Distribution and Assembly Processes of Soil Fungal Communities along an Altitudinal Gradient in Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area and Soil Sample Collection
2.2. Soil Physicochemical Analysis
2.3. Soil Genomic DNA Extraction and Determination of Quality and Quantity
2.4. ITS1 Gene Amplification, Purification, Library Construction and Sequencing
2.5. Processing of Fungal Sequencing Data and Diversity Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.7. Null Model Based Phylogenetic Diversity Matrices and Ecological Assembly Analysis
2.8. Evidence of Neutral and Niche Processes by Sloan’s Neutral Community Model for Fungal Community
2.9. Statistical Analyses
3. Results
3.1. Soil Physicochemical Analysis
3.2. Quantification of Fungal Gene Abundance
3.3. Sequence Analysis and Fungal Community Composition
3.4. Alpha Diversity Analysis
3.5. Quantification of Neutral Processes and Migration Rate
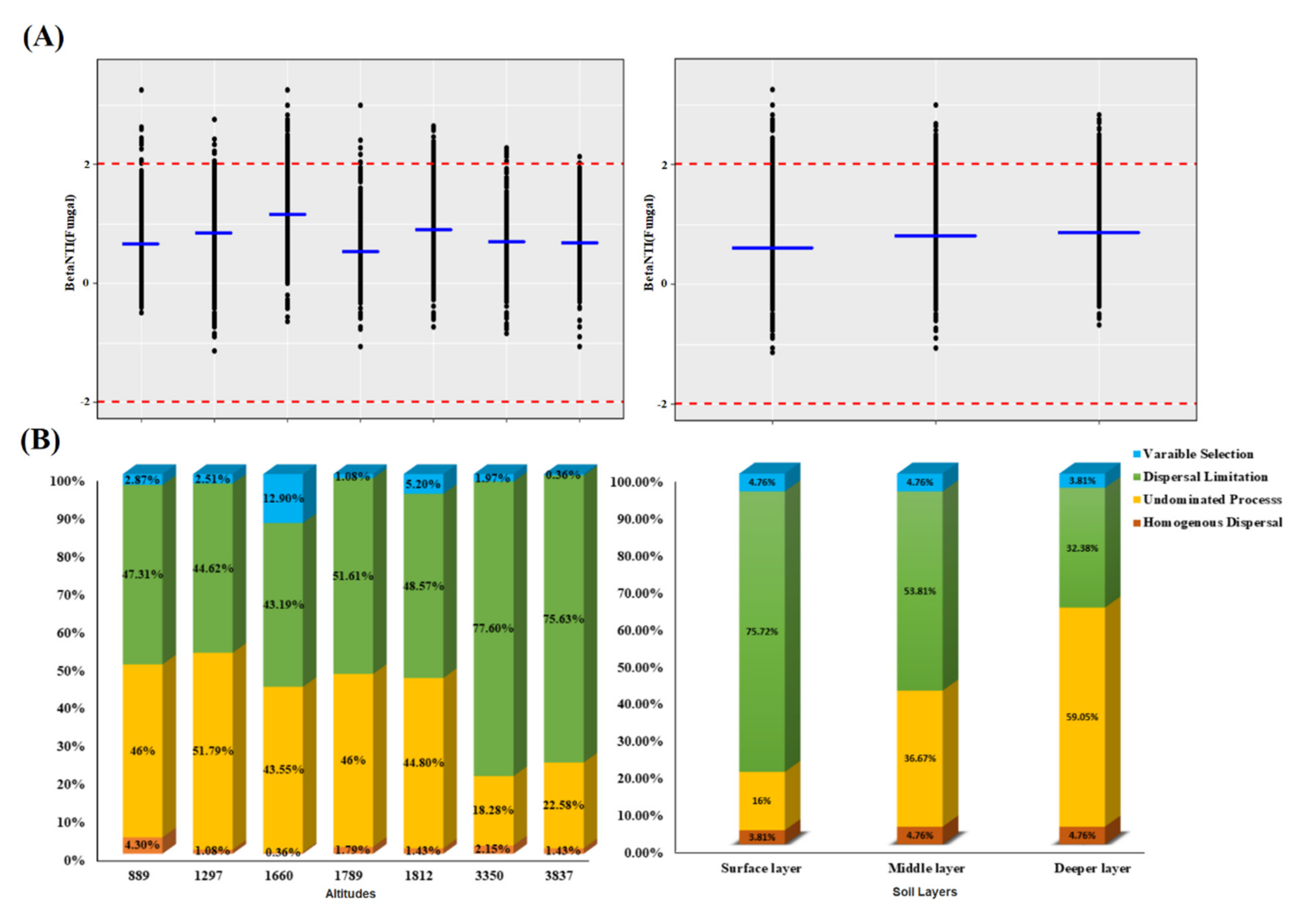
3.6. Assembly Processes of the Fungal Community and Contribution of Deterministic and Stochastic Processes
3.7. Integration of Environmental and Spatial Distance in Shaping Fungal Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 6213. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Nakano, T.; Hattori, M.; Nara, K. The mid-domain effect in ectomycorrhizal fungi: Range overlap along an elevation gradient on Mount Fuji, Japan. ISME J. 2014, 8, 1739–1746. [Google Scholar] [CrossRef]
- Linnaeus, C. On the increase of the habitable earth. Amoenitates Acad. 1781, 2, 17–27. [Google Scholar]
- Lomolino, M.V.; Sax, D.F.; Brown, J.H. Foundations of Biogeography: Classic Papers with Commentaries; University of Chicago Press: Chicago, IL, USA, 2004. [Google Scholar]
- Lomolino, M.V. Elevation gradients of species-density: Historical and prospective views. Glob. Ecol. Biogeogr. 2001, 10, 3–13. [Google Scholar] [CrossRef]
- Herzog, S.K.; Kessler, M.; Bach, K. The elevational gradient in Andean bird species richness at the local scale: A foothill peak and a high-elevation plateau. Ecography 2005, 28, 209–222. [Google Scholar] [CrossRef]
- Zheng, Z.; Gong, D.J.; Sun, C.X.; Li, X.J.; Li, W.J. Altitudinal patterns of species richness and species range size of vascular plants in Xiaolongshan Reserve of Qinling Mountain: A test of Rapoport’s rule. Yingyong Shengtai Xuebao 2014, 25. [Google Scholar]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Srivastava, D.S.; Anderson, K.M.; Brown, C.D.; Jankowski, J.E.; Kleynhans, E.J.; Kraft, N.J.; Letaw, A.D.; Macdonald, A.A.M.; Maclean, J.E. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 2014, 123, 1420–1430. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Ofiţeru, I.D.; Lunn, M.; Curtis, T.P.; Wells, G.F.; Criddle, C.S.; Francis, C.A.; Sloan, W.T. Combined niche and neutral effects in a microbial wastewater treatment community. Proc. Natl. Acad. Sci. USA 2010, 107, 15345–15350. [Google Scholar] [CrossRef]
