matrix remained intact (Figure 2a). The plasma membrane had a linear shape, and the cell organelles, such as vacuoles, mitochondria, and nuclei appeared normal. The untreated mycelia had long strands of smooth hyphae with an intact cell wall. The hypha had a clear thick cell wall, and the cytoplasmic matrix remained unaltered (Figure 2b). In mycelia treated with clove EO (500 ppm), the main changes observed concerned the conidia and hypha. Conidia lost their normal shape and size, disintegrated cell surface, and lost their structural integrity in comparison to the control (Figure 2c). The treated hyphae suffered major alterations such as lysis of the cell wall, cytoplasmic disruption, the disintegration of mitochondria, and destruction of the nucleus. The hyphal wall appeared markedly thinner, and extensive vacuolization of the cytoplasmic matrix was clearly visible (Figure 2d). Treated hyphae also showed irregular septa (Figure 2e) and shrinkage of the cytoplasmic content (Figure 2f). Structural damage of mitochondria was prominent, and other cell organelles, including the nuclear content, were absent (Figure 2g).
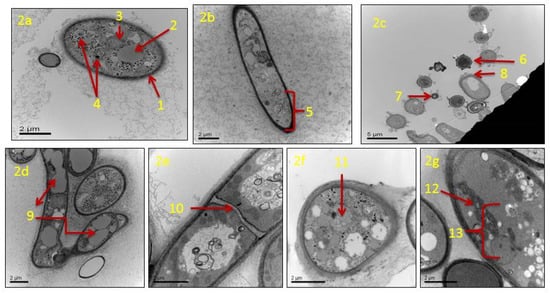
Figure 2.
Transmission electron micrographs of Aspergillus flavus treated with 500 ppm of clove essential oil after seven days of incubation: (a) untreated healthy conidium with cell wall and cell-matrix intact; cell wall (1), vacuole (2), mitochondria (3), and nuclei (4); (b) untreated hypha with a clear thick cell wall and cytoplasmic matrix intact (5); (c) cross section of treated fungal conidia with an irregular shape (6), size (7) and disintegrated cell surface (8); (d) treated hyphae with extensive vacuolization in the cytoplasmic matrix (9); (e) treated hyphae with irregular septa (10); (f) treated hyphal cell with shrunken cytoplasmic contents (11); (g) treated hyphal cell with irregular/disrupted mitochondria (12), the absence of the nucleus and other cellular organelles (13).
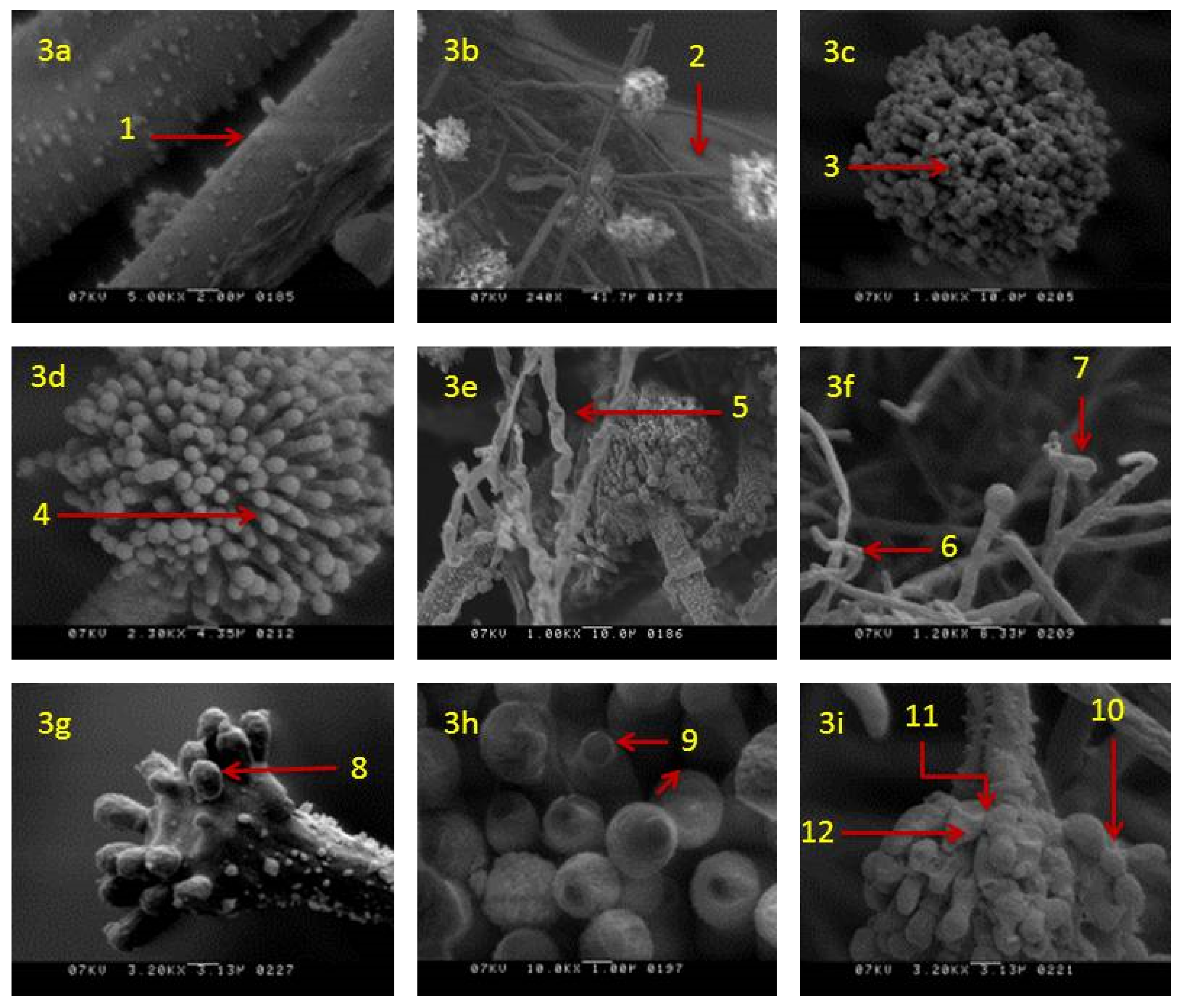
SEM analysis showed that in actively growing mycelia of A. flavus, untreated with clove EO, the morphology of hyphae and conidia remained unaltered. Mycelium had long strands of linear, regular, rough-surfaced healthy hyphae (Figure 3a) with healthy conidiophores (Figure 3b) bearing healthy conidia (Figure 3c) and phialides (Figure 3d). Morphological changes were noticed in colonies incubated in media supplemented with clove EO. In these colonies, morphological alterations were prominent in both somatic and reproductive structures. The mycelia treated with clove EO (500 ppm) showed blistering of hypha (Figure 3e), with disrupted conidiophores bearing deformed vesicles (Figure 3f). In addition, in a few conidiophores, the chains of conidia were not visible and had fewer or destroyed phialides (Figure 3g). Affected phialides showed discrete damage or shrunken apex (Figure 3h). Overall, conidiophores showed a disrupted conidial chain, loss of conidia and collapsed vesicles in all treated mycelium (Figure 3i).
