Cryptococcus in Wildlife and Free-Living Mammals
Abstract
:1. Introduction
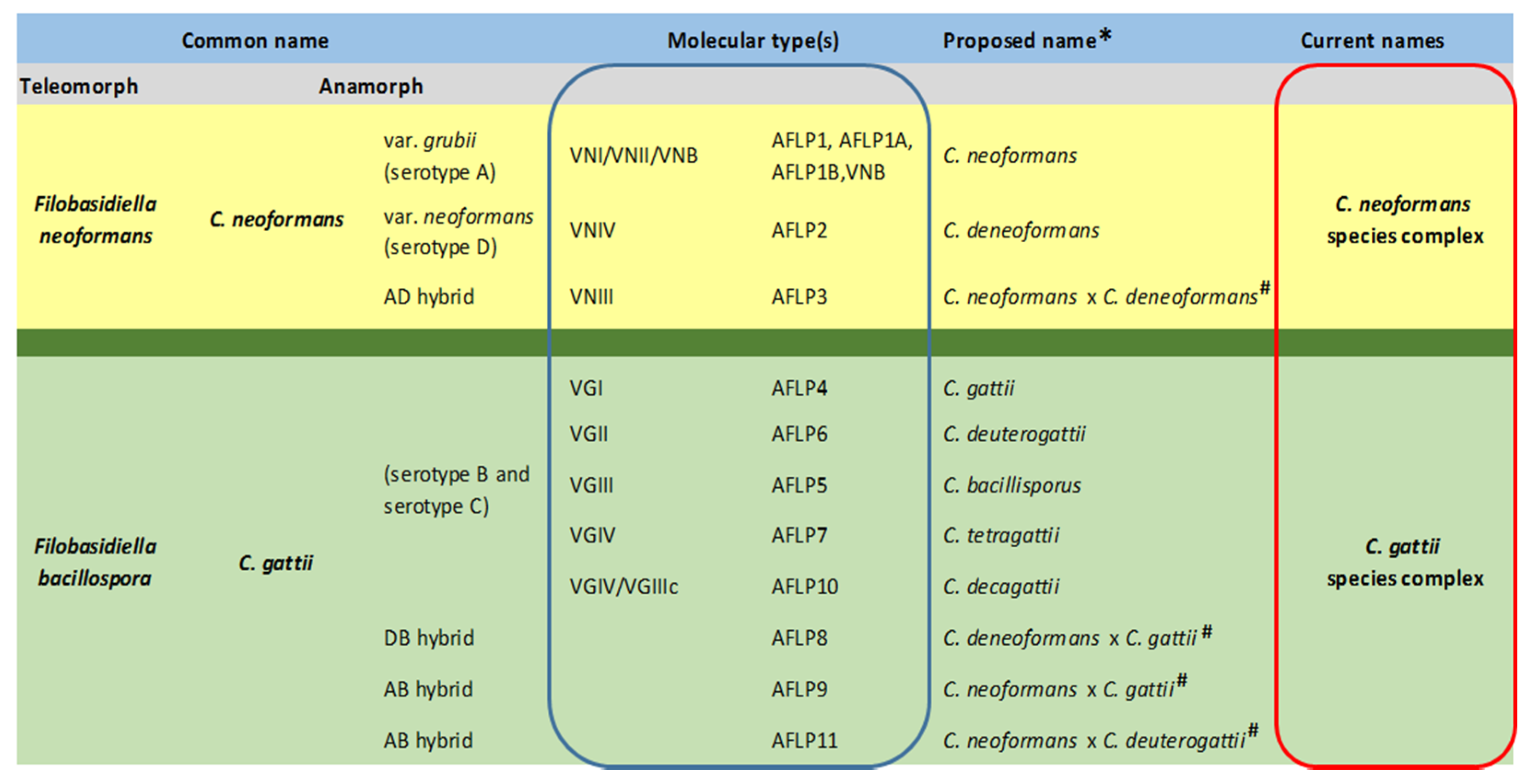
2. Nomenclature
3. Epidemiology
4. Pathogenesis and Virulence Factors
5. A Possible Environmental Niche
6. Wild Mammals
6.1. Cryptococcosis in Wild Felids
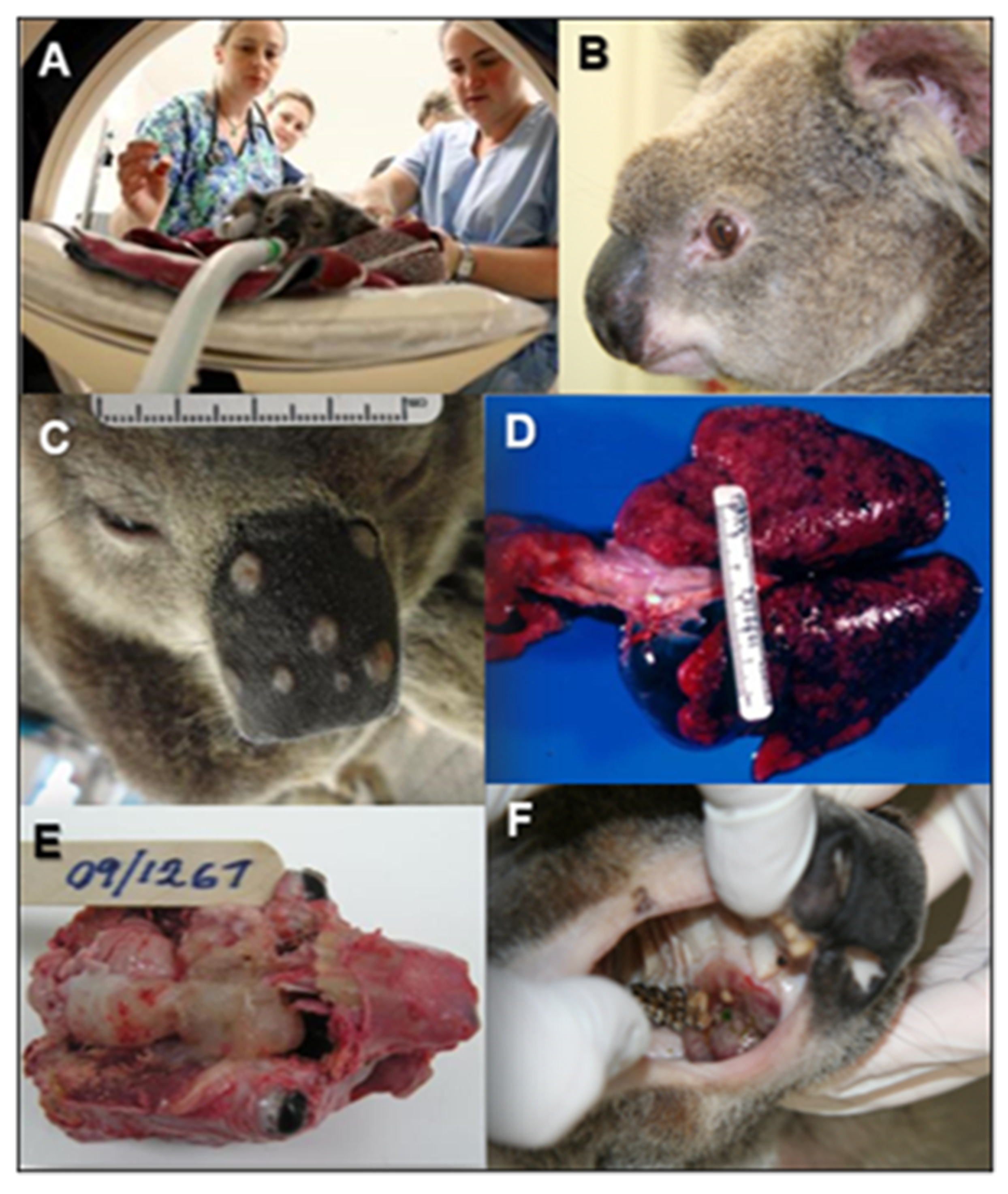
6.2. Cryptococcosis in Koalas
6.3. Cryptococcosis in Marine Mammals
6.4. Cryptococcosis in Free-Living Ungulates
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iatta, R.; Immediato, D.; Puttilli, M.R.; Danesi, P.; Passantino, G.; Parisi, A.; Mallia, E.; Otranto, D.; Cafarchia, C. Cryptococcus neoformans in the respiratory tract of squirrels, Callosciurus finlaysonii (Rodentia, Sciuridae). Med. Mycol. 2015, 53, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cafarchia, C.; Romito, D.; Iatta, R.; Camarda, A.; Montagna, M.T.; Otranto, D. Role of birds of prey as carriers and spreaders of Cryptococcus neoformans and other zoonotic yeasts. Med. Mycol. 2006, 44, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon-Chung, K.J.; Bennett, J.E.; Wickes, B.L.; Meyer, W.; Cuomo, C.A.; Wollenburg, K.R.; Bicanic, T.A.; Castañeda, E.; Chang, Y.C.; Chen, J.; et al. The Case for Adopting the “Species Complex” Nomenclature for the Etiologic Agents of Cryptococcosis. mSphere 2017, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.Á.L.; Wang, Z.A.; Janbon, G.; Idnurm, A.; Bahn, Y.S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2015, 4, a019760. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.E.; Bennett, J.E.; Bailey, J.W. Serologic Grouping of Cryptococcus neoformans. Proc. Soc. Exp. Biol Med. 1968, 127, 820–823. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J. A new genus, filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 1975, 67, 1197–1200. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J. A New Species of Filobasidiella, the Sexual State of Cryptococcus neoformans B and C Serotypes. Mycologia 1976, 68, 942. [Google Scholar] [CrossRef]
- D’Souza, C.; Kronstad, J.; Taylor, G.; Warren, R.; Yuen, M.; Hu, G.; Jung, W.; Sham, A.; Kidd, S.; Tangen, K.; et al. Genome Variation in Cryptococcus gattii, an Emerging Pathogen of. MBio 2011, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kwon-Chung, K.J.; Boekhout, T.; Fell, J.W.; Diaz, M. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon 2002, 51, 804–806. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Varma, A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006, 6, 574–587. [Google Scholar] [CrossRef] [Green Version]
- Meyer, W.; Castañeda, A.; Jackson, S.; Huynh, M.; Castañeda, E.; Arechavala, A.; Davel, G.; Rodero, L.; Perrotta, D.; Lazera, M.; et al. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect. Dis. 2003, 9, 189–195. [Google Scholar] [CrossRef]
- Boekhout, T.; Theelen, B.; Diaz, M.; Fell, J.W.; Hop, W.C.J.; Abeln, E.C.A.; Dromer, F.; Meyer, W. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 2001, 147, 891–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, W.; Aanensen, D.M.; Boekhout, T.; Cogliati, M.; Diaz, M.R.; Esposto, M.C.; Fisher, M.; Gilgado, F.; Hagen, F.; Kaocharoen, S.; et al. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 2009, 47, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Meyer, W. Cryptococcus gattii in the age of whole-genome sequencing. mBio 2015, 6, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuomo, C.A.; Rhodes, J.; Desjardins, C.A. Advances in cryptococcus genomics: Insights into the evolution of pathogenesis. Mem. Inst. Oswaldo Cruz 2018, 113, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bovers, M.; Hagen, F.; Kuramae, E.E.; Boekhout, T. Six monophyletic lineages identified within Cryptococcus neoformans and Cryptococcus gattii by multi-locus sequence typing. Fungal Genet. Biol. 2008, 45, 400–421. [Google Scholar] [CrossRef] [PubMed]
