Augmenting the Activity of Chlorhexidine for Decolonization of Candida auris from Porcine skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organisms and Inoculum
2.2. Reagents
2.3. Porcine Skin Model
2.4. Histopathology
2.5. In Vitro Assessment of Antimicrobial Activity
2.6. Biofilm Minimal Inhibitory Concentrations Testing (BMIC)
2.7. Antiseptic Combinations Studies
3. Results
3.1. C. auris Persists on Porcine Skin following the Application of Hospital Antiseptic Cleansers
3.2. Isopropanol Augments the Activity of Chlorhexidine for C. auris Skin Decolonization
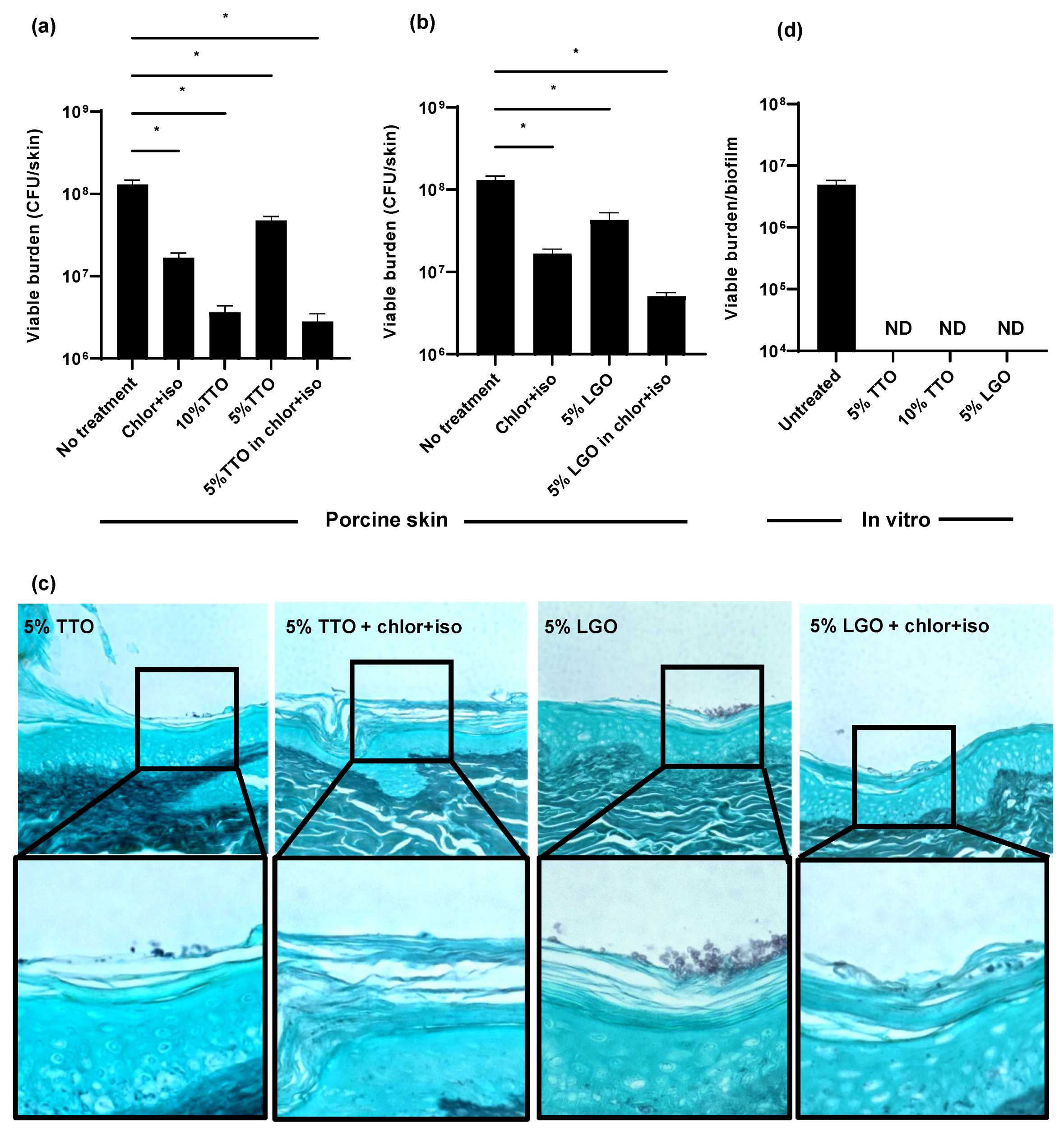
3.3. Tea Tree Oil (TTO) and Lemongrass Oil (LGO) Enhance the Activity of Chlor+Iso for C. auris Decolonization of Skin
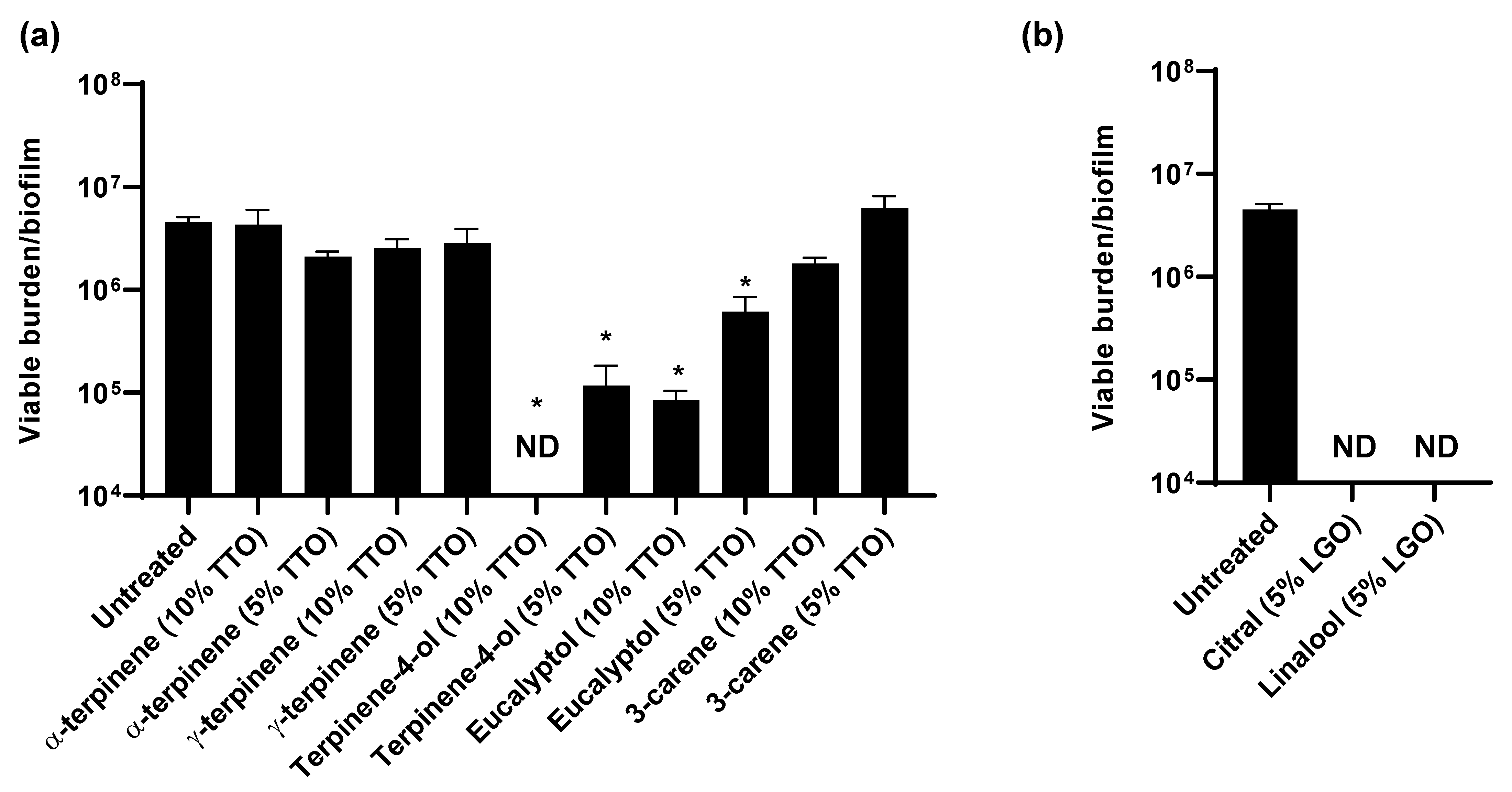
3.4. TTO and LGO and Their Active Components Are Synergistic with Chlorhexidine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [Green Version]
- van Schalkwyk, E.; Mpembe, R.S.; Thomas, J.; Shuping, L.; Ismail, H.; Lowman, W.; Karstaedt, A.S.; Chibabhai, V.; Wadula, J.; Avenant, T.; et al. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017. Emerg. Infect. Dis. 2019, 25, 1698–1707. [Google Scholar] [CrossRef] [Green Version]
- Mathur, P.; Hasan, F.; Singh, P.K.; Malhotra, R.; Walia, K.; Chowdhary, A. Five-year profile of candidaemia at an Indian trauma centre: High rates of Candida auris blood stream infections. Mycoses 2018, 61, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Rudramurthy, S.M.; Chakrabarti, A.; Paul, R.A.; Sood, P.; Kaur, H.; Capoor, M.R.; Kindo, A.J.; Marak, R.S.K.; Arora, A.; Sardana, R.; et al. Candida auris candidaemia in Indian ICUs: Analysis of risk factors. J. Antimicrob. Chemother. 2017, 72, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control 2016, 5, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamoth, F.; Kontoyiannis, D.P. The Candida auris alert: Facts and perspectives. J. Infect. Dis. 2018, 217, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, G.; Bloch, D.; Murray, K.; Kratz, M.; Parton, H.; Ackelsberg, J.; Antwi, M.; Del Rosso, P.; Dorsinville, M.; Kubinson, H.; et al. Candida auris colonization after discharge to a community setting: New York City, 2017–2019. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2021; Volume 8, p. ofaa620. [Google Scholar] [CrossRef]
