COVID-19-Associated Pulmonary Aspergillosis in Russia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- CAPA develops mainly on the background of diabetes (33%), hematological or oncological diseases (31%), and COPD (13%).
- The probability of CAPA significantly increases with lymphocytopenia for >10 days (OR = 8.156 (3.056–21.771), p = 0.001), decompensated diabetes (29% vs. 7%, (OR = 5.688 (1.991–16.246), p = 0.001), use of steroids at a prednisone-equivalent dose > 60 mg/day (OR = 4.493 (1.896–10.647), p = 0.001) and monoclonal antibodies to IL-1ß and IL-6 (OR = 2.880 (1.272–6.518), p = 0.01)
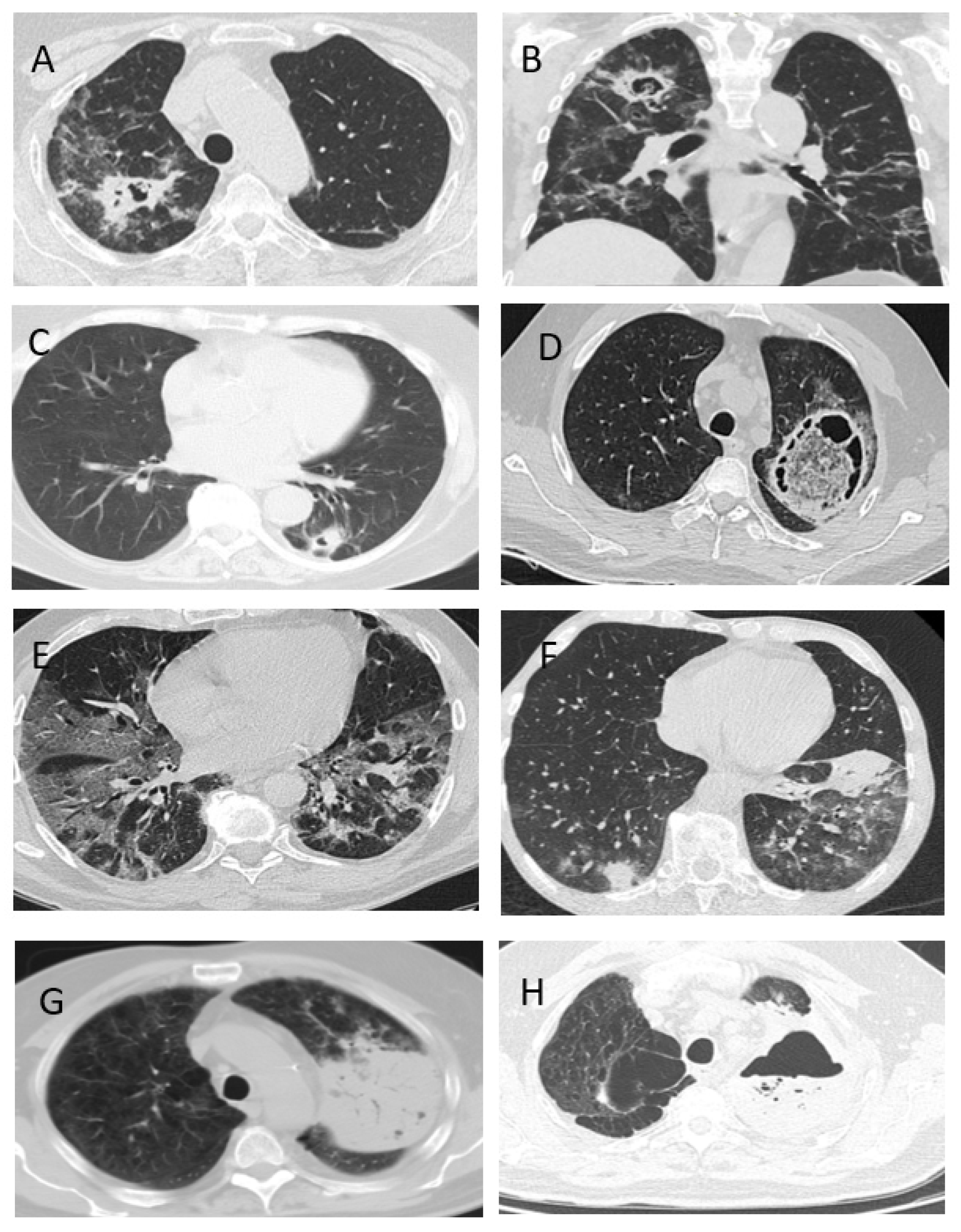
- CAPA is characterized by pulmonary involvement (100%), and rarely characterized by trachea and bronchi (7%).