- Ngamskulrungroj, P.; Gilgado, F.; Faganello, J.; Litvintseva, A.P.; Leal, A.L.; Tsui, K.M.; Mitchell, T.G.; Vainstein, M.H.; Meyer, W. Genetic diversity of the Cryptococcus species complex suggests that Cryptococcus gattii deserves to have varieties. PLoS ONE 2009, 4, e5862. [Google Scholar] [CrossRef]
- Hagen, F.; Khayhan, K.; Theelen, B.; Kolecka, A.; Polacheck, I.; Sionov, E.; Falk, R.; Parnmen, S.; Lumbsch, H.T.; Boekhout, T. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, T.G.; Castañeda, E.; Nielsen, K.; Wanke, B.; Lazéra, M.S. Environmental Niches for Cryptococcus neoformans and Cryptococcus gattii. In Cryptococcus: From Human Pathogen to Model Yeast; Heitman, J., Kozel, T., Kwon-Chung, K., Perfect, J., Casadevall, A., Eds.; American Society for Microbiology: Washington, DC, USA, 2011; pp. 237–259. ISBN 9781555816858. [Google Scholar]
- Staib, F. Cryptococcus neoformans in canaries. Zentralbl. Bakteriol. 1962, 185, 129–134. [Google Scholar]
- Casadevall, A.; Freij, J.B.; Hann-Soden, C.; Taylor, J. Continental Drift and Speciation of the Cryptococcus neoformans and Cryptococcus gattii Species Complexes. mSphere 2017, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Engelthaler, D.M.; Meyer, W. Furthering the Continental Drift Speciation Hypothesis in the Pathogenic Cryptococcus Species Complexes. mSphere 2017, 2, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogliati, M.; D’Amicis, R.; Zani, A.; Montagna, M.T.; Caggiano, G.; De Giglio, O.; Balbino, S.; De Donno, A.; Serio, F.; Susever, S.; et al. Environmental distribution of Cryptococcus neoformans and C. gattii around the Mediterranean basin. FEMS Yeast Res. 2016, 16, fow045. [Google Scholar] [CrossRef] [Green Version]
- Mörner, T.; Avenäs, A.; Mattsson, R. Adiaspiromycosis in a European beaver from Sweden. J. Wildl. Dis. 1999, 35, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Camarda, A.; Romito, D.; Campolo, M.; Quaglia, N.C.; Tullio, D.; Otranto, D. Occurrence of yeasts in cloacae of migratory birds. Mycopathologia 2006, 161, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.J.; Mohan, R.; Heitman, J. Plants promote mating and dispersal of the human pathogenic fungus Cryptococcus. PLoS ONE 2017, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Farrer, R.A. Pathogen and Host Genetics Underpinning Cryptococcal Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 105, ISBN 9780128216859. [Google Scholar]
- Chen, S.C.A.; Meyer, W.; Sorrell, T.C. Cryptococcus gattii infections. Clin. Microbiol. Rev. 2014, 27, 980–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, R.; Krockenberger, M.B.; O’Brien, C.R.; Carter, D.A.; Meyer, W.; Canfield, P.J. Veterinary Insights into Cryptococcosis Caused by Cryptococcus neoformans and Cryptococcus gattii. In Cryptococcus: From Human Pathogen to Model Yeast; Heitman, J., Kozel, T., Kwon-Chung, K., Perfect, J., Casadevall, A., Eds.; American Society for Microbiology: Washington, DC, USA, 2011; pp. 489–504. ISBN 9781555816858. [Google Scholar]
