Phylogenetic Relationships, Speciation, and Origin of Armillaria in the Northern Hemisphere: A Lesson Based on rRNA and Elongation Factor 1-Alpha
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Specimens and Isolates
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Nucleotide Alignment and Data Matrices
2.4. Phylogenetic Analyses and Species Delimitation
2.5. Pairwise Homoplasy Index Test
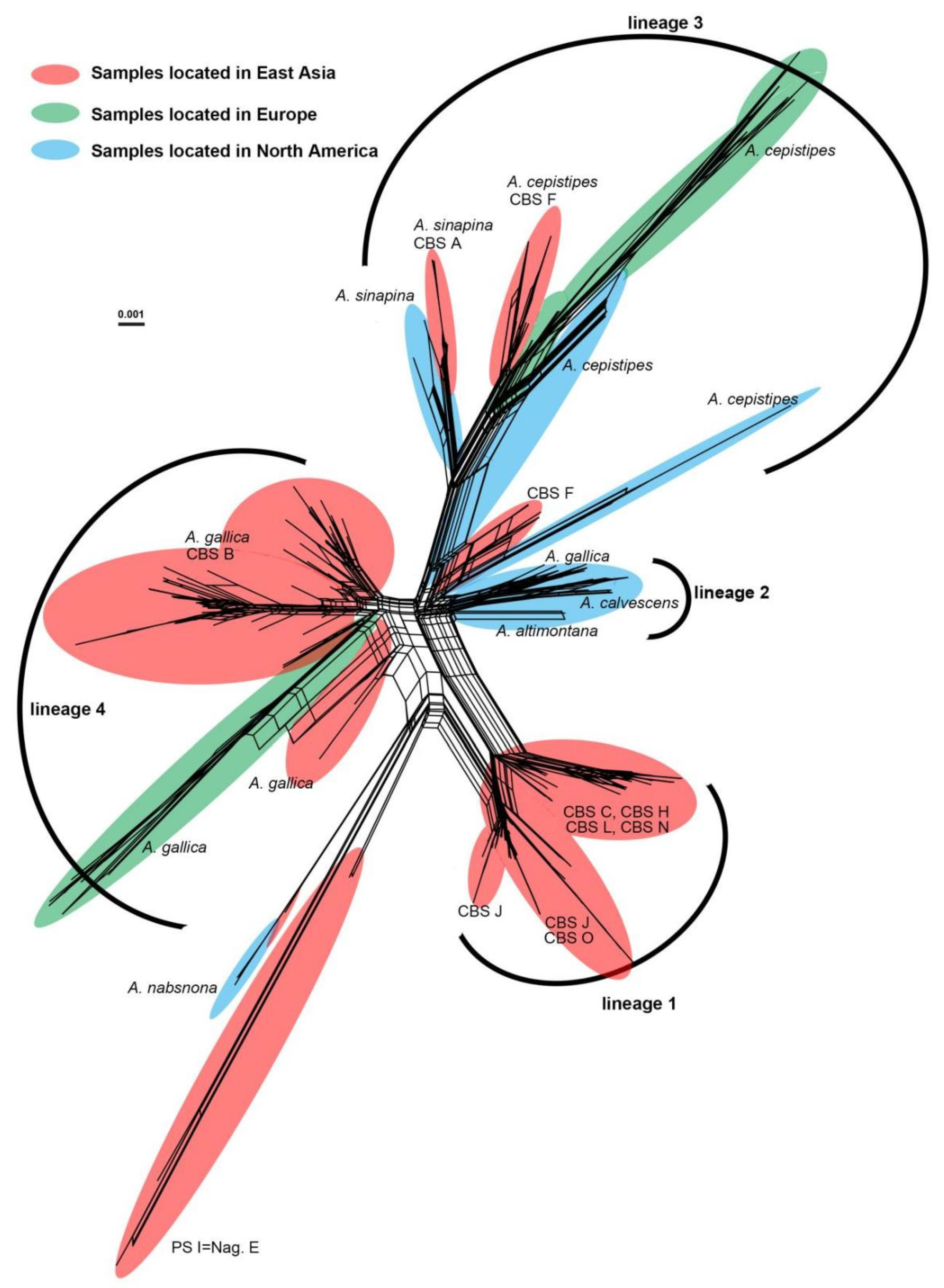
2.6. Neighbor-net Network Analysis
2.7. Molecular Dating Analysis
2.8. Ancestral Area Reconstruction
3. Results
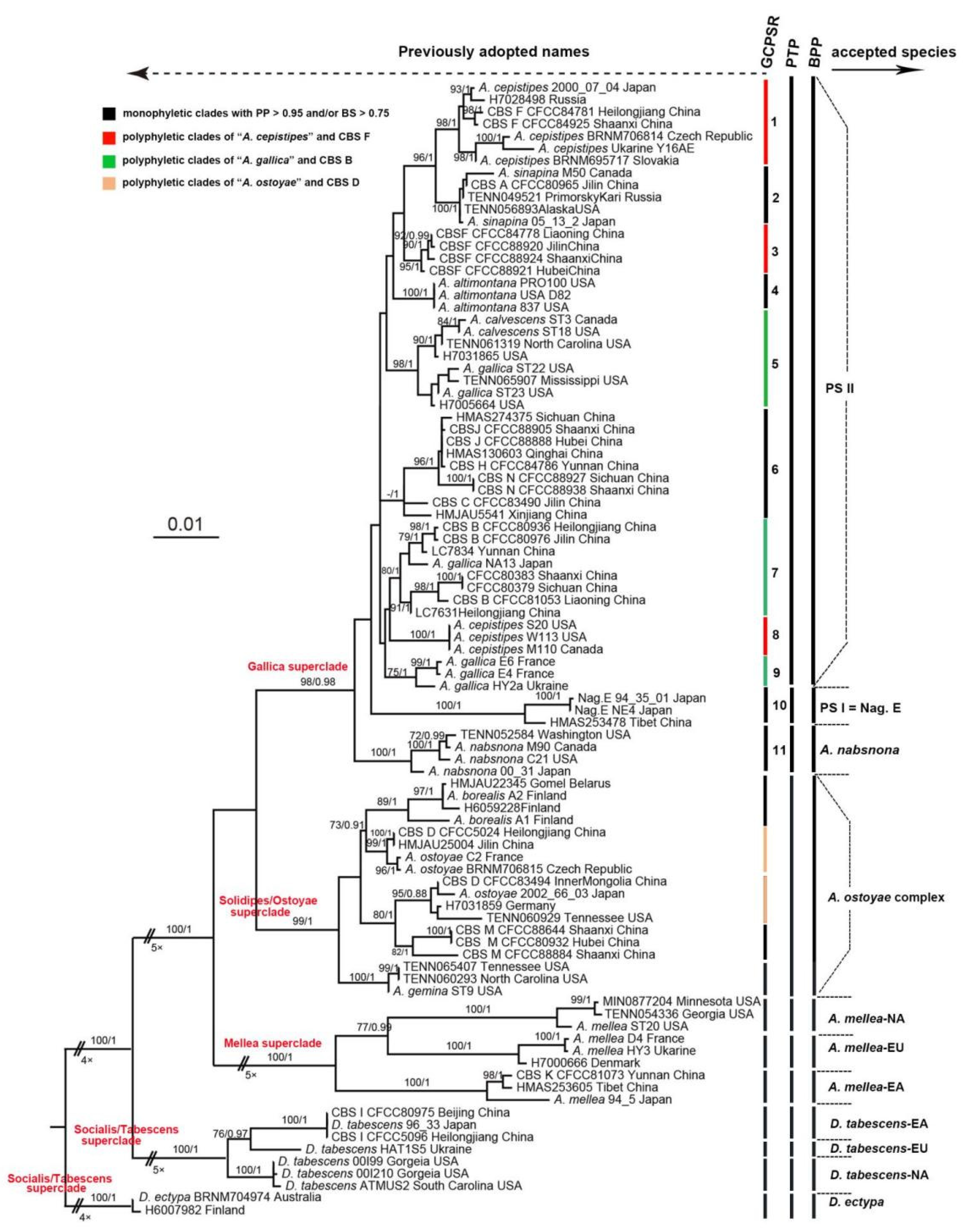
3.1. Phylogenetic Analyses and Species Delimitation
3.2. Phylogenetic Relationships in PS II
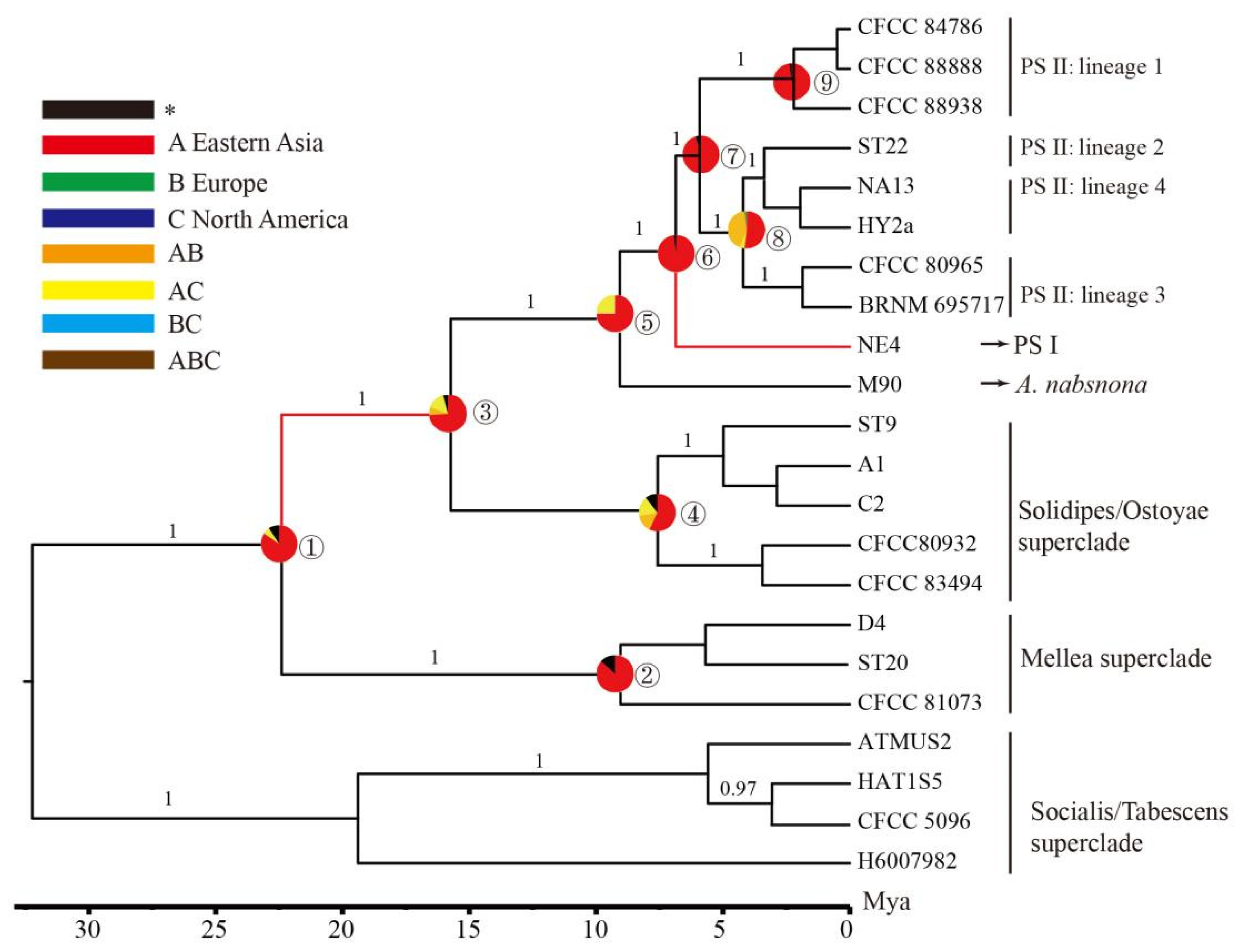
3.3. Estimation of Divergence Time
3.4. Ancestral Areas of Armillaria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berbee, M.L.; Taylor, J.W. Dating the molecular clock in fungi-how close are we? Fungal Biol. Rev. 2010, 24, 1–16. [Google Scholar] [CrossRef]
- Tharreau, D.; Fudal, I.; Andriantsimialona, D.; Santoso, U.D.; Fournier, E.; Lebrun, M.H.; Nottéghem, J.L. World population structure and migration of the rice blast fungus, Magnaporthe oryzae. In Advances in Genetics, Genomics and Control of Rice Blast Disease; Wang, G.L., Valent, B., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 209–215. [Google Scholar]
- Saleh, D.; Milazzo, J.; Adreit, H.; Fournier, E.; Tharreau, D. South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytol. 2014, 201, 1440–1456. [Google Scholar] [CrossRef] [Green Version]
- Manos, P.S.; Stanford, A.M. The Historical Biogeography of Fagaceae: Tracking the Tertiary History of Temperate and Subtropical Forests of the Northern Hemisphere. Int. J. Plant Sci. 2001, 162, S77–S93. [Google Scholar] [CrossRef]
- Taylor, J.W.; Berbee, M.L. Dating divergences in the Fungal Tree of Life: Review and new analyses. Mycologia 2006, 98, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Percy, D.M.; Garver, A.M.; Wagner, W.L.; James, H.; Cunningham, C.; Miller, S.; Fleischer, R.C. Progressive island colonization and ancient origin of Hawaiian Metrosideros (Myrtaceae). Proc. R. Soc. B Boil. Sci. 2008, 275, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.L.; Feng, B.; Wu, G.; Halling, R.E.; Buyck, B.; Yorou, N.S.; Wbika, S.T.N.; Yang, Z.L. African origin and global distribution patterns: Evidence inferred from phylogenetic and biogeographical analyses of ectomycorrhizal fungal genus Strobilomyces. J. Biogeogr. 2018, 45, 201–212. [Google Scholar] [CrossRef]
