Biomass and Cordycepin Production by the Medicinal Mushroom Cordyceps militaris—A Review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus
Abstract
:1. Introduction
2. The Effect of Culture Degeneration on the Yield and Quality of Fruit Bodies Produced by C. militaris
3. Substrates, Nutrient Requirements and Treatments Related with the Cultivation of C. militaris
4. Genetics, Genomics, and Genetic Engineering of C. militaris
5. Molecular, Nutritional, and Environmental Aspects of Cordycepin Biosynthesis and Ascomata Formation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikoh, N.; Fukatsu, T. Interkingdom Host Jumping Underground: Phylogenetic Analysis of Entomoparasitic Fungi of the Genus Cordyceps. Mol. Biol. Evol. 2000, 17, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The Genus Cordyceps: An Extensive Review of Its Traditional Uses, Phytochemistry and Pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Mains, E.B. North American Entomogenous Species of Cordyceps. Mycologia 1958, 50, 169–222. [Google Scholar] [CrossRef]
- Wang, M.; Meng, X.Y.; Le Yang, R.; Qin, T.; Wang, X.Y.; Zhang, K.Y.; Fei, C.Z.; Li, Y.; Hu, Y.L.; Xue, F.Q. Cordyceps militaris Polysaccharides Can Enhance the Immunity and Antioxidation Activity in Immunosuppressed Mice. Carbohydr. Polym. 2012, 89, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Duan, Y.; Zhang, H.; Ma, H. Advance in Cordyceps militaris (Linn) Link Polysaccharides: Isolation, Structure, and Bioactivities: A Review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Panda, M.K.; Mishra, A.P.; Nigam, M.; Saklani, S. Cordyceps spp.: A Review on Its Immune-Stimulatory and Other Biological Potentials. Front. Pharmacol. 2021, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Gu, L.; Xiong, W.-T.; Wang, H.-Z.; Lian, D.-H.; Zheng, Y.-M.; Zhou, S.; Zhou, W.; Gu, J.-L.; Shen, J.-H. 1H NMR Spectroscopy-Based Metabolic Profiling of Ophiocordyceps sinensis and Cordyceps militaris in Water-Boiled and 50% Ethanol-Soaked Extracts. J. Pharm. Biomed. Anal. 2020, 180, 113038. [Google Scholar] [CrossRef]
- Schwenzer, H.; De Zan, E.; Elshani, M.; Van Stiphout, R.; Kudsy, M.; Morris, J.; Ferrari, V.; Um, I.H.; Chettle, J.; Kazmi, F. The Novel Nucleoside Analogue ProTide NUC-7738 Overcomes Cancer Resistance Mechanisms in Vitro and in a First-in-Human Phase 1 Clinical Trial. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.-H.; Wu, W.-S.; Tzeng, Y.-M. Constituents Isolated from Cordyceps militaris Suppress Enhanced Inflammatory Mediator’s Production and Human Cancer Cell Proliferation. J. Ethnopharmacol. 2010, 131, 363–367. [Google Scholar] [CrossRef]
- Chiu, C.-P.; Liu, S.-C.; Tang, C.-H.; Chan, Y.; El-Shazly, M.; Lee, C.-L.; Du, Y.-C.; Wu, T.-Y.; Chang, F.-R.; Wu, Y.-C. Anti-Inflammatory Cerebrosides from Cultivated Cordyceps militaris. J. Agric. Food Chem. 2016, 64, 1540–1548. [Google Scholar] [CrossRef]
- Lee, J.; Cho, K.; Shin, S.G.; Bae, H.; Koo, T.; Han, G.; Hwang, S. Nutrient Recovery of Starch Processing Waste to Cordyceps militaris: Solid State Cultivation and Submerged Liquid Cultivation. Appl. Biochem. Biotechnol. 2016, 180, 274–288. [Google Scholar] [CrossRef]
- Lin, Q.; Long, L.; Wu, L.; Zhang, F.; Wu, S.; Zhang, W.; Sun, X. Evaluation of Different Agricultural Wastes for the Production of Fruiting Bodies and Bioactive Compounds by Medicinal Mushroom Cordyceps militaris. J. Sci. Food Agric. 2017, 97, 3476–3480. [Google Scholar] [CrossRef]
- Cui, J.D. Biotechnological Production and Applications of Cordyceps militaris, a Valued Traditional Chinese Medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Han, Z.; Qiu, P.; Zhou, Z.; Tang, Y.; Zhao, Y.; Zheng, S.; Xu, C.; Zhang, X.; Yin, P. Salinity-Induced Anti-Angiogenesis Activities and Structural Changes of the Polysaccharides from Cultured Cordyceps militaris. PLoS ONE 2014, 9, e103880. [Google Scholar]
- Tang, J.; Qian, Z.; Wu, H. Enhancing Cordycepin Production in Liquid Static Cultivation of Cordyceps militaris by Adding Vegetable Oils as the Secondary Carbon Source. Bioresour. Technol. 2018, 268, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Wu, J.-Y.; Chang, C.-Y.; Yu, S.-T.; Liu, Y.-C. Enhanced Exopolysaccharide Production by Cordyceps militaris Using Repeated Batch Cultivation. J. Biosci. Bioeng. 2019, 127, 499–505. [Google Scholar] [CrossRef]
- Guo, M.; Guo, S.; Huaijun, Y.; Bu, N.; Dong, C. Comparison of Major Bioactive Compounds of the Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes), Fruiting Bodies Cultured on Wheat Substrate and Pupae. Int. J. Med. Mushrooms 2016, 18, 327–336. [Google Scholar] [CrossRef]
- Shi, K.; Yang, G.; He, L.; Yang, B.; Li, Q.; Yi, S. Purification, Characterization, Antioxidant, and Antitumor Activity of Polysaccharides Isolated from Silkworm Cordyceps. J. Food Biochem. 2020, 44, e13482. [Google Scholar] [CrossRef]