- Horton, M.V.; Johnson, C.J.; Kernien, J.F.; Patel, T.D.; Lam, B.C.; Cheong, J.Z.A.; Meudt, J.J.; Shanmuganayagam, D.; Kalan, L.R.; Nett, J.E. Candida auris forms high-burden biofilms in skin niche conditions and on porcine skin. mSphere 2020, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, E.; Quinn, M.; Tsay, S.; Poirot, E.; Chaturvedi, S.; Southwick, K.; Greenko, J.; Fernandez, R.; Kallen, A.; Vallabhaneni, S.; et al. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg. Infect. Dis. 2018, 24, 1816–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdolrasouli, A.; Armstrong-James, D.; Ryan, L.; Schelenz, S. In vitro efficacy of disinfectants utilised for skin decolonisation and environmental decontamination during a hospital outbreak with Candida auris. Mycoses 2017, 60, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Mulet Bayona, J.V.; Tormo Palop, N.; Salvador Garcia, C.; Herrero Rodriguez, P.; Abril Lopez de Medrano, V.; Ferrer Gomez, C.; Gimeno Cardona, C. Characteristics and management of candidaemia episodes in an established Candida auris outbreak. Antibiotics 2020, 9, 558. [Google Scholar] [CrossRef]
- Biswal, M.; Rudramurthy, S.M.; Jain, N.; Shamanth, A.S.; Sharma, D.; Jain, K.; Yaddanapudi, L.N.; Chakrabarti, A. Controlling a possible outbreak of Candida auris infection: Lessons learnt from multiple interventions. J. Hosp. Infect. 2017, 97, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.Y.; Shang, H.T.; Liu, C.E.; Wang, Y.; Niu, R.; Wu, J.; Wei, H. Light microscopic, electron microscopic, and immunohistochemical comparison of Bama minipig (Sus scrofa domestica) and human skin. Comp. Med. 2010, 60, 142–148. [Google Scholar] [PubMed]
