The Evolution of Life Modes in Stictidaceae, with Three Novel Taxa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phenotypic Analysis
2.2. DNA Extraction, PCR Amplification, and Gene Sequencing
2.3. Phylogenetic Analyses and Species Recognition
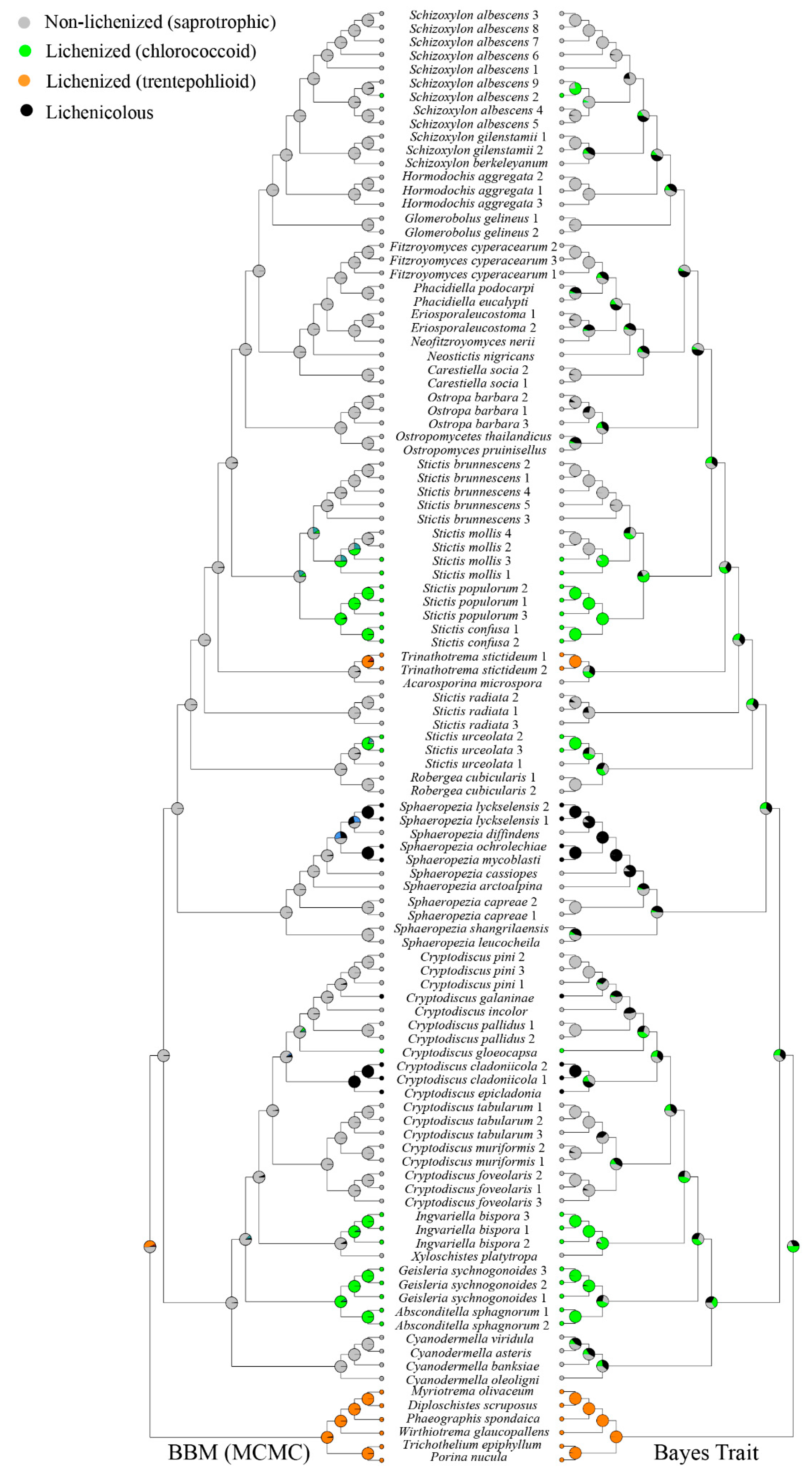
2.4. Ancestral Character State Analyses
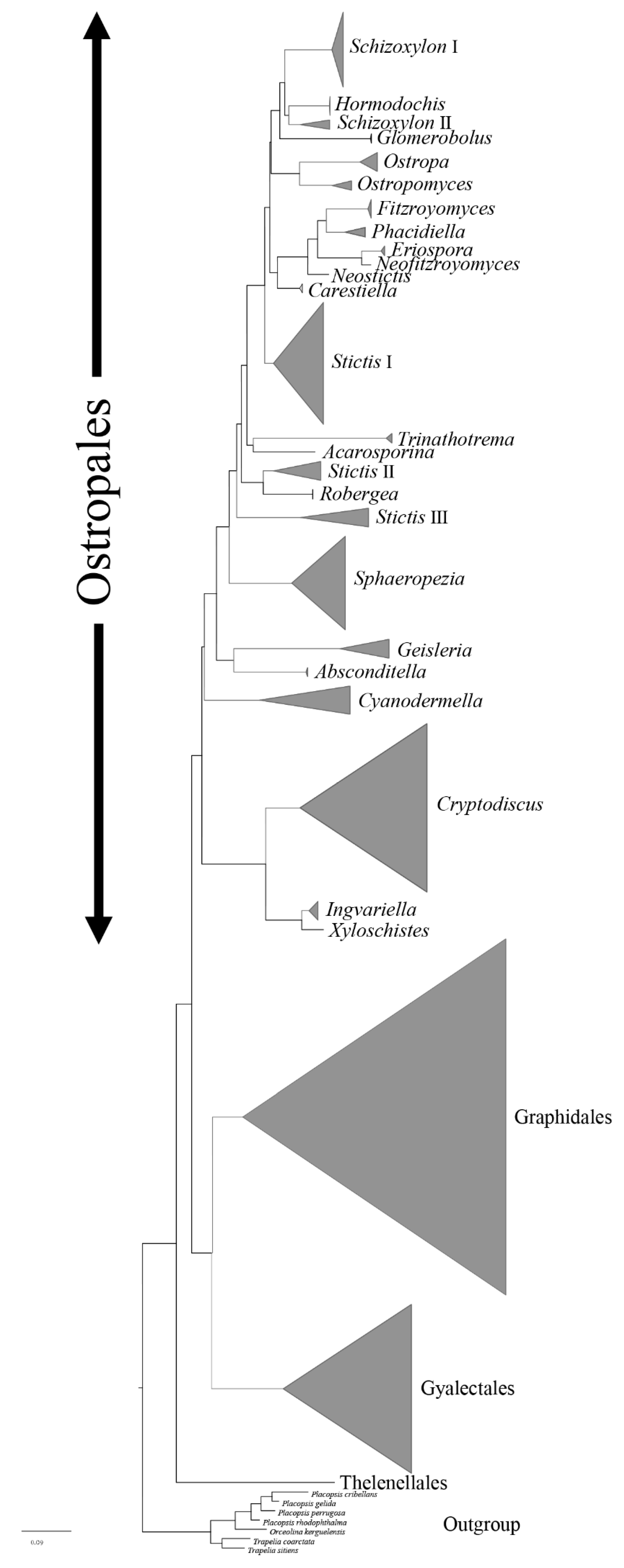
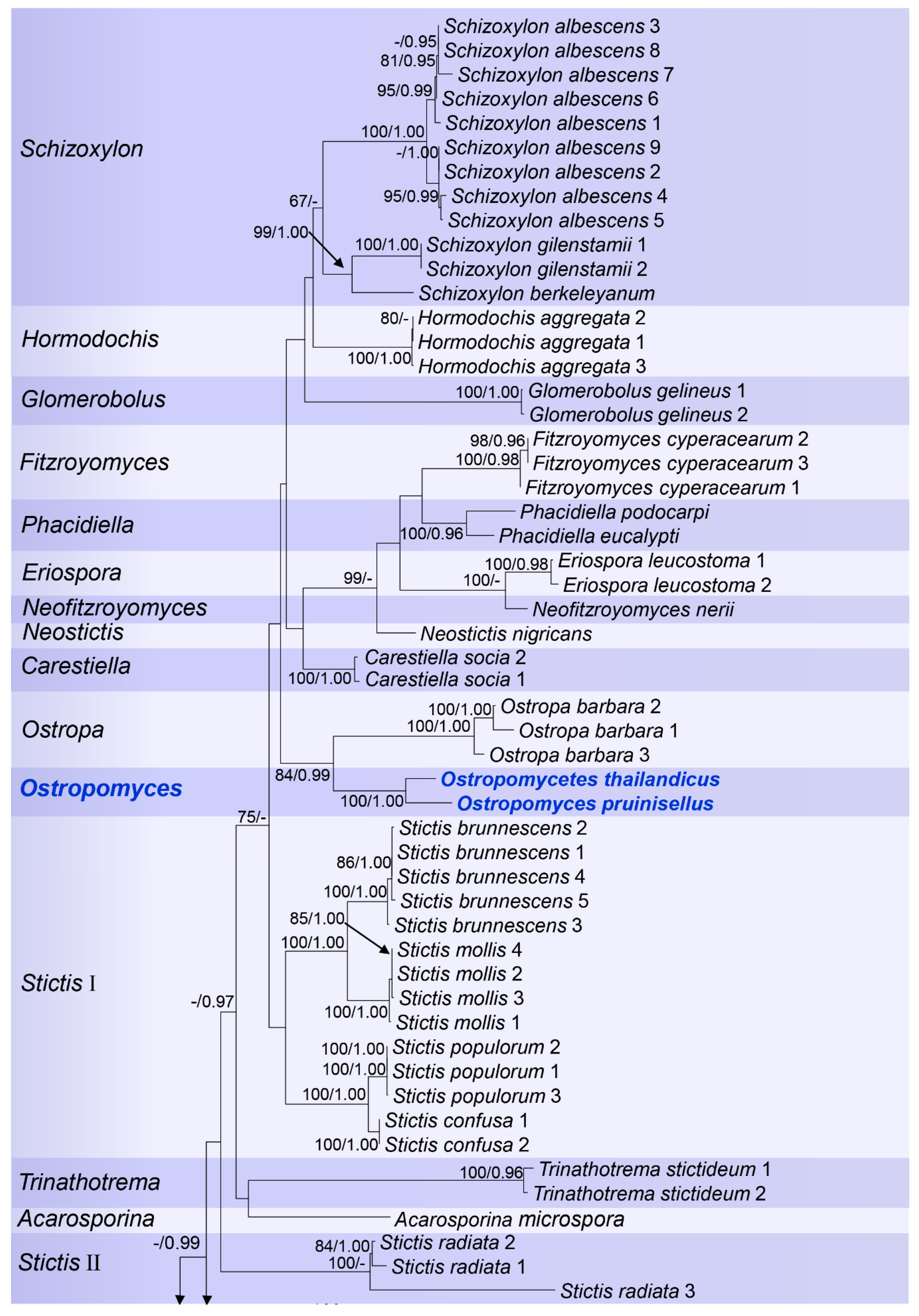
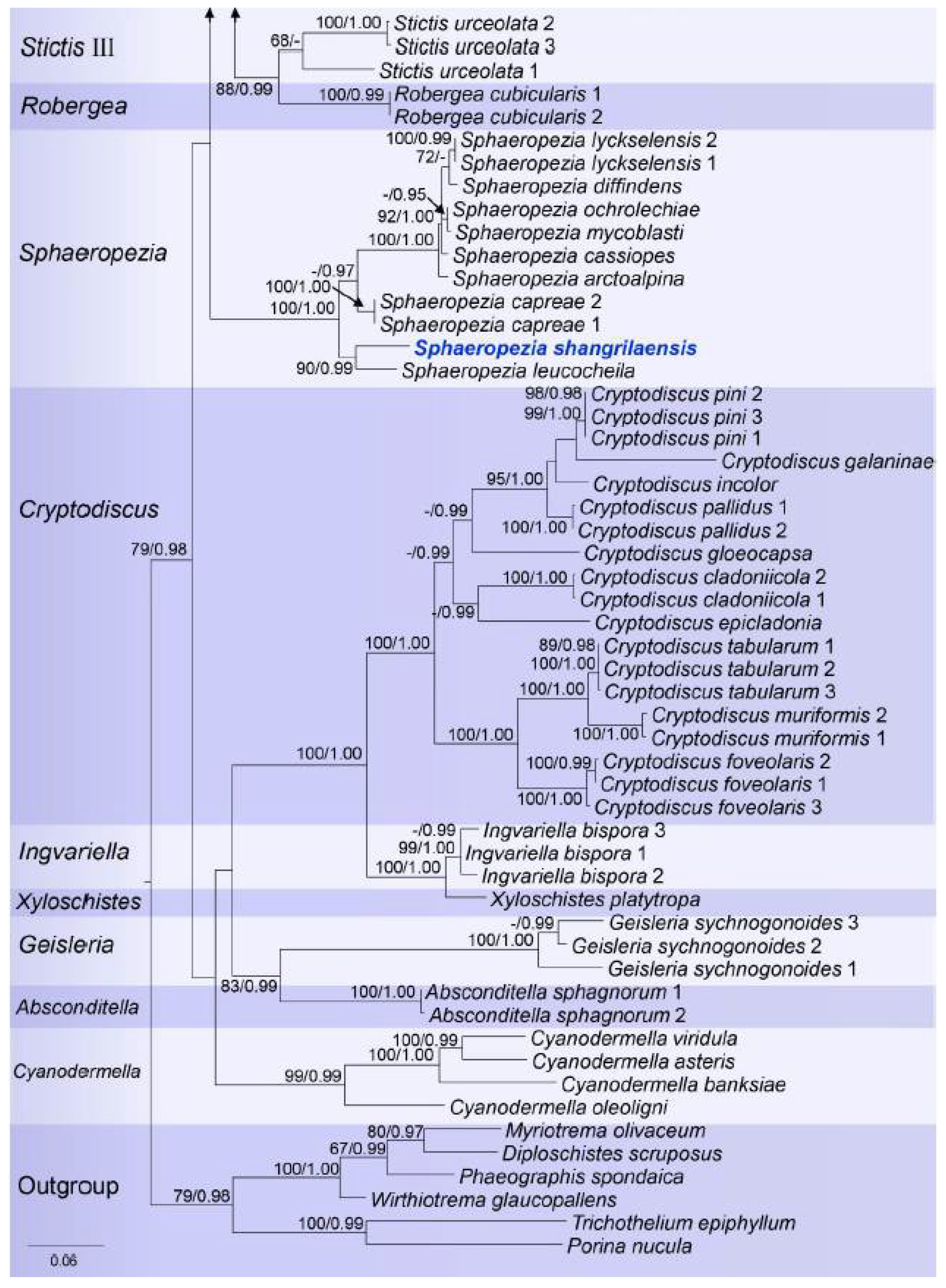
3. Results
3.1. Phylogenetic Analyses
3.2. Ancestral Character State Analysis
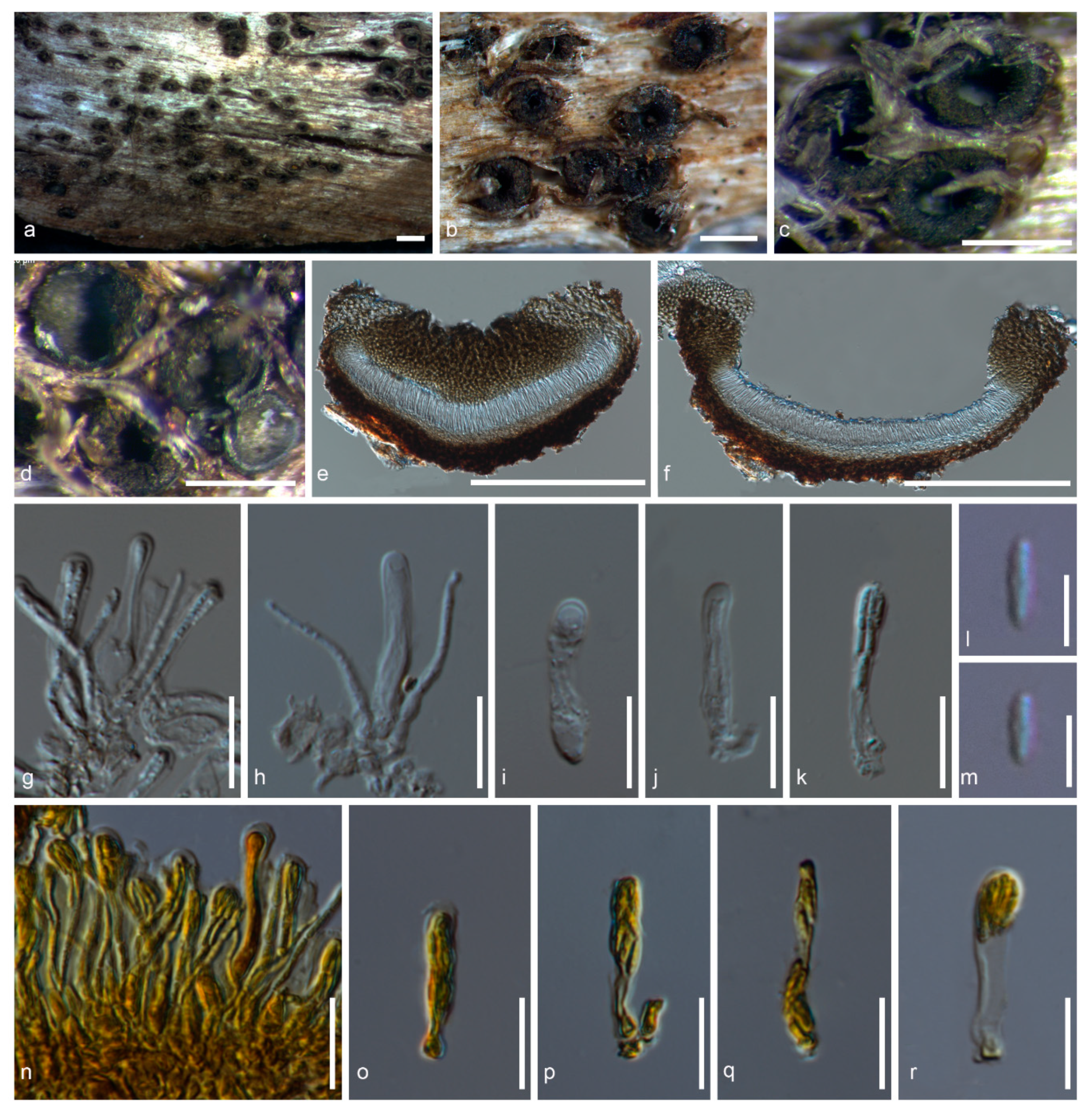
3.3. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota–Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Lücking, R.; Nelsen, M.P. Ediacarans, protolichens, and lichen-derived Penicillium: A critical reassessment of the evolution of lichenization in fungi. Transform. Paleobotany 2018, 551–590. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Lücking, R.; Boyce, C.K.; Lumbsch, H.T.; Ree, R.H. The macroevolutionary dynamics of symbiotic and phenotypic diversification in lichens. Proc. Natl. Acad. Sci. USA 2020, 117, 21495–21503. [Google Scholar] [CrossRef]
- Gargas, A.; DePriest, P.T.; Grube, M.; Tehler, A. Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science 1995, 268, 1492–1495. [Google Scholar] [CrossRef] [Green Version]
- Lawrey, J.D.; Lücking, R.; Sipman, H.J.; Chaves, J.L.; Redhead, S.A.; Bungartz, F.; Sikaroodi, M.; Gillevet, P.M. High concentration of basidiolichens in a single family of agaricoid mushrooms (Basidiomycota: Agaricales: Hygrophoraceae). Mycol. Res. 2009, 113, 1154–1171. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, M.; Lücking, R.; Grube, M.; Mbatchou, J.; Muggia, L.; Plata, E.R.; Lumbsch, H. Unravelling the phylogenetic relationships of lichenised fungi in Dothideomyceta. Stud. Mycol. 2009, 64, 135–144. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Lücking, R.; Mbatchou, J.S.; Andrew, C.J.; Spielmann, A.A.; Lumbsch, H.T. New insights into relationships of lichen-forming Dothideomycetes. Fungal Divers. 2011, 51, 155–162. [Google Scholar] [CrossRef]
- Schoch, C.L.; Sung, G.-H.; López-Giráldez, F.; Townsend, J.P.; Miadlikowska, J.; Hofstetter, V.; Robbertse, B.; Matheny, P.B.; Kauff, F.; Wang, Z.; et al. The Ascomycota tree of life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 2009, 58, 224–239. [Google Scholar] [CrossRef]