- Trivedi, S.R.; Malik, R.; Meyer, W.; Sykes, J.E. Feline cryptococcosis. Impact of current research on clinical management. J. Feline Med. Surg. 2011, 13, 163–172. [Google Scholar] [CrossRef]
- Ma, H.; May, R.C. Virulence in Cryptococcus species. Adv. Appl. Microbiol. 2009, 67, 131–190. [Google Scholar] [CrossRef]
- Casadevall, A.; Steenbergen, J.N.; Nosanchuk, J.D. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi--the Cryptococcus neoformans paradigm. Curr. Opin. Microbiol. 2003, 6, 332–337. [Google Scholar] [CrossRef]
- Casadevall, A.; Fu, M.S.; Guimaraes, A.J.; Albuquerque, P. The ‘Amoeboid Predator-Fungal Animal Virulence’ Hypothesis. J. Fungi 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Castellani, A. Phagocytic and destructive action of Hartmanella castellanii (Amoeba castellanii) on pathogenic encapsulated yeast-like fungi Torulopsis neoformans (Cryptococcus neoformans). Ann. Inst. Pasteur. 1955, 89, 1–7. [Google Scholar]
- Chrisman, C.J.; Albuquerque, P.; Guimaraes, A.J.; Nieves, E.; Casadevall, A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog. 2011, 7, e1002047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dromer, F.; Casadevall, A.; Perfect, J.; Sorrell, T. Cryptococcus neoformans: Latency and Disease. In Cryptococcus: From Human Pathogen to Model Yeast; Heitman, J., Kozel, T., Kwon-Chung, K., Perfect, J., Casadevall, A., Eds.; American Society for Microbiology: Washington, DC, USA, 2011; pp. 431–439. ISBN 9781555816858. [Google Scholar]
- Perfect, J.R.; Casadevall, A. Cryptococcosis. Infect. Dis Clin. N. Am. 2002, 16, 837–874. [Google Scholar] [CrossRef]
- Boyce, K.J.; Andrianopoulos, A. Fungal dimorphism: The switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol. Rev. 2015, 39, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Heitman, J.; Sun, S.; James, T.Y. Evolution of fungal sexual reproduction. Mycologia 2013, 105, 1–27. [Google Scholar] [CrossRef]
- Coelho, C.; Bocca, A.L.; Casadevall, A. The intracellular life of Cryptococcus neoformans. Annu. Rev. Pathol. 2014, 9, 219–238. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, R.; Hsueh, Y.P.; Geunes-Boyer, S.; Wright, J.R.; Heitman, J. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 2009, 77, 4345–4355. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Lin, J.; Fan, Y.; Lin, X. Life cycle of Cryptococcus neoformans. Annu. Rev. Microbiol. 2019, 73, 17–42. [Google Scholar] [CrossRef]
- Ellis, D.H.; Pfeiffer, T.J. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 1990, 336, 923–925. [Google Scholar] [CrossRef]
- Halliday, C.L.; Bui, T.; Krockenberger, M.; Malik, R.; Ellis, D.H.; Carter, D.A. Presence of α and a mating types in environmental and clinical collections of Cryptococcus neoformans var. gattii strains from Australia. J. Clin. Microbiol. 1999, 37, 2920–2926. [Google Scholar] [CrossRef] [Green Version]
- Littman, M.L.; Borok, R. Relation of the pigeon to cryptococcosis: Natural carrier state, heat resistance and survival of Cryptococcus neoformans. Mycopathol. Mycol. Appl. 1968, 35, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Konicek, C.; Vodrážka, P.; Barták, P.; Knotek, Z.; Hess, C.; Račka, K.; Hess, M.; Troxler, S. Detection of zoonotic pathogens in wild birds in the cross-border region Austria–Czech Republic. J. Wildl. Dis. 2016, 52, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.; De Obaldia, A.L.; Heitman, J. Cryptococcus neoformans mates on pigeon guano: Implications for the realized ecological niche and globalization. Eukaryot Cell 2007, 6, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Krockenberger, M.; Stalder, K.; Malik, R.; Canfield, P. Cryptococcosis in Australian Wildlife. Microbiol. Aust. 2005, 26, 69–73. [Google Scholar] [CrossRef]