- Eaglstein, W.H.; Mertz, P.M. New methods for assessing epidermal wound healing: The effects of triamcinolone acetonide and polyethelene film occlusion. J. Invest. Dermatol. 1978, 71, 382–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Valle Arevalo, A.; Fishburn, A.; Johnson, A.D.; Nobile, C.J. Assessment and optimizations of Candida albicans in vitro biofilm assays. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Graninger, W.; Presterl, E. In vitro activity of antifungal combinations against Candida albicans biofilms. J. Antimicrob. Chemother. 2010, 65, 271–274. [Google Scholar] [CrossRef] [Green Version]
- Schomberg, D.T.; Tellez, A.; Meudt, J.J.; Brady, D.A.; Dillon, K.N.; Arowolo, F.K.; Wicks, J.; Rousselle, S.D.; Shanmuganayagam, D. Miniature swine for preclinical modeling of complexities of human disease for translational scientific discovery and accelerated development of therapies and medical devices. Toxicol. Pathol. 2016, 44, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Digison, M.B. A review of anti-septic agents for pre-operative skin preparation. Plast. Surg. Nurs. 2007, 27, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Mimoz, O.; Lucet, J.C.; Kerforne, T.; Pascal, J.; Souweine, B.; Goudet, V.; Mercat, A.; Bouadma, L.; Lasocki, S.; Alfandari, S.; et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): An open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 2015, 386, 2069–2077. [Google Scholar] [CrossRef]
- Wade, R.G.; Burr, N.E.; McCauley, G.; Bourke, G.; Efthimiou, O. The comparative efficacy of chlorhexidine gluconate and povidone-iodine antiseptics for the prevention of infection in clean surgery: A systematic review and network meta-analysis. Ann. Surg. 2020. [Google Scholar] [CrossRef]
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal Infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Ergin, A.; Arikan, S. Comparison of microdilution and disc diffusion methods in assessing the in vitro activity of fluconazole and Melaleuca alternifolia (tea tree) oil against vaginal Candida isolates. J. Chemother. 2002, 14, 465–472. [Google Scholar] [CrossRef]
