Long-Term Nitrogen Deposition Alters Ectomycorrhizal Community Composition and Function in a Poplar Plantation
Abstract
:1. Introduction
- (1)
- In poplar plantations, the long-term addition of N alters the EMF colonization rate, diversity, and community structure;
- (2)
- Variable soil properties (e.g., nutrient availability and C flow) induced by N inputs contribute to variations in EMF communities;
- (3)
- Differences in strategies and capacities to take up N between EMF give rise to various functional EMF community structure responses to N additions.
2. Materials and Methods
2.1. Site Description and Experimental Set Up
2.2. Sample Collection
2.3. Soil and Root Chemistry Analysis
2.4. Morphotyping
2.5. rDNA-ITS Region Sequencing
2.6. Data Analysis
3. Results
3.1. Effect of N Addition on Soil Properties
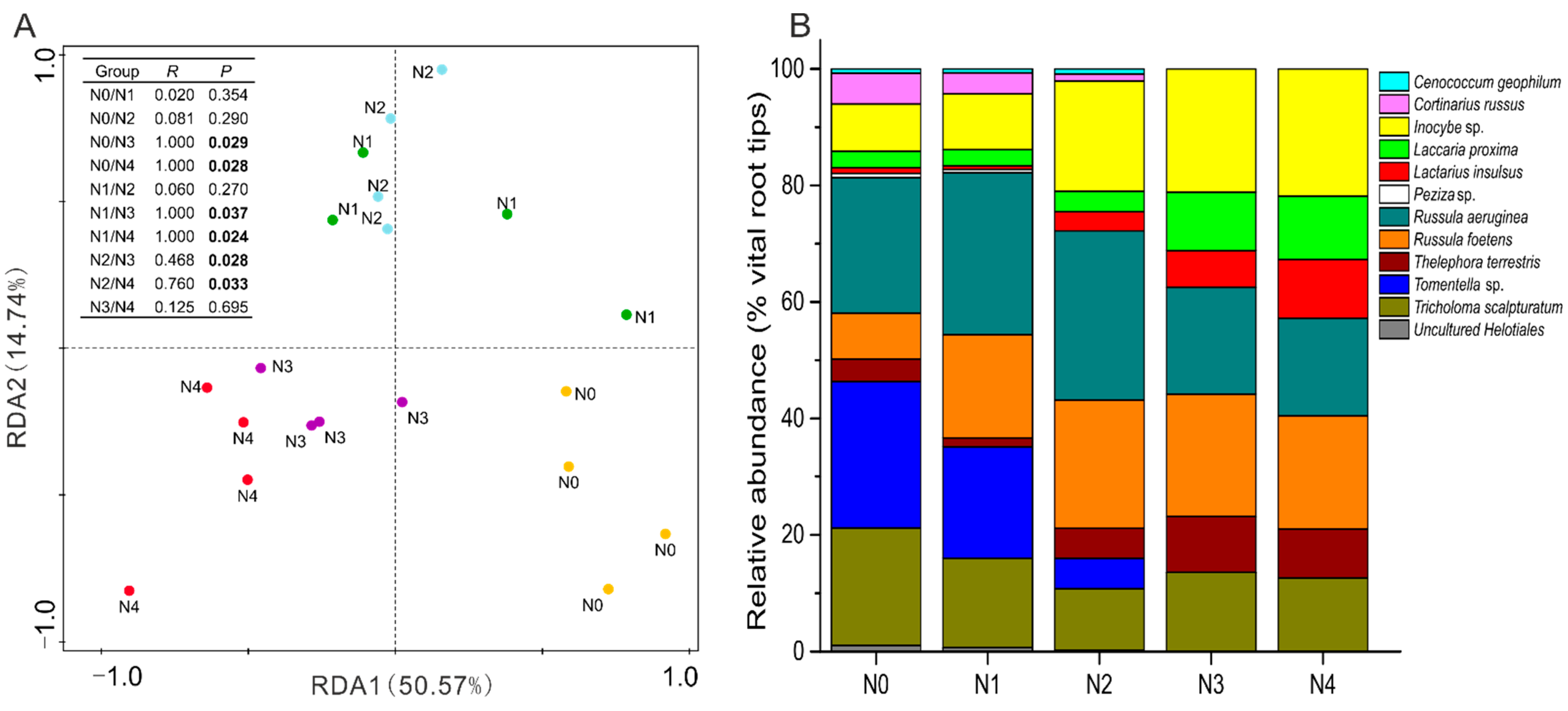
3.2. Ectomycorrhizal Fungal Diversity under Variation in Soil N Addition
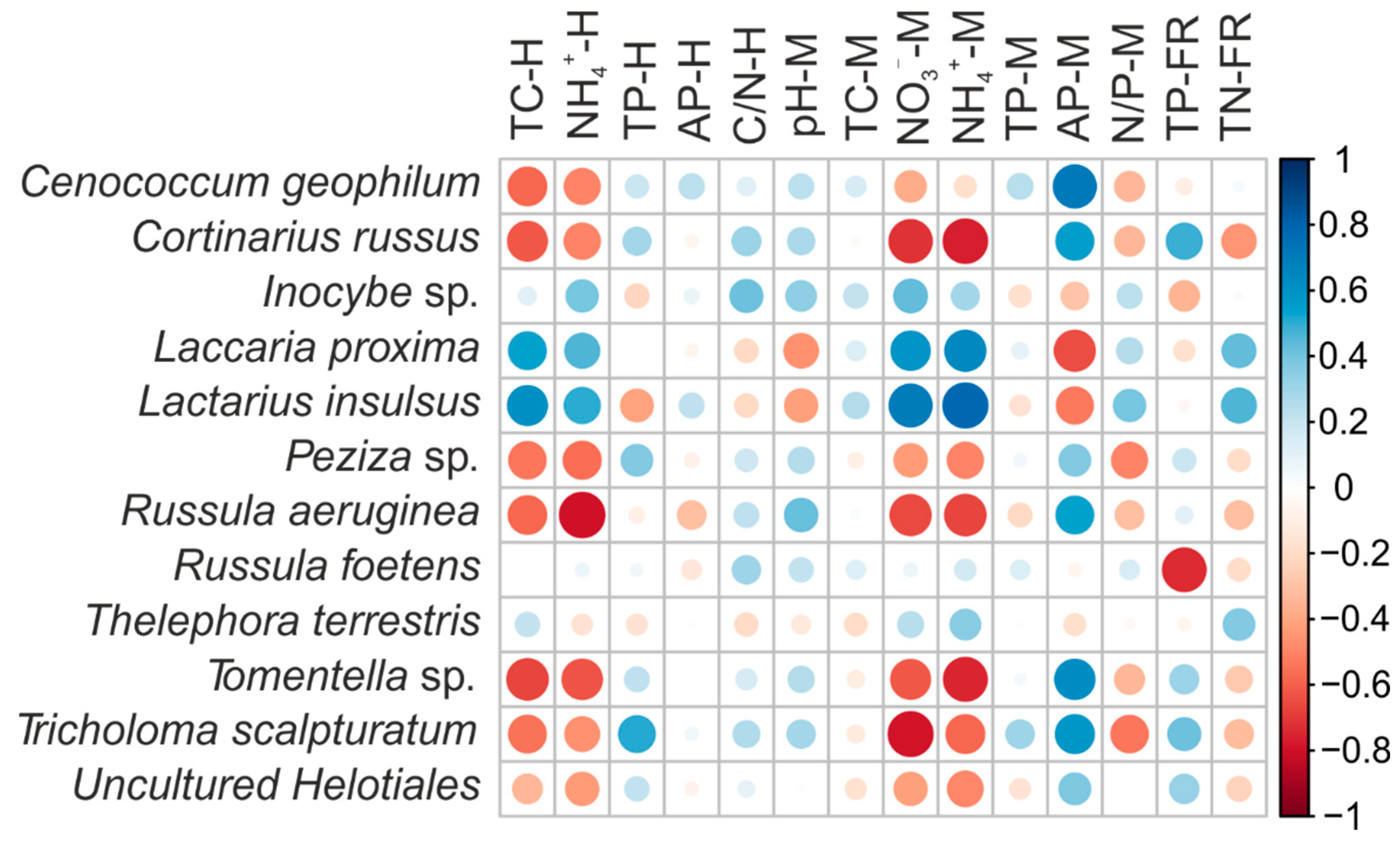
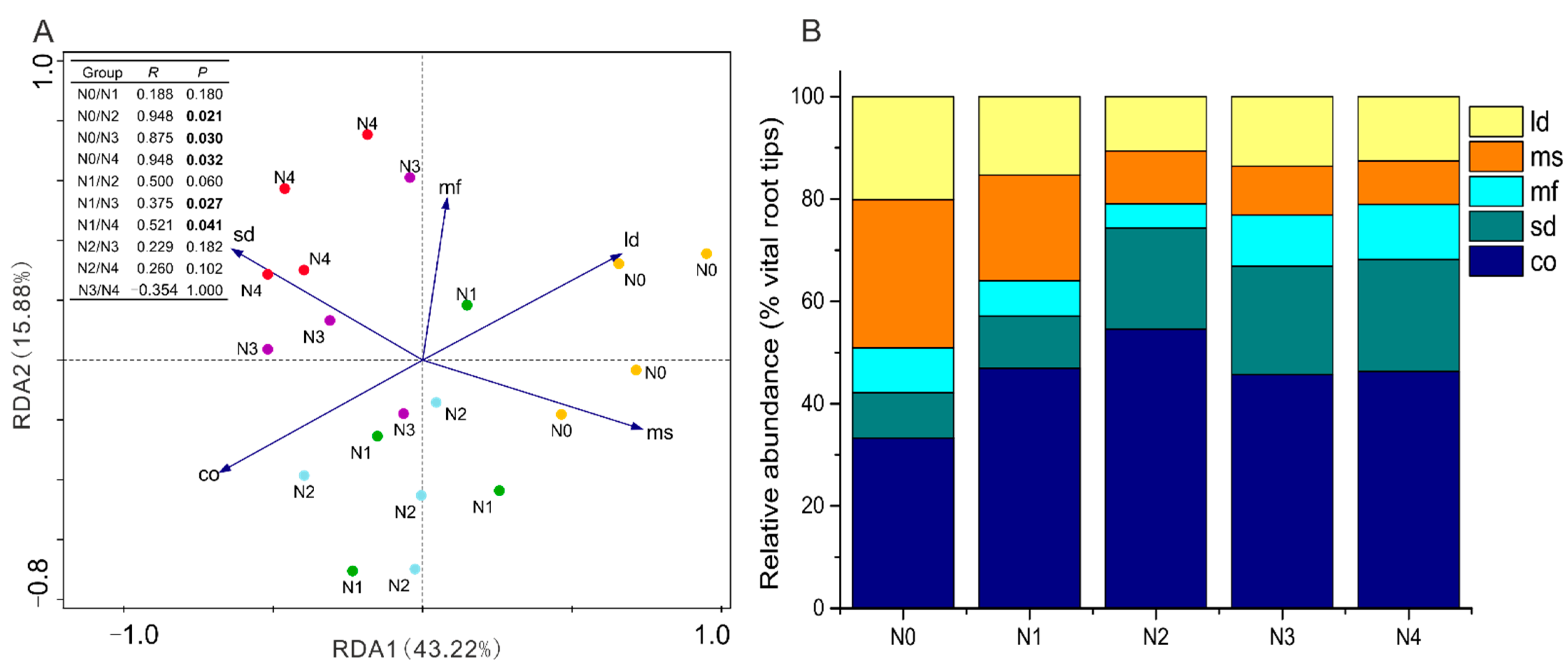
3.3. Ectomycorrhizal Fungal Community Structure in Relation to Soil Chemistry
3.4. Variation of EMF Functional Traits across Different Soil N Additions
4. Discussion
4.1. Changed Soil Properties under N Addition
4.2. N Addition Changed the Abundance and Diversity of Ectomycorrhizal Fungi
4.3. Alterations of EMF Community Structure and Function Traits under N Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.I.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an ecological understanding of biological nitrogen fixation. In The Nitrogen Cycle at Regional to Global Scales; Springer: Dordrecht, The Nederlands, 2002; Volume 57, pp. 1–45. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Du, E.; Jiang, L.; Ma, S.; Zeng, W.; Zou, A.; Feng, C.; Xu, L.; Xing, A.; Wang, W.; et al. Responses of forest ecosystems to increasing N deposition in China: A critical review. Environ. Pollut. 2018, 243, 75–86. [Google Scholar] [CrossRef]
- Du, E.; Jiang, Y.; Fang, J.; de Vries, W. Inorganic nitrogen deposition in China’s forests: Status and characteristics. Atmos. Environ. 2014, 98, 474–482. [Google Scholar] [CrossRef]
- Aber, J.D.; Magill, A.H. Chronic nitrogen additions at the Harvard forest (USA): The first 15 years of a nitrogen saturation experiment. For. Ecol. Manag. 2004, 196, 1–5. [Google Scholar] [CrossRef]
- Liu, X.; Duan, L.; Mo, J.; Du, E.; Shen, J.; Lu, X.; Zhang, Y.; Zhou, X.; He, C.; Zhang, F. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef]
- Shantz, A.A.; Lemoine, N.P.; Burkepile, D.E. Nutrient loading alters the performance of key nutrient exchange mutualisms. Ecol. Lett. 2016, 19, 20–28. [Google Scholar] [CrossRef]