- Ferrenberg, S.; O’neill, S.P.; Knelman, J.E.; Todd, B.; Duggan, S.; Bradley, D.; Robinson, T.; Schmidt, S.K.; Townsend, A.R.; Williams, M.W. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME J. 2013, 7, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, W.; Deng, Y.; Jiang, Y.-H.; Xue, K.; He, Z.; Nostrand, J.D.V.; Wu, L.; Yang, Y.; Wang, A.; et al. Stochastic assembly leads to alternative communities with distinct functions in a bioreactor microbial community. MBio 2013, 4, e00584-12. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Martiny, J. Dispersal and the microbiome. Microbe 2015, 10, 191–196. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, S.P. The Neutral Theory of Biodiversity and Biogeography and Stephen Jay Gould. Paleobiology 2005, 31, 122–132. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Põlme, S.; Kõljalg, U.; Zarre, S.; Tedersoo, L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol. 2012, 193, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Lentendu, G.; Zinger, L.; Manel, S.; Coissac, E.; Choler, P.; Geremia, R.A.; Melodelima, C. Assessment of soil fungal diversity in different alpine tundra habitats by means of pyrosequencing. Fungal Divers. 2011, 49, 113–123. [Google Scholar] [CrossRef]
- Wang, J.-T.; Zheng, Y.-M.; Hu, H.-W.; Zhang, L.-M.; Li, J.; He, J.-Z. Soil pH determines the alpha diversity but not beta diversity of soil fungal community along altitude in a typical Tibetan forest ecosystem. J. Soils Sediments 2015, 15, 1224–1232. [Google Scholar] [CrossRef]
- Gao, C.; Shi, N.-N.; Liu, Y.-X.; Peay, K.G.; Zheng, Y.; Ding, Q.; Mi, X.-C.; Ma, K.-P.; Wubet, T.; Buscot, F.; et al. Host plant genus-level diversity is the best predictor of ectomycorrhizal fungal diversity in a Chinese subtropical forest. Mol. Ecol. 2013, 22, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Adams, J.M.; Shi, Y.; Sun, H.; Cheng, L.; Zhang, Y.; Chu, H. Fungal community assemblages in a high elevation desert environment: Absence of dispersal limitation and edaphic effects in surface soil. Soil Biol. Biochem. 2017, 115, 393–402. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y.; Dong, Y.; Zhao, H.; Dong, S.; Zhao, S.; Beazley, R. The spatio-temporal patterns of the topsoil organic carbon density and its influencing factors based on different estimation models in the grassland of Qinghai-Tibet Plateau. PLoS ONE 2019, 14, e0225952. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Kawashima, S.; Yonemura, S.; Zhang, X.; Chen, S. Mutual influence between human activities and climate change in the Tibetan Plateau during recent years. Glob. Planet. Chang. 2004, 41, 241–249. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Liu, L.; Zhang, W.; Wang, Z.; Bai, W. The relationship between NDVI and precipitation on the Tibetan Plateau. J. Geogr. Sci. 2007, 17, 259–268. [Google Scholar] [CrossRef]
- Chu, H.; Sun, H.; Tripathi, B.M.; Adams, J.M.; Huang, R.; Zhang, Y.; Shi, Y. Bacterial community dissimilarity between the surface and subsurface soils equals horizontal differences over several kilometers in the western Tibetan Plateau. Environ. Microbiol. 2016, 18, 1523–1533. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Shi, Z.; Rossel, R.; Wu, Y.U. Interactive effects of elevation and land use on soil bacterial communities in the Tibetan Plateau. Pedosphere 2020, 30, 817–831. [Google Scholar] [CrossRef]
