Aspergillus Goes Viral: Ecological Insights from the Geographical Distribution of the Mycovirome within an Aspergillus flavus Population and Its Possible Correlation with Aflatoxin Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Growth Conditions
2.2. Chemotype Characterization of A. flavus Strains
2.3. Sclerotia Biogenesis Evaluation
2.4. A. flavus gDNA Extraction and RAPD-PCR Profile Characterization
2.5. RNA Extraction and Viral Sequences’ Detection
2.6. Validation of In Silico Detected Viruses
2.7. Virus Particles’ Purification and Electron Microscopy Observation
2.8. Data Analysis
3. Results
3.1. Selection of A. flavus Strains and Chemotype/Sclerotia Production Characterization of the Population
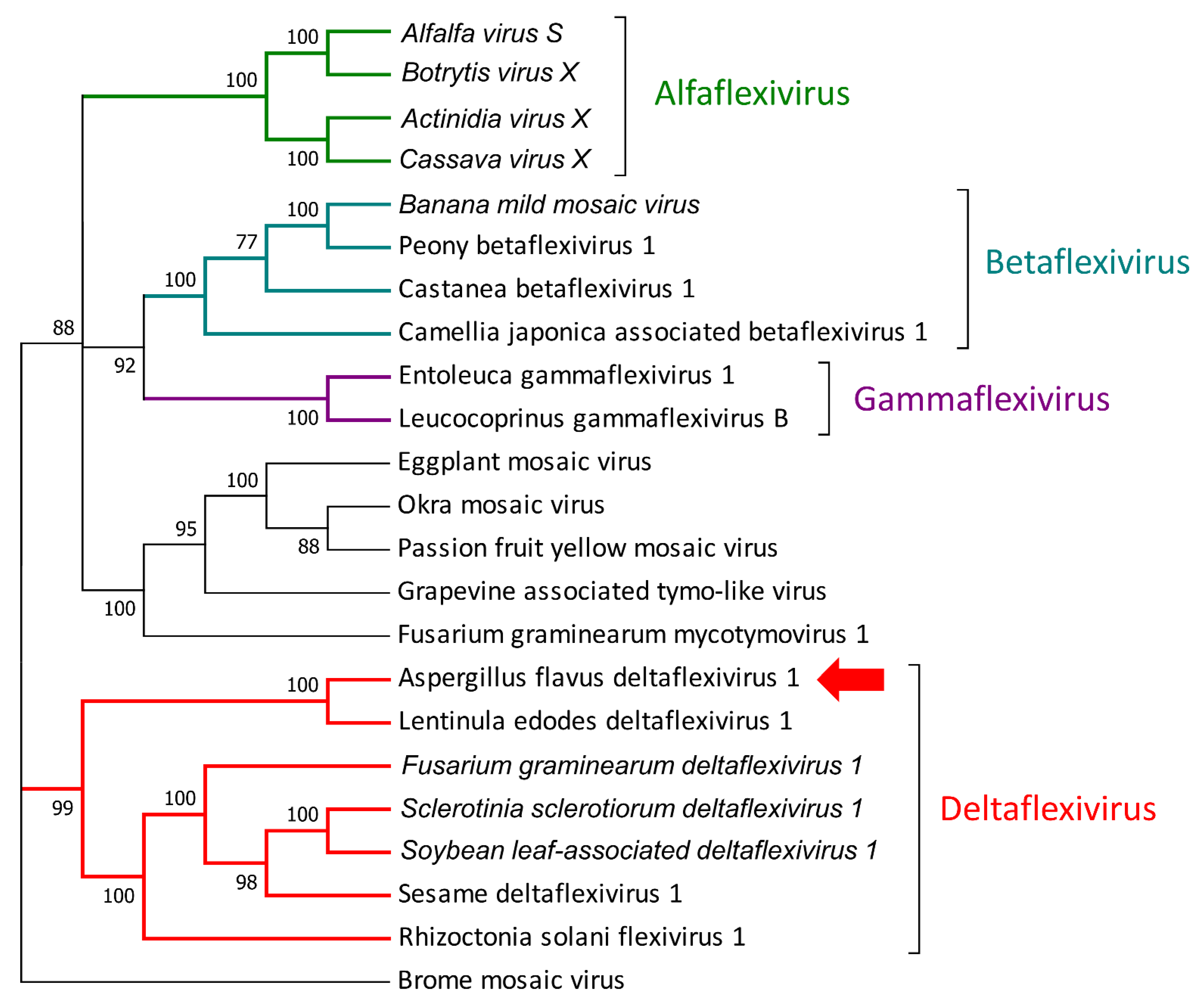
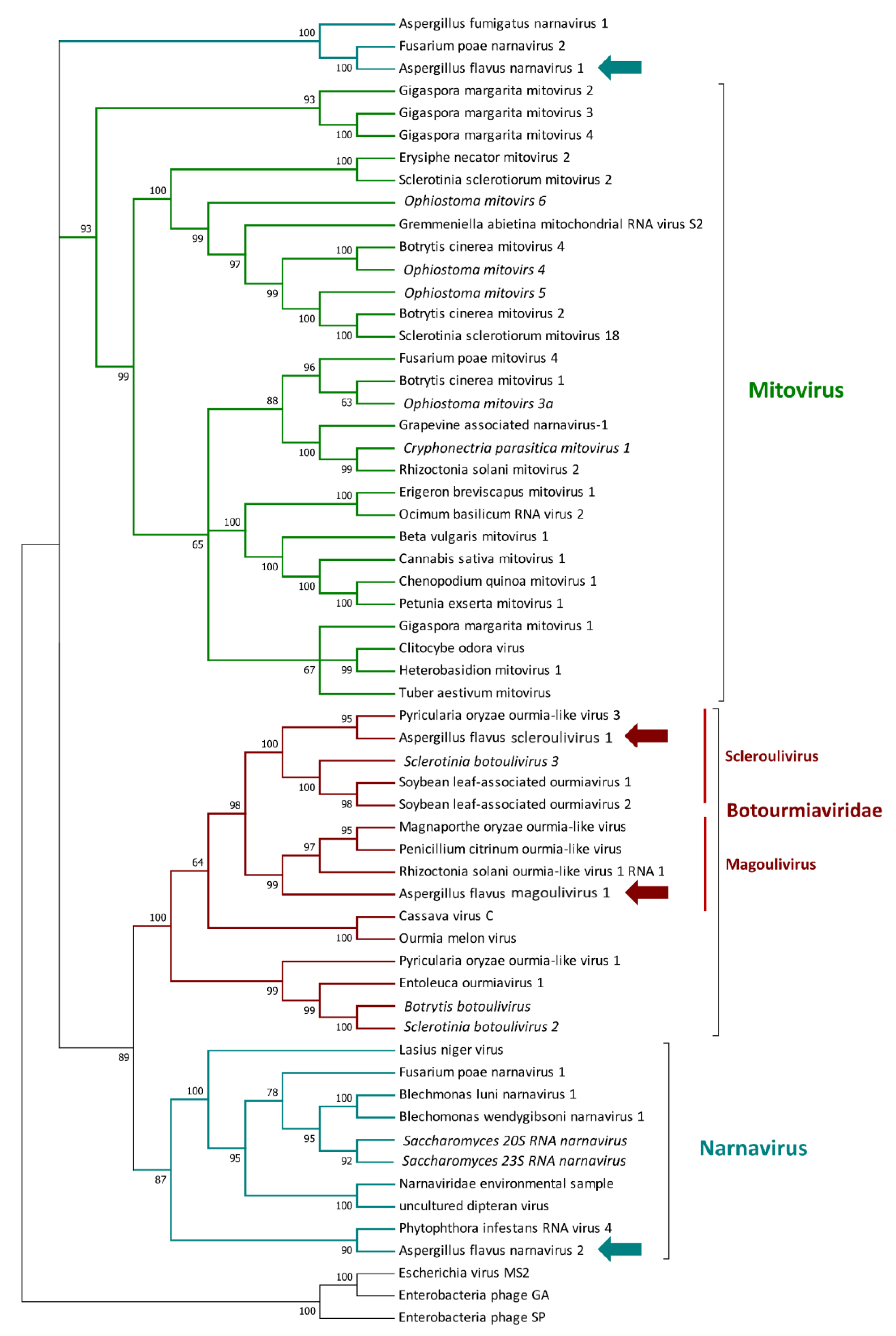
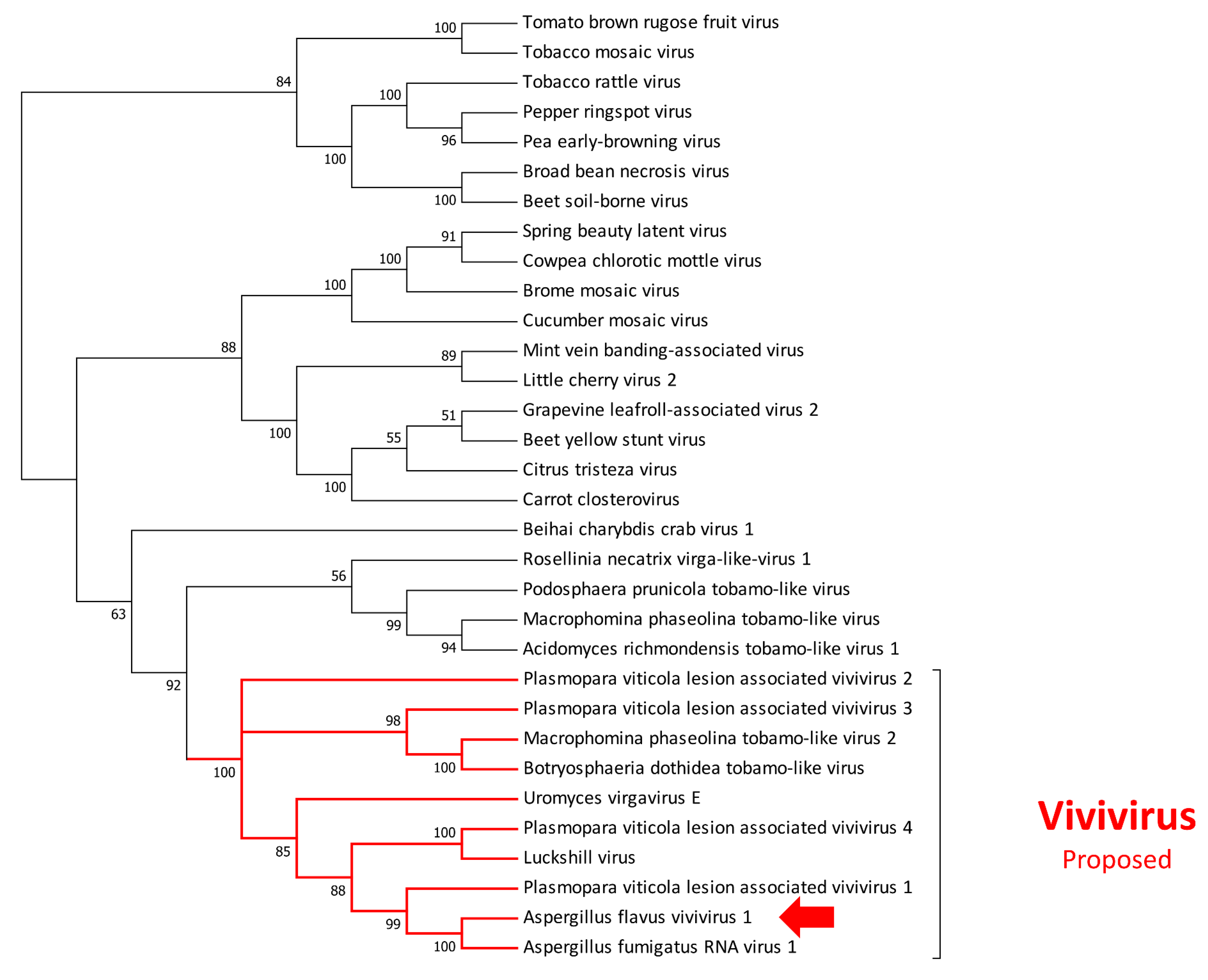
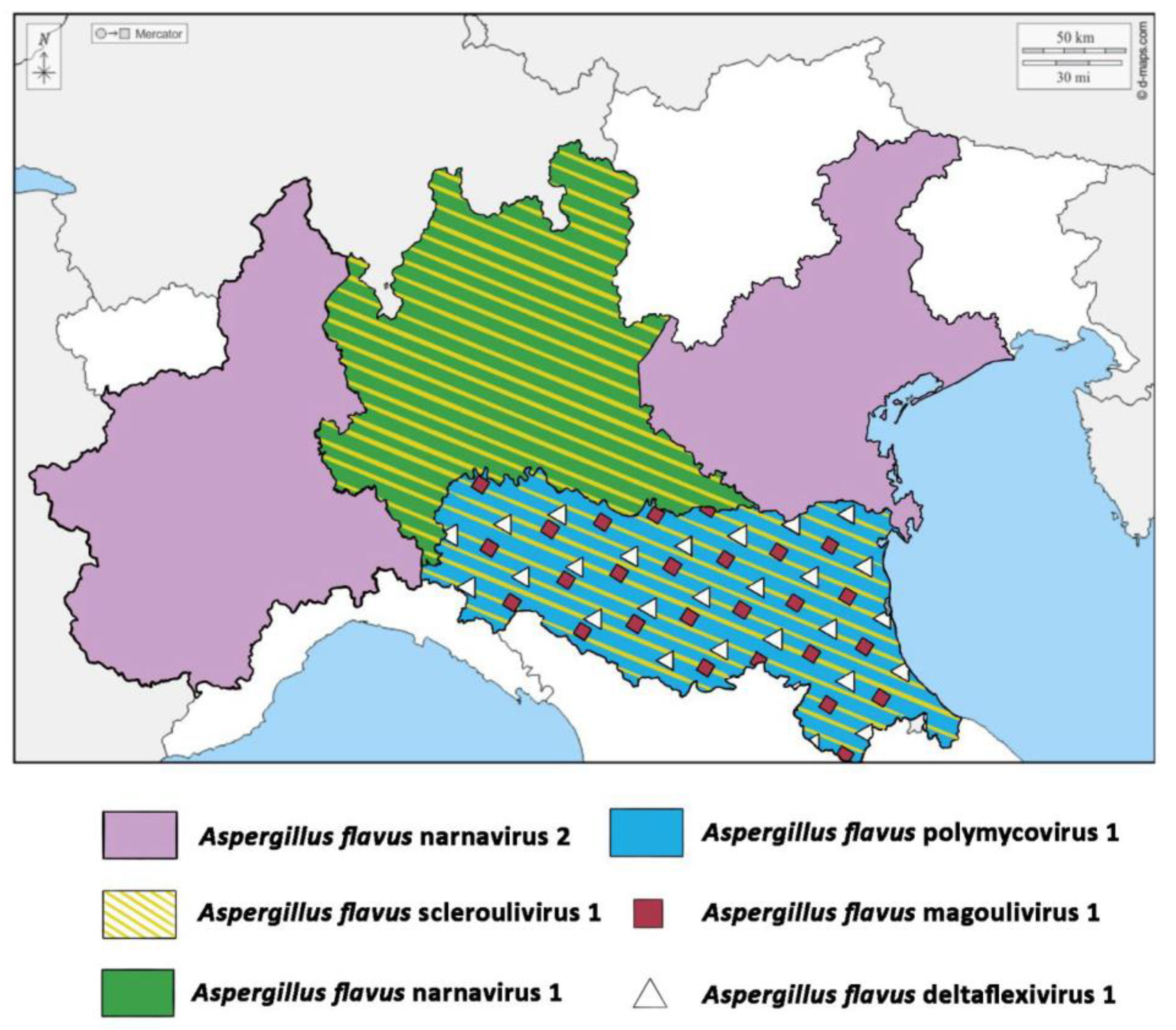
3.2. Mycovirome Characterization
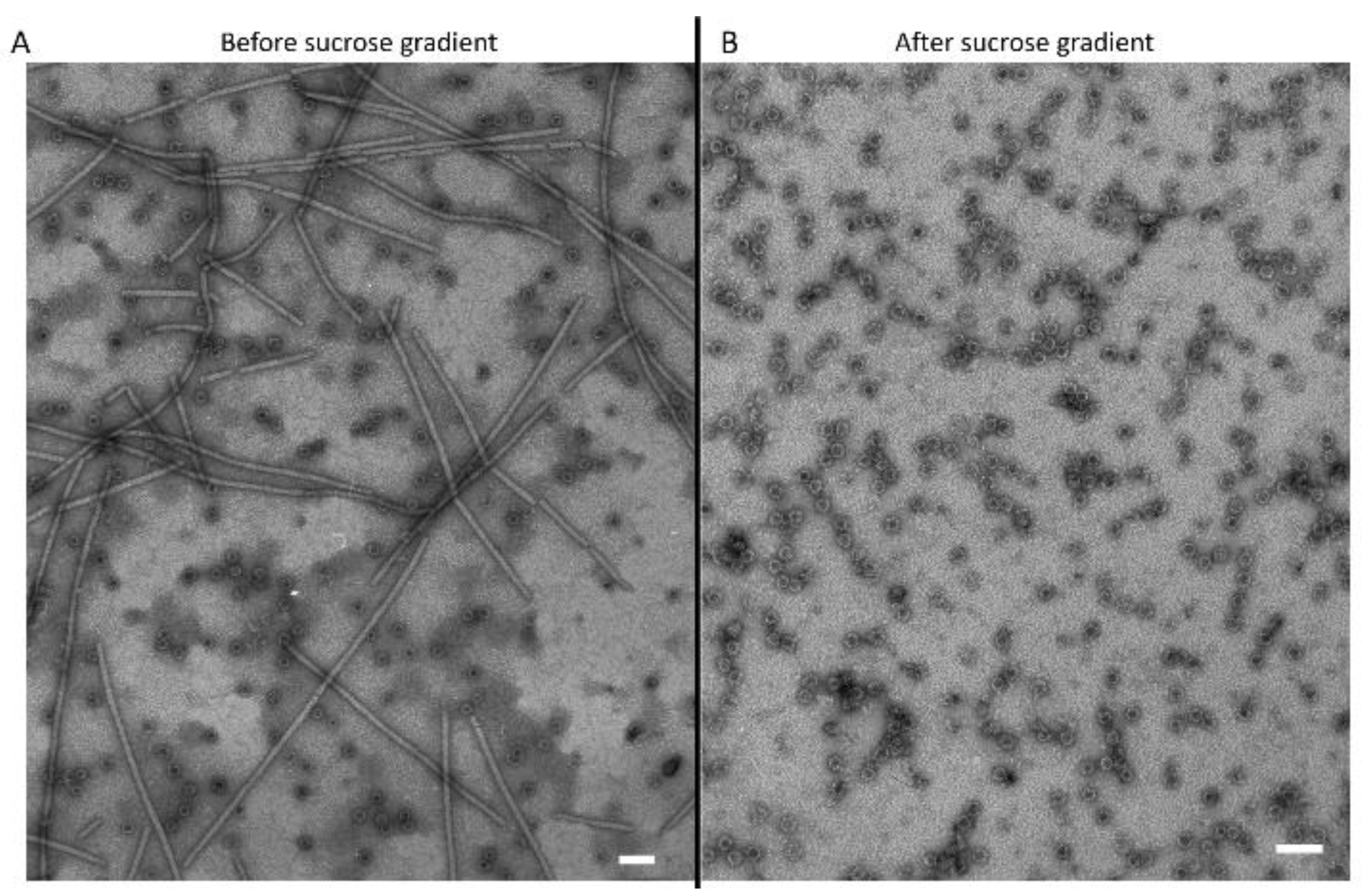
3.3. Transmission Electron Microscopy Observation of Viral Particles
3.4. Correlation between AF Production and Viral Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019; ISBN 9491751182. [Google Scholar]
- De Lucca, A.J. Harmful fungi in both agriculture and medicine. Rev. Iberoam. Micol. 2007, 24, 3. [Google Scholar] [CrossRef]
- Yadav, A.N.; Mishra, S.; Kour, D.; Yadav, N.; Kumar, A. Agriculturally Important Fungi for Sustainable Agriculture; Springer: Heidelberg, Germany, 2020; ISBN 3030459705. [Google Scholar]
- Balestrini, R.; Lumini, E. Focus on mycorrhizal symbioses. Appl. Soil Ecol. 2018, 123, 299–304. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant. Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Mannino, G.; Nerva, L.; Gritli, T.; Novero, M.; Fiorilli, V.; Bacem, M.; Bertea, C.M.; Lumini, E.; Chitarra, W.; Balestrini, R. Effects of different microbial inocula on tomato tolerance to water deficit. Agronomy 2020, 10, 170. [Google Scholar] [CrossRef]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Fungal Kingd. 2017, 5, 701–726. [Google Scholar]
- Powell, W.; Van Alfen, N. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J. Bacteriol. 1987, 169, 5324–5326. [Google Scholar] [CrossRef]
- Son, M.; Yu, J.; Kim, K.-H. Five questions about mycoviruses. PLoS Pathog. 2015, 11, e1005172. [Google Scholar] [CrossRef]