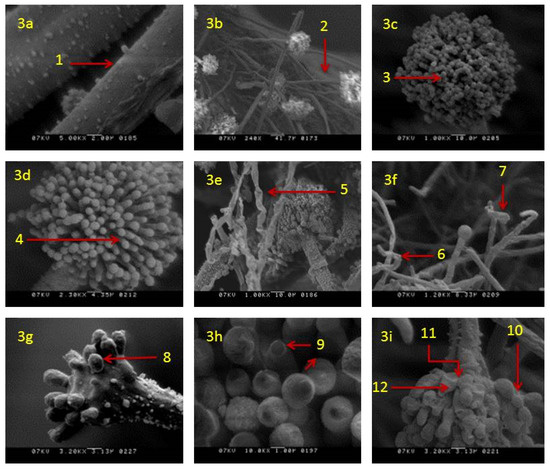
Figure 3.
Scanning electron micrographs of A. flavus treated with 500 ppm of clove essential oil after seven days of incubation: (a) Healthy hyphae rough surface (1); (b) healthy hyphae with healthy conidiophore (2); (c,d) healthy conidium (3) and phialides (4); (e) treated mycelia with blistering hyphae (5); (f) treated hyphae with disrupted conidiophores (6) and deformed vesicles (7); (g) treated conidiophore with fewer or destroyed phialides on vesicle (8); (h) treated phialides with damage/shrunken apex (9); (i) treated conidiophores with disrupted conidial chain (10) collapsed vesicles (11) and phialides (12).
3.5. Detection of Fungus Aflatoxin
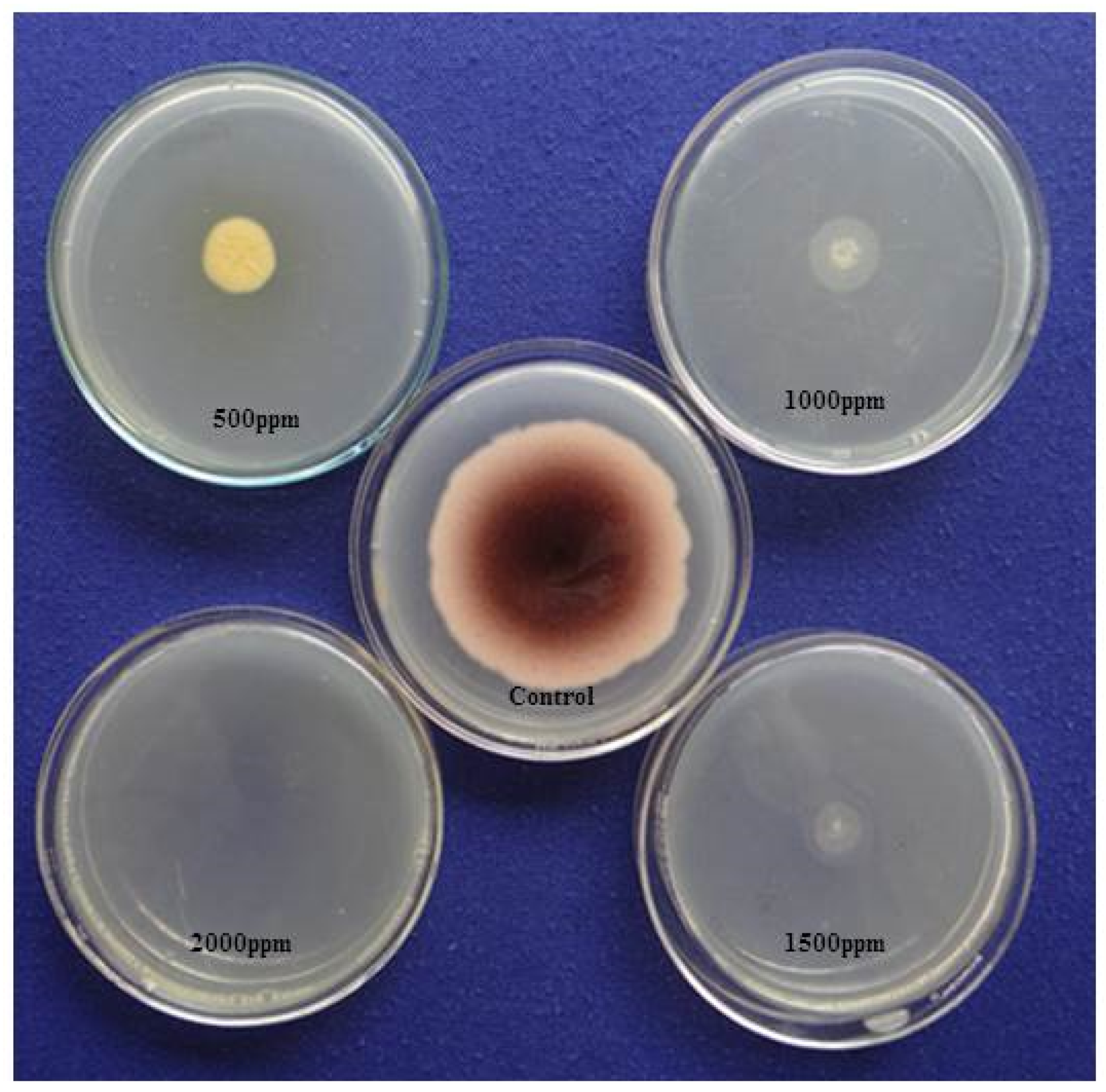
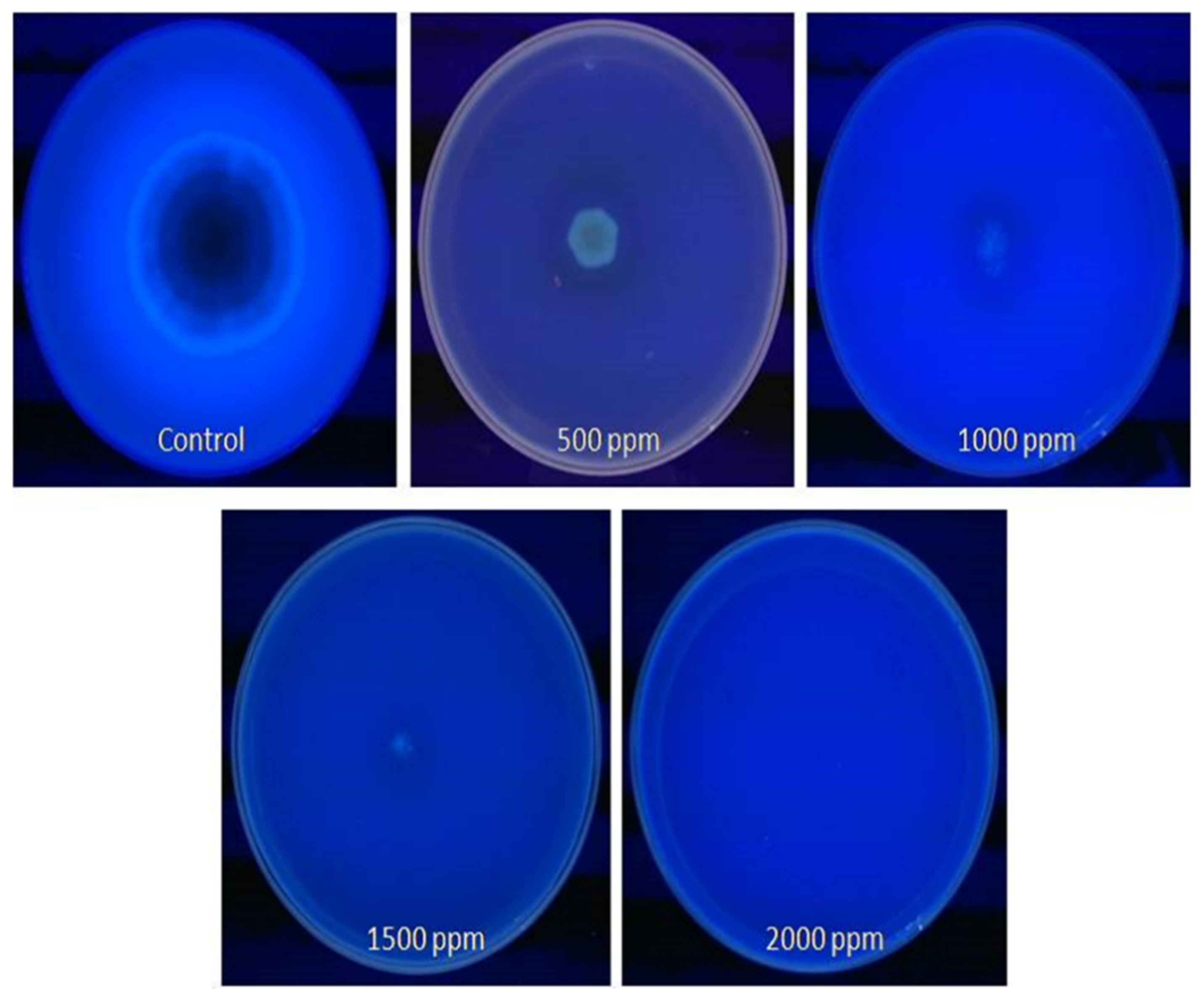
Using the AV method, we detected the aflatoxigenic form of A. flavus in peanuts, variety Tifguard. After exposure to ammonia vapor followed by one-hour incubation, the five-day-old mycelia and spores on YES media showed a plum-red colour change on the undersides of the plated colonies (Figure 4). This was an indication of aflatoxin producing strain of A. flavus isolate. YES plates, which served as the control, showed no colour change. We also observed that in plates impregnated with different concentrations of clove EO (500–2000 ppm), there was a significant