- Severe disease course with prolonged (median—15.5 (5–60) days) stays in the ICU (71%), mechanical ventilation (52%) and ARDS (31%) are typical in CAPA patients.
- The clinical signs of CAPA are nonspecific, but typically include: fever (98%), cough (89%) and hemoptysis (36%). The radiological signs of CAPA are foci of consolidation (89%) and destruction (47%), and hydrothorax (26%).
- The most effective method of diagnosing CAPA is the GM test in BAL.
- The overall 12-week survival rate of patients with CAPA was 47.2%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van De Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis Complicating Severe Coronavirus Disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Cornely, O.A.; Böttiger, B.W.; Dusse, F.; Eichenauer, D.A.; Fuchs, F.; Hallek, M.; Jung, N.; Klein, F.; Persigehl, T.; et al. COVID-19 associated pulmonary aspergillosis. Mycoses 2020, 63, 528–534. [Google Scholar] [CrossRef] [PubMed]
- van Arkel, A.L.; Rijpstra, T.A.; Belderbos, H.N.; Van Wijngaarden, P.; Verweij, P.E.; Bentvelsen, R.G. COVID-19 associated pulmonary aspergillosis. Am. J. Respir. Crit. Care Med. 2020, 202, 132–135. [Google Scholar] [CrossRef]
- Alanio, A.; Dellière, S.; Fodil, S.; Bretagne, S.; Mégarbane, B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020, 8, e48–e49. [Google Scholar] [CrossRef]
- Nasir, N.; Farooqi, J.; Mahmood, S.F.; Jabeen, K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses 2020, 63, 766–770. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Ho, Y.-L.; Besen, B.A.M.P.; Malbouisson, L.M.S.; Taniguchi, L.U.; Mendes, P.V.; Costa, E.L.V.; Park, M.; Daltro-Oliveira, R.; Roepke, R.M.L.; et al. Additional file 1 of Protective ventilation and outcomes of critically ill patients with COVID-19: A cohort study. Ann. Intensive Care 2021, 11, 11. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Mostert, C.; Hentschker, C.; Voshaar, T.; Malzahn, J.; Schillinger, G.; Klauber, J.; Janssens, U.; Marx, G.; Weber-Carstens, S.; et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020, 8, 853–862. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis Among Intubated Patients With COVID-19: A Prospective Study. Clin. Infect. Dis. 2020, ciaa1065. [Google Scholar] [CrossRef]
- Gusarov, V.G.; Zamyatin, M.N.; Kamyshova, D.A.; Fomina, V.S.; Abovich, Y.A.; Lovtsevich, N.V.; Bronov, O.Y.; Petrova, L.V.; Sysoeva, T.S.; Vasilashko, V.I.; et al. Invasive pulmonary aspergillosis in COVID-19 patients. J. Infektologii 2021, 13, 38–49. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Dis-ease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Van De Veerdonk, F.L.; Jenks, J.D.; Koehler, P.; Krause, R.; Cornely, O.A.; Perlin, D.S.; Lass-Flörl, C.; Hoenigl, M. COVID-19 Associated Pulmonary Aspergillosis (CAPA)—From Immunology to Treatment. J. Fungi 2020, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Salmanton-Garcia, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19–Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, H.; Kuno, T.; Takagi, H.; Patrawalla, P. Incidence and mortality of COVID-19-associated pulmonary aspergillosis: A systematic review and meta-analysis. Mycoses 2021, 64, 993–1001. [Google Scholar] [CrossRef]
- Dellière, S.; Dudoignon, E.; Fodil, S.; Voicu, S.; Collet, M.; Oillic, P.-A.; Salmona, M.; Dépret, F.; Ghelfenstein-Ferreira, T.; Plaud, B.; et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: A French multicentric retrospective cohort. Clin. Microbiol. Infect. 2020, 27, 790.e1–790.e5. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Dannaoui, E.; Fekkar, A.; Luyt, C.E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Pharm, S.H.; Timsit, J.-F.; et al. Characterization of Fungal Infections in COVID-19 Infected Mechanically Ventilated Patients in I.C.U. the MY-CO-VID Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04368221 (accessed on 8 October 2021).