- Brusini, J.; Wayne, M.L.; Franc, A.; Robin, C. The Impact of parasitism on resource allocation in a fungal host: The case of Cryphonectria parasitica and its mycovirus, Cryphonectria hypovirus 1. Ecol. Evol. 2017, 7, 5967–5976. [Google Scholar] [CrossRef]
- Gupta, T.; Kumari, C.; Vanshika; Kulshrestha, S. Biology and mycovirus-assisted biological control of Sclerotinia sclerotiorum infecting vegetable and oilseed crops. Arch. Phytopathol. Plant Prot. 2019, 52, 1049–1067. [Google Scholar] [CrossRef]
- Nerva, L.; Chitarra, W.; Siciliano, I.; Gaiotti, F.; Ciuffo, M.; Forgia, M.; Varese, G.C.; Turina, M. Mycoviruses Mediate Mycotoxin Regulation in Aspergillus ochraceus. Environ. Microbiol. 2019, 21, 1957–1968. [Google Scholar] [CrossRef]
- Okada, R.; Ichinose, S.; Takeshita, K.; Urayama, S.-I.; Fukuhara, T.; Komatsu, K.; Arie, T.; Ishihara, A.; Egusa, M.; Kodama, M. Molecular characterization of a novel mycovirus in Alternaria alternata manifesting two-sided effects: Down-regulation of host growth and up-regulation of host plant pathogenicity. Virology 2018, 519, 23–32. [Google Scholar] [CrossRef]
- Jiang, D.; Fu, Y.; Guoqing, L.; Ghabrial, S.A. Viruses of the plant pathogenic fungus Sclerotinia sclerotiorum. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 86, pp. 215–248. ISBN 0065-3527. [Google Scholar]
- Nuss, D.L. Hypovirulence: Mycoviruses at the fungal–Plant interface. Nat. Rev. Microbiol. 2005, 3, 632–642. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Tang, Y.; Zhang, S.; She, X.; Lan, G.; Varsani, A.; He, Z. Identification and molecular characterization of a single-stranded circular DNA Virus with similarities to sclerotinia Sclerotiorum hypovirulence-Associated DNA Virus 1. Arch. Virol. 2014, 159, 1527–1531. [Google Scholar] [CrossRef]
- Choi, G.H.; Dawe, A.L.; Churbanov, A.; Smith, M.L.; Milgroom, M.G.; Nuss, D.L. Molecular characterization of vegetative incompatibility genes that restrict hypovirus transmission in the chestnut blight fungus Cryphonectria parasitica. Genetics 2012, 190, 113–127. [Google Scholar] [CrossRef]
- Short, D.P.G.; Double, M.; Nuss, D.L.; Stauder, C.M.; MacDonald, W.; Kasson, M.T. Multilocus PCR assays elucidate vegetative incompatibility gene profiles of Cryphonectria parasitica in the United States. Appl. Environ. Microbiol. 2015, 81, 5736–5742. [Google Scholar] [CrossRef]