reduction in mycelial growth, and no colour change was observed on the undersides of the plated colonies (Figure 4). In contrast, we observed a change in colour from pale white to dark pink in our EO untreated plates, indicative of an interaction between ammonia vapors and aflatoxin diffused into the YES medium.
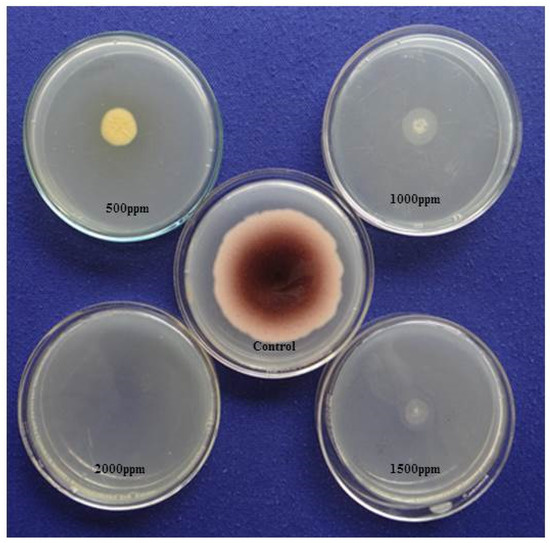
Figure 4.
Effect of clove essential oil on aflatoxin (AFB1) production by A. flavus on yeast extract sucrose (YES) medium exposed to ammonia vapor.
Using the CMA method, we confirmed an aflatoxigenic strain of A. flavus in peanuts, variety Tifguard. We also observed that in plates impregnated with different concentrations (500–2000 ppm) of clove EO, the five-day-old mycelia and spores showed no blue fluorescence in the CMA plates (Figure 5). Furthermore, the 500 ppm concentration of clove EO appeared to be most effective in inhibiting aflatoxin production by this fungus. The blue fluorescence surrounding the colony was only detected in plates that served as a control. The absence of fluorescence was indicative of the action of clove EO on aflatoxin production by A. flavus in the CMA-treated plates.
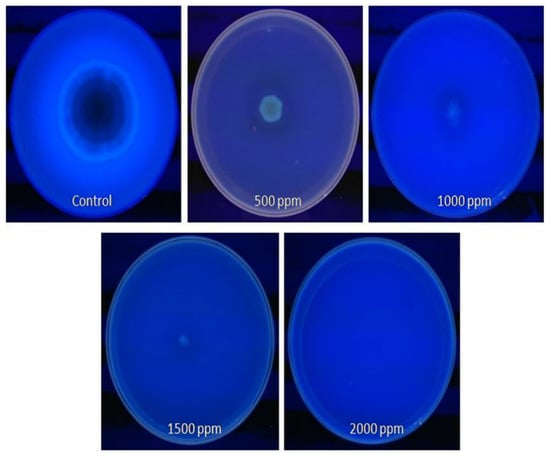
Figure 5.
Detection of aflatoxin (AFB1) production by A. flavus treated with clove essential oil on CMA observed under UV radiations.