- Bahr, A.; Ellstrom, M.; Bergh, J.; Wallander, H. Nitrogen leaching and ectomycorrhizal nitrogen retention capacity in a Norway spruce forest fertilized with nitrogen and phosphorus. Plant Soil 2015, 390, 323–335. [Google Scholar] [CrossRef]
- Cairney, J.W.G. Ectomycorrhizal fungi: The symbiotic route to the root for phosphorus in forest soils. Plant Soil 2011, 344, 51–71. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Pena, R.; Tejedor, J.; Zeller, B.; Dannenmann, M.; Polle, A. Interspecific temporal and spatial differences in the acquisition of litter-derived nitrogen by ectomycorrhizal fungal assemblages. New Phytol. 2013, 199, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.; Aerts, R.; Cerabolini, B.; Werger, M.; van der Heijden, M. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 2001, 129, 611–619. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants: Tansley Review. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Courty, P.-E.; Pouysegur, R.; Buée, M.; Garbaye, J. Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol. Biochem. 2006, 38, 1219–1222. [Google Scholar] [CrossRef]
- Pena, R.; Lang, C.; Naumann, A.; Polle, A. Ectomycorrhizal identification in environmental samples of tree roots by Fourier-transform infrared (FTIR) spectroscopy. Front. Plant Sci. 2014, 5, 229. [Google Scholar] [CrossRef] [Green Version]
- Pena, R.; Polle, A. Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J. 2014, 8, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotter, P.; Loreau, M.; de Mazancourt, C. Why do forests respond differently to nitrogen deposition? A modelling approach. Ecol. Model. 2020, 425, 109034. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales: Tansley Review. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Johnson, D.; Gilbert, L. Interplant signalling through hyphal networks. New Phytol. 2015, 205, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Avis, P.G. Ectomycorrhizal iconoclasts: The ITS rDNA diversity and nitrophilic tendencies of fetid Russula. Mycologia 2012, 104, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Avis, P.G.; McLaughlin, D.J.; Dentinger, B.C.; Reich, P.B. Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytol. 2003, 160, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baum, C.; Weih, M.; Verwijst, T.; Makeschin, F. The effects of nitrogen fertilization and soil properties on mycorrhizal formation of Salix viminalis. For. Ecol. Manag. 2002, 160, 35–43. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Hicks, L.C.; Ccahuana, A.J.Q.; Salinas, N.; Bååth, E.; Meir, P. Nutrient limitations to bacterial and fungal growth during cellulose decomposition in tropical forest soils. Biol. Fertil. Soils 2018, 54, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Lilleskov, E.A.; Kuyper, T.W.; Bidartondo, M.I.; Hobbie, E.A. Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: A review. Environ. Pollut. 2019, 246, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Van der Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi, E.; Atkinson, B.; Benham, S.; Carroll, C.; Cools, N.; et al. Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 2018, 558, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Jiang, L.; Ma, S.; Fang, W.; Schmid, B.; Xu, L.; Zhu, J.; Li, P.; Losapio, G.; Jing, X.; et al. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China. Sci. Total. Environ. 2017, 607-608, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.P.; Rosenstock, N.P.; Forsmark, B.; Bergh, J.; Wallander, H. Ectomycorrhizal community composition and function in a spruce forest transitioning between nitrogen and phosphorus limitation. Fungal Ecol. 2019, 40, 20–31. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Metcalfe, D.B.; Inselsbacher, E.; Stangl, Z.; Oren, R.; Näsholm, T.; Högberg, P. Greater carbon allocation to mycorrhizal fungi reduces tree nitrogen uptake in a boreal forest. Ecology 2015, 97, 1012–1022. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trowbridge, J.; Mandyam, K.; Johnson, L. Nitrogen enrichment causes minimal changes in arbuscular mycorrhizal colonization but shifts community composition—Evidence from rDNA data. Biol. Fertil. Soils 2005, 41, 217–224. [Google Scholar] [CrossRef]
- Lilleskov, E.A.; Bruns, T.D. Root colonization dynamics of two ectomycorrhizal fungi of contrasting life history strategies are mediated by addition of organic nutrient patches. New Phytol. 2003, 159, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.-Z. Silviculture of poplar plantation in China: A review. Chin. J. Appl. Ecol. 2008, 19, 2308–2316. [Google Scholar] [CrossRef]
- Vet, R.; Artz, R.S.; Carou, S.; Shaw, M.; Ro, C.-U.; Aas, W.; Baker, A.; Bowersox, V.C.; Dentener, F.; Galy-Lacaux, C.; et al. A global assessment of precipitation chemistry and deposition of sulfur, nitrogen, sea salt, base cations, organic acids, acidity and pH, and phosphorus. Atmos. Environ. 2014, 93, 3–100. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In SSSA Book Series; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 775–833. ISBN 978-0-89118-865-0. [Google Scholar]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Yang, N.; Butenschoen, O.; Rana, R.; Köhler, L.; Hertel, D.; Leuschner, C.; Scheu, S.; Polle, A.; Pena, R. Leaf litter species identity influences biochemical composition of ectomycorrhizal fungi. Mycorrhiza 2019, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, W. Decomposing the process of species accumulation into area dependent and time dependent parts. Ecol. Res. 2006, 21, 578–585. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Grömping, U. Estimators of relative importance in linear regression based on variance decomposition. Am. Stat. 2007, 61, 139–147. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer Series in Statistics; Springer International Publishing: Cham, Germany, 2015; ISBN 978-3-319-19424-0. [Google Scholar]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- O’Connor, R.J. Multivariate analysis of ecological communities. Trends Ecol. Evol. 1988, 3, 121. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Bai, X.; Grace, J.B.; Bai, Y. Evidence that acidification-induced declines in plant diversity and productivity are mediated by changes in below-ground communities and soil properties in a semi-arid steppe. J. Ecol. 2013, 101, 1322–1334. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, T.; Cheng, Z.; Zhao, B.; Mulder, J.; Larssen, T.; Wang, S.; Duan, L. Is surface water acidification a serious regional issue in China? Sci. Total. Environ. 2017, 584–585, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Chang. Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Deng, M.; Liu, L.; Sun, Z.; Piao, S.; Ma, Y.; Chen, Y.; Wang, J.; Qiao, C.; Wang, X.; Li, P. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperate Larix principis-rupprechtii plantations. New Phytol. 2016, 212, 1019–1029. [Google Scholar] [CrossRef] [Green Version]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 2009, 153, 231–240. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 2010, 13, 819–828. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; He, H.; Zhao, X.; Datta, A.; Ma, W.; Zhang, Y.; Liu, X.; Han, W.; Wilson, M.C.; et al. Long-term changes in soil pH across major forest ecosystems in China: Soil pH dynamics in forests. Geophys. Res. Lett. 2015, 42, 933–940. [Google Scholar] [CrossRef]
- Börjesson, G.; Menichetti, L.; Kirchmann, H.; Kätterer, T. Soil microbial community structure affected by 53 years of nitrogen fertilisation and different organic amendments. Biol. Fertil. Soils 2012, 48, 245–257. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123705266. [Google Scholar]
- Wolf, K.; Veldkamp, E.; Homeier, J.; Martinson, G.O. Nitrogen availability links forest productivity, soil nitrous oxide and nitric oxide fluxes of a tropical montane forest in southern Ecuador. Glob. Biogeochem. Cycles 2011, 25, GB4009. [Google Scholar] [CrossRef]
- Cline, L.C.; Huggins, J.A.; Hobbie, S.E.; Kennedy, P.G. Organic nitrogen addition suppresses fungal richness and alters community composition in temperate forest soils. Soil Biol. Biochem. 2018, 125, 222–230. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 2013, 363, 411–419. [Google Scholar] [CrossRef]