- Zheng, Z.; Qiu, X.; Han, R. Identification of the Genes Involved in the Fruiting Body Production and Cordycepin Formation of Cordyceps militaris Fungus. Mycobiology 2015, 43, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Lin, J.; Guo, L.; Wang, X.; Tian, S.; Liu, C.; Zhao, Y.; Zhao, R. Advances in Research on Cordyceps militaris Degeneration. Appl. Microbiol. Biotechnol. 2019, 103, 7835–7841. [Google Scholar] [CrossRef]
- Lin, Q.; Qiu, X.; Zheng, Z.; Xie, C.; Xu, Z.; Han, R. Characteristics of the Degenerate Strains of Cordyceps militaris. Mycosystema 2010, 29, 670–677. [Google Scholar]
- Xiong, C.; Xia, Y.; Zheng, P.; Wang, C. Increasing Oxidative Stress Tolerance and Subculturing Stability of Cordyceps militaris by Overexpression of a Glutathione Peroxidase Gene. Appl. Microbiol. Biotechnol. 2013, 97, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-X.; Wei, T.; Xue, L.-N.; Zheng, Q.-W.; Ye, Z.-W.; Zou, Y.; Yang, Y.; Yun, F.; Guo, L.-Q.; Lin, J.-F. Transcriptome Analysis Reveals the Flexibility of Cordycepin Network in Cordyceps militaris Activated by L-Alanine Addition. Front. Microbiol. 2020, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, Y.; Shao, Y.; Huang, B. A Novel Technique for Rejuvenation of Degenerated Caterpillar Medicinal Mushroom, Cordyceps militaris (Ascomycetes), a Valued Traditional Chinese Medicine. Int. J. Med. Mushrooms 2017, 19, 87–91. [Google Scholar] [CrossRef]
- He, L.; Han, C.; Li, P.; Chen, Y.; Liu, D.; Geng, L. Effect of Mineral Elements on Colony Types of Cordyceps militaris in Subculturing. J. Shenyang Agric. Univ. 2009, 40, 672–677. [Google Scholar]
- Wang, H.; Wei, J.; Lin, N.; Feng, A.; Chen, M.; Bao, D. Distribution of Mating-Type Genes in Fruiting and Non-Fruiting Forms of Cordyceps militaris. Acta Edulis Fungi 2010, 17, 1–4. [Google Scholar]
- Xin, X.; Yin, J.; Zhang, B.; Li, Z.; Zhao, S.; Gui, Z. Genome-Wide Analysis of DNA Methylation in Subcultured Cordyceps militaris. Arch. Microbiol. 2019, 201, 369–375. [Google Scholar] [CrossRef]
- Sun, H.; Hu, T.; Guo, Y.; Liang, Y. Preservation Affects the Vgetative Growth and Fruiting Body Production of Cordyceps militaris. World J. Microbiol. Biotechnol. 2018, 34, 166. [Google Scholar] [CrossRef]
- Lee, H.-H.; Kang, N.; Park, I.; Park, J.; Kim, I.; Kim, J.; Kim, N.; Lee, J.-Y.; Seo, Y.-S. Characterization of Newly Bred Cordyceps militaris Strains for Higher Production of Cordycepin through HPLC and URP-PCR Analysis. J. Microbiol. Biotechnol. 2017, 27, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Z.; Liu, C.; Wang, S.; Huang, B. Genome-Wide Analysis of DNA Methylation in the Sexual Stage of the Insect Pathogenic Fungus Cordyceps militaris. Fungal Biol. 2015, 119, 1246–1254. [Google Scholar] [CrossRef]
- Meina, L.; Chunyan, L.; Bosen, F.; Qingwei, L. Molecular Analysis of Degeneration of Artificial Flanted Cordyceps militaris. Jun Wu Xi Tong Mycosystema 2003, 22, 277–282. [Google Scholar]
- Raethong, N.; Wang, H.; Nielsen, J.; Vongsangnak, W. Optimizing Cultivation of Cordyceps militaris for Fast Growth and Cordycepin Overproduction Using Rational Design of Synthetic Media. Comput. Struct. Biotechnol. J. 2020, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Matías, R.; Ordaz-Hernández, A.; Angel-Cuapio, A.; Colin-Bonifacio, Y.; Garcia-Garcia, R.E.; Ángel-Sahagún, C.A.; Arredondo-Bernal, H.C. Principal Component Analysis of the Biological Characteristics of Entomopathogenic Fungi in Nutrient-limited and Cuticle-based Media. J. Basic Microbiol. 2021, 61, 147–156. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Liang, X.; Zhao, J.; Wang, Y.; Li, S.-P. Dynamic Analysis of Nucleosides and Carbohydrates during Developmental Stages of Cordyceps militaris in Silkworm (Bombyx mori). J. AOAC Int. 2019, 102, 741–747. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhang, H.; Xiao, L.; Lei, Z.; Kaul, S.C.; Wadhwa, R.; Zhang, Z. Low Dose of Fluoride in the Culture Medium of Cordyceps militaris Promotes Its Growth and Enhances Bioactives with Antioxidant and Anticancer Properties. J. Fungi 2021, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Q.; Qiu, X.-H.; Cao, L.; Han, R.-C. Scratching Stimuli of Mycelia Influence Fruiting Body Production and ROS-Scavenging Gene Expression of Cordyceps militaris. Mycobiology 2018, 46, 382–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Xia, Y.; Luo, F.; Dong, C.; Wang, C. Functional Convergence and Divergence of Mating-Type Genes Fulfilling in Cordyceps militaris. Fungal Genet. Biol. 2016, 88, 35–43. [Google Scholar] [CrossRef]
- Li, W.; Zhao, S.; Chen, H.; Yuan, H.; Wang, T.; Huang, X. High-Yielding Cordycepin in Cordyceps militaris Modified by Low-Energy Ion Beam. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2009, 25, 1725–1731. [Google Scholar]