- Xing, P.; Tao, Y.; Jeppesen, E.; Wu, Q.L. Comparing microbial composition and diversity in freshwater lakes between Greenland and the Tibetan Plateau. Limnol. Oceanogr. 2021, 66, S142–S156. [Google Scholar] [CrossRef]
- Jiang, H.; Deng, S.; Huang, Q.; Dong, H.; Yu, B. Response of Aerobic Anoxygenic Phototrophic Bacterial Diversity to Environment Conditions in Saline Lakes and Daotang River on the Tibetan Plateau, NW China. Geomicrobiol. J. 2010, 27, 400–408. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, H.; Yu, B.; Liu, X.; Li, Y.; Ji, S.; Zhang, C.L. Microbial response to salinity change in Lake Chaka, a hypersaline lake on Tibetan plateau. Environ. Microbiol. 2007, 9, 2603–2621. [Google Scholar] [CrossRef]
- Xing, P.; Hahn, M.W.; Wu, Q.L. Low Taxon Richness of Bacterioplankton in High-Altitude Lakes of the Eastern Tibetan Plateau, with a Predominance of Bacteroidetes and Synechococcus spp. Appl. Environ. Microbiol. 2009, 75, 7017–7025. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, Q.; Li, D.; Cheng, G.; Mu, J.; Wu, Q.; Niu, F.; An, L.; Feng, H. Diversity and community structure of fungi through a permafrost core profile from the Qinghai-Tibet Plateau of China. J. Basic Microbiol. 2014, 54, 1331–1341. [Google Scholar] [CrossRef]
- Shi, T.; Reeves, R.H.; Gilichinsky, D.A.; Friedmann, E.I. Characterization of Viable Bacteria from Siberian Permafrost by 16S rDNA Sequencing. Microb. Ecol. 1997, 33, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Schulte, E.; Hoskins, B. Recommended soil organic matter tests. Recomm. Soil Test. Proced. North. East. USA Northeast. Reg. Publ. 1995, 493, 52–60. [Google Scholar]
- Bremner, J.M. Nitrogen-total. Methods Soil Anal. Part. 3 Chem. Methods 1996, 5, 1085–1121. [Google Scholar]
- Eckert, D.; Sims, J.T. Recommended soil pH and lime requirement tests. Recomm. Soil Test. Proced. Northeast. United States Northeast. Reg. Bull. 1995, 493, 11–16. [Google Scholar]
- Bao, S.D. Agricultural and Chemistry Analysis of Soil; China Agricultural Press: Beijing, China, 2005; pp. 355–356. [Google Scholar]
- Yun, T.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Lu, Z. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Kempers, A.J.; Luft, A.G. Re-examination of the determination of environmental nitrate as nitrite by reduction with hydrazine. Analyst 1988, 113, 1117–1120. [Google Scholar] [CrossRef]
- White, T.; Bruns, T.; Lee, S.; Taylor, F.; White, T.; Lee, S.H.; Taylor, L.; Shawetaylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kembel, S.W. Disentangling niche and neutral influences on community assembly: Assessing the performance of community phylogenetic structure tests. Ecol. Lett. 2010, 12, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Chemidlin Prévost-Bouré, N.; Christen, R.; Dequiedt, S.; Mougel, C.; Lelièvre, M.; Jolivet, C.; Shahbazkia, H.R.; Guillou, L.; Arrouays, D.; Ranjard, L. Validation and application of a PCR primer set to quantify fungal communities in the soil environment by real-time quantitative PCR. PLoS ONE 2011, 6, e24166. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Konopka, A.E.; Fredrickson, J.K. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J. 2012, 6, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shen, J.; Wu, Y.; Tu, C.; Soininen, J.; Stegen, J.C.; He, J.; Liu, X.; Zhang, L.; Zhang, E. Phylogenetic beta diversity in bacterial assemblages across ecosystems: Deterministic versus stochastic processes. ISME J. 2013, 7, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Stegen, J.C.; Fredrickson, J.K.; Wilkins, M.J.; Konopka, A.E.; Nelson, W.C.; Arntzen, E.V.; Chrisler, W.B.; Chu, R.K.; Danczak, R.E.; Fansler, S.J.; et al. Groundwater–surface water mixing shifts ecological assembly processes and stimulates organic carbon turnover. Nat. Commun. 2016, 7, 11237. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Kraft, N.J.; Smith, K.G.; Vellend, M.; Inouye, B.D. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Burns, A.R.; Stephens, W.Z.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016, 10, 655–664. [Google Scholar] [CrossRef]
- Sloan, W.T.; Woodcock, S.; Lunn, M.; Head, I.M.; Curtis, T.P. Modeling Taxa-Abundance Distributions in Microbial Communities using Environmental Sequence Data. Microb. Ecol. 2007, 53, 443–455. [Google Scholar] [CrossRef]
- Roguet, A.; Laigle, G.S.; Therial, C.; Bressy, A.; Soulignac, F.; Catherine, A.; Lacroix, G.; Jardillier, L.; Bonhomme, C.; Lerch, T.Z.; et al. Neutral community model explains the bacterial community assembly in freshwater lakes. FEMS Microbiol. Ecol. 2015, 91, fiv125. [Google Scholar] [CrossRef]
- Östman, Ö.; Drakare, S.; Kritzberg, E.S.; Langenheder, S.; Logue, J.B.; Lindström, E.S. Regional invariance among microbial communities. Ecol. Lett. 2010, 13, 118–127. [Google Scholar] [CrossRef]
- Ling, F.; Whitaker, R.; LeChevallier, M.W.; Liu, W.-T. Drinking water microbiome assembly induced by water stagnation. ISME J. 2018, 12, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Bassis, C.M.; Beck, J.M.; Young, V.B.; Curtis, J.L.; Huffnagle, G.B.; Schmidt, T.M. Application of a neutral community model to assess structuring of the human lung microbiome. MBio 2015, 6, e02284-14. [Google Scholar] [CrossRef]
- Preston, F.W. The commonness, and rarity, of species. Ecology 1948, 29, 254–283. [Google Scholar] [CrossRef]
- Kevan, P.G.; Greco, C.F.; Belaoussoff, S. Log-normality of biodiversity and abundance in diagnosis and measuring of ecosystemic health: Pesticide stress on pollinators on blueberry heaths. J. Appl. Ecol. 1997, 34, 1122–1136. [Google Scholar] [CrossRef]
- Huber, J.A.; Welch, D.B.M.; Morrison, H.G.; Huse, S.M.; Neal, P.R.; Butterfield, D.A.; Sogin, M.L. Microbial Population Structures in the Deep Marine Biosphere. Science 2007, 318, 97–100. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.O.; Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Community Ecology Package. R Package Version 2.0-5. 2014. Available online: http://cran.r-project.org/package=vegan (accessed on 15 November 2021).