- Rutsaert, L.; Steinfort, N.; Van Hunsel, T.; Bomans, P.; Naesens, R.; Mertes, H.; Dits, H.; Van Regenmortel, N. COVID-19-associated invasive pulmonary aspergillosis. Ann. Intensiv. Care 2020, 10, 528–534. [Google Scholar] [CrossRef]
- Janssen, N.A.F.; Nyga, R.; Vanderbeke, L.; Jacobs, C.; Ergün, M.; Buil, J.B.; van Dijk, K.; Altenburg, J.; Bouman, C.S.C.; van der Spoel, H.I.; et al. Multinational Observational Cohort Study of COVID-19—Associated Pulmonary Aspergillosis. Emerg. Infect. Dis. 2021, 27, 2892. [Google Scholar] [CrossRef] [PubMed]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Salmanton-García, J.; Maertens, J.; Bourgeois, M.; Reynders, M.; Rutsaert, L.; Van Regenmortel, N.; Lormans, P.; et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clin. Microbiol. Infect. 2021, 27, 2892. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019—Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Zhang, P.; Sheng, J.; Zhou, J.; Qu, T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: A retrospective case series. Crit. Care 2020, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Buil, J.B.; Calandra, T.; Chiller, T.; Clancy, C.J.; Cornely, O.A.; et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensiv. Care Med. 2021, 47, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Lahmer, T.; Kriescher, S.; Herner, A.; Rothe, K.; Spinner, C.D.; Schneider, J.; Mayer, U.; Neuenhahn, M.; Hoffmann, D.; Geisler, F.; et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: Results from the prospective AspCOVID-19 study. PLoS ONE 2021, 16, e0238825. [Google Scholar] [CrossRef]
- Hatzl, S.; Reisinger, A.C.; Posch, F.; Prattes, J.; Stradner, M.; Pilz, S.; Eller, P.; Schoerghuber, M.; Toller, W.; Gorkiewicz, G.; et al. Antifungal prophylaxis for prevention of COVID-19-associated pulmonary asper-gillosis in critically ill patients: An observational study. Crit. Care. 2021, 25, 335. [Google Scholar] [CrossRef] [PubMed]

| CAPA | COVID-19 without IA | p-Value | |||
|---|---|---|---|---|---|
| n = 45 | % | n = 90 | % | ||
| Demographics | |||||
| males | 31 | 69% | 60 | 67% | 0.66 |
| females | 14 | 31% | 30 | 33% | |
| age median (years) | 34–82 62 | 25–82 63 | 0.67 | ||
| background diseases | |||||
| hematological diseases | 9 | 20% | 11 | 12% | 0.2 |
| lymphomas acute leukemia MM CLL others | 6 1 2 - - | 13% 2% 4% | 6 1 1 2 1 | 7% 1% 1% 1% | |
| oncology | 5 | 11% | 5 | 5.5% | 0.1 |
| active hematological/oncological disease | 11 | 24% | 2 | 2% | 0.03 |
| DM decompensated DM | 15 13 | 33% 29% | 17 6 | 19% 7% | 0.06 0.0003 |
| COPD | 6 | 13% | 5 | 5.5% | 0.09 |
| ARF/CRF | 5 | 11% | 8 | 9% | 0.6 |
| COVID-IA | COVID-19 without IA | p-Value | |||
|---|---|---|---|---|---|
| n/N | % | n/N | % | ||
| neutropenia <0.5 × 109/L | 4/45 | 9% | 0/90 | 0.006 | |
| duration (min-max)/ Me (days) | 5–25 10 | - | |||