- Singpoonga, N.; Rittiron, R.; Seang-On, B.; Chaiprasart, P.; Bantadjan, Y. Determination of Adenosine and Cordycepin Concentrations in Cordyceps militaris Fruiting Bodies Using Near-Infrared Spectroscopy. ACS Omega 2020, 5, 27235–27244. [Google Scholar] [CrossRef]
- Fan, D.; Wang, W.; Zhong, J.-J. Enhancement of Cordycepin Production in Submerged Cultures of Cordyceps militaris by Addition of Ferrous Sulfate. Biochem. Eng. J. 2012, 60, 30–35. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.H.; Kim, H.R.; Chun, Y.; Lee, J.H.; Yoo, H.Y.; Park, C.; Kim, S.W. Improved Cordycepin Production by Cordyceps militaris KYL05 Using Casein Hydrolysate in Submerged Conditions. Biomolecules 2019, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.-B.; Eksriwong, T.; Chauvatcharin, S.; Zhong, J.-J. Optimization of Carbon Source and Carbon/Nitrogen Ratio for Cordycepin Production by Submerged Cultivation of Medicinal Mushroom Cordyceps militaris. Process Biochem. 2005, 40, 1667–1672. [Google Scholar] [CrossRef]
- Masuda, M.; Urabe, E.; Honda, H.; Sakurai, A.; Sakakibara, M. Enhanced Production of Cordycepin by Surface Culture Using the Medicinal Mushroom Cordyceps militaris. Enzyme Microb. Technol. 2007, 40, 1199–1205. [Google Scholar] [CrossRef]
- Masuda, M.; Das, S.K.; Hatashita, M.; Fujihara, S.; Sakurai, A. Efficient Production of Cordycepin by the Cordyceps militaris Mutant G81-3 for Practical Use. Process. Biochem. 2014, 49, 181–187. [Google Scholar] [CrossRef]
- Hu, T.; Liang, Y.; Zhao, G.; Wu, W.; Li, H.; Guo, Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019, 187, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Shih, L.; Tsai, K.-L.; Hsieh, C. Effects of Culture Conditions on the Mycelial Growth and Bioactive Metabolite Production in Submerged Culture of Cordyceps militaris. Biochem. Eng. J. 2007, 33, 193–201. [Google Scholar] [CrossRef]
- Jiapeng, T.; Yiting, L.; Li, Z. Optimization of Fermentation Conditions and Purification of Cordycepin from Cordyceps militaris. Prep. Biochem. Biotechnol. 2014, 44, 90–106. [Google Scholar] [CrossRef]
- Lou, H.; Ye, Z.; Yun, F.; Lin, J.; Guo, L.; Chen, B.; Mu, Z. Targeted Gene Deletion in Cordyceps militaris Using the Split-Marker Approach. Mol. Biotechnol. 2018, 60, 380–385. [Google Scholar] [CrossRef]
- Lou, H.-W.; Ye, Z.-W.; Yu, Y.-H.; Lin, J.-F.; Guo, L.-Q.; Chen, B.-X.; Tang, H.-B.; Wei, T.; Chen, L.-T.; Yun, F. The Efficient Genetic Transformation of Cordyceps militaris by Using Mononuclear Protoplasts. Sci. Hortic. 2019, 243, 307–313. [Google Scholar] [CrossRef]
- Lou, H.-W.; Zhao, Y.; Chen, B.-X.; Yu, Y.-H.; Tang, H.-B.; Ye, Z.-W.; Lin, J.-F.; Guo, L.-Q. Cmfhp Gene Mediates Fruiting Body Development and Carotenoid Production in Cordyceps militaris. Biomolecules 2020, 10, 410. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.-X.; Wei, T.; Ye, Z.-W.; Yun, F.; Kang, L.-Z.; Tang, H.-B.; Guo, L.-Q.; Lin, J.-F. Efficient CRISPR-Cas9 Gene Disruption System in Edible-Medicinal Mushroom Cordyceps militaris. Front. Microbiol. 2018, 9, 1157. [Google Scholar] [CrossRef]
- Butt, T.M.; Goettel, M.S. Bioassays of Entomogenous Fungi. In Bioassays of Entomopathogenic Microbes and Nematodes; Centre for Agriculture and Bioscience International: Wallingford, UK, 2000; pp. 141–195. [Google Scholar]
- Butt, T.M.; Wang, C.; Shah, F.A.; Hall, R. Degeneration of Entomogenous Fungi. In An Ecological and Societal Approach to Biological Control; Springer: Berlin/Heidelberg, Germany, 2006; pp. 213–226. [Google Scholar]
- Kawakami, K. On the Changes of Characteristics of the Silkworm Muscardines through Successive Cultures. Bull. Seric. Exp. Stn. Jpn. 1960, 16, 83–99. [Google Scholar]
- Nagaich, B.B. Verticillium sp. Pathogenic on Aphids. Indian Phytopathol. 1973, 26, 163–165. [Google Scholar]
- Ibrahim, L.; Jenkinson, P. Effect of Artificial Culture Media on Germination, Growth, Virulence and Surface Properties of the Entomopathogenic Hyphomycete Metarhizium anisopliae. Mycol. Res. 2002, 106, 705–715. [Google Scholar] [CrossRef]
- Sun, S.-J.; Deng, C.-H.; Zhang, L.-Y.; Hu, K.-H. Molecular Analysis and Biochemical Characteristics of Degenerated Strains of Cordyceps Militaris. Arch. Microbiol. 2017, 199, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xin, X.; Weng, Y.; Gui, Z. Transcriptome-Wide Analysis Reveals the Progress of Cordyceps militaris Subculture Degeneration. PLoS ONE 2017, 12, e0186279. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; He, X.; Zhao, R.; Ren, G.; Liu, Y. Comparison of Cordyceps militaris Spawn Rejuvenation Method. North. Hortic. 2016, 40, 137–139. [Google Scholar]