- Zhang, M.-S.; Li, W.; Zhang, W.-G.; Li, Y.-T.; Li, J.-Y.; Gao, Y. Agricultural land-use change exacerbates the dissemination of antibiotic resistance genes via surface runoffs in Lake Tai Basin, China. Ecotoxicol. Environ. Saf. 2021, 220, 112328. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Freeman, K.R.; Pescador, M.Y.; Reed, S.C.; Costello, E.K.; Robeson, M.S.; Schmidt, S.K. Soil CO2 flux and photoautotrophic community composition in high-elevation, ‘barren’ soil. Environ. Microbiol. 2009, 11, 674–686. [Google Scholar] [CrossRef]
- Schmidt, S.; Nemergut, D.; Miller, A.; Freeman, K.; King, A.; Seimon, A. Microbial activity and diversity during extreme freeze–thaw cycles in periglacial soils, 5400 m elevation, Cordillera Vilcanota, Perú. Extremophiles 2009, 13, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.R.; Martin, A.P.; Karki, D.; Lynch, R.C.; Mitter, M.S.; Meyer, A.F.; Longcore, J.E.; Simmons, D.R.; Schmidt, S.K. Evidence that chytrids dominate fungal communities in high-elevation soils. Proc. Natl. Acad. Sci. USA 2009, 106, 18315. [Google Scholar] [CrossRef]
- Geml, J.; Morgado, L.N.; Semenova-Nelsen, T.A.; Schilthuizen, M. Changes in richness and community composition of ectomycorrhizal fungi among altitudinal vegetation types on Mount Kinabalu in Borneo. New Phytol. 2017, 215, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hart, M.M.; Zhang, J.; Cai, X.; Gai, J.; Christie, P.; Li, X.; Klironomos, J.N. Altitudinal distribution patterns of AM fungal assemblages in a Tibetan alpine grassland. FEMS Microbiol. Ecol. 2015, 91, fiv078. [Google Scholar] [CrossRef]
- Gómez-Hernández, M.; Williams-Linera, G.; Guevara, R.; Lodge, D.J. Patterns of macromycete community assemblage along an elevation gradient: Options for fungal gradient and metacommunity analyse. Biodivers. Conserv. 2012, 21, 2247–2268. [Google Scholar] [CrossRef]
- Gai, J.; Tian, H.; Yang, F.; Christie, P.; Li, X.; Klironomos, J. Arbuscular mycorrhizal fungal diversity along a Tibetan elevation gradient. Pedobiologia 2012, 55, 145–151. [Google Scholar] [CrossRef]
- Siles, J.A.; Öhlinger, B.; Cajthaml, T.; Kistler, E.; Margesin, R. Characterization of soil bacterial, archaeal and fungal communities inhabiting archaeological human-impacted layers at Monte Iato settlement (Sicily, Italy). Sci. Rep. 2018, 8, 1903. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Yang, Y.; Yang, L. Seasonal variations in soil fungal communities and co-occurrence networks along an altitudinal gradient in the cold temperate zone of China: A case study on Oakley Mountain. CATENA 2021, 204, 105448. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Kou, Y.; Yao, M.; He, Z.; Li, X. Distinct mechanisms shape soil bacterial and fungal co-occurrence networks in a mountain ecosystem. FEMS Microbiol. Ecol. 2020, 96, fiaa030. [Google Scholar] [CrossRef] [PubMed]
- Shigyo, N.; Umeki, K.; Hirao, T. Seasonal Dynamics of Soil Fungal and Bacterial Communities in Cool-Temperate Montane Forests. Front. Microbiol. 2019, 10, 1944. [Google Scholar] [CrossRef]
- Tanaka, D.; Sato, K.; Goto, M.; Fujiyoshi, S.; Maruyama, F.; Takato, S.; Shimada, T.; Sakatoku, A.; Aoki, K.; Nakamura, S. Airborne Microbial Communities at High-Altitude and Suburban Sites in Toyama, Japan Suggest a New Perspective for Bioprospecting. Front. Bioeng. Biotechnol. 2019, 7, 12. [Google Scholar] [CrossRef]