- Byrnes, E.J., 3rd; Bildfell, R.J.; Dearing, P.L.; Valentine, B.A.; Heitman, J. Cryptococcus gattii with bimorphic colony types in a dog in western Oregon: Additional evidence for expansion of the Vancouver Island outbreak. J. Vet. Diagn. Invest. 2009, 21, 133–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, C. The Emergence of Cryptococcus gattii in British Columbia: Veterinary Aspects. Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, June 2005. [Google Scholar]
- Duncan, C.; Schwantje, H.; Stephen, C.; Campbell, J.; Bartlett, K. Cryptococcus gattii in wildlife of Vancouver Island, British Columbia, Canada. J. Wildl. Dis. 2006, 42, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephen, C.; Lester, S.; Black, W.; Fyfe, M.; Raverty, S. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can. Vet. J. 2002, 43, 792–794. [Google Scholar] [PubMed]
- Farrer, R.A.; Chang, M.; Davis, M.J.; van Dorp, L.; Yang, D.H.; Shea, T.; Sewell, T.R.; Meyer, W.; Balloux, F.; Edwards, H.M.; et al. A new lineage of Cryptococcus gattii (VGV) discovered in the central zambezian miombo woodlands. mBio 2019, 10, e02306-19. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef]
- Scott, L. Hyrax (Procaviidae) and dassie rat (Petromuridae) middens in palaeoenvironmental studies in Africa. In Packrat middens: The Last 40,000 Years of Biotic Change; Jilio, L., Betancourt, T.R., van Devender, T.R., Martin, P.S., Eds.; University of Arizona Press: Tucson, AZ, USA, 1990; pp. 408–427. [Google Scholar]
- Chase, B.M.; Scott, L.; Meadows, M.E.; Gil-Romera, G.; Boom, A.; Carr, A.S.; Reimer, P.J.; Truc, L.; Valsecchi, V.; Quick, L.J. Rock hyrax middens: A palaeoenvironmental archive for southern African drylands. Quat. Sci. Rev. 2012, 56, 107–125. [Google Scholar] [CrossRef]
- Illnait-Zaragozí, M.T.; Hagen, F.; Fernández-Andreu, C.M.; Martínez-Machín, G.F.; Polo-Leal, J.L.; Boekhout, T.; Klaassen, C.H.; Meis, J.F. Reactivation of a Cryptococcus gattii infection in a cheetah (Acinonyx jubatus) held in the National Zoo, Havana, Cuba. Mycoses 2011, 54, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Millward, I.R.; Williams, M.C. Cryptococcus neoformans granuloma in the lung and spinal cord of a free-ranging cheetah (Acinonyx jubatus). A clinical report and literature review. J. S. Afr. Vet. Assoc. 2005, 76, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, L.A.; Lobetti, R.G.; Evezard, D.N.; Picard, J.A.; Nesbit, J.W.; Van Heerden, J.; Burroughs, R.E.J. Cryptococcosis in captive cheetah (Acinonyx jubatus): Two cases. J. S. Afr. Vet. Assoc. 1999, 70, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, W.L.; Jardine, J.E.; Espie, I.W. Pulmonary cryptococcoma and cryptococcal meningoencephalomyelitis in a king cheetah (Acinonyx jubatus). J. Zoo Wildl. Med. 1997, 28, 485–490. [Google Scholar] [PubMed]
- Polo Leal, J.L.; Fernández Andreu, C.M.; Martínez Machín, G.; Illnait Zaragozi, M.T.; Perurena Lancha, M.R. Cyptococcus gattii isolated from a cheetah (Acinonyx jubatus) in the National Zoo of Cuba. Rev. Cubana Med. Trop. 2010, 62, 257–260. [Google Scholar]
- Beehler, B.A. Oral therapy for nasal cryptococcosis in a cheetah. J. Am. Vet. Med. Assoc. 1982, 181, 1400–1401. [Google Scholar] [PubMed]