- Wyatt, G.A.K.; Kiers, T.; Gardner, A.; West, S. A biological market analysis of the plant-mycorrhizal symbiosis: Mycorrhizal symbiosis as a biological market. Evolution 2014, 68, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Wallander, H.; Johansson, U.; Sterkenburg, E.; Durling, M.B.; Lindahl, B.D. Production of ectomycorrhizal mycelium peaks during canopy closure in Norway spruce forests. New Phytol. 2010, 187, 1124–1134. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Kårén, O.; Nylund, J.-E. Effects of ammonium sulphate on the community structure and biomass of ectomycorrhizal fungi in a Norway spruce stand in southwestern Sweden. Can. J. Bot. 1997, 75, 1628–1642. [Google Scholar] [CrossRef]
- Corrêa, A.; Strasser, R.J.; Martins-Loução, M.A. Response of plants to ectomycorrhizae in N-limited conditions: Which factors determine its variation? Mycorrhiza 2008, 18, 413–427. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.-S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus carbon drives allocation and plant–soil interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Näsholm, T.; Högberg, P.; Franklin, O.; Metcalfe, D.; Keel, S.G.; Campbell, C.; Hurry, V.; Linder, S.; Högberg, M.N. Are ectomycorrhizal fungi alleviating or aggravating nitrogen limitation of tree growth in boreal forests? New Phytol. 2013, 198, 214–221. [Google Scholar] [CrossRef]
- Konvalinková, T.; Püschel, D.; Řezáčová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi-potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447. [Google Scholar] [CrossRef]
- Rineau, F.; Garbaye, J. Does forest liming impact the enzymatic profiles of ectomycorrhizal communities through specialized fungal symbionts? Mycorrhiza 2005, 19, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Bödeker, I.T.M.; Clemmensen, K.; de Boer, W.; Martin, F.; Olson, Å.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The plant cell wall-decomposing machinery underlies the functional diversity of forest fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delavaux, C.S.; Camenzind, T.; Homeier, J.; Jiménez-Paz, R.; Ashton, M.; Queenborough, S.A. Nutrient enrichment effects on mycorrhizal fungi in an Andean tropical montane Forest. Mycorrhiza 2017, 27, 311–319. [Google Scholar] [CrossRef]
- Zavišić, A.; Nassal, P.; Yang, N.; Heuck, C.; Spohn, M.; Marhan, S.; Pena, R.; Kandeler, E.; Polle, A. Phosphorus availabilities in beech (Fagus sylvatica L.) forests impose habitat filtering on ectomycorrhizal communities and impact tree nutrition. Soil Biol. Biochem. 2016, 98, 127–137. [Google Scholar] [CrossRef]
- Cox, F.; Barsoum, N.; Lilleskov, E.A.; Bidartondo, M.I. Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients: Effects of nitrogen on mycorrhizas. Ecol. Lett. 2010, 13, 1103–1113. [Google Scholar] [CrossRef]