- Suzuki, N.; Cornejo, C.; Aulia, A.; Shahi, S.; Hillman, B.I.; Rigling, D. In-tree behavior of diverse viruses harbored in the chestnut blight fungus, Cryphonectria parasitica. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Nerva, L.; Forgia, M.; Ciuffo, M.; Chitarra, W.; Chiapello, M.; Vallino, M.; Varese, G.; Turina, M. The mycovirome of a fungal collection from the sea cucumber Holothuria polii. Virus Res. 2019, 273, 197737. [Google Scholar] [CrossRef]
- Nerva, L.; Turina, M.; Zanzotto, A.; Gardiman, M.; Gaiotti, F.; Gambino, G.; Chitarra, W. Isolation, molecular characterization and virome analysis of culturable wood fungal endophytes in esca symptomatic and asymptomatic grapevine plants. Environ. Microbiol. 2019, 21, 2886–2904. [Google Scholar] [CrossRef] [PubMed]
- Banks, G.; Buck, K.; Chain, E.; Darbyshire, J.E.; Himmelweit, F.; Ratti, G.; Sharpe, T.; Planterose, D. Antiviral activity of double stranded RNA from a virus isolated from Aspergillus foetidus. Nature 1970, 227, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Kotta-Loizou, I.; Coutts, R.H. Mycoviruses in Aspergilli: A comprehensive review. Front. Microbiol. 2017, 8, 1699. [Google Scholar] [CrossRef]
- Hammond, T.M.; Andrewski, M.D.; Roossinck, M.J.; Keller, N.P. Aspergillus mycoviruses are targets and suppressors of RNA silencing. Eukaryot. Cell 2008, 7, 350–357. [Google Scholar] [CrossRef]
- Hammond, T.M.; Keller, N.P. RNA silencing in the Aspergilli. In The Aspergilli, 1st ed.; CRC PRESS: Boca Raton, FL, USA, 2007; p. 197. [Google Scholar]
- Schmidt, F.R. The RNA Interference—Virus Interplay: Tools of nature for gene modulation, morphogenesis, evolution and a possible mean for aflatoxin control. Appl. Microbiol. Biotechnol. 2009, 83, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Yang, H.-E.; Kim, D.-H. Identification of a novel partitivirus of Trichoderma harzianum NFCF319 and evidence for the related antifungal activity. Front. Plant Sci. 2018, 9, 1699. [Google Scholar] [CrossRef]
- Degola, F.; Berni, E.; Restivo, F.M. Laboratory tests for assessing efficacy of atoxigenic Aspergillus flavus strains as biocontrol agents. Int. J. Food Microbiol. 2011, 146, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.R.; Lemke, P.A.; Esser, K. Viral influences on aflatoxin formation by Aspergillus flavus. Appl. Microbiol. Biotechnol. 1986, 24, 248–252. [Google Scholar] [CrossRef]
- Silva, V.N.; Durigon, E.L.; de Pires, M.F.C.; Lourenço, A.; de Faria, M.J.; Corrêa, B. Time course of virus-like particles (VLPs) double-stranded Rna accumulation in toxigenic and non-toxigenic strains of Aspergillus flavus. Braz. J. Microbiol. 2001, 32, 56–60. [Google Scholar] [CrossRef][Green Version]