- Grognet, P.; Bidard, F.; Kuchly, C.; Tong, L.C.H.; Coppin, E.; Benkhali, J.A.; Couloux, A.; Wincker, P.; Debuchy, R.; Silar, P. Maintaining Two Mating Types: Structure of the Mating Type Locus and Its Role in Heterokaryosis in Podospora anserina. Genetics 2014, 197, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Ni, M.; Feretzaki, M.; Sun, S.; Wang, X.; Heitman, J. Sex in Fungi. Annu. Rev. Genet. 2011, 45, 405–430. [Google Scholar] [CrossRef]
- Yin, J.; Xin, X.-D.; Weng, Y.-J.; Li, S.-H.; Jia, J.-Q.; Gui, Z.-Z. Genotypic Analysis of Degenerative Cordyceps militaris Cultured in the Pupa of Bombyx mori. Entomol. Res. 2018, 48, 137–144. [Google Scholar] [CrossRef]
- Yokoyama, E.; Yamagishi, K.; Hara, A. Development of a PCR-Based Mating-Type Assay for Clavicipitaceae. FEMS Microbiol. Lett. 2004, 237, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Li, M.F.; He, J.; Ding, L.; Kang, J.; Zhang, Q.; Zheng, Q. Single Spore Strains without Producing Fruit Body Isolated from Cordyceps militaris and Their RAPD Analysis. Southwest China J. Agric. Sci. 2007, 20, 547–550. [Google Scholar]
- Sulewska, A.; Niklinska, W.; Kozlowski, M.; Minarowski, L.; Naumnik, W.; Niklinski, J.; Dabrowska, K.; Chyczewski, L. DNA Methylation in States of Cell Physiology and Pathology. Folia Histochem. Cytobiol. 2007, 45, 149–158. [Google Scholar]
- Lee, T.; Zhai, J.; Meyers, B.C. Conservation and Divergence in Eukaryotic DNA Methylation. Proc. Natl. Acad. Sci. USA 2010, 107, 9027–9028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selker, E.U.; Tountas, N.A.; Cross, S.H.; Margolin, B.S.; Murphy, J.G.; Bird, A.P.; Freitag, M. The Methylated Component of the Neurospora crassa Genome. Nature 2003, 422, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Choi, J.; Lee, G.-W.; Park, S.-Y.; Huh, A.; Dean, R.A.; Lee, Y.-H. Genome-Wide Profiling of DNA Methylation Provides Insights into Epigenetic Regulation of Fungal Development in a Plant Pathogenic Fungus, Magnaporthe oryzae. Sci. Rep. 2015, 5, 8567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for Health and Wellbeing: A Potent Bioactive Metabolite of an Entomopathogenic Medicinal Fungus Cordyceps with Its Nutraceutical and Therapeutic Potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal Uses of the Mushroom Cordyceps militaris: Current State and Prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Zheng, Q.; Wei, T.; Lin, Y.; Ye, Z.-W.; Lin, J.-F.; Guo, L.-Q.; Yun, F.; Kang, L. Developing a Novel Two-Stage Process for Carotenoid Production by Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2019, 21, 47–57. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of Lignocellulosic Residues by Agrocybe Cylindracea and Pleurotus Ostreatus Mushroom Fungi–Assessment of Their Effect on the Final Product and Spent Substrate Properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of Olive Mill Wastewater and Bioconversion of Olive Crop Residues into High-Value-Added Biomass by the Choice Edible Mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannatos, D.; Koutrotsios, G.; Xekalaki, S.; Tarantilis, P.; Zervakis, G.I. Cultivation of the Entomopathogenic Fungus Cordyceps militaris in Insect-Enriched Substrates. In Proceedings of the ISMS e-Congress, Virtual, 14–17 September 2021. [Google Scholar]
- Czaja, W.; Miller, K.Y.; Skinner, M.K.; Miller, B.L. Structural and Functional Conservation of Fungal MatA and Human SRY Sex-Determining Proteins. Nat. Commun. 2014, 5, 5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzenberg, R.L.; Glass, N.L. Mating Type and Mating Strategies in Neurospora. Bioessays 1990, 12, 53–59. [Google Scholar] [CrossRef]
- Kang, N.; Lee, H.-H.; Park, I.; Seo, Y.-S. Development of High Cordycepin-Producing Cordyceps militaris Strains. Mycobiology 2017, 45, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Liang, Y. Improvement of Fruiting Body Production in Cordyceps militaris by Molecular Assessment. Arch. Microbiol. 2013, 195, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, C.; Cao, L.; Xie, C.; Han, R. Agrobacterium tumefaciens-Mediated Transformation as a Tool for Insertional Mutagenesis in Medicinal Fungus Cordyceps militaris. Fungal Biol. 2011, 115, 265–274. [Google Scholar] [CrossRef]
- Yang, T.; Guo, M.; Yang, H.; Guo, S.; Dong, C. The Blue-Light Receptor CmWC-1 Mediates Fruit Body Development and Secondary Metabolism in Cordyceps militaris. Appl. Microbiol. Biotechnol. 2016, 100, 743–755. [Google Scholar] [CrossRef]
- Dong, J.Z.; Wang, S.H.; Ai, X.R.; Yao, L.; Sun, Z.W.; Lei, C.; Wang, Y.; Wang, Q. Composition and Characterization of Cordyxanthins from Cordyceps militaris Fruit Bodies. J. Funct. Foods 2013, 5, 1450–1455. [Google Scholar] [CrossRef]