- Kranabetter, J.M.; Durall, D.M.; MacKenzie, W.H. Diversity and species distribution of ectomycorrhizal fungi along productivity gradients of a southern boreal forest. Mycorrhiza 2009, 19, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Ayer, F.; Egli, S. Nitrogen addition in a Norway spruce stand altered macromycete sporocarp production and below-ground ectomycorrhizal species composition. New Phytol. 2001, 149, 311–325. [Google Scholar] [CrossRef]
- Suz, L.M.; Kallow, S.; Reed, K.; Bidartondo, M.I.; Barsoum, N. Pine mycorrhizal communities in pure and mixed pine-oak forests: Abiotic environment trumps neighboring oak host effects. For. Ecol. Manag. 2017, 406, 370–380. [Google Scholar] [CrossRef]
- Suz, L.M.; Barsoum, N.; Benham, S.; Dietrich, H.-P.; Fetzer, K.D.; Fischer, R.; García, P.; Gehrman, J.; Kristöfel, F.; Manninger, M.; et al. Environmental drivers of ectomycorrhizal communities in Europe’s temperate oak forests. Mol. Ecol. 2014, 23, 5628–5644. [Google Scholar] [CrossRef]
- Dong, L.; Berg, B.; Sun, T.; Wang, Z.; Han, X. Response of fine root decomposition to different forms of N deposition in a temperate grassland. Soil Biol. Biochem. 2020, 147, 107845. [Google Scholar] [CrossRef]
- Toljander, J.F.; Eberhardt, U.; Toljander, Y.K.; Paul, L.R.; Taylor, A.F.S. Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol. 2006, 170, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The mycorrhizal-associated nutrient economy: A new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]
- Clausing, S.; Pena, R.; Song, B.; Müller, K.; Mayer-Gruner, P.; Marhan, S.; Grafe, M.; Schulz, S.; Krüger, J.; Lang, F.; et al. Carbohydrate depletion in roots impedes phosphorus nutrition in young forest trees. New Phytol. 2021, 229, 2611–2624. [Google Scholar] [CrossRef]
- Hauenstein, S.; Neidhardt, H.; Lang, F.; Krüger, J.; Hofmann, D.; Pütz, T.; Oelmann, Y. Organic layers favor phosphorus storage and uptake by young beech trees (Fagus sylvatica L.) at nutrient poor ecosystems. Plant Soil 2018, 432, 289–301. [Google Scholar] [CrossRef]
- Pena, R.; Offermann, C.; Simon, J.; Naumann, P.S.; Geßler, A.; Holst, J.; Dannenmann, M.; Mayer, H.; Kögel-Knabner, I.; Rennenberg, H.; et al. Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest. Appl. Environ. Microbiol. 2010, 76, 1831–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clausing, S.; Polle, A. Mycorrhizal phosphorus efficiencies and microbial competition drive root P uptake. Front. For. Glob. Chang. 2020, 3, 54. [Google Scholar] [CrossRef]