- Schmidt, F.R.; Davis, N.D.; Diener, U.L.; Lemke, P. Cycloheximide induction of aflatoxin synthesis in a nontoxigenic strain of Aspergillus flavus. Biotechnology 1983, 1, 794–795. [Google Scholar] [CrossRef]
- Degola, F.; Bisceglie, F.; Pioli, M.; Palmano, S.; Elviri, L.; Pelosi, G.; Lodi, T.; Restivo, F.M. Structural modification of cuminaldehyde thiosemicarbazone increases inhibition specificity toward aflatoxin biosynthesis and sclerotia development in Aspergillus flavus. Appl. Microbiol. Biotechnol. 2017, 101, 6683–6696. [Google Scholar] [CrossRef]
- Nerva, L.; Varese, G.C.; Turina, M. Different approaches to discover mycovirus associated to marine organisms. In Viral Metagenomics; Springer: Heidelberg, Germany, 2018; pp. 97–114. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M. De Novo Transcript Sequence Reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with burrows–wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Ciuffo, M.; Vallino, M.; Margaria, P.; Varese, G.; Gnavi, G.; Turina, M. Multiple approaches for the detection and characterization of viral and plasmid symbionts from a collection of marine fungi. Virus Res. 2016, 219, 22–38. [Google Scholar] [CrossRef]
- Hillman, B.I.; Cai, G. The family Narnaviridae: Simplest of RNA viruses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 86, pp. 149–176. ISBN 00653527. [Google Scholar]
- Sutela, S.; Forgia, M.; Vainio, E.J.; Chiapello, M.; Daghino, S.; Vallino, M.; Martino, E.; Girlanda, M.; Perotto, S.; Turina, M. The Virome from a Collection of Endomycorrhizal Fungi Reveals New Viral Taxa with Unprecedented Genome Organization. Virus Evol. 2020, 6, veaa076. [Google Scholar] [CrossRef]
- Chiapello, M.; Rodríguez-Romero, J.; Nerva, L.; Forgia, M.; Chitarra, W.; Ayllón, M.; Turina, M. Putative new plant viruses associated with Plasmopara viticola-infected grapevine samples. Ann. Appl. Biol. 2020, 176, 180–191. [Google Scholar] [CrossRef]
- Ayllón, M.A.; Turina, M.; Xie, J.; Nerva, L.; Marzano, S.-Y.L.; Donaire, L.; Jiang, D.; Consortium, I.R. ICTV virus taxonomy profile: Botourmiaviridae. J. Gen. Virol. 2020, 101, 454. [Google Scholar] [CrossRef]
- Holmes, E.C. The expanding virosphere. Cell Host Microbe 2016, 20, 279–280. [Google Scholar] [CrossRef]