- Lou, H.-W.; Zhao, Y.; Tang, H.-B.; Ye, Z.-W.; Wei, T.; Lin, J.-F.; Guo, L.-Q. Transcriptome Analysis of Cordyceps militaris Reveals Genes Associated with Carotenoid Synthesis and Identification of the Function of the Cmtns Gene. Front. Microbiol. 2019, 10, 2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frappier, F.; Pais, M.; Elsworth, J.F.; Grove, J.F. Revised Structure of Beauvellide, a Cyclodepsipeptide from Beauveria tenella. Phytochemistry 1978, 17, 545–546. [Google Scholar] [CrossRef]
- Mochizuki, K.; Ohmori, K.; Tamura, H.; Shizuri, Y.; Nishiyama, S.; Miyoshi, E.; Yamamura, S. The Structures of Bioactive Cyclodepsipeptides, Beauveriolides I and II, Metabolites of Entomopathogenic Fungi Beauveria sp. Bull. Chem. Soc. Jpn. 1993, 66, 3041–3046. [Google Scholar] [CrossRef]
- Namatame, I.; Tomoda, H.; Tabata, N.; SI, S.; Omura, S. Structure Elucidation of Fungal Beauveriolide III, a Novel Inhibitor of Lipid Droplet Formation in Mouse Macrophages. J. Antibiot. 1999, 52, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namatame, I.; Tomoda, H.; Si, S.; Yamaguchi, Y.; Masuma, R.; Omura, S. Beauveriolides, Specific Inhibitors of Lipid Droplet Formation in Mouse Macrophages, Produced by Beauveria sp. FO-6979. J. Antibiot. 1999, 52, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Namatame, I.; Tomoda, H.; Ishibashi, S.; Omura, S. Antiatherogenic Activity of Fungal Beauveriolides, Inhibitors of Lipid Droplet Accumulation in Macrophages. Proc. Natl. Acad. Sci. USA 2004, 101, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, Y.-L.; Zhang, M.-L.; Zhang, H.-D.; Huang, J.-Z.; Li, L. Genome Mining and Biosynthesis of the Acyl-CoA: Cholesterol Acyltransferase Inhibitor Beauveriolide I and III in Cordyceps militaris. J. Biotechnol. 2020, 309, 85–91. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical Composition and Nutritional and Medicinal Value of Fruit Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Manson, W.; Spring, F.S.; Hutchinson, S.A. Cordycepin, a Metabolic Product Isolated from Cultures of Cordyceps militaris (Linn.) Link. Nature 1950, 166, 949. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Cordyceps—A Traditional Chinese Medicine and Another Fungal Therapeutic Biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, Y. The Strategies for Increasing Cordycepin Production of Cordyceps militaris by Liquid Fermentation. Fungal Genom. Biol. 2016, 6, 134. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.-J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I. Cordycepin Inhibits Lipopolysaccharide-Induced Inflammation by the Suppression of NF-ΚB through Akt and P38 Inhibition in RAW 264.7 Macrophage Cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef]
- Noh, E.-M.; Kim, J.-S.; Hur, H.; Park, B.-H.; Song, E.-K.; Han, M.-K.; Kwon, K.-B.; Yoo, W.-H.; Shim, I.-K.; Lee, S.J. Cordycepin Inhibits IL-1β-Induced MMP-1 and MMP-3 Expression in Rheumatoid Arthritis Synovial Fibroblasts. Rheumatology 2009, 48, 45–48. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Li, Y.; Shen, Y.; Li, Q.; Wang, Q.; Feng, L. Apoptosis and Inhibition of Proliferation of Cancer Cells Induced by Cordycepin. Oncol. Lett. 2015, 10, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuli, H.S.; Sandhu, S.S.; Sharma, A.K. Pharmacological and Therapeutic Potential of Cordyceps with Special Reference to Cordycepin. 3 Biotech 2014, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Cai, G.; He, Y.I.; Tong, G. Separation of Cordycepin from Cordyceps militaris Fermentation Supernatant Using Preparative HPLC and Evaluation of Its Antibacterial Activity as an NAD+-Dependent DNA Ligase Inhibitor. Exp. Ther. Med. 2016, 12, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal Cordycepin Biosynthesis Is Coupled with the Production of the Safeguard Molecule Pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballario, P.; Vittorioso, P.; Magrelli, A.; Talora, C.; Cabibbo, A.; Macino, G. White Collar-1, a Central Regulator of Blue Light Responses in Neurospora, Is a Zinc Finger Protein. EMBO J. 1996, 15, 1650–1657. [Google Scholar] [CrossRef]
- Dunlap, J.C. Proteins in the Neurospora Circadian Clockworks. J. Biol. Chem. 2006, 281, 28489–28493. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Dunlap, J.C.; Loros, J.J. Neurospora Illuminates Fungal Photoreception. Fungal Genet. Biol. 2010, 47, 922–929. [Google Scholar] [CrossRef] [Green Version]
- Corrochano, L.M. Fungal Photoreceptors: Sensory Molecules for Fungal Development and Behaviour. Photochem. Photobiol. Sci. 2007, 6, 725–736. [Google Scholar] [CrossRef]
- Linden, H.; Ballario, P.; Macino, G. Blue Light Regulation In Neurospora crassa. Fungal Genet. Biol. 1997, 22, 141–150. [Google Scholar] [CrossRef]
- Linden, H. A White Collar Protein Senses Blue Light. Science 2002, 297, 777–778. [Google Scholar] [CrossRef]