3.6. Quantification of Aflatoxin (AFB1)
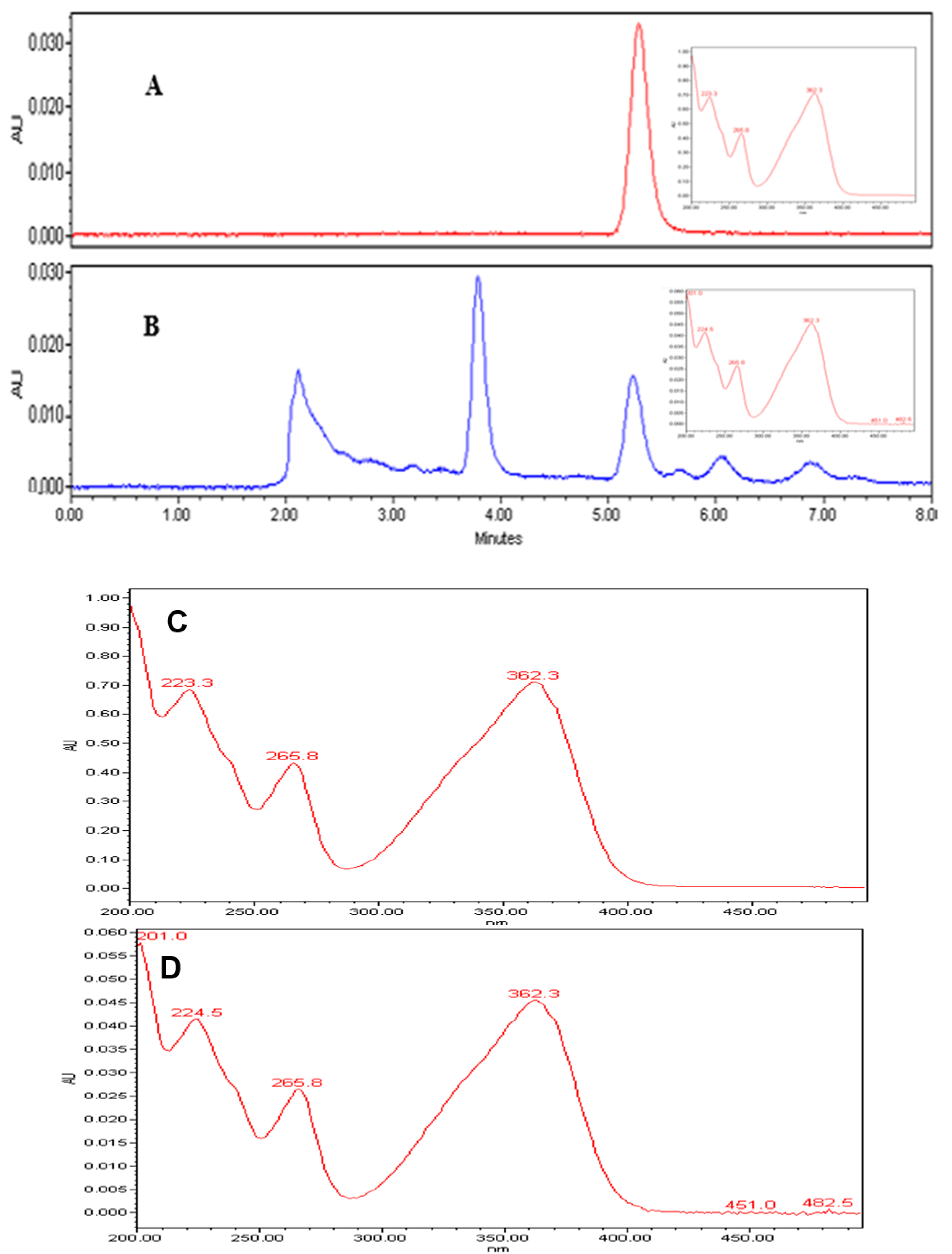
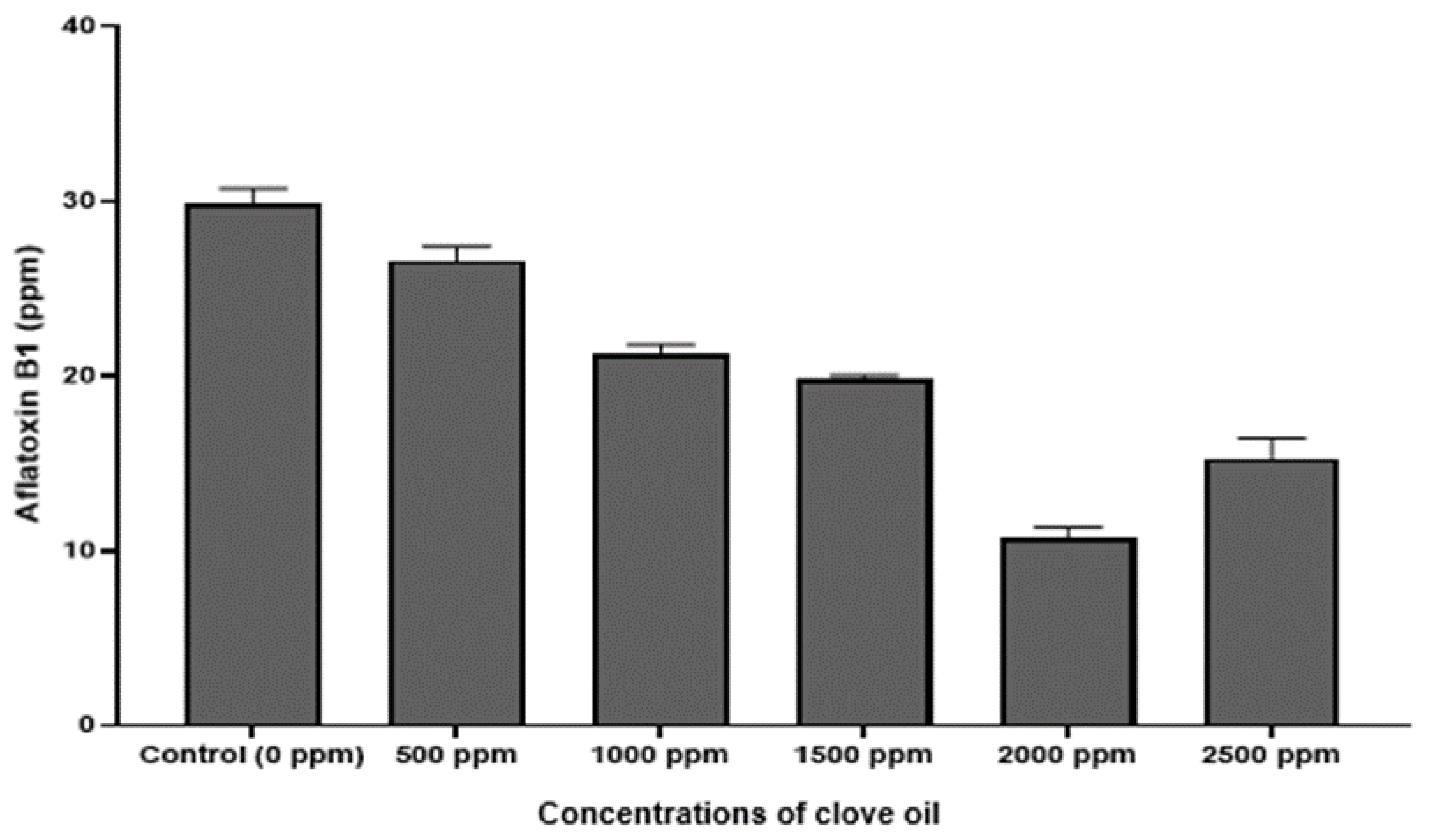
Qualitative analysis of the chromatograms in our study, indicated that the peaks (Figure 6A,B) appearing at retention times 5.23 min, confirmed the identification of AFB1 in the extracts, based on the AFB1 reference standard. Further, UV spectrum of AFB1 reference standard (Figure 6C), is identical to the UV spectrum (Figure 6D) of the clove EO-treated mycelia and spore samples of A. flavus, confirming the presence of AFB1 in the extracts. We also detected two unidentified peaks from the sample extracts. In addition, a plot of increasing clove EO versus AFB1 concentrations in sample extracts revealed a 10% decrease in the AFB1 concentration at 500 ppm clove EO compared to control, and to approximately 46% decrease at 2000 ppm clove EO, with a negative slope of −3.248 (ppm clove/ppm AFB1; Figure 7). Moreover, we established a correlation of 0.95, which is significant in terms of the effective suppression of AFB1 by clove EO. For quantitation of AFB1 to generate the results in Figure 7, the AFB1 calibration curve Y = 169758X + 80,464 with a regression coefficient of R2 = 0.9957 was based on the data provided (Supplementary Materials Table S1 and Figure S1). The calibration equation (Figure S1) obtained from Table S1a was used to calculate residual aflatoxin B1 based on the peak areas (Table S1b) of the clove oil treated samples.
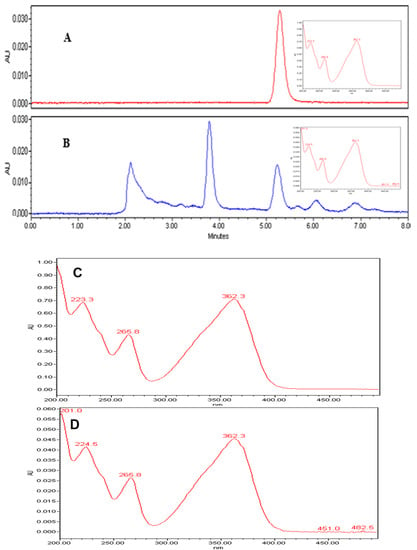
Figure 6.
Chromatogram (A)—the peak of AFB1 standard at 40 ppm; chromatogram (B)—the peak of AFB1 from the extract at 500 ppm clove oil; the retention times of AFB1 average at 5.23 min in both chromatograms and additional peaks in chromatogram B were not identified in this study. UV spectra (C,D) are identical to the peaks eluting at 5.23 min in chromatograms A and B, confirming the presence of AFB1 in the extracts.
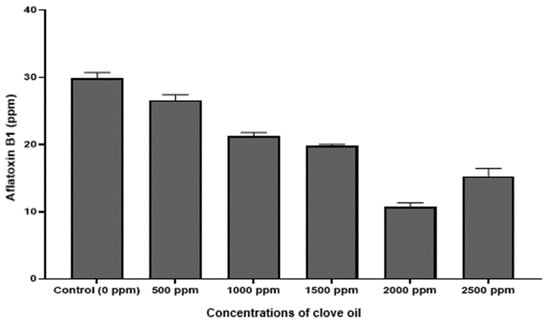
Figure 7.
Column plot of the relation between the clove oil used versus residual aflatoxin (AFB1) after exposure of A. flavus mycelium to clove oil at different concentrations (ppm).
4. Discussion
Fungal contamination of peanuts is of utmost concern globally as peanuts are a rich and economical source of protein. The use of synthetic fungicides remains the common measure to reduce fungal contamination in the peanut industry in the US and elsewhere. It is widely known that these synthetic fungicides not only affect peanut consumers, but they can also be detrimental to the environment. Many