- Cai, Q.; Tulloss, R.E.; Tang, L.P.; Tolgor, B.; Zhang, P.; Chen, Z.H.; Yang, Z.L. Multi-locus phylogeny of lethal amanitas: Implications for species diversity and historical biogeography. BMC Evol. Biol. 2014, 14, 143. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Cui, B.K. Phylogeny, divergence time and historical biogeography of Laetiporus (Basidiomycota, Polyporales). BMC Evol. Biol. 2017, 17, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, C.; Sanchez-Ramirez, S.; Kuhar, F.; Kaplan, Z.; Smith, M.E. The Gondwanan connection-Southern temperate Amanita lineages and the description of the first sequestrate species from the Americas. Fungal Biol. 2017, 121, 638–651. [Google Scholar] [CrossRef] [Green Version]
- Boluda, C.G.; Rico, V.J.; Naciri, Y.; Hawksworth, D.L.; Scheidegger, C. Phylogeographic reconstructions can be biased by ancestral shared alleles: The case of the polymorphic lichen Bryoria fuscescens in Europe and North Africa. Mol. Ecol. 2021, 30, 4845–4865. [Google Scholar] [CrossRef]
- Baumgartner, K.; Coetzee, M.; Hoffmeister, D. Secrets of the subterranean pathosystem of Armillaria. Mol. Plant Pathol. 2011, 12, 515–534. [Google Scholar] [CrossRef]
- Smith, M.; Bruhn, J.N.; Anderson, J.B. The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nat. Cell Biol. 1992, 356, 428–431. [Google Scholar] [CrossRef]
- Hobbs, C. Medicinal Mushrooms: An Exploration of Tradition, Healing and Culture; Botanica Press: Summertown, TN, USA, 2003. [Google Scholar]
- Kikuchi, G.; Yamaji, H. Identification of Armillaria species associated with Polyporus umbellatus using ITS sequences of nuclear ribosomal DNA. Mycoscience 2010, 51, 366–372. [Google Scholar] [CrossRef]
- Coetzee, M.P.A.; Bloomer, P.; Wingfield, M.J.; Wingfield, B. Paleogene Radiation of a Plant Pathogenic Mushroom. PLoS ONE 2011, 6, e28545. [Google Scholar] [CrossRef]
- Koch, R.A.; Wilson, A.W.; Séné, O.; Henkel, T.W.; Aime, M.C. Resolved phylogeny and biogeography of the root pathogen Armillaria and its gasteroid relative, Guyanagaster. BMC Evol. Biol. 2017, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Watling, R.; Kile, G.; Gregory, N.M. The genus Armillaria-nomenclature, typification, the identity of Armillaria mellea and species differentiation. Trans. Br. Mycol. Soc. 1982, 78, 271–285. [Google Scholar] [CrossRef]