- Girvan, M.S.; Bullimore, J.; Pretty, J.N.; Osborn, A.M.; Ball, A.S. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl. Environ. Microbiol. 2003, 69, 1800–1809. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Anderson, T.-H. Interactive effects of pH and substrate quality on the fungal-to-bacterial ratio and qCO2 of microbial communities in forest soils. Soil Biol. Biochem. 1998, 30, 1269–1274. [Google Scholar] [CrossRef]
- Frey, S.D.; Knorr, M.; Parrent, J.L.; Simpson, R.T. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For. Ecol. Manag. 2004, 196, 159–171. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z.; et al. Litterfall Production Along Successional and Altitudinal Gradients of Subtropical Monsoon Evergreen Broadleaved Forests in Guangdong, China. Plant. Ecol. 2007, 188, 77–89. [Google Scholar] [CrossRef]
- Tang, M.; Li, L.; Wang, X.; You, J.; Li, J.; Chen, X. Elevational is the main factor controlling the soil microbial community structure in alpine tundra of the Changbai Mountain. Sci. Rep. 2020, 10, 12442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Zhao, L.; Xu, S.J., Jr.; Liu, Y.Z.; Liu, H.Y.; Cheng, G.D. Soil moisture effect on bacterial and fungal community in Beilu River (Tibetan Plateau) permafrost soils with different vegetation types. J. Appl. Microbiol. 2013, 114, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Štursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar] [CrossRef]
- Veach, A.M.; Stokes, C.E.; Knoepp, J.; Jumpponen, A.; Baird, R. Fungal Communities and Functional Guilds Shift Along an Elevational Gradient in the Southern Appalachian Mountains. Microb. Ecol. 2018, 76, 156–168. [Google Scholar] [CrossRef]
- Soininen, J.; McDonald, R.; Hillebrand, H. The distance decay of similarity in ecological communities. Ecography 2007, 30, 3–12. [Google Scholar] [CrossRef]
- Liu, Y.-R.; Eldridge, D.J.; Zeng, X.-M.; Wang, J.; Singh, B.K.; Delgado-Baquerizo, M. Global diversity and ecological drivers of lichenised soil fungi. New Phytol. 2021, 231, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Wubet, T.; Christ, S.; Schöning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in Soil Fungal Communities between European Beech (Fagus sylvatica L.) Dominated Forests Are Related to Soil and Understory Vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef] [PubMed]
- Toljander, J.F.; Eberhardt, U.; Toljander, Y.K.; Paul, L.R.; Taylor, A.F.S. Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol. 2006, 170, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, S.G.; Woodward, S.; Taylor, A.F.S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol. 2015, 206, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Du, B.; Tan, H.; Liu, X.; Su, L.; Saeedur, R.; Ali, M.; Liu, C.; Sun, N.; Hui, N. Phosphorus elevation erodes ectomycorrhizal community diversity and induces divergence of saprophytic community composition between vegetation types. Sci. Total. Environ. 2021, 793, 148502. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lü, G.; Jiang, H.; Shi, D.-N.; Liu, Z. Diversity and distribution of soil micro-fungi along an elevation gradient on the north slope of Changbai Mountain. J. For. Res. 2017, 28, 831–839. [Google Scholar] [CrossRef]