- Bernstein, J.J. Cryptococcus osteomyelitis in a cheetah. Feline Pract. 1979, 9, 23–25. [Google Scholar]
- Sorrell, T.C.; Brownlee, A.G.; Ruma, P.; Malik, R.; Pfeiffer, T.J.; Ellis, D.H. Natural environmental sources of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 1996, 34, 1261–1263. [Google Scholar] [CrossRef] [Green Version]
- Illnait-Zaragozí, M.T.; Martínez-Machín, G.F.; Fernández-Andreu, C.M.; Boekhout, T.; Meis, J.F.; Klaassen, C.H. Microsatellite typing of clinical and environmental Cryptococcus neoformans var. grubii isolates from Cuba shows multiple genetic lineages. PLoS ONE 2010, 5, e9124. [Google Scholar] [CrossRef]
- Malik, R. Genetic diseases of cats. J. Feline Med. Surg. 2001, 3, 109–113. [Google Scholar] [CrossRef]
- Miller-Edge, M.A.; Worley, M.B. In vitro responses of cheetah mononuclear cells to feline herpesvirus-1 and Cryptococcus neoformans. Vet. Immunol. Immunopathol. 1992, 30, 261–274. [Google Scholar] [CrossRef]
- Kuiken, T.; Höfle, U.; Bennett, P.M.; Allchin, C.R.; Kirkwood, J.K.; Baker, J.R.; Appleby, E.C.; Lockyer, C.H.; Walton, M.J.; Sheldrick, M.C. Adrenocortical hyperplasia, disease and chlorinated hydrocarbons in the harbour porpoise (Phocoena phocoena). Mar. Pollut. Bull. 1993, 26, 440–446. [Google Scholar] [CrossRef]
- McColl, K.A. Pathology in captive platypus (Ornithorhynchus anatinus) in Victoria, Australia. J. Wildl. Dis. 1983, 19, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Munson, L. Diseases of captive cheetahs (Acinonyx jubatus): Results of the cheetah research council pathology survey, 1989–1992. Zoo Biol. 1993, 12, 105–124. [Google Scholar] [CrossRef]
- Rideout, B.A.; Gause, G.E.; Benirschke, K.; Lasley, B.L. Stress-induced adrenal changes and their relation to reproductive failure in captive nine-banded armadillos (Dasypus novemcinctus). Zoo Biol. 1985, 4, 129–137. [Google Scholar] [CrossRef]
- Schmertmann, L.J.; Stalder, K.; Hudson, D.; Martin, P.; Makara, M.; Meyer, W.; Malik, R.; Krockenberger, M.B. Cryptococcosis in the koala (Phascolarctos cinereus): Pathogenesis and treatment in the context of two atypical cases. Med. Mycol. 2018, 56, 926–936. [Google Scholar] [CrossRef]
- Krockenberger, M.B.; Canfield, P.J.; Malik, R. Cryptococcus neoformans var. gattii in the koala (Phascolarctos cinereus): A review of 43 cases of cryptococcosis. Med. Mycol. 2003, 41, 225–234. [Google Scholar] [CrossRef]
- Wynne, J.; Klause, S.; Stadler, C.K.; Pye, G.W.; Meyer, W.; Sykes, J.E. Preshipment testing success: Resolution of a nasal sinus granuloma in a captive koala (Phascolarctos cinereus) caused by Cryptococcus gattii. J. Zoo Wildl. Med. 2012, 43, 939–942. [Google Scholar] [CrossRef]
- Martínez-Nevado, E.; Alonso-Alegre, E.G.; Martínez, M.Á.; Rodríguez-Álvaro, A.; de Merlo, E.M.; García, J.G.; Real, I.G. Atypical presentation of Cryptococcus neoformans in a koala (Phascolarctos cinereus): A magnetic resonance imaging and computed tomography study. J. Zoo Wildl. Med. 2017, 48, 250–254. [Google Scholar] [CrossRef]
- Waugh, C.A.; Hanger, J.; Loader, J.; King, A.; Hobbs, M.; Johnson, R.; Timms, P. Infection with koala retrovirus subgroup B (KoRV-B), but not KoRV-A, is associated with chlamydial disease in free-ranging koalas (Phascolarctos cinereus). Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Krockenberger, M.B. (The University of Sydney, Sydney, Australia). Personal communication, 2020.