- Volk, T.J.; Burdsall, H.H. A Nomenclatural Study of Armillaria and Armillariella Species: Basidiomycotina, Tricholomataceae; Fungiflora Press: Oslo, Norway, 1995. [Google Scholar]
- Korhonen, K. Interfertility and clonal size in the Armillaria mellea complex. Karstenia 1978, 18, 31–42. [Google Scholar] [CrossRef]
- Qin, G.F.; Zhao, J.; Korhonen, K. A study on intersterility groups of Armillaria in China. Mycologia 2007, 99, 430–441. [Google Scholar] [CrossRef]
- Nixon, K.C.; Wheeler, Q.D. An Amplification of the Phylogenetic Species Concept. Cladistics 1990, 6, 211–223. [Google Scholar] [CrossRef]
- O’Donnell, K. Molecular phylogeny of the Nectria haematococca–Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.; Subandiyah, S.; Crous, P.; Kema, G. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef]
- Weir, B.; Johnston, P.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, M.P.; Wingfield, B.; Zhao, J.; van Coller, S.J.; Wingfield, M.J. Phylogenetic relationships among biological species of Armillaria from China. Mycoscience 2015, 56, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Klopfenstein, N.B.; Stewart, J.E.; Ota, Y.; Hanna, J.W.; Richardson, B.A.; Ross-Davis, A.L.; Elías-Román, R.D.; Korhonen, K.; Keča, N.; Iturritxa, E.; et al. Insights into the phylogeny of Northern Hemisphere Armillaria: Neighbor-net and Bayesian analyses of translation elongation factor 1-α gene sequences. Mycologia 2017, 109, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.P.; Wingfield, B.D.; Wingfield, M.J. Armillaria Root-Rot Pathogens: Species Boundaries and Global Distribution. Pathogens 2018, 7, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonín, V.; Stewart, J.E.; Ortiz, R.M.; Kim, M.-S.; Bonello, P.; Tomšovský, M.; Klopfenstein, N.B. Desarmillaria caespitosa, a North American vicariant of D. tabescens. Mycologia 2021, 113, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.J.; Burdsall, H.H.; Banik, M.T. Armillaria nabsnona, a New Species from Western North America. Mycologia 1996, 88, 484–491. [Google Scholar] [CrossRef]
- Berube, J.A.; Dessureault, M. Morphological studies of the Armillaria mellea complex: Two new species, A. gemina and A. calvescens. Mycologia 1989, 81, 216–225. [Google Scholar] [CrossRef]