- Devi, L.S.; Khaund, P.; Nongkhlaw, F.M.W.; Joshi, S.R. Diversity of Culturable Soil Micro-fungi along Altitudinal Gradients of Eastern Himalayas. Mycobiology 2012, 40, 151–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, W.; Zheng, Y.; Gao, C.; Duan, J.-C.; Wang, S.-P.; Guo, L.-D. Arbuscular mycorrhizal fungal community composition affected by original elevation rather than translocation along an altitudinal gradient on the Qinghai-Tibet Plateau. Sci. Rep. 2016, 6, 36606. [Google Scholar] [CrossRef]
- Cho, J.C.; Tiedje, J.M. Biogeography and Degree of Endemicity of Fluorescent Pseudomonas Strains in Soil. Appl. Environ. Microbiol. 2000, 66, 5448–5456. [Google Scholar] [CrossRef]
- Margesin, R.; Jud, M.; Tscherko, D.; Schinner, F. Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol. Ecol. 2009, 67, 208–218. [Google Scholar] [CrossRef]
- Newsham, K.K.; Hopkins, D.W.; Carvalhais, L.C.; Fretwell, P.T.; Rushton, S.P.; O’Donnell, A.G.; Dennis, P.G. Relationship between soil fungal diversity and temperature in the maritime Antarctic. Nat. Clim. Chang. 2016, 6, 182–186. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Y.; Yang, J.; Chen, H.; Holohan, B.; Vaudrey, J.; Lin, S.; McManus, G.B. The diversity and biogeography of abundant and rare intertidal marine microeukaryotes explained by environment and dispersal limitation. Environ. Microbiol. 2018, 20, 462–476. [Google Scholar] [CrossRef]
- Chen, W.; Pan, Y.; Yu, L.; Yang, J.; Zhang, W. Patterns and processes in marine microeukaryotic community biogeography from Xiamen coastal waters and intertidal sediments, southeast China. Front. Microbiol. 2017, 8, 1912. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, W.; Yang, J.; Lin, Y.; Yu, Z.; Lin, S. Biogeographic patterns of abundant and rare bacterioplankton in three subtropical bays resulting from selective and neutral processes. ISME J. 2018, 12, 2198–2210. [Google Scholar] [CrossRef]
- Lindström, E.S.; Langenheder, S. Local and regional factors influencing bacterial community assembly. Environ. Microbiol. Rep. 2012, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nabout, J.C.; Siqueira, T.; Bini, L.M.; Nogueira, I.d.S. No evidence for environmental and spatial processes in structuring phytoplankton communities. Acta Oecol. 2009, 35, 720–726. [Google Scholar] [CrossRef]
- Attayde, J.L.; Bozelli, R.L. Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Can. J. Fish. Aquat. Sci. 1998, 55, 1789–1797. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Faust, K.; Henry, N.; Decelle, J.; Colin, S.; Carcillo, F.; Chaffron, S.; Ignacio-Espinosa, J.C.; Roux, S.; Vincent, F. Determinants of community structure in the global plankton interactome. Science 2015, 348, 6237. [Google Scholar] [CrossRef]
- Wei, G.; Li, M.; Li, F.; Li, H.; Gao, Z. Distinct distribution patterns of prokaryotes between sediment and water in the Yellow River estuary. Appl. Microbiol. Biotechnol. 2016, 100, 9683–9697. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, B.; Bennett, J.R. Partitioning variation in ecological communities: Do the numbers add up? J. Appl. Ecol. 2010, 47, 1071–1082. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.A.; Fuhrman, J.A.; Horner-Devine, M.C.; Martiny, J.B.H. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, A.; Wang, J.; Liu, S.; Jiang, X.; Dang, C.; Ma, T.; Liu, S.; Chen, Q.; Xie, S. Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Logares, R.; Lindström, E.S.; Langenheder, S.; Logue, J.B.; Paterson, H.; Laybourn-Parry, J.; Rengefors, K.; Tranvik, L.; Bertilsson, S. Biogeography of bacterial communities exposed to progressive long-term environmental change. ISME J. 2013, 7, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Gao, C.; Chen, L.; Ji, N.-N.; Wu, B.-W.; Li, X.-C.; Lü, P.-P.; Zheng, Y.; Guo, L.-D. Host plant phylogeny and geographic distance strongly structure Betulaceae-associated ectomycorrhizal fungal communities in Chinese secondary forest ecosystems. FEMS Microbiol. Ecol. 2019, 95, fiz037. [Google Scholar] [CrossRef]
- Wu, B.-W.; Gao, C.; Chen, L.; Buscot, F.; Goldmann, K.; Purahong, W.; Ji, N.-N.; Wang, Y.-L.; Lü, P.-P.; Li, X.-C.; et al. Host Phylogeny Is a Major Determinant of Fagaceae-Associated Ectomycorrhizal Fungal Community Assembly at a Regional Scale. Front. Microbiol. 2018, 9, 2409. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.I.; Peay, K.G.; Talbot, J.M.; Smith, D.P.; Chung, J.A.; Taylor, J.W.; Vilgalys, R.; Bruns, T.D. A continental view of pine-associated ectomycorrhizal fungal spore banks: A quiescent functional guild with a strong biogeographic pattern. New Phytol. 2015, 205, 1619–1631. [Google Scholar] [CrossRef]
- Finlay, B.J. Global Dispersal of Free-Living Microbial Eukaryote Species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef]
- Moeller, H.V.; Peay, K.G.; Tadashi, F. Ectomycorrhizal fungal traits reflect environmental conditions along a coastal California edaphic gradient. Fems Microbiol. Ecol. 2014, 87, 797–806. [Google Scholar] [CrossRef]
- Truong, C.; Gabbarini, L.A.; Corrales, A.; Mujic, A.B.; Escobar, J.M.; Moretto, A.; Smith, M.E. Ectomycorrhizal fungi and soil enzymes exhibit contrasting patterns along elevation gradients in southern Patagonia. New Phytol. 2019, 222, 1936–1950. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Dumbrell, A.J.; Liu, M.; Li, G.; Wu, M.; Jiang, C.; Li, Z.; Shank, E.A. Spatial Variation in Soil Fungal Communities across Paddy Fields in Subtropical China. mSystems 2020, 5, e00704-19. [Google Scholar] [CrossRef]







| Factors | Fungal Gene Abundance (Log Copies) | |
|---|---|---|
| F | p | |
| SL | 5.813 | 0.006 |
| A | 5.881 | 0.000 |
| SL × A | 7.746 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Liu, H.; Liu, S.; Yin, Y.; Yuan, Z.; Zhao, Y.; Cao, H. Distribution and Assembly Processes of Soil Fungal Communities along an Altitudinal Gradient in Tibetan Plateau. J. Fungi 2021, 7, 1082. https://doi.org/10.3390/jof7121082
Hussain S, Liu H, Liu S, Yin Y, Yuan Z, Zhao Y, Cao H. Distribution and Assembly Processes of Soil Fungal Communities along an Altitudinal Gradient in Tibetan Plateau. Journal of Fungi. 2021; 7(12):1082. https://doi.org/10.3390/jof7121082
Chicago/Turabian StyleHussain, Sarfraz, Hao Liu, Senlin Liu, Yifan Yin, Zhongyuan Yuan, Yuguo Zhao, and Hui Cao. 2021. "Distribution and Assembly Processes of Soil Fungal Communities along an Altitudinal Gradient in Tibetan Plateau" Journal of Fungi 7, no. 12: 1082. https://doi.org/10.3390/jof7121082
APA StyleHussain, S., Liu, H., Liu, S., Yin, Y., Yuan, Z., Zhao, Y., & Cao, H. (2021). Distribution and Assembly Processes of Soil Fungal Communities along an Altitudinal Gradient in Tibetan Plateau. Journal of Fungi, 7(12), 1082. https://doi.org/10.3390/jof7121082