- Kohler, J.; Yang, N.; Pena, R.; Raghavan, V.; Polle, A.; Meier, I.C. Ectomycorrhizal fungal diversity increases phosphorus uptake efficiency of European beech. New Phytol. 2018, 220, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Lilleskov, E.A.; Hobbie, E.A.; Horton, T.R. Conservation of ectomycorrhizal fungi: Exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol. 2011, 4, 174–183. [Google Scholar] [CrossRef]
- Morrison, E.W.; Frey, S.D.; Sadowsky, J.J.; van Diepen, L.T.A.; Thomas, W.K.; Pringle, A. Chronic nitrogen additions fundamentally restructure the soil fungal community in a temperate forest. Fungal Ecol. 2016, 23, 48–57. [Google Scholar] [CrossRef]
- Karst, J.; Wasyliw, J.; Birch, J.D.; Franklin, J.; Chang, S.X.; Erbilgin, N. Long-term nitrogen addition does not sustain host tree stem radial growth but doubles the abundance of high-biomass ectomycorrhizal fungi. Glob. Chang. Biol. 2021, 27, 4125–4138. [Google Scholar] [CrossRef]
- Heijden, M.G.A.; Martin, F.M.; Selosse, M.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Taylor, A.F.S.; Martin, F.; Read, D.J. Fungal diversity in ectomycorrhizal communities of Norway spruce (Picea abies (L.) Karst.) and Beech (Fagus sylvatica L.) in forests along north-south transects in Europe. In Carbon and Nitrogen Cycling in European Forest Ecosystems, Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2000; pp. 343–365. [Google Scholar]
- Lilleskov, E.A.; Fahey, T.J.; Horton, T.R.; Lovett, G.M. Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 2002, 83, 104–115. [Google Scholar] [CrossRef]

| N Addition Treatments | pH | TC (g/kg) | TN (g/kg) | NO3− (mg/kg) | NH4+ (mg/kg) | TP (g/kg) | AP (mg/kg) | C/N | N/P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Humus layer | N0 | 7.77 ± 0.01 a | 25.3 ± 2.00 c | 2.25 ± 0.20 c | 16.8 ± 2.45 b | 55.7 ± 3.71 b | 0.99 ± 0.03 a | 13.5 ± 0.95 a | 11.2 ±0.29 a | 2.27 ± 0.25 b |
| N1 | 7.73 ± 0.05 a | 27.6 ±2.75 bc | 2.50 ± 0.28 bc | 16.3 ± 2.39 b | 55.2 ± 9.84 b | 0.95 ± 0.02 a | 12.4 ± 0.61 a | 11.0 ±0.29 a | 2.63 ± 0.33 b | |
| N2 | 7.73 ± 0.01 a | 25.6 ± 1.94 c | 2.25 ± 0.24 c | 17.1 ± 2.14 b | 60.3 ± 5.64 b | 0.92 ± 0.02 a | 12.5 ± 0.67 a | 11.5 ± 0.37 a | 2.47 ± 0.31 b | |
| N3 | 7.74 ± 0.03 a | 36.4 ±1.79 b | 3.30 ± 0.18 b | 21.7 ± 0.82 b | 64.5 ± 4.69 ab | 0.96 ± 0.03 a | 12.4 ± 0.85 a | 11.1 ± 0.35 a | 3.46 ± 0.27 ab | |
| N4 | 7.80 ± 0.06 a | 48.3 ± 5.37 a | 4.50 ± 0.51 a | 40.3 ± 5.02 a | 79.1 ± 6.49 a | 0.93 ± 0.02 a | 13.3 ± 1.05 a | 10.7 ±0.07 a | 4.87 ±0.64 a | |
| Mineral soil | N0 | 8.38 ± 0.07 A | 14.9 ± 0.77 AB | 1.28 ± 0.04 A | 3.92 ± 0.66 B | 12.2 ± 0.69 C | 0.83 ± 0.03 A | 5.22 ± 0.30 AB | 11.7 ±1.00 a | 1.54 ± 0.04 a |
| N1 | 8.54 ± 0.12 A | 16.1 ± 0.49 AB | 1.33 ±0.10 A | 4.33 ± 0.97 B | 15.5 ± 0.97 BC | 0.83 ± 0.02 A | 5.61 ± 0.68 A | 12.4 ±1.00 a | 1.61 ± 0.15 a | |