- Schmertmann, L.J.; Irinyi, L.; Malik, R.; Powell, J.R.; Meyer, W.; Krockenberger, M.B. The mycobiome of Australian tree hollows in relation to the Cryptococcus gattii and C. neoformans species complexes. Ecol. Evol. 2019, 9, 9684–9700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotstein, D.S.; West, K.; Levine, G.; Lockhart, S.R.; Raverty, S.; Morshed, M.G.; Rowles, T. Cryptococcus gattii VGI in a spinner dolphin (Stenella longirostris) from Hawaii. J. Zoo Wildl. Med. 2010, 41, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Gales, N.; Wallace, G.; Dickson, J. Pulmonary cryptococcosis in a striped dolphin (Stenella coeruleoalba). J. Wildl. Dis. 1985, 21, 443–446. [Google Scholar] [CrossRef]
- Mouton, M.; Reeb, D.; Botha, A.; Best, P. Yeast infection in a beached southern right whale (Eubalaena australis) neonate. J. Wildl. Dis. 2009, 45, 692–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcleland, S.; Duncan, C.; Spraker, T.; Wheeler, E.; Lockhart, S.R.; Gulland, F. Cryptococcus albidus infection in a California sea lion (Zalophus californianus). J. Wildl. Dis. 2012, 48, 1030–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migaki, G.; Gunnels, R.D.; Casey, H.W. Pulmonary cryptococcosis in an Atlantic bottlenosed dolphin (Tursiops truncatus). Lab. Anim. Sci. 1978, 28, 603–606. [Google Scholar]
- Field, C.L.; Tuttle, A.D.; Sidor, I.F.; Nyaoke, A.; Deering, K.M.; Gilbert-Marcheterre, K.; Risatti, G.; Spoon, T.; Meegan, J.; Romano, T.A.; et al. Systemic mycosis in a California sea lion (Zalophus californianus) with detection of cystofilobasidiales DNA. J. Zoo Wildl. Med. 2012, 43, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Man, S.; Balbimie, A.; Mithani, S.; Wong, Q.; Zabek, E.; Raverty, S.; Hoang, L.M. Molecular Characteristics of Cryptococcus isolates from Marine Mammals stranded along the Pacific Northwest Coast. In Proceedings of the Infectious Diseases Society of America (IDSA) 48th Annual Meeting, Vancouver, BC, Canada, 21–24 October 2010. [Google Scholar]
- Miller, W.G.; Padhye, A.A.; Van Bonn, W.; Jensen, E.; Brandt, M.E.; Ridgway, S.H. Cryptococcosis in a bottlenose dolphin (Tursiops truncatus) caused by Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 2002, 40, 721–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, J.F.; Haulena, M.; Hoang, L.M.N.; Morshed, M.; Zabek, E.; Raverty, S.A. Cryptococcus gattii type VGIIa infection in harbor seals (Phoca vitulina) in British Columbia, Canada. J. Wildl. Dis. 2016, 52, 677–681. [Google Scholar] [CrossRef]
- Norman, S.A.; Raverty, S.; Zabek, E.; Etheridge, S.; Ford, J.K.; Hoang, L.M.; Morshed, M. Maternal–Fetal Transmission of Cryptococcus gattii in Harbor Porpoise. Emerg. Infect. Dis. 2011, 17, 304–305. [Google Scholar] [CrossRef]
- Huckabone, S.E.; Gulland, F.M.D.; Johnson, S.M.; Colegrove, K.M.; Dodd, E.M.; Pappagianis, D.; Dunkin, R.C.; Casper, D.; Carlson, E.L.; Sykes, J.E.; et al. Coccidioidomycosis and other systemic mycoses of marine mammals stranding along the central California, USA coast: 1998–2012. J. Wildl. Dis 2015, 51, 295–308. [Google Scholar] [CrossRef]
- Reidarson, T.H.; McBain, J.F.; Dalton, J.M.; Rinaldi, M.G. Marine Mammal Mycoses. In CRC Handbook of Marine Mammal Medicine; Dierauf, L.A., Gulland, F., Eds.; CRC Press: New York, NY, USA, 2001; pp. 337–356. ISBN 9781498796873. [Google Scholar]
- Overy, D.P.; McBurney, S.; Muckle, A.; Lund, L.; Lewis, P.J.; Strang, R. Cryptococcus gattii VGIIb-like variant in white-tailed deer, Nova Scotia, Canada. Emerg. Infect. Dis. 2016, 22, 1131–1133. [Google Scholar] [CrossRef] [Green Version]
- Hamir, A.N.; Miller, J.M.; Sonn, R.J. Meningoencephalitis and pneumonia associated with Cryptococcus neoformans infection in a free-ranging elk in the USA. Vet. Rec. 2002, 151, 332–333. [Google Scholar] [CrossRef]
- Olsson, M.P.O.; Cox, J.; Larkin, J.L.; Maehr, D.S.; Widen, P.; Wichrowski, M.W. Movement and Activity Patterns of Translocated Elk (Cervus elaphus Nelsoni) on an Active Coal Mine in Kentucky. Wildl. Biol. Pract. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Morera, N.; Hagen, F.; Juan-Sallés, C.; Artigas, C.; Patricio, R.; Serra, J.I.; Colom, M.F. Ferrets as sentinels of the presence of pathogenic Cryptococcus species in the Mediterranean environment. Mycopathologia. 2014, 178, 145–151. [Google Scholar] [CrossRef]
- Headley, S.A.; Pimentel, L.A.; Michelazzo, M.Z.; Toma, H.S.; Pretto-Giordano, L.G.; Marcasso, R.A.; Amude, A.M.; Oliveira, T.E.; Santos, M.D.; Krockenberger, M. Pathologic, histochemical, and immunohistochemical findings in pulmonary and encephalitic cryptococcosis in a goat. J. Vet. Diagn. Invest. 2019, 31, 69–73. [Google Scholar] [CrossRef]
- Baró, T.; Torres-Rodríguez, J.M.; De Mendoza, M.H.; Morera, Y.; Alía, C. First identification of autochthonous Cryptococcus neoformans var. gattii isolated from goats with predominantly severe pulmonary disease in Spain. J. Clin. Microbiol. 1998, 36, 458–461. [Google Scholar] [CrossRef] [Green Version]