- Brazee, N.J.; Ortiz-Santana, B.; Banik, M.T.; Lindner, D.L. Armillaria altimontana, a new species from the western interior of North America. Mycologia 2012, 104, 1200–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.Y.; Sung, J.M.; Igarashi, T. Biological species and morphological characteristics of Armillaria mellea complex in Hokkaido: A. sinapina and two new species, A. jezoensis and A. singula. Mycoscience 1994, 35, 39–47. [Google Scholar] [CrossRef]
- Pons, J.; Barraclough, T.; Gómez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P. Sequence-Based Species Delimitation for the DNA Taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: A revised method and evaluation on simulated data sets. Syst. Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Rannala, B. Bayesian species identification under the multispecies coalescent provides significant improvements to DNA barcoding analyses. Mol. Ecol. 2017, 26, 3028–3036. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Tian, S.; Wang, Y.; Qin, G. A method of single spore isolation and culture for Armillaria genetic test. Microbiology 1999, 26, 207–209. [Google Scholar]
- Coetzee, M.P.A.; Wingfield, B.D.; Harrington, T.C.; Dalevi, D.; Coutinho, T.A.; Wingfield, M.J. Geographical diversity of Ar-millaria mellea s. s. based on phylogenetic analysis. Mycologia 2000, 92, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hsiau, T.W. The Taxonomy and Phylogeny of the Mycangial Fungi from Dendroctonus brevicomis and D. frontalis (Coleoptera: Scolytidae). Ph.D. Thesis, Lowa State university, Ames, IA, USA, 1996. [Google Scholar]
- Duchesne, L.C.; Anderson, J.B. Location and direction of transcription of the 5S rRNA gene in Armillaria. Mycol. Res. 1990, 94, 266–269. [Google Scholar] [CrossRef]
- Kauserud, H.; Schumacher, T. Outcrossing or inbreeding: DNA markers provide evidence for type of reproductive mode in Phellinus nigrolimitatus (Basidiomycota). Mycol. Res. 2001, 105, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D. MAFFT multiple sequence alignment software version improvements in performance and usability. Mol. Biol. Evo. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Alzohairy, A.M. Bioedit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.31. 2017. Available online: https://www.mesquiteproject.org (accessed on 6 November 2021).