- Idnurm, A.; Verma, S.; Corrochano, L.M. A Glimpse into the Basis of Vision in the Kingdom Mycota. Fungal Genet. Biol. 2010, 47, 881–892. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Song, X.; Dong, X.; Zhang, J.; Dong, C. DASH-Type Cryptochromes Regulate Fruiting Body Development and Secondary Metabolism Differently than CmWC-1 in the Fungus Cordyceps militaris. Appl. Microbiol. Biotechnol. 2017, 101, 4645–4657. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Q.; Zhang, J.; Liu, K.; Li, K.; Liu, G.; Dong, C. Comparative Transcriptome Analysis between a Spontaneous Albino Mutant and Its Sibling Strain of Cordyceps militaris in Response to Light Stress. Front. Microbiol. 2018, 9, 1237. [Google Scholar] [CrossRef]
- Jiaojiao, Z.; Fen, W.; Kuanbo, L.; Qing, L.; Ying, Y.; Caihong, D. Heat and Light Stresses Affect Metabolite Production in the Fruit Body of the Medicinal Mushroom Cordyceps militaris. Appl. Microbiol. Biotechnol. 2018, 102, 4523–4533. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tang, J.; Chen, S.; Wu, Y.; Wang, K.; Ma, B.; Zhou, Q.; Chen, A.; Wang, Y. MilR4 and MilR16 Mediated Fruiting Body Development in the Medicinal Fungus Cordyceps militaris. Front. Microbiol. 2019, 10, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.-Y.; Cao, H.-F.; Liu, S.-K.; Wu, M.; Li, S.-S.; Zhang, Z.-Q.; Chen, A.-J.; Shen, G.-H.; Wu, H.-J.; Li, M.-L. Novel Liquid Fermentation Medium of Cordyceps militaris and Optimization of Hydrothermal Reflux Extraction of Cordycepin. J. Asian Nat. Prod. Res. 2020, 22, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Pan, M.-C.; Chang, C.-K.; Chang, S.-W.; Hsieh, C.-W. Optimization of Ultrasonic-Assisted Extraction of Cordycepin from Cordyceps militaris Using Orthogonal Experimental Design. Molecules 2014, 19, 20808–20820. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Chen, S.-T.; Chang, J.-C.; Hsu, T.-Y.; Cheng, C.-C.; Chang, H.-S.; Liu, C.-S.; Wu, Y.-C.; Liang, Z.-C. Radical Scavenging and Antiproliferative Effects of Cordycepin-Rich Ethanol Extract from Brown Rice—Cultivated Cordyceps militaris (Ascomycetes) Mycelium on Breast Cancer Cell Lines. Int. J. Med. Mushrooms 2019, 21, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Lei, C.; Zheng, X.J.; Ai, X.R.; Wang, Y.; Wang, Q. Light Wavelengths Regulate Growth and Active Components of Cordyceps militaris Fruit Bodies. J. Food Biochem. 2013, 37, 578–584. [Google Scholar] [CrossRef]
- Dong, J.Z.; Lei, C.; Ai, X.R.; Wang, Y. Selenium Enrichment on Cordyceps militaris Link and Analysis on Its Main Active Components. Appl. Biochem. Biotechnol. 2012, 166, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.C.; Li, G.R.; Kang, J.C.; Kang, C.; Hyde, K.D. Optimization of Solid-State Fermentation for Fruiting Body Growth and Cordycepin Production by Cordyceps militaris. Chiang Mai J. Sci. 2014, 41, 858–872. [Google Scholar]
- Kang, C.; Wen, T.-C.; Kang, J.-C.; Meng, Z.-B.; Li, G.-R.; Hyde, K.D. Optimization of Large-Scale Culture Conditions for the Production of Cordycepin with Cordyceps militaris by Liquid Static Culture. Sci. World J. 2014, 2014, 510627. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.-Y.; Kuo, H.-P.; Tsai, S.-F.; Huang, S.-T.; Yang, M.-J.; Lee, S.-S.; Chang, C.-C. Doubled Production of Cordycepin Analogs in Cultured Cordyceps militaris by Addition of Andrea Droppings. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Suparmin, A.; Kato, T.; Dohra, H.; Park, E.Y. Insight into Cordycepin Biosynthesis of Cordyceps militaris: Comparison between a Liquid Surface Culture and a Submerged Culture through Transcriptomic Analysis. PLoS ONE 2017, 12, e0187052. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.C.; Kang, J.C.; Lei, B.X.; Li, G.R.; He, J. Enhanced Production of Cordycepin by Submerged Culture Using Additives in Cordyceps militaris. Food Sci. 2010, 31, 175–179. [Google Scholar]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. The Methanolic Extract of Cordyceps militaris (L.) Link Fruiting Body Shows Antioxidant, Antibacterial, Antifungal and Antihuman Tumor Cell Lines Properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Chen, Y.-H.; Pan, B.-S.; Chang, M.-M.; Huang, B.-M. Functional Study of Cordyceps sinensis and Cordycepin in Male Reproduction: A Review. J. Food Drug Anal. 2017, 25, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Kunhorm, P.; Chaicharoenaudomrung, N.; Noisa, P. Enrichment of Cordycepin for Cosmeceutical Applications: Culture Systems and Strategies. Appl. Microbiol. Biotechnol. 2019, 103, 1681–1691. [Google Scholar] [CrossRef]
- Lee, C.-T.; Huang, K.-S.; Shaw, J.-F.; Chen, J.-R.; Kuo, W.-S.; Shen, G.; Grumezescu, A.M.; Holban, A.M.; Wang, Y.-T.; Wang, J.-S. Trends in the Immunomodulatory Effects of Cordyceps militaris: Total Extracts, Polysaccharides and Cordycepin. Front. Pharmacol. 2020, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Shinozuka, K.; Yoshikawa, N. Anticancer and Antimetastatic Effects of Cordycepin, an Active Component of Cordyceps sinensis. J. Pharmacol. Sci. 2015, 127, 53–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.Y.; Park, S.J.; Park, Y.J. The Anticancer Properties of Cordycepin and Their Underlying Mechanisms. Int. J. Mol. Sci. 2018, 19, 3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