| lymphocytopenia <1.0 × 109/L | 38/43 | 88% | 68/88 | 77% | 0.1 |
| duration (min-max)/ Me (days) | 5–100 15 | 2–42 9 | 0.00002 | ||
| lymphocytopenia >10 days | 29/35 | 83% | 32/86 | 37% | 0.006 |
| glucocorticoids (GCS): | 38/43 | 88% | 77/88 | 88% | 0.7 |
| GCS >60 mg/d in prednisone-equivalent dose | 17/37 | 46% | 14/88 | 16% | 0.01 |
| inhibitors of receptors IL-1β and IL-6 | 18/43 | 42% | 16/80 | 20% | 0.01 |
| Risk Factors | CAPA | COVID-19 without IA | OR (95% CI) | p-Value |
|---|---|---|---|---|
| n/N(%) | n/N(%) | |||
| decompensated DM | 13/45 (29%) | 6/90 (7%) | 5.688 (1.991–16.246) | 0.001 |
| lymphocytopenia >10 days | 29/35 (83%) | 32/86 (37%) | 8.156 (3.056–21.771) | 0.0001 |
| GCS >60 mg/d in prednisone-equivalent dose | 17/37 (46%) | 14/88 (16%) | 4.493 (1.896–10.647) | 0.001 |
| inhibitors of receptors IL-1β and IL-6 | 18/43 (42%) | 16/80 (20%) | 2.880 (1.272–6.518) | 0.01 |
| Features | CAPA n = 45 | COVID-19 without IA n = 90 | p-Value | ||
|---|---|---|---|---|---|
| N | % | n | % | ||
| fever | 44 | 98% | 62/73 | 85% | 0.007 |
| cough | 40 | 89% | 42/53 | 72% | 0.002 |
| chest pain | 10/42 | 24% | 4/45 | 9% | 0.05 |
| respiratory failure 2-3-4 (requiring O2 or ventilation) | 28 | 62% | 54 | 60% | 0.7 |
| ARDS | 14 | 31% | 16 | 18% | 0.02 |
| hemoptysis | 16 | 36% | 3/87 | 3% | 0.0001 |
| ICU | 32 | 71% | 57 | 63% | 0.4 |
| total days in ICU Me | 5–60 15.5 | 1–55 6 | 0.0004 | ||
| mechanical ventilation | 14 | 52% | 8/54 | 15% | 0.004 |
| CT-signs | |||||
| bilateral lesion | 42 | 93% | 75/80 | 94% | 0.8 |
| infiltrations | 40 | 89% | 37/63 | 59% | 0.004 |
| the “frosted glass” symptom | 33 | 73% | 64/80 | 80% | 0.3 |
| destruction cavity | 21 | 47% | 1 | 1% | 0.00001 |
| the “halo” symptom | - | - | - | - | |
| hydrothorax | 10/38 | 26% | 10/88 | 11% | 0.03 |
| Method | Result | |
|---|---|---|
| n | % | |
| microscopy (+) | 11 | 24% |
| culture (+) | 14 | 31% |
| GM in blood (+) | 3 | 7% |
| GM in BAL (+) | 25 | 56% |
| histology (+) | 3 | 7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shadrivova, O.; Gusev, D.; Vashukova, M.; Lobzin, D.; Gusarov, V.; Zamyatin, M.; Zavrazhnov, A.; Mitichkin, M.; Borzova, Y.; Kozlova, O.; et al. COVID-19-Associated Pulmonary Aspergillosis in Russia. J. Fungi 2021, 7, 1059. https://doi.org/10.3390/jof7121059
Shadrivova O, Gusev D, Vashukova M, Lobzin D, Gusarov V, Zamyatin M, Zavrazhnov A, Mitichkin M, Borzova Y, Kozlova O, et al. COVID-19-Associated Pulmonary Aspergillosis in Russia. Journal of Fungi. 2021; 7(12):1059. https://doi.org/10.3390/jof7121059
Chicago/Turabian StyleShadrivova, Olga, Denis Gusev, Maria Vashukova, Dmitriy Lobzin, Vitaliy Gusarov, Mikhail Zamyatin, Anatoliy Zavrazhnov, Mikhail Mitichkin, Yulia Borzova, Olga Kozlova, and et al. 2021. "COVID-19-Associated Pulmonary Aspergillosis in Russia" Journal of Fungi 7, no. 12: 1059. https://doi.org/10.3390/jof7121059