strategies, including natural control, biological control, control of insect pests, development of resistant cultivar, have been studied for the management of contamination and aflatoxin production in crops. According to Reddy et al. [34], health hazards from exposure to toxic chemicals and economic considerations make natural plant extracts ideal alternatives to protect food and feed from fungal contamination. Plant-based antimicrobial compounds such as essential oils (EOs) and their bioactive compounds have been identified as potential biological control agents in the reduction/control of fungal contamination and toxin production, and more specifically as alternatives to synthetic fungicides against toxin-producing molds [35,36,37,38].
In our study, we screened fifteen essential oils at different concentrations against the mycelial growth of A. flavus in peanuts. The results demonstrate a dose-dependent inhibitory activity of all the EOs tested on the target fungus. Strong antifungal activity was observed in most of the EOs, demonstrated by the growth inhibition zones and minimal inhibitory concentration (MIC) values on the agar media. However, clove EO was the most effective of the EOs tested, against A. flavus mycelial growth and development, with a fungicidal effect at concentration 500 ppm. The commercial fungicide, prothioconazole, completely inhibited A. flavus at all tested concentrations ranging from 125 to 4000 ppm. In fact, the fungicide completely inhibited fungal growth at concentrations far below the reported ones. Similarly, previous studies have also demonstrated antifungal, inhibitory, and fungicidal action of different plants, including clove, thyme, cinnamon, lemongrass, tea tree, citronella, peppermint, and oregano oils [38,39,40,41,42,43,44]. Furthermore, earlier reports have demonstrated greater antifungal activity of thyme, lemongrass, and tea tree EOs against Aspergillus spp. compared to clove EO [42,45,46]. This is in contrast with our findings, as clove EO was more effective than all tested EOs and caused total inhibition of the mycelial growth. It is worth noting that our study focused on A. flavus in contaminated peanuts, which is different from several other studies that have investigated the antifungal activity of EOs against Aspergillus spp. in other hosts. To our knowledge, this is the first time that the antifungal activity of clove EOs has been demonstrated against A. flavus in contaminated peanuts, variety Tifguard, and our data pave the way to investigate other varieties of peanuts in Georgia.