| N2 | 8.59 ± 0.09 A | 16.8 ± 0.69 A | 1.38 ± 0.03 A | 5.77 ± 1.09 B | 14.3 ± 1.58 C | 0.81 ± 0.02 A | 4.60 ±0.47 AB | 12.3 ±0.88 a | 1.69 ± 0.07 a | |
| N3 | 8.43 ± 0.10 A | 13.9 ± 0.39 B | 1.30 ± 0.07 A | 7.50 ± 1.02 B | 18.4 ± 1.15 AB | 0.83 ± 0.02 A | 3.91 ± 0.35 AB | 10.8 ±0.63 a | 1.57 ± 0.06 a | |
| N4 | 8.16 ± 0.10 A | 17.1 ± 1.25 A | 1.53 ± 0.11 A | 30.8 ± 4.36 A | 20.7 ± 0.86 A | 0.83 ± 0.02 A | 3.17 ± 0.30 B | 11.3 ±0.74 a | 1.85 ±0.17 a | |
| Alpha Diversity | N0 | N1 | N2 | N3 | N4 |
|---|---|---|---|---|---|
| Richness | 11.0 ± 0.41 a | 10.3 ± 0.48 a | 9.25 ± 0.25 b | 7.00 ± 0.00 c | 7.00 ± 0.00 c |
| Shannon | 1.88 ± 0.08 a | 1.83 ± 0.02 a | 1.82 ± 0.05 a | 1.81 ±0.02 a | 1.85 ± 0.01 a |
| Simpson | 0.80 ± 0.02 a | 0.80 ± 0.01 a | 0.79 ± 0.01 a | 0.82 ± 0.01 a | 0.83 ± 0.003 a |
| Pielou | 0.79 ± 0.02 b | 0.79 ± 0.02 b | 0.82 ± 0.01 b | 0.93 ± 0.01 a | 0.95 ± 0.01 a |
| Type | Predictor Variables | Slope (SE) | t-Value | p | Variance * |
|---|---|---|---|---|---|
| Richness | (Intercept) | −1.802 (0.501) | −3.542 | 0.002 | |
| TC-H | −0.492 (0.123) | −3.992 | 0.001 | 41.49 | |
| AP-M | 0.281 (0.101) | 2.793 | 0.013 | 34.28 | |
| TP-FR | 0.001 (0.000) | 1674 | 0.114 | 4.95 | |
| Richness ~TC-H + AP-M + TP-FR | |||||
| AIC = −28.27, residual standard error = 0.0406, multiple R2 = 0.8072, adjusted R2 = 0.771, F = 22.34, on 17 degrees of freedom, p ≤ 0.000. * Percentage of variance explained by each predictor. | |||||
| Simpson | (Intercept) | 0.737 (0.017) | 42.9 | <0.000 | |
| NH4+-M | 0.004 (0.001) | 4.33 | <0.000 | 48.33 | |
| N/P-M | −0.006 (0.003) | −1.73 | 0.101 | 4. 84 | |
| Simpson ~NH4+-M + N/P-M | |||||
| AIC = −159.73, residual standard error = 0.01, multiple R2 = 0.53, adjusted R2 = 0.47, F = 9.65, on 17 degrees of freedom, p = 0.001. * Percentage of variance explained by each predictor. | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Wang, B.; Liu, D.; Wang, X.; Li, X.; Zhang, Y.; Xu, Y.; Peng, S.; Ge, Z.; Mao, L.; et al. Long-Term Nitrogen Deposition Alters Ectomycorrhizal Community Composition and Function in a Poplar Plantation. J. Fungi 2021, 7, 791. https://doi.org/10.3390/jof7100791
Yang N, Wang B, Liu D, Wang X, Li X, Zhang Y, Xu Y, Peng S, Ge Z, Mao L, et al. Long-Term Nitrogen Deposition Alters Ectomycorrhizal Community Composition and Function in a Poplar Plantation. Journal of Fungi. 2021; 7(10):791. https://doi.org/10.3390/jof7100791
Chicago/Turabian StyleYang, Nan, Bo Wang, Dong Liu, Xuan Wang, Xiuxiu Li, Yan Zhang, Yaming Xu, Sili Peng, Zhiwei Ge, Lingfeng Mao, and et al. 2021. "Long-Term Nitrogen Deposition Alters Ectomycorrhizal Community Composition and Function in a Poplar Plantation" Journal of Fungi 7, no. 10: 791. https://doi.org/10.3390/jof7100791