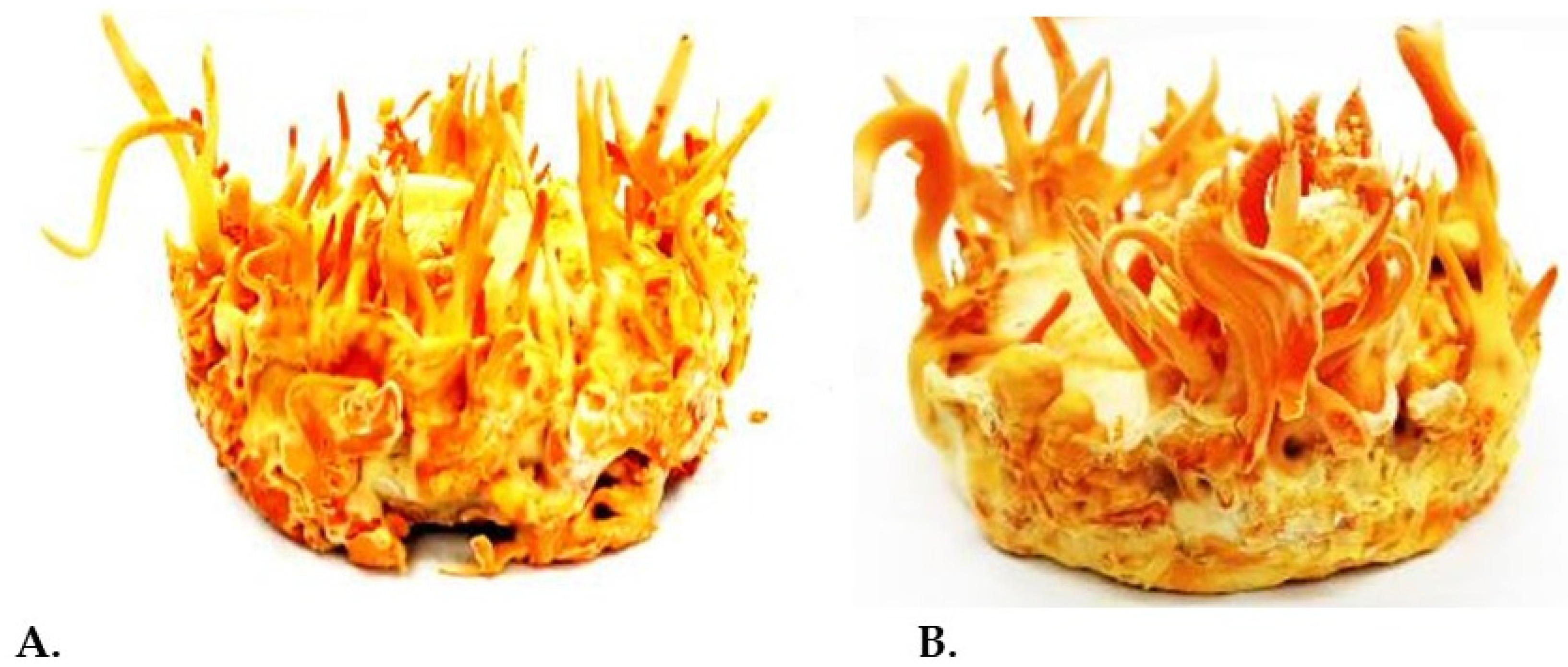
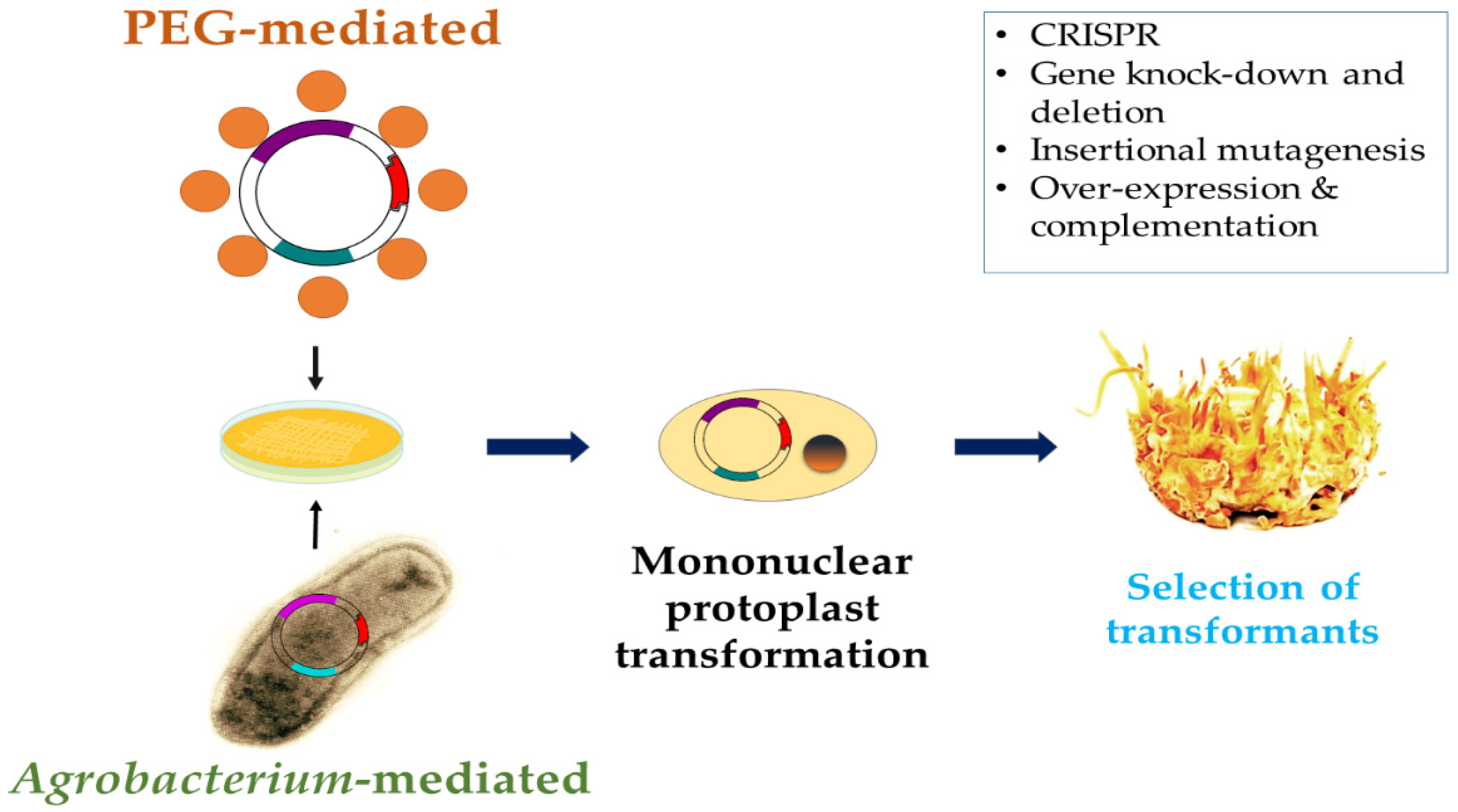
| Factors | Effects on Culture Degeneration: P: Promotes; H: Hinders | Treatment | References |
|---|---|---|---|
| K+, Ca2+, Zn2+ | H | Addition in culture | [25] |
| Mn2+, Mg2+ | P | Avoid usage | [25] |
| Preservation | H | Preservation at 4 °C | [28] |
| Cellular accumulation of reactive oxygen species (ROS) | P | [22] | |
| Subculturing | P | Avoid subculturing after the third generation Rejuvenation by mating single ascospore isolates and producing new heterokaryons | [58] |
| Culture temperature-medium | P | Degenerated strains could be rejuvenated by using an insect host | [59] |
| Homokaryosis | P | Rejuvenation every 6 months Appropriate light regime during preservation | [21,24,26,29] |
| DNA methylation | P | [27,30] | |
| Genetic mutations | P | [27,31] |
| Substrates, Nutrients, and Treatments in the Cultivation of C. militaris | Cultivation-Related Practices | Effects on Ascomata Production or Mycelium Growth | References |
|---|---|---|---|
| Serotonin, adrenaline, and dopamine catabolism to amino acids | Addition of insect-based supplements in culture | Induces amino acids production | [32] |
| Chitin, glucosamine, and GABA catabolism in low C/N availability | Addition of insect-based supplements Addition of rice Addition of chitosan | Highest growth rate and improved cordycepin production | [32,33,34] |
| Sucrose utilization | Addition (instead of glucose) | Highest growth rate | [32] |
| C/N ratio | 12.7:1 | Optimal | [32] |
| Cottonseed shells, corn cobs particles at ratios of 8:1:1 (w/w/w) with rice and wheat | - | Increased fruit body yield and improved cordycepin levels | [12] |
| Vegetable oils | Addition in liquid culture | Cell growth and cordycepin production enhancement | [15] |
| Fluoride | Addition of 0.01 mM | Growth promotion and bioactive substance enhancement; stronger anti-proliferation effects of ascomata extracts on U2OS cancer cells | [35] |
| Aerial mycelia scratching | Scratching C. militaris degenerated mycelia before stromata differentiation | Shortening growth periods of C. militaris fruit bodies by at least 5 days | [36] |
| Strain Code (or N/A When No Strain Code Appears) | Cultivation Conditions | Type of Biomass and/or Substrate Measured | Cordycepin Concentration | References |
|---|---|---|---|---|
| Ν/A | Effects of various light wavelengths (darkness, red, pink blue, and daylight) | M | 1.15–1.98 mg g−1 | [115] |
| NBRC 9787 | Addition of 1 g L−1 adenine plus glycine or l-glutamine in batch operation Addition of 1 g L−1 adenine plus glycine or l-glutamine repeated batch operation | M | 19.7–33.7 mg L−1 62.4–98.2 mg L−1 | [43] |
| NBRC 9787 G81-3 | Various strains and autoclaving techniques | M | 76–237 mg g−1 | [44] |
| Ν/A | Optimized carbon and nitrogen sources, i.e., 42.0 g L−1 glucose and 15.8 g L−1 peptone | M | Up to 345.4 mg L−1 | [42] |
| KYL05 | pH 6, 25 °C, 150 rpm, culture period of 6 days, carbon source: casein hydrolysate at 2% | M | Up to 445 mg L−1 | [41] |
| Ν/A | pH 6, 25 °C, 110 rpm, culture period of 15–20 days, addition of 1 g L−1 FeSO4 at day 0 | M | Up to 596.6 mg L−1 | [40] |
| CICC 14014 | Addition of 30 g L−1 peanut oil in standard medium | M | Up to 5290 mg L−1 | [15] |
| Ν/A | Effects of sodium selenite (0–18 ppm) | F | 0–0.6 mg g−1 | [116] |
| CGMCC2459 | Effect of various mineral salts: K2HPO4, KH2PO4, Ca(NO3)2, CaCl2, KCl, MgSO47H2O, FeSO4 | F | 0.95–5.72 mg g−1 | [117] |
| CGMCC33.16322 | Wheat standard substrate and pupal (B. mori) injection | F | ~1 mg g−1 ~1.2 mg g−1 | [17] |
| CGMCC2459 | Effect of different nitrogen sources (wheat bran, soybean oil meal, beef extract, peptone, yeast extract, silkworm pupa, NH4NO3) | F | 1.78–10.90 mg g−1 | [117] |
| CGMCC2459 | 20 g of brown rice, millet, sorghum, corn, wheat, and glutinous rice as fruiting medium supplemented with 32 mL of nutrient solution | F | 2.42–5.62 mg g−1 | [117] |
| CGMCC2459 | Effect of various growth factors (vitamins B1, B9, α-naphthyl acetic acid, 2,4-dichlorophenoxyacetic acid, indole-3-butytric acid | F | 2.92–6.21 mg g−1 | [117] |
| CGMCC3.16321 | Generation of 498 (CGMCC 5.2190) sibling normal strain, generation of 505 (CGMCC 5.2191) albino strain by spontaneous mutation | F | 3.09 mg g−1 for the normal sibling strain 6.70 mg g−1 for the albino strain | [109] |