- Silva Cde, B.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lulekal, E.; Tesfaye, S.; Gebrechristos, S.; Dires, K.; Zenebe, T.; Zegeye, N.; Feleke, G.; Kassahun, A.; Shiferaw, Y.; Mekonnen, A. Phytochemical analysis and evaluation of skin irritation, acute and sub-acute toxicity of Cymbopogon citratus essential oil in mice and rabbits. Toxicol. Rep. 2019, 6, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Krzysko-Lupicka, T.; Sokol, S.; Piekarska-Stachowiak, A.A. Evaluation of fungistatic activity of eight selected essential oils on four heterogeneous Fusarium isolates obtained from cereal grains in Southern Poland. Molecules 2020, 25, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Liu, G.; Li, J.; Chen, J.; Li, L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.F.; Zhang, S. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Front. Cell Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef]
- Lepak, A.J.; Zhao, M.; Berkow, E.L.; Lockhart, S.R.; Andes, D.R. Pharmacodynamic optimization for treatment of invasive Candida auris infection. Antimicrob. Agents Chemother. 2017, 61, S73. [Google Scholar] [CrossRef] [Green Version]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forgacs, L.; Borman, A.M.; Prepost, E.; Toth, Z.; Kardos, G.; Kovacs, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in vivo pathogenicity of four Candida auris clades in a neutropenic bloodstream infection murine model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.; Hager, C.; Chandra, J.; Mukherjee, P.K.; Retuerto, M.; Salem, I.; Long, L.; Isham, N.; Kovanda, L.; Borroto-Esoda, K.; et al. The emerging pathogen Candida auris: Growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpanen, T.J.; Worthington, T.; Conway, B.R.; Hilton, A.C.; Elliott, T.S.; Lambert, P.A. Penetration of chlorhexidine into human skin. Antimicrob. Agents Chemother. 2008, 52, 3633–3636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Hurabielle, C.; Drummond, R.A.; Bouladoux, N.; Desai, J.V.; Sim, C.K.; Belkaid, Y.; Lionakis, M.S.; Segre, J.A. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe 2021, 29, 210–221 e216. [Google Scholar] [CrossRef]
- Moore, G.; Schelenz, S.; Borman, A.M.; Johnson, E.M.; Brown, C.S. Yeasticidal activity of chemical disinfectants and antiseptics against Candida auris. J. Hosp. Infect. 2017, 97, 371–375. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Cal, K.; Kupiec, K.; Sznitowska, M. Effect of physicochemical properties of cyclic terpenes on their ex vivo skin absorption and elimination kinetics. J. Dermatol. Sci. 2006, 41, 137–142. [Google Scholar] [CrossRef]