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in Fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Rannala, B. Bayesian species delimitation using multilocus sequence data. Proc. Natl. Acad. Sci. USA 2010, 107, 9264–9269. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hoehna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest 2.3 README, 22 May 2008. Syst. Zool. 2008, 3–4. [Google Scholar]
- Dettman, J.R.; Jacobson, D.J.; Taylor, J.W. Multilocus sequence data reveal extensive phylogenetic species diversity within the Neurospora discreta complex. Mycologia 2006, 98, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. The BPP program for species tree estimation and species delimitation. Curr. Zoöl. 2015, 61, 854–865. [Google Scholar] [CrossRef]
- Quaedvlieg, W.; Binder, M.; Groenewald, J.; Summerell, B.; Carnegie, A.; Burgess, T.; Crous, P. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia, Mol. Phylogeny Evol. Fungi 2014, 33, 1–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huson, D.H.; Kloepper, T.; Bryant, D. SplitsTree 4.0-Computation of phylogenetic trees and networks. Bioinformatics 2008, 14, 68–73. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbett, D.; Grimaldi, D.; Donoghue, M. Fossil mushrooms from Miocene and Cretaceous ambers and the evolution of Homobasidiomycetes. Am J. Bot. 1997, 84, 981–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.Y.; Currah, R.S.; Stockey, R.A. Cretaceous and Eocene poroid hymenophores from Vancouver Island, British Co-lumbia. Mycologia 2004, 96, 180–186. [Google Scholar] [CrossRef]
- Drummond, R.B.A.J.; Rambaut, A.A. Beast: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2017, 7, 214. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Ree, R.H.; Smith, S. Maximum Likelihood Inference of Geographic Range Evolution by Dispersal, Local Extinction, and Cladogenesis. Syst. Biol. 2008, 57, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Harris, A.; Blair, C.; He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenetics Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Tiffney, B.H. The Eocene North Atlantic land bridge:its importance in Tertiary and modern phytogeography of the Northern Hemisphere. J. Arnold Arboretum. 1985, 66, 243–273. [Google Scholar] [CrossRef]
- Nagasawa, E. Taxonomic reassessment of Armillaria mellea in Japan. Report for a Grant-in-Aid for Scientific Research. 1991. 63560155. Available online: https://kaken.nii.ac.jp/en/grant/KAKENHI-PROJECT-63560155/ (accessed on 4 November 2021).