- Urayama, S.; Kondo, F.; Chiba, Y.; Takaki, Y.; Hirai, M.; Minegishi, Y.; Hagiwara, D.; Nunoura, T. Diverged and active partitiviruses in lichen. Front. Microbiol. 2020, 11, 2576. [Google Scholar] [CrossRef]
- Vainio, E.J.; Chiba, S.; Ghabrial, S.A.; Maiss, E.; Roossinck, M.; Sabanadzovic, S.; Suzuki, N.; Xie, J.; Nibert, M. ICTV virus taxonomy profile: Partitiviridae. J. Gen. Virol. 2018, 99, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Oiki, S.; Yaguchi, T.; Urayama, S.; Hagiwara, D. Discovery of Divided RdRp sequences and a hitherto unknown genomic complexity in fungal viruses. Virus Evol. 2021, 7, veaa101. [Google Scholar] [CrossRef]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive aspergillosis by Aspergillus flavus: Epidemiology, diagnosis, antifungal resistance, and management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Gilbert, M.K.; Mack, B.M.; OBrian, G.R.; Rodriguez, A.; Bhatnagar, D.; Payne, G.; Magan, N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and Aflatoxin B1 production. Int. J. Food Microbiol. 2017, 256, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.G.; Rokas, A. The function and evolution of the Aspergillus genome. Trends Microbiol. 2013, 21, 14–22. [Google Scholar] [CrossRef]
- Degola, F. Aspergilli, more than just fungi: Shaping the last decades of model systems. Encycl. Mycol. 2021, 1, 156–163. [Google Scholar]
- Carding, S.R.; Davis, N.; Hoyles, L. The Human intestinal virome in health and disease. Aliment. Pharmacol. Ther. 2017, 46, 800–815. [Google Scholar] [CrossRef]
- Neil, J.A.; Cadwell, K. The intestinal virome and immunity. J. Immunol. 2018, 201, 1615–1624. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. The healthy human virome: From virus–Host symbiosis to disease. Curr. Opin. Virol. 2021, 47, 86–94. [Google Scholar] [CrossRef]
- Kumata, R.; Ito, J.; Takahashi, K.; Suzuki, T.; Sato, K. A tissue level atlas of the healthy human virome. BMC Biol. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Schoebel, C.N.; Prospero, S.; Gross, A.; Rigling, D. Detection of a conspecific mycovirus in two closely related native and introduced fungal hosts and evidence for interspecific virus transmission. Viruses 2018, 10, 628. [Google Scholar] [CrossRef]
- Dawe, A.L.; Van Voorhies, W.A.; Lau, T.A.; Ulanov, A.V.; Li, Z. Major impacts on the primary metabolism of the plant pathogen Cryphonectria parasitica by the virulence-attenuating virus CHV1-EP713. Microbiology 2009, 155, 3913–3921. [Google Scholar] [CrossRef]