- Maestrale, C.; Masia, M.; Pintus, D.; Lollai, S.; Kozel, T.R.; Gates-Hollingsworth, M.A.; Cancedda, M.G.; Cabras, P.; Pirino, S.; D’Ascenzo, V.; et al. Genetic and pathological characteristics of Cryptococcus gattii and Cryptococcus neoformans var. neoformans from meningoencephalitis in autochthonous goats and mouflons, Sardinia, Italy. Vet. Microbiol. 2015, 177, 409–413. [Google Scholar] [CrossRef]
- Rosa, F.B.; Rubin, M.; Olinda, R.G.; Leal, P.; Lima, S.; Pupin, R.C.; Gomes, D.; Lemos, R.; Martins, T.B.; Rodrigues-Hoffmann, A.; et al. Granulomatous leptomeningitis in a goat associated with infection by Cryptococcus neoformans. Braz. J. Vet. Pathol. 2016, 9, 98–102. [Google Scholar]
- Makimura, K.; Karasawa, M.; Hosoi, H.; Kobayashi, T.; Kamijo, N.; Kobayashi, K.; Hiramatsu, H.; Akikawa, T.; Yabe, T.; Yamaguchi, A.; et al. A Queensland koala kept in a Japanese zoological park was carrier of an imported fungal pathogen, Filobasidiella neoformans var. bacillispora (Cryptococcus neoformans var. gattii). Jpn. J. Infect. Dis. 2002, 55, 31–32. [Google Scholar]
- Colom, M.F.; Hagen, F.; Gonzalez, A.; Mellado, A.; Morera, N.; Linares, C.; García, D.F.; Peñataro, J.S.; Boekhout, T.; Sánchez, M. Ceratonia siliqua (carob) trees as natural habitat and source of infection by Cryptococcus gattii in the Mediterranean environment. Med. Mycol. 2012, 50, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Silva-Pando, F.J.; Pino-Pérez, R. Introduction of Eucalyptus into Europe. Aust For. 2016, 79, 283–291. [Google Scholar] [CrossRef]
- Malik, R.; Krockenberger, M.B.; Cross, G.; Doneley, R.; Madill, D.N.; Black, D.; Mcwhirter, P.; Rozenwax, A.; Rose, K.; Alley, M.; et al. Avian cryptococcosis. Med. Mycol. 2003, 41, 115–124. [Google Scholar] [CrossRef]
- Secombe, C.J.; Lester, G.D.; Krockenberger, M.B. Equine Pulmonary Cryptococcosis: A Comparative Literature Review and Evaluation of Fluconazole Monotherapy. Mycopathologia 2017, 182, 413–423. [Google Scholar] [CrossRef]
- Danesi, P.; Falcaro, C.; Dukik, K.; Jiang, Y.; Rizzoli, A.P.; Allavena, R.; Simpson, V.; Ravagnan, S.; Zanardello, C.; Capelli, G.; et al. Molecular Diagnosis of Emmonsia-Like Fungi Occurring in Wild Animals. Mycopathologia 2020, 185, 51–65. [Google Scholar] [CrossRef]
- McGill, S.; Malik, R.; Saul, N.; Beetson, S.; Secombe, C.; Robertson, I.; Irwin, P. Cryptococcosis in domestic animals in Western Australia: A retrospective study from 1995–2006. Med. Mycol. 2009, 47, 625–639. [Google Scholar] [CrossRef] [Green Version]
- Engelthaler, D.M.; Casadevall, A. On the emergence of Cryptococcus gattii in the pacific northwest: Ballast Tanks, Tsunamis, and black swans. mBio 2019, 10, e02193-19. [Google Scholar] [CrossRef] [Green Version]
- Kido, N.; Makimura, K.; Kamegaya, C.; Shindo, I.; Shibata, E.; Omiya, T.; Yamamoto, Y. Long-term surveillance and treatment of subclinical cryptococcosis and nasal colonization by Cryptococcus neoformans and C. gattii species complex in captive koalas (Phascolarctes cinereus). Med. Mycol. 2012, 50, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.F.; Tansie, S.M.; Shahan, J.R.; Seipelt-Thiemann, R.L.; McClelland, E.E. Serial Passage of Cryptococcus neoformans in Galleria mellonella results in increased capsule and intracellular replication in hemocytes, but not increased resistance to hydrogen peroxide. Pathogens 2020, 9, 732. [Google Scholar] [CrossRef]
- Rabinowitz, P.M.; Kock, R.; Kachani, M.; Kunkel, R.; Thomas, J.; Gilbert, J.; Wallace, R.; Blackmore, C.; Wong, D.; Karesh, W.; et al. Toward proof of concept of a one health approach to disease prediction and control. Emerg. Infect. Dis. 2013, 19, e130265. [Google Scholar] [CrossRef]
- Canfield, P.J.; Krockenberger, M.B.; Higgins, D.P. Koalas. In Proceedings of the Wildlife Pathology Short Course, Taronga Zoo, Sydney, Australia, 21–24 August 2008. [Google Scholar]



| Case | Diagnosis | Species/Variety | Outcome (Treatment, Necropsy) | Location | Date | References |
|---|---|---|---|---|---|---|
| 1 | Pulmonary cryptococcoma and meningomyelitis (spinal cord) | Cryptococcus gattii | Necropsy | South Africa | 2005 | [58] |
| 2 | Pulmonary cryptococcoma and cryptococcal meningoencephalomyelitis | Cryptococcus neoformans | Necropsy | South Africa | 1997 | [60] |
| 3 | Pulmonary cryptococcomas and extensive meningoencephalomyelitis | Cryptococcus neoformans | Necropsy | South Africa | 1999 | [59] |
| 4 | Pulmonary cryptococcomas and extensive meningoencephalomyelitis | Cryptococcus gattii | Necropsy | South Africa | 1999 | [59] |
| 5 | Shortness of breath and nasal discharge | Cryptococcus gattii | The animal was taken to the veterinary clinic for sampling and died before any diagnosis could be made | Cuba | 2010 | [61] |
| 6 | Shortness of breath and nasal discharge | Cryptococcus gattii AFLP4/VGI | Cuba | 2011 | [57] | |
| 7 | Nasal cryptococcosis | Abstract not available | USA | 1982 | [62] | |
| 8 | Osteomyelitis | Abstract not available | [63] |
| Case | Mammal Species | Diagnosis | Species/Variety | Outcome (Treatment, Necropsy) | Location | Date | References |
|---|---|---|---|---|---|---|---|
| 1 | Whale (Eubalaena australis) neonate | Skin lesion culture positive for Candida zeylanoides | Cryptococcus neoformans | Necropsy | South Africa | 2009 | [81] |