It is widely known that the antimicrobial activity of essential oils is caused by various components that vary in their composition within the specific oil or other factors, which include plant type, seasonality, region, or location of where the plant is grown. Prindle and Wright [28] reported that the effect of essential oils (phenolic compounds) is concentration-dependent. At low concentrations, phenolic compounds affect enzyme activity, and at higher concentrations, they cause protein denaturation. Our GC-MS analysis showed that the clove EO was characterized by high amounts of eugenol (83.25%), with E-caryophyllene (13.36%) and α-humulene (2.18%) as marker compounds comprising approximately 99% of the whole oil. The predominantly high concentration of the eugenol is similar to that observed in the study by Pinto et al. [47]. In a recent study, the authors [44] found the IC50 of clove and cinnamon EOs to be >2000 µg/mL; however, the concentration of eugenol in the clove EO was determined to be 54% of the whole EO. This might explain the variations in the dose-responses between their study and ours, with a four-fold difference in the effective dose of clove EO (ppm) required for inhibition of mycelial growth of the A. flavus.
We further investigated the effects of the clove EO on mycelia and conidia of A. flavus by electron microscopy. A crucial observation of the ultrastructural changes, under TEM analysis, was cell wall lysis, thinning of the cell wall, cell membranes, and extreme vacuolization of the cytoplasmic matrix of hyphal cells. The most prominent observation was the damage to mitochondria and complete disintegration of other cell organelles, and most importantly, the nucleus was absent. Our observations corroborate the findings by Khosravi et al. [48]. These authors found changes in the cellular contents, including the cell wall, plasma membrane, and membranous organelles such as the nuclei and mitochondria of A. fumigatus and A. flavus, following exposure to the different EOs. According to [49], the antifungal activity of dill oil results from its ability to disrupt the permeability barrier of the plasma membrane and from the mitochondrial dysfunction-induced reactive oxygen species (ROS) accumulation in A. flavus. They further stated that mitochondrial damage leads to loss of ATP synthesis process in the cell, which is required for the fungi. We noticed excess vacuolization in our clove EO treated cells. It is reported that fungal vacuoles are responsible for the synthesis of many enzymes involved in glyoxylate pathways and fatty acid oxidation; thus, damage to vacuoles results in loss of these functions [50]. The review by Miri et al. [51] on the effect of essential oils on growth and aflatoxin production by A. flavus summarized that EOs could also coagulate the cytoplasm and damage lipids, proteins, cell walls and membranes that can lead to the leakage of macromolecules and the lysis. Data from our TEM analysis clearly showed that clove EO caused ultrastructural modifications of A. flavus hyphal cells leading to destruction and disintegration of the cellular matrix, and these changes may be attributed to a disruption in the enzymatic system of these cells by clove EO. Destruction of the cytoplasmic content might have certainly resulted in disruptions in metabolic activities of affected hyphal cells, ultimately affecting normal mycelial growth as observed in our study. In addition, since in our study, clove EO showed a negative impact on the conidial structural integrity, such changes certainly might have contributed to the mycelial growth of A. flavus.
The negative impact of clove EO on the mycelia and conidia of A. flavus was also observed in our SEM study. Our findings demonstrate that morphological alterations were prominent in both somatic and reproductive structures. Zambonelli et al. [52] observed inhibition of fungal growth, in addition to degeneration of fungal hyphae after treating them with Thymus vulgaris, Lavandula R.C. hybrid, and Mentha piperita essential oils. This type of damage to the hyphal morphology of plant pathogenic fungi following exposure to essential oils has also been reported by others [53,54] after exposure to EOs of the Cymbopogon species. Similar to our observations, the SEM study of A. ochraceus cells fumigated with natural cinnamaldehyde, citral, and eugenol showed alteration in the morphology of the hyphae, which appeared collapsed, with abnormal branching of hyphae in the apical region and loss of linearity [55].
Another key finding of our study was that growth inhibitions of the A. flavus were consistent with corresponding morphological changes of the hyphae and reproductive structures exposed to clove EO. The changes in hyphal morphology were certainly related to the loss of structural integrity of the cell wall supported by our TEM analysis, and further suggests that clove EO might have interfered with fungal wall synthesis directly or indirectly. It has been reported that several compounds of EOs, due to their lipophilic nature, cross fungal cell walls and membranes, interacting with membrane proteins and enzymes, disrupting cell metabolism, ultimately leading to cell death [56,57]. That clove EO, an antifungal agent, resulted in both morphological and ultrastructural alterations of A. flavus mycelium and spores is demonstrated for the first time by electron microscopy in our contaminated peanuts, variety Tifguard.
In addition, after confirming the presence of aflatoxin producing isolates of A. flavus in the contaminated peanuts by qualitative methods, we further established that clove EO also had an inhibitory effect on AFB1 production by this fungus. Similar to our studies, HPLC has been employed previously to detect aflatoxin production by A. flavus cultured on CMA and other differential media exposed to ammonia vapor pressure [58,59,60]. Other reports using such techniques demonstrated the potential of antifungal plant extracts against A. flavus growth and aflatoxin production in crops of economic importance [16,34,37,61,62,63,64]. Among these studies, some have found that clove oil inhibited the mycelial growth and aflatoxin production of A. flavus in rice [16,65]. The findings in our study support the conclusions of the previous research on the effect of clove EO on other crops, and at 500 ppm and above, a dose-dependent effect was observed, leading to a significant reduction in mycelial growth and AFB1 production. Hence, our study showed for the first time that clove EO showed both antifungal and antitoxigenic properties against A. flavus in the contaminated peanuts, variety Tifguard.
In the United States, the State of Georgia is recognized for approximately 50% of the national peanut supply, thus aiding the global recognition of the US as the third largest peanut producer [66]. Despite extensive research by the peanut industry in the US, the use of synthetic fungicides to combat contamination and aflatoxin production is very much in use on a large scale. The roles of essential oils as a biocontrol agent against Aspergillus spp. growth and toxin production in peanuts are largely unexplored, and our study offers new insight into this area. In addition, none of the earlier reports indicate that clove EOs have been tested for their fungi-toxic and antiaflatoxigenic potential against A. flavus in peanut growing states. Moreover, we are not aware of any published studies investigating the antifungal effect of essential oils or, in particular, clove against A. flavus in peanuts of the variety, Tifguard. Hence, we demonstrated for the first time the implication of clove EO on the deleterious morphological and ultrastructural alterations of somatic and reproductive structures of A. flavus and its ability to reduce AFB1 production by A. flavus in one variety of peanut. Our findings increase the possibility of exploiting the clove EO as an effective alternative to synthetic chemicals and as an effective bio-control and non-toxic bio-preservative to increase the quality and safety of peanuts varieties in the US and possibly around the globe. Most importantly, eugenol, the main compound in clove EO, can be further exploited to determine the EO mechanism of action and obtained data on its in vitro efficacy in both liquid and vapor form, and possibly finds its way as a lead compound in integrated pest management (IPM) to control A. flavus and to eliminate the carcinogenic aflatoxin B1 in peanuts.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/6/4/383/s1, Figure S1: Calibration equation generated by linear regression from Table S1 data, Table S1: (a) Raw data used to generate the calibration equation shown in Figure S1 below; Stock reference standard was 1 µg/mL Aflatoxin B1 (Sigma Aldrich, St. Louis, MO, USA). (b) Raw data for sample analysis. Control = not Clove Oil treated. Column four was calculated based on the calibration equation above.