- van Diepeningen, A.D.; Debets, A.J.; Hoekstra, R.F. Dynamics of DsRNA mycoviruses in black Aspergillus populations. Fungal Genet. Biol. 2006, 43, 446–452. [Google Scholar] [CrossRef]
- Varga, J.; Rinyu, E.; Kevei, É.; Tóth, B.; Kozakiewicz, Z. Double-stranded RNA mycoviruses in species of Aspergillus sections Circumdati and Fumigati. Can. J. Microbiol. 1998, 44, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.A.; Bozarth, R.F.; Adler, J.; Mackenzie, D.W. Proteinaceous virus-like particles from an isolate of Aspergillus flavus. J. Virol. 1974, 13, 532–534. [Google Scholar] [CrossRef]
- Elias, K.S.; Cotty, P.J. Incidence and stability of infection by double-stranded RNA genetic elements in Aspergillus section Flavi and effects on aflatoxigenicity. Can. J. Bot. 1996, 74, 716–725. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Yang, B.; Wang, Q.; Zhou, J.; Yu, W. Molecular characterization of a debilitation-associated partitivirus infecting the pathogenic fungus Aspergillus flavus. Front. Microbiol. 2019, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Ko, Y.-H.; Kim, D.-H. Transcriptome analysis of Cryphonectria parasitica infected with Cryphonectria hypovirus 1 (CHV1) reveals distinct genes related to fungal metabolites, virulence, antiviral RNA-silencing, and their regulation. Front. Microbiol. 2020, 11, 1711. [Google Scholar] [CrossRef]
- Ninomiya, A.; Urayama, S.; Suo, R.; Itoi, S.; Fuji, S.; Moriyama, H.; Hagiwara, D. Mycovirus-induced tenuazonic acid production in a rice blast fungus Magnaporthe oryzae. Front. Microbiol. 2020, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.K.; Mackey, B.; Cotty, P.J. Population dynamics of Aspergillus flavus in the air of an intensively cultivated region of South-West Arizona. Plant. Pathol. 2004, 53, 422–433. [Google Scholar] [CrossRef]
- van de Sande, W.W.; Vonk, A.G. Mycovirus therapy for invasive pulmonary aspergillosis? Med. Mycol. 2019, 57, S179–S188. [Google Scholar] [CrossRef] [PubMed]


| A. flavus Isolates | ||
|---|---|---|
| Strain | AF | Sclerotia |
| CR1 | - | L |
| CR5 | - | L |
| CR8 | - | - |
| CR9 | - | L |
| Ven7 | - | - |
| Pie6 | - | - |
| Fri7 | - | L |
| Fri3 | yes | L |
| OZ7 | - | L |
| OZ11 | - | - |
| OZ15 | - | L |
| Lom7 | - | - |
| MN1 | - | - |
| OZ1 | yes | - |
| OZ2 | yes | L |
| OZ3 | yes | L |
| OZ6 | yes | - |
| OZ8 | yes | L |