- Anderson, J.B.; Ullrich, R.C. Biological Species of Armillaria mellea in North America. Mycologia 1979, 71, 402. [Google Scholar] [CrossRef]
- Anderson, J.B.; Korhonen, K.; Ullrich, R.C. Relationships between European and North American biological species of Armillaria mellea. Exp. Mycol. 1980, 4, 78–86. [Google Scholar] [CrossRef]
- Banik, M.T.; Burdsall, H.H. Assessment of Compatibility among Armillaria cepistipes, A. sinapina, and North American Biological Species X and XI, Using Culture Morphology and Molecular Biology. Mycologia 1998, 90, 798. [Google Scholar] [CrossRef]
- Ota, Y.; Matsushita, N.; Nagasawa, E.; Terashita, T.; Fukuda, K.; Suzuki, K. Biological Species of Armillaria in Japan. Plant Dis. 1998, 82, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, H.C.; Xue, W.Q.; Zhao, J.; Yang, Z.L. Phylogenetic analyses of Armillaria reveal at least 15 phylogenetic Lineages in China, seven of which are associated with cultivated Gastrodia elata. PLoS ONE 2016, 11, e0154794. [Google Scholar] [CrossRef] [Green Version]
- Kellner, R.; Vollmeister, E.; Feldbrügge, M.; Begerow, D. Interspecific sex in grass smuts and the genetic diversity of their pheromone-receptor System. PLoS Genet. 2011, 7, e1002436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menkis, A.; Bastiaans, E.; Jacobson, D.J.; Johannesson, H. Phylogenetic and biological species diversity within the Neurospora tetrasperma complex. J. Evol. Biol. 2009, 22, 1923–1936. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, J.; Qin, G.F.; Liu, T.H. Cultivation of fruiting bodies using hybrid diploids of Armillaria ostoyae. Forest Pest Dis. 2003, 22, 13–16. (In Chinese) [Google Scholar]
- Ford, K.L.; Baumgartner, K.; Henricot, B.; Bailey, A.M.; Foster, G.D. A reliable in vitro fruiting system for Armillaria mellea for evaluation of Agrobacterium tumefaciens transformation vectors. Fungal Biol. 2015, 119, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J. Fungal species concepts in the genomics era. Genome 2020, 63, 459–468. [Google Scholar] [CrossRef]
- Matute, D.R.; Sepúlveda, V. Fungal species boundaries in the genomics era. Fungal Genet. Biol. 2019, 131, 103249. [Google Scholar] [CrossRef]
- Wolfe, J.A. Some Aspects of Plant Geography of the Northern Hemisphere During the Late Cretaceous and Tertiary. Ann. Mo. Bot. Gard. 1975, 62, 264. [Google Scholar] [CrossRef]
- Ramstein, G.; Fluteau, F.; Besse, J.; Joussaume, S. Effect of orogeny, plate motion and land–sea distribution on Eurasian climate change over the past 30 million years. Nat. Cell Biol. 1997, 386, 788–795. [Google Scholar] [CrossRef]
- Chillali, M.; Wipf, D.; Guillaumin, J.; Mohammed, C.; Botton, B. Delineation of the European Armillaria species based on the sequences of the internal transcribed spacer (ITS) of ribosomal DNA. New Phytol. 1998, 138, 553–561. [Google Scholar] [CrossRef]
- Baumgartner, K.; Travadon, R.; Bruhn, J.; Bergemann, S.E. Contrasting patterns of genetic diversity and population structure of Armillaria mellea sensu stricto in the eastern and western United States. Phytopathology 2010, 100, 708–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaumin, J.J.; Mohammed, C.; Abomo, N.S. Vegetative incompatibility and sexual systems of Armillaria isolates from tropical Africa. In Proceedings of the Eighth International Conference on Root and Butt Rots, Haikko, Finland, 9–16 August 1993; pp. 349–354. [Google Scholar]
- Elías-Román, R.D.; Guzmán-Plazola, R.A.; Klopfenstein, N.B.; Alvarado-Rosales, D.; Calderón-Zavala, G.; Mora-Aguilera, J.A.; Kim, M.-S.; García-Espinosa, R. Incidence and phylogenetic analyses of Armillaria spp. associated with root disease in peach orchards in the State of Mexico, Mexico. For. Pathol. 2013, 43, 390–401. [Google Scholar] [CrossRef]