Author Contributions
P.N.A. and P.Q. screened EOs and performed SEM and TEM; M.Y.S. and H.N. detected aflatoxin by qualitative methods; H.Z.M. quantified and analysed AFB1; A.S. quantified EOs. All authors contributed to analyis of rest of the data and write up of the manuscript. P.N.A. and E.C.A. did the final editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mentor Protégé, Kennesaw State University, Grant Number 08036.
Acknowledgments
The authors would like to thank undergraduate students, Yoossef, M; Yawa Z and S Khorsandi for their contribution in maintaining aflatoxin strain and overseeing HPLC protocols, and Department of Molecular and Cellular Biology, Kennesaw State University, Kennesaw, Georgia, USA, for partially funding undergraduate research and Department of Chemistry and Biochemistry for Biochemical assays. In addition, this study was supported by the Robert P Apkarian Integrated Electron Microscopy Core (IEMC) at Emory University, Atlanta, which is subsidized by the School of Medicine and Emory College of Arts and Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 20 June 2020).
- Cullen, J.M.; Newberne, P.M. Acute hepatotoxicity of aflatoxins. In The Toxicology of Aflatoxins; Elsevier: Amsterdam, The Netherlands, 1994; pp. 3–26. [Google Scholar]
- World Health Organization; International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; World Health Organization: Geneva, Switzerland, 1993; Volume 56. [Google Scholar]
- N’dede, C.B.; Jolly, C.M.; Vodouhe, S.D.; Jolly, P.E. Economic risks of aflatoxin contamination in marketing of peanut in benin. Econ. Res. Int. 2012, 1–12. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Faustinelli, P.C.; Wang, X.M.; Palencia, E.R.; Arias, R.S. Genome sequences of eight Aspergillus flavus spp. and one A. parasiticus sp., isolated from peanut seeds in Georgia: Table 1. Genome Announc. 2016, 4, e00278-16. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rajendran, S.; Srivastava, A.; Sharma, S.; Kundu, B. Antifungal activities of selected essential oils against Fusarium oxysporum f. sp. lycopersici 1322, with emphasis on Syzygium aromaticum essential oil. J. Biosci. Bioeng. 2017, 123, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lawley, R. Aflatoxins. Food Safety Watch. 2013. Available online: http://www.foodsafetywatch.org/factsheets/aflatoxins/ (accessed on 21 August 2020).
- Pitt, J.I.; Dyer, S.K.; McCammon, S. Systemic invasion of developing peanut plants by Aspergillus flavus. Lett. Appl. Microbiol. 1991, 13, 16–20. [Google Scholar] [CrossRef]
- Achar, P.N.; Hermetz, K.; Rao, S.; Apkarian, R.; Taylor, J. Microscopic studies on the Aspergillus flavus infected kernels of commercial peanuts in Georgia. Ecotoxicol. Environ. Saf. 2009, 72, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Suszkiw, J.; Dorner, J.W.; Lamb, M.C. Protecting peanuts from aflatoxins. Environ. Health Perspect. 2004, 112, A987. [Google Scholar]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Basilico, M.Z.; Basilico, J.C. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999, 29, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Salleh, B.; Saad, B.; Abbas, H.; Abel, C.; Shier, W. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Dorner, J.W. Management and prevention of mycotoxins in peanuts. Food Addit. Contam. Part A 2008, 25, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W. Biological control of aflatoxin contamination of crops. J. Toxicol. Toxin Rev. 2004, 23, 425–450. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of aflatoxin in peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef]
- Čvek, D.; Markov, K.; Frece, J.; Dragičević, T.; Majica, M.; Delaš, F. Growth inhibition of Aspergillus ochraceus ZMPBF 318 and Penicillium expansum ZMPBF 565 by four essential oils. Arch. Ind. Hyg. Toxicol. 2010, 61, 191–196. [Google Scholar] [CrossRef]
- Passone, M.A.; Girardi, N.S.; Etcheverry, M. Evaluation of the control ability of five essential oils against Aspergillus section Nigri growth and ochratoxin A accumulation in peanut meal extract agar conditioned at different water activities levels. Int. J. Food Microbiol. 2012, 159, 198–206. [Google Scholar] [CrossRef]
- Císarová, M.; Tančinová, D.; Medo, J.; Kačániová, M. The in vitro effect of selected essential oils on the growth and mycotoxin production of Aspergillus species. J. Environ. Sci. Health Part B 2016, 51, 668–674. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Ying, G.; Yang, M.; Nian, Y.; Wei, F.; Kong, W. Antifungal evaluation of plant essential oils and their major components against toxigenic fungi. Ind. Crops Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- US Department of Agriculture. New Peanut Variety Resistant to Nematodes, Virus. ScienceDaily. 2008. Available online: www.sciencedaily.com/releases/2008/05/080521101458.htm (accessed on 11 June 2020).