| OZ16 | yes | - |
| OZ19 | yes | - |
| OZ4 | - | L |
| OZ5 | - | - |
| OZ9 | - | L |
| OZ10 | - | - |
| OZ14 | - | L |
| OZ17 | - | L |
| OZ18 | - | - |
| Emi4 | yes | L |
| Emi5 | - | L |
| BO | yes | L |
| GR | yes | L |
| CSPT2 | yes | L |
| CSPT7 | - | L |
| Ven2 | yes | L |
| Ven4 | yes | - |
| Ven8 | yes | L |
| Ven1 | - | - |
| VRε | - | L |
| Fri2 | yes | L |
| Fri6 | yes | - |
| Fri8 | yes | - |
| UD1 | yes | - |
| UD4 | yes | - |
| Fri5 | - | - |
| UD3 | - | - |
| AT1 | yes | - |
| Lom6 | yes | L |
| TNβ | yes | - |
| TNδ | yes | - |
| TNµ | yes | - |
| Pie10 | yes | L |
| Pie1 | - | L |
| Pie9 | - | - |
| TOα | - | - |
| PRα | yes | - |
| CR6 | yes | L |
| CR10 | yes | L |
| CR16 | yes | L |
| CR20 | yes | - |
| CR18 | yes | L |
| CR7 | - | L |
| CR14 | - | - |
| A. flavus Strain | Virus Detected |
|---|---|
| CR1 | Aspergillus flavus polymycovirus 1 |
| Aspergillus flavus magoulivirus 1 | |
| CR5 | Aspergillus flavus scleroulivirus 1 |
| Aspergillus flavus deltaflexivirus 1 | |
| CR8 | Aspergillus flavus scleroulivirus 1 |
| CR9 | Aspergillus flavus polymycovirus 1 |
| Ven7 | Aspergillus flavus narnavirus 2 |
| Pie 6 | Aspergillus flavus narnavirus 2 |
| Fri7 | Aspergillus flavus partitivirus 2 |
| Fri3 | Aspergillus flavus partitivirus 2 |
| OZ7 | Aspergillus flavus deltaflexivirus 1 |
| OZ15 | Aspergillus flavus scleroulivirus 1 Aspergillus flavus deltaflexivirus 1 |
| OZ11 | Aspergillus flavus scleroulivirus 1 |
| Lom7 | Aspergillus flavus scleroulivirus 1 |
| Aspergillus flavus narnavirus 1 | |
| MN1 | Aspergillus flavus narnavirus 1 |
| Aspergillus flavus vivivirus 1 |
| First Hit in NCBI | Identity | Putative Function | Viral Group | Proposed Name | Host (Isolate Acronym) | |
|---|---|---|---|---|---|---|
| Accession | Organism | |||||
| QNQ74055.1 | Plasmopara viticola lesion associated vivivirus 1 | 37.63% | RdRP | Unclassified | Aspergillus flavus vivivirus 1 | CR9 |
| QNQ74056.1 | Plasmopara viticola lesion associated vivivirus 1 | 30.26% | Methyltransferase | |||
| QDH90368.1 | Riboviria sp. | 48.86% | RdRP | Deltaflexivirus | Aspergillus flavus deltaflexivirus 1 | CR5 |
| QIR30284.1 | Plasmopara viticola lesion associated narnavirus 5 | 63.68% | RdRP | Narnavirus | Aspergillus flavus narnavirus 1 | Lom7, MN1 |
| QIR30311.1 | Plasmopara viticola lesion associated narnavirus 32 | 55.58% | RdRP | Narnavirus | Aspergillus flavus narnavirus 2 | Ven7, Pie6 |