- Owaid, M.N.; Muslat, M.M.; Tan, W.C. First collection and identification of wild mushrooms in western Iraq. J. Adv. Res. Biol. 2014, 5, 29–34. [Google Scholar]
- Koch, R.A.; Herr, J.R. Global distribution and richness of Armillaria and related species inferred from public databases and amplicon sequencing datasets. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Gavrilets, S. Perspective: Models of speciation: What have we learned in 40 years? Evolution 2003, 57, 2197–2215. [Google Scholar] [CrossRef] [PubMed]
- Schluter, D. Evidence for Ecological Speciation and Its Alternative. Science 2009, 323, 737–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipos, G.; Prasanna, A.N.; Walter, M.C.; O’Connor, E.; Bálint, B.; Krizsán, K.; Kiss, B.; Hess, J.; Varga, T.; Slot, J.; et al. Genome expansion and lineage-specific genetic innovations in the forest path-ogenic fungi Armillaria. Nat. Ecol. Evol. 2017, 1, 1931–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, E.; Ota, Y.; Hattori, T.; Kikuchi, T. 2010. Sequence based identification of Japanese Armillaria species using elonga-tion factor-1 alpha gene. Mycologia 2010, 102, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Powell, C.M.; An, Z.S.; Zhou, J.; Dong, G.R. Pliocene uplift of the northern Tibetan plateau. Geology 2000, 28, 715. [Google Scholar] [CrossRef]
- Huntington, K.W.; Blythe, A.E.; Hodges, K.V. Climate change and Late Pliocene acceleration of erosion in the Himalaya. Earth Planet. Sci. Lett. 2006, 252, 107–118. [Google Scholar] [CrossRef]
- Song, C.; Hu, S.; Han, W.; Zhang, T.; Fang, X.; Gao, J.; Wu, F. Middle Miocene to earliest Pliocene sedimentological and geo-chemical records of climate change in the western Qaidam Basin on the NE Tibetan Plateau. Palaeogeogr. Palaeocl. 2014, 395, 67–76. [Google Scholar] [CrossRef]
- Bing, J.; Han, P.J.; Liu, W.Q.; Wang, Q.M.; Bai, F.Y. Evidence for a Far East Asian origin of lager beer yeast. Curr. Biol. 2014, 24, R380–R381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Zheng, Q.J.; Qian, Z.Q.; Yang, J.; Zhang, Y.P.; Li, Z.H.; Zhao, G.F. Genetic structure and evolutionary history of three alpine sclerophyllous Oaks in East Himalaya-Hengduan Mountains and adjacent regions. Front. Plant Sci. 2016, 7, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Li, S. Extinction vs. Rapid radiation: The juxtaposed evolutionary histories of Coelotine spiders support the Eocene-Oligocene Orogenesis of the Tibetan Plateau. Syst. Biol. 2017, 66, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gladenkov, A.Y.; Oleinik, A.E.; Marincovich, L.; Barinov, K.B. A refined age for the earliest opening of Bering Strait. Palaeogeogr. Palaeoclim. Palaeoecol. 2002, 183, 321–328. [Google Scholar] [CrossRef]
- Leal, I.; Bergeron, M.-J.; Feau, N.; Tsui, C.; Foord, B.; Pellow, K.; Hamelin, R.; Sturrock, R.N. Cryptic speciation in Western North America and Eastern Eurasia of the pathogens responsible for Laminated root rot. Phytopathology 2019, 109, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Li, D.W. Release and dispersal of basidiospores from Amanita muscaria var. alba and their infiltration into a residence. Mycol. Res. 2005, 109, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Priors | Posterior Probability | Posterior Probability for Delimited Species | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. mella –EA | A. mella –NA | A. mella –EA | A. mella –EU | PS I /Clade 10 | PS II | Clade 9 | Clade 8 | Clade 7 | Clade 6 | Clade 5 | Clade 4 | Clade 3 | Clade 2 | Clade 1 | ||