- Carson, C.F.; Mee, B.J.; Riley, T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents Chemother. 2002, 46, 1914–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, B.H.; Kim, Y.S.; Yoo, S.; Hur, M.H. Antimicrobial and hand hygiene effects of Tea Tree Essential Oil disinfectant: A randomised control trial. Int. J. Clin. Pract. 2021, e14206. [Google Scholar] [CrossRef]


| Component % in 5% Oil (v/v) | Modified MIC (%) | FICI (Oil/Component + Chlorhexidine) | FICI Interpretation | |
|---|---|---|---|---|
| Tea tree oil | 0.5 | 0.375 | Synergistic | |
| terpinen-4-ol | 1.9 | 0.25 | 0.625 | Additive/indifferent |
| eucalyptol | 0.7 | 2 | 0.22 | Synergistic |
| Lemongrass oil | 0.0625 | 0.376 | Synergistic | |
| citral | 3.4 | 0.03 | 0.372 | Synergistic |
| linalool | 0.285 | 0.19 | 0.5 | Additive/indifferent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, C.J.; Eix, E.F.; Lam, B.C.; Wartman, K.M.; Meudt, J.J.; Shanmuganayagam, D.; Nett, J.E. Augmenting the Activity of Chlorhexidine for Decolonization of Candida auris from Porcine skin. J. Fungi 2021, 7, 804. https://doi.org/10.3390/jof7100804
Johnson CJ, Eix EF, Lam BC, Wartman KM, Meudt JJ, Shanmuganayagam D, Nett JE. Augmenting the Activity of Chlorhexidine for Decolonization of Candida auris from Porcine skin. Journal of Fungi. 2021; 7(10):804. https://doi.org/10.3390/jof7100804
Chicago/Turabian StyleJohnson, Chad J., Emily F. Eix, Brandon C. Lam, Kayla M. Wartman, Jennifer J. Meudt, Dhanansayan Shanmuganayagam, and Jeniel E. Nett. 2021. "Augmenting the Activity of Chlorhexidine for Decolonization of Candida auris from Porcine skin" Journal of Fungi 7, no. 10: 804. https://doi.org/10.3390/jof7100804
APA StyleJohnson, C. J., Eix, E. F., Lam, B. C., Wartman, K. M., Meudt, J. J., Shanmuganayagam, D., & Nett, J. E. (2021). Augmenting the Activity of Chlorhexidine for Decolonization of Candida auris from Porcine skin. Journal of Fungi, 7(10), 804. https://doi.org/10.3390/jof7100804