- Holbrook, C.C.; Timper, P.; Culbreath, A.K.; Kvien, C.K. Registration of ‘Tifguard’ peanut. J. Plant Regist. 2008, 2, 92–94. [Google Scholar] [CrossRef]
- Lopez, A.; Alzamora, S.M.; Guerrero, S. Natural antimicrobials from plants. In Minimally Processed Fruits and Vegetables. Fundamentals Aspects and Applications; Alzamora, S.M., Tapia, M.S., Lopez-Malo, A., Eds.; Aspen Publishers: Gaithersburg, MD, USA, 2000; pp. 237–264. [Google Scholar]
- Raper, K.B.; Fennell, D.I. The Genus Aspergillus; Williams & Wilkins: Baltimore, MD, USA; E. & S. Livingstone: Edinburgh, UK, 1965. [Google Scholar]
- Prindle, R.F.; Wright, E.S. Phenolic compounds. In Disinfection, Sterilization and Preservation; Lawrence, C.A., Block, S.S., Eds.; Lea & Febiger: Pennsylvania, PA, USA, 1977; pp. 115–118. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species; CBS: Utrecht, The Netherlands, 2002; ISBN 9070351463. [Google Scholar]
- Gqaleni, N.; Smith, J.E.; Lacey, J.; Gettinby, G. Effects of temperature, water activity, and incubation time on production of aflatoxins and cyclopiazonic acid by an isolate of Aspergillus flavus in surface agar culture. Appl. Environ. Microbiol. 1997, 63, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Machida, S. A rapid identification method for aflatoxin-producing strains of Aspergillus flavus and A. parasiticus by ammonia vapor. Mycoscience 1999, 40, 205–208. [Google Scholar] [CrossRef]
- Davis, N.D.; Iyer, S.K.; Diener, U.L. Improved method of screening for aflatoxin with a coconut agar medium. Appl. Environ. Microbiol. 1987, 53, 1593–1595. [Google Scholar] [CrossRef] [PubMed]
- Vosough, M.; Bayat, M.; Salemi, A. Matrix-free analysis of aflatoxins in pistachio nuts using parallel factor modeling of liquid chromatography diode-array detection data. Anal. Chim. Acta 2010, 663, 11–18. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Reddy, C.S.; Muralidharan, K. Potential of botanicals and biocontrol agents on growth and aflatoxin production by Aspergillus flavus infecting rice grains. Food Control 2009, 20, 173–178. [Google Scholar] [CrossRef]
- Soliman, K.; Badeaa, R. Effect of oil extracted from some medicinal plants on different mycotoxigenic fungi. Food Chem. Toxicol. 2002, 40, 1669–1675. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Rezaee, M.-B.; Jaimand, K.; Alinezhad, S.; Saberi, R.; Yoshinari, T. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control 2009, 20, 1018–1024. [Google Scholar] [CrossRef]
- Sreenivasa, M.Y.; Dass, R.S.; Charith Raj, A.P.; Nagendra Prasad, M.N.; Achar, P.N.; Janardhana, G.R. Assessment of the growth inhibiting effect of some plant essential oils on different Fusarium species isolated from sorghum and maize grains. J. Plant Dis. Prot. 2011, 118, 208–213. [Google Scholar] [CrossRef]
- Prasad, N.; Bhat, S. Antifungal activity of essential oils against Phomopsis azadirachtae—The causative agent of die-back disease of neem. J. Agric. Technol. 2010, 6, 127–133. [Google Scholar]
- Adukwu, E.C.; Bowles, M.; Edwards-Jones, V.; Bone, H. Antimicrobial activity, cytotoxicity and chemical analysis of lemongrass essential oil (Cymbopogon flexuosus) and pure citral. Appl. Microbiol. Biotechnol. 2016, 100, 9619–9627. [Google Scholar] [CrossRef] [PubMed]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Elcocks, E.R.; Spencer-Phillips, P.T.N.; Adukwu, E.C. Rapid bactericidal effect of cinnamon bark essential oil against Pseudomonas aeruginosa. J. Appl. Microbiol. 2020, 128, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 2020, 202, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.L.; Zhang, Y.S.; Zhang, J.G.; Wei, Z.J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Minooeianhaghighi, M.H.; Shokri, H.; Emami, S.A.; Alavi, S.M.; Asili, J. The potential inhibitory effect of Cuminum cyminum, Ziziphora clinopodioides and Nigella sativa essential oils on the growth of Aspergillus fumigatus and Aspergillus flavus. Braz. J. Microbiol. 2011, 42, 216–224. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The mechanism of antifungal action of essential oil from dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Waterham, H.R.; Ferdinandusse, S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front. Cell Dev. Biol. 2016, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ben Miri, Y.; Belasli, A.; Djenane, D.; Ariño, A. Prevention by essential oils of the occurrence and growth of Aspergillus flavus and aflatoxin B1 production in food systems: Review. Aflatoxin B1 Occur. Detect. Toxicol. Eff. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Zambonelli, A.; d’Aulerio, A.Z.; Bianchi, A.; Albasini, A. Effects of essential oils on phytopathogenic fungi in vitro. J. Phytopathol. 1996, 144, 491–494. [Google Scholar] [CrossRef]
- De Billerbeck, V.G.; Roques, C.G.; Bessière, J.M.; Fonvieille, J.L.; Dargent, R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can. J. Microbiol. 2001, 47, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.A.; Sarhan, M.M.; Abu Shahla, A.N.K.; Abou El-Khair, E.K. Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain. J. Basic Microbiol. 2006, 46, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory effect of essential oils on Aspergillus ochraceus growth and ochratoxin a production. PLoS ONE 2014, 9, e108285. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Gemeda, N.; Woldeamanuel, Y.; Asrat, D.; Debella, A. Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: A potential source of botanical food preservative. Asian Pac. J. Trop. Biomed. 2014, 4, S373–S381. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Weaver, M.A.; Horn, B.W.; Xie, W.; Shier, W.T. Comparison of cultural and analytical methods for determination of aflatoxin production by Mississippi Delta Aspergillus isolates. Can. J. Microbiol. 2004, 50, 193–199. [Google Scholar] [CrossRef]
- Naik, M.K.; Guru Prasad, G.R.; Jadhav, H.P.; Hashem, A.; Abd_allah, E.F.; Sayyed, R.Z. Differentiation of toxigenic and atoxigenic Aspergillus flavus: Polyphasic approach, a new dimension. Indian J. Exp. Biol. 2018, 56, 892–898. [Google Scholar]
- Kushiro, M.; Hatabayashi, H.; Yabe, K.; Loladze, A. Detection of aflatoxigenic and atoxigenic Mexican Aspergillus strains by the dichlorvos–ammonia (DV–AM) method. Toxins 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, M.M.; Omer, E.A. Effect of essential oils of some medicinal plants on phytonematodes. Anz. Schädlingskd. Pflanzenschutz Umweltschutz 1995, 68, 82–84. [Google Scholar] [CrossRef]
- Mondali, N.K.; Mojumdar, A.; Chattterji, S.K.; Banerjee, A.; Datta, J.K.; Gupta, S. Antifungal activities and chemical characterization of Neem leaf extracts on the growth of some selected fungal species in vitro culture medium. J. Appl. Sci. Environ. Manag. 2009, 13, 49–53. [Google Scholar]
- Latif, M.A.; Saleh, A.K.M.; Khan, M.A.I.; Rahman, H.; Hossain, M.A. Efficacy of some plant extracts in controlling seed-borne fungal infections of mustard. Bangladesh J. Microbiol. 1970, 23, 168–170. [Google Scholar] [CrossRef]
- Sitara, U.; Niaz, I.; Naseem, J.; Sultana, N. Antifungal effect of essential oils on in vitro growth of pathogenic fungi. Pak. J. Bot. 2008, 40, 409–414. [Google Scholar]
- Mabrouk, S.S.; El-Shayeb, N.M.A. Inhibition of aflatoxin formation by some spices. Z. Lebensm. Unters. Forsch. 1980, 171, 344–347. [Google Scholar] [CrossRef]
- Chenault, K.D.; Burns, J.A.; Melouk, H.A.; Payton, M.E. Hydrolase activity in transgenic peanut. Peanut Sci. 2002, 29, 89–95. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).