| CGMCC2459 | Effect of various carbon sources (glucose, sucrose, amidulin, lactose, maltose, mannose) | F | 3.77–6.50 mg g−1 | [117] |
| CGMCC2459 | Effect of initial pH (5.0–8.0) | F | 4.39–7.40 mg g−1 | [117] |
| CGMCC 3.16321 | Heat stress treatment at 25 °C, light at 1700 lx | F | Up to 5.56 mg g−1 | [110] |
| KSP8 | Single spore mating of SPNU 1006xKACC44455 | F | Up to 6.63 mg g−1 | [118] |
| N/A | Addition of tea leaves or Andraca theae droppings in basal media | F | 8.35–12.85 mg g−1 | [119] |
| Cm09 | Generation of ΔMAT1-1-2; injection of 107 ΔMAT1-1-2xΜAΤ1-2 spores/mL into the Chinese Tussah silkworm pupae | F | Up to 16.77 mg g−1 | [37] |
| NO. 20130508 | Corn cob particles/wheat bran/rice bran (8:1:1) + 20 g L−1 glucose, and 5 g L−1 peptone Cottonseed shells/wheat bran/rice (8:1:1) + 20 g L−1 glucose, and 5 g L−1 peptone; 20 g rice+ 20 g L−1 glucose, and 5 g L−1 peptone | F | 26.9 mg g−1 23.4 mg g−1 34.5 mg g−1 | [12] |
| Ν/A | 20 g rice and 20 mL potato dextrose medium + selenate or selenite or selenomethionine at a concentration of 40 μg g−1 in rice | F | 43.3 mg g−1 69.3 mg g−1 77.4 mg g−1 | [45] |
| CM10 | Basal medium: 20 g L−1 peptone, 24.7 g L−1 sucrose, 1.11 g L−1 K2HPO4⋅3H2O, 0.90 g L−1 MgSO4⋅7H2O, 0.01 g L−1 vitamin B1 plus 8 g L−1 L-alanine, and overexpression of CCM_02568 and CCM_01481 transcription factors | L | 30.04–99.83 mg L−1 | [23] |
| Ν/A | Liquid fermentation in basal media containing silkworm pupae powder, wheat, or silkworm pupae powder, plus wheat by applying different extraction methodologies (heat, frequency, solvents, and resins) | L | Up to 39.40 mg L−1 | [112] |
| CCRC 32219 | Multifactorial analysis (four factors): pH 4 to 7, various nitrogen sources, varying yeast extract content, shake vs. static conditions | L | 44.4–1375.6 mg L−1 | [46] |
| TBRC6039 | Rational design of synthetic media | L | 65.7–377 mg L−1 | [32] |
| GACP08Y5 GACP08Y1 GACP0746 | 20 g L−1 sucrose, 20 g L−1 peptone, 1 g L−1 KH2PO4, and 0.5 g L−1 MgSO4·7H2O in static liquid bioreactors of different culture volumes | L | 271–4376 mg L−1 | [118] |
| NBRC 9787 | Addition of 1 g L−1 adenine plus glycine or l-glutamine in batch operation Addition of 1 g L−1 adenine plus glycine or l-glutamine repeated batch operation | L | 542.4–2500 mg L−1 3400–14,100 mg L−1 | [43] |
| CGMCC2459 | Optimized medium (20 g L−1 peptone, 24.7 g L−1 sucrose, 1.11 g L−1 K2HPO4·3H2O, 0.90 g L−1 MgSO4·7H2O, 10 mg L−1 vitamin B1, 5.45 g L−1 hypoxanthine, and 12.23 g L−1 L-alanine) | L | Up to 2008 mg L−1 | [118] |
| NBRC 9787 G81-3 | Various strains and autoclaving conditions | L | 2400–10,900 mg L−1 | [44] |
| BCRC34380 | Effect of porcine liver extracts (0.5 g L−1, 1 g L−1, 5 g L−1, 7.5 g L−1, and 10 g L−1) | L | Up to 2452 mg L−1 | [12] |
| BCRC34380 | Effect of blue light irradiation (0, 8, 16, and 24 h d−1) | L | Up to 3483 mg L−1 | [12] |
| NBRC 103752 | 72.5 g L−1 yeast extract, 62.6 g L−1 glucose (pH 5.6), and Vogel’s medium at 1:10 concentration | L | Up to ~5000 mg L−1 | [120] |
| CM14014 | 60 g L−1 glucose, 0.7 g L−1 KH2PO4, 0.7 g L−1 MgSO4 7H2O, 9.00 g L−1 yeast extract, and 17.10 g L−1 tryptone at 27.1 °C; seed age, 3 days; inoculum size, 10% | L | Up to ~7350 mg L−1 | [47] |
| NBRC 9787 | Surface liquid culture and mutagenesis by ion beam irradiation | L | Up to 8570 mg L−1 | [38] |
| CM016 | Solid state fermentation on rice-based medium containing 20 g L−1 sucrose, 10 g L−1 peptone, 0.1 g L−1 MgSO4 7H2O, and 0.1 g L−1 KH2PO4 using a four-factor, five-leveled central composite against glucose, peptone, adenine, histidine | F | 1.92–20.86 mg g−1 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontogiannatos, D.; Koutrotsios, G.; Xekalaki, S.; Zervakis, G.I. Biomass and Cordycepin Production by the Medicinal Mushroom Cordyceps militaris—A Review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus. J. Fungi 2021, 7, 986. https://doi.org/10.3390/jof7110986
Kontogiannatos D, Koutrotsios G, Xekalaki S, Zervakis GI. Biomass and Cordycepin Production by the Medicinal Mushroom Cordyceps militaris—A Review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus. Journal of Fungi. 2021; 7(11):986. https://doi.org/10.3390/jof7110986
Chicago/Turabian StyleKontogiannatos, Dimitrios, Georgios Koutrotsios, Savvina Xekalaki, and Georgios I. Zervakis. 2021. "Biomass and Cordycepin Production by the Medicinal Mushroom Cordyceps militaris—A Review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus" Journal of Fungi 7, no. 11: 986. https://doi.org/10.3390/jof7110986
APA StyleKontogiannatos, D., Koutrotsios, G., Xekalaki, S., & Zervakis, G. I. (2021). Biomass and Cordycepin Production by the Medicinal Mushroom Cordyceps militaris—A Review of Various Aspects and Recent Trends towards the Exploitation of a Valuable Fungus. Journal of Fungi, 7(11), 986. https://doi.org/10.3390/jof7110986