| 2 | Spinner dolphin (Stenella longirostris) | Skin, lymph nodes and several organs (stomach, adrenal gland, kidney and spleen) | Cryptococcus gattii—VGI | Necropsy | Hawaii | 2010 | [79] |
| 3 | Striped Dolphin (Stenella coeruleoalba) | Pulmonary cryptococcosis (lung and mediastinal lymph gland) and gastric mucosa. Stomach with moderate number of nematodes (Anisakis simplex) | Cryptococcus spp. | Necropsy | Western Australia | 1985 | [80] |
| 4 | Bottlenose dolphin (Tursiops truncatus) | Pulmonary Cryptococcosis Bronchopneumonia with pleuritis | Cryptococcus gattii | Necropsy Itraconazole therapy | California, USA | 2000 | [86] |
| 5 | Atlantic bottlenosed dolphin (Tursiops truncatus) | Pulmonary cryptococcosis | Cryptococcus spp. | Necropsy | Not reported | 1978 | [83] |
| 6 | Dall’s porpoise (Phocoenoides dalli) | Pulmonary cryptococcosis with generalized lymphadenopathy | Cryptococcus gattii | Necropsy | Vancouver Island, Canada | 2000–2001 | [52] |
| 7 | Dall’s porpoise (Phocoenoides dalli) | Pulmonary cryptococcosis with generalized lymphadenopathy | Cryptococcus gattii | Necropsy | Vancouver Island, Canada | 2000–2001 | [52] |
| 8 | Dall’s porpoise (Phocoenoides dalli) | Pulmonary cryptococcosis with generalized lymphadenopathy | Cryptococcus neoformans | Necropsy | Vancouver Island, Canada | 2000–2001 | [52] |
| 9 | Dall’s porpoise (Phocoenoides dalli) | Pulmonary cryptococcosis with generalized lymphadenopathy | Cryptococcus neoformans | Necropsy | Vancouver Island, Canada | 2000–2001 | [52] |
| 10 | Dall’s porpoise (Phocoenoides dalli) | Pulmonary cryptococcosis with generalized lymphadenopathy | Cryptococcus neoformans | Necropsy | Vancouver Island, Canada | 2000–2001 | [52] |
| 11 | Harbor porpoise (Phocoena phocoena) | Pulmonary cryptococcosis with generalized lymphadenopathy | Cryptococcus neoformans | Necropsy | Vancouver Island, Canada | 2000–2001 | [52] |
| 12 | Adult and foetus Harbor porpoise (Phocoena phocoena) | Maternal–Foetal transmission of Cryptococcus | Cryptococcus gattii | Necropsy | USA | 2007 | [88] |
| 13 | Porpoise | Not reported | Cryptococcus gattii—VGI | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 14 | Porpoise | Not reported | Cryptococcus gattii—VGIIa | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 15 | Porpoise | Not reported | Cryptococcus gattii—VGIIa | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 16 | Porpoise | Not reported | Cryptococcus gattii—VGIIa | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 17 | Dall’s porpoise (Phocoenoides dalli) | Pyogranulomatous pneumonia and lymphadenitis | Cryptococcus gattii | Necropsy | California, USA | 2014 | [89] |
| 18 | California sea lion (Zalophus californianus) | Systemic mycosis (skin lesions, dermal nodules, severe lymphadenopathy) | Cryptococcus spp. | Necropsy—combination of antibiotic (enrofloxacin, amoxicillin trihydrate-clavulanate potassium), low-dose steroid (prednisone, furosemide), anti-inflammatory (carprofren), oral diphenhydramine, itraconazole and voriconazole. Growth of Escherichia coli. | USA | 2012 | [84] |
| 19 | Juvenile Californian sea lion (Zalophus californianus) | Pneumonia with concurrent fungal and bacterial infection | Cryptococcus albidus | Necropsy | California, USA | 2009 | [82] |
| 20 | Young (3 weeks) harbor seal (Phoca vitulina) | Systemic cryptococcosis: generalized lymphadenopathy, bronchopneumonia, meningoencephalitis, fungemia | Cryptococcus gattii—VGIIa | Necropsy | Vancouver Island, Canada | 2016 | [87] |
| 21 | Dolphin | Not reported | Cryptococcus gattii—VGI | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 22 | Dolphin | Not reported | Cryptococcus gattii—VGI | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 23 | Dolphin | Not reported | Cryptococcus gattii—VGI | Culture. Multi-Locus Sequence Typing (MLST) | Pacific Northwest Coast, Canada | 2010 | [85] |
| 24 | 22× living harbor seals (Phoca vitulina), juvenile | Not reported | Nasal swabs (19×) and lung culture (3×) negative for Cryptococcus spp. | Culture | Vancouver Island, Canada | Between February and August 2004 | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danesi, P.; Falcaro, C.; Schmertmann, L.J.; de Miranda, L.H.M.; Krockenberger, M.; Malik, R. Cryptococcus in Wildlife and Free-Living Mammals. J. Fungi 2021, 7, 29. https://doi.org/10.3390/jof7010029
Danesi P, Falcaro C, Schmertmann LJ, de Miranda LHM, Krockenberger M, Malik R. Cryptococcus in Wildlife and Free-Living Mammals. Journal of Fungi. 2021; 7(1):29. https://doi.org/10.3390/jof7010029
Chicago/Turabian StyleDanesi, Patrizia, Christian Falcaro, Laura J. Schmertmann, Luisa Helena Monteiro de Miranda, Mark Krockenberger, and Richard Malik. 2021. "Cryptococcus in Wildlife and Free-Living Mammals" Journal of Fungi 7, no. 1: 29. https://doi.org/10.3390/jof7010029
APA StyleDanesi, P., Falcaro, C., Schmertmann, L. J., de Miranda, L. H. M., Krockenberger, M., & Malik, R. (2021). Cryptococcus in Wildlife and Free-Living Mammals. Journal of Fungi, 7(1), 29. https://doi.org/10.3390/jof7010029