| QQZ00864.1 | Spodoptera exempta virus TenAfr-2017 | 48.49% | RdRP | Botourmiaviridae | Aspergillus flavus magoulivirus 1 | CR1 |
| QNN89181.1 | Oidiodendron maius ourmia-like virus 1 | 43.31% | RdRP | Botourmiaviridae | Aspergillus flavus scleroulivirus 1 | CR5, OZ15, OZ11, Lom7 |
| APG78241.1 | Hubei partiti-like virus 27 | 55.40% | RdRP | Partitiviridae | Aspergillus flavus partitivirus 2 | Fri3, Fri7 |
| BCD56394.1 | Lichen partiti-like RNA virus sp. | 34.22% | Coat protein | |||
| AYP71801.1 | Penicillium brevicompactum tetramycovirus 1 | 60.34% | RdRP | Polymycovirus | Aspergillus flavus polymycovirus 1 | CR9 |
| AYP71802.1 | Penicillium brevicompactum tetramycovirus 1 | 54.63% | PAS-rich protein | |||
| AYP71803.1 | Penicillium brevicompactum tetramycovirus 1 | 50.82% | Methyltransferase | |||
| AYP71804.1 | Penicillium brevicompactum tetramycovirus 1 | 52.42% | Hypotetical protein | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degola, F.; Spadola, G.; Forgia, M.; Turina, M.; Dramis, L.; Chitarra, W.; Nerva, L. Aspergillus Goes Viral: Ecological Insights from the Geographical Distribution of the Mycovirome within an Aspergillus flavus Population and Its Possible Correlation with Aflatoxin Biosynthesis. J. Fungi 2021, 7, 833. https://doi.org/10.3390/jof7100833
Degola F, Spadola G, Forgia M, Turina M, Dramis L, Chitarra W, Nerva L. Aspergillus Goes Viral: Ecological Insights from the Geographical Distribution of the Mycovirome within an Aspergillus flavus Population and Its Possible Correlation with Aflatoxin Biosynthesis. Journal of Fungi. 2021; 7(10):833. https://doi.org/10.3390/jof7100833
Chicago/Turabian StyleDegola, Francesca, Giorgio Spadola, Marco Forgia, Massimo Turina, Lucia Dramis, Walter Chitarra, and Luca Nerva. 2021. "Aspergillus Goes Viral: Ecological Insights from the Geographical Distribution of the Mycovirome within an Aspergillus flavus Population and Its Possible Correlation with Aflatoxin Biosynthesis" Journal of Fungi 7, no. 10: 833. https://doi.org/10.3390/jof7100833
APA StyleDegola, F., Spadola, G., Forgia, M., Turina, M., Dramis, L., Chitarra, W., & Nerva, L. (2021). Aspergillus Goes Viral: Ecological Insights from the Geographical Distribution of the Mycovirome within an Aspergillus flavus Population and Its Possible Correlation with Aflatoxin Biosynthesis. Journal of Fungi, 7(10), 833. https://doi.org/10.3390/jof7100833