| θ ~ G(1, 10), τ0 ~ G(1, 10) | P[3] = 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | – | – | – | – | – | – | – | – | – |
| θ ~ G(1, 10), τ0 ~ G(2, 1000) | P[3] = 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | – | – | – | – | – | – | – | – | – |
| θ ~ G(2, 1000), τ0 ~ G(1, 10) | P[3] = 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | – | – | – | – | – | – | – | – | – |
| θ ~ G(2, 1000), τ0 ~ G(2, 1000) | P[3] = 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | – | – | – | –– | – | – | – | – | – |
| θ ~ G(1, 10), τ0 ~ G(1, 10) | P[11] = 0.215 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | – | 0.691 | 1.000 | 0.694 | 0.990 | 1.000 | 0.998 | 0.497 | 0.335 | 0.780 |
| θ ~ G(1, 10), τ0 ~ G(2, 1000) | P[11] = 0.373 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | – | 0.961 | 1.000 | 0.961 | 0.783 | 1.000 | 1.000 | 0.951 | 0.595 | 0.427 |
| Species | PS II a | PS I | A. nabsnona | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clade 1 b | Clade 2 | Clade 3 | Clade 4 | Clade 5 | Clade 6 | Clade 7 | Clade 8 | Clade 9 | Clade 10 | Clade 11 | |
| A. Cepistipes EA&EU c | A. sinapina | CBS F | A. altimontana | A. calvescens | CBS C, CBS J, CBS L, CBS H, CBS N | A. gallica EA | A. cepistipe NA | A. gallica EU | Nag. E | A. nabsnona | |
| Clade 1 | 0.03 | 0.94 | 0.7 | 0.305 | 0.001 | 0.138 | 0.18 | 0.048 | 0.091 | 0.067 | |
| Clade 2 | 0.30 | 0.35 | 0.107 | 0.001 | 0.027 | 0.37 | 0.219 | 0.085 | 0.277 | ||
| Clade 3 | 1 | 0.39 | 0.003 | 0.306 | 1 | 1 | 0.092 | 0.159 | |||
| Clade 4 | 0.065 | 0.031 | 0.322 | 1 | 1 | 0.091 | 0.329 | ||||
| Clade 5 | 0.021 | 0.089 | 0.15 | 0.15 | 0.051 | 0.269 | |||||
| Clade 6 | 0.008 | 0.001 | 0.001 | 0.108 | 0.037 | ||||||
| Clade 7 | 0.001 | 0.001 | 0.254 | 0.081 | |||||||
| Clade 8 | 1 | 0.096 | 0.12 | ||||||||
| Clade 9 | 0.237 | 0.156 | |||||||||
| Clade 10 | 0.089 | ||||||||||
| Clade 11 | |||||||||||
| Node | Species/Lineage | Mean Divergence Time (95% HPD Mya) | Ancestral Area Reconstruction (Area/Relative Probability) | |
|---|---|---|---|---|
| BBM * | DEC * | |||
| 1 | Armillaria | 21.8 (13.1–32.6) | A/0.84 | ABC/0.3 |
| 2 | Mellea superclade | 9.0 (1.5–21.6) | A/0.87 | ABC/1 |
| 3 | Solidipes/Ostoyae superclade+A. nabsnona+PS I+PS II | 15.7 (3.5–36.2) | A/0.74 | ABC/0.48 |
| 4 | Solidipes/Ostoyae superclade | 7.6 (0.8–18.1) | A/0.57 | ABC/1 |
| 5 | Gallica superclade | 9.1 (1.2–21.4) | A/0.73 | A/0.71 |
| 6 | PS I+PS II | 6.9 (1.1–16.2) | A/0.98 | A/1 |
| 7 | PS II | 5.9 (0.8–14.0) | A/0.95 | A/0.74 |
| 8 | PS II: lineage 2, lineage 3 and lineage 4 | 4.2 (0.6–10.1) | A/0.51 | ABC/1 |
| 9 | PS II: lineage 1 | 2.2 (0.1–6.2) | A/0.99 | A/0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Pecoraro, L.; Cai, L.; Yuan, Z.; Zhao, P.; Tsui, C.K.M.; Zhang, Z. Phylogenetic Relationships, Speciation, and Origin of Armillaria in the Northern Hemisphere: A Lesson Based on rRNA and Elongation Factor 1-Alpha. J. Fungi 2021, 7, 1088. https://doi.org/10.3390/jof7121088
Liang J, Pecoraro L, Cai L, Yuan Z, Zhao P, Tsui CKM, Zhang Z. Phylogenetic Relationships, Speciation, and Origin of Armillaria in the Northern Hemisphere: A Lesson Based on rRNA and Elongation Factor 1-Alpha. Journal of Fungi. 2021; 7(12):1088. https://doi.org/10.3390/jof7121088
Chicago/Turabian StyleLiang, Junmin, Lorenzo Pecoraro, Lei Cai, Zhilin Yuan, Peng Zhao, Clement K. M. Tsui, and Zhifeng Zhang. 2021. "Phylogenetic Relationships, Speciation, and Origin of Armillaria in the Northern Hemisphere: A Lesson Based on rRNA and Elongation Factor 1-Alpha" Journal of Fungi 7, no. 12: 1088. https://doi.org/10.3390/jof7121088