- Prieto, M.; Wedin, M. Dating the diversification of the major lineages of Ascomycota (Fungi). PLoS ONE 2013, 8, e65576. [Google Scholar] [CrossRef] [Green Version]
- Hodkinson, B.P.; Moncada, B.; Lücking, R. Lepidostromatales, a new order of lichenized fungi (Basidiomycota, Agaricomycetes), with two new genera, Ertzia and Sulzbacheromyces, and one new species, Lepidostroma winklerianum. Fungal Divers. 2014, 64, 165–179. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Lichenization: The origins of a fungal life-style. Recent Adv. Lichenol. 2015, 2, 1–10. [Google Scholar]
- Lutzoni, F.; Nowak, M.D.; Alfaro, M.E.; Reeb, V.; Miadlikowska, J.; Krug, M.; Arnold, A.E.; Lewis, L.A.; Swofford, D.L.; Hibbett, D. Contemporaneous radiations of fungi and plants linked to symbiosis. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kraichak, E.; Huang, J.-P.; Nelsen, M.; Leavitt, S.D.; Lumbsch, H.T. A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot. J. Linn. Soc. 2018, 188, 233–249. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Rajeshkumar, K.C.; Hawksworth, D.L.; Madrid, H.; Kirk, P.M.; Braun, U.; Singh, R.V.; Crous, P.W.; Kukwa, M.; et al. Notes for genera: Ascomycota. Fungal Divers. 2017, 86, 1–594. [Google Scholar]
- Wijayawardene, N.; Hyde, K.; Al-Ani, L.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.; Zhao, R.; Aptroot, A.; Leontyev, D.; Saxena, R.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Voglmayr, H.; Fournier, J.; Jaklitsch, W. Two new classes of Ascomycota: Xylobotryomycetes and Candelariomycetes. Pers. Mol. Phylogeny Evol. Fungi 2019, 42, 36. [Google Scholar] [CrossRef] [Green Version]
- Lücking, R.; Grube, M. Facultative parasitism and reproductive strategies in Chroodiscus (Ascomycota, Ostropales). Stapfia 2002, 80, 267–292. [Google Scholar]
- Baloch, E.; Gilenstam, G.; Wedin, M. The relationships of Odontotrema (Odontotremataceae) and the resurrected Sphaeropezia (Stictidaceae)—New combinations and three new Sphaeropezia species. Mycologia 2013, 105, 384–397. [Google Scholar] [CrossRef]
- Baloch, E.; Lücking, R.; Lumbsch, H.T.; Wedin, M. Major clades and phylogenetic relationships between lichenized and non-lichenized lineages in Ostropales (Ascomycota: Lecanoromycetes). Taxon 2010, 59, 1483–1494. [Google Scholar] [CrossRef]
- Lutzoni, F.; Pagel, M.; Reeb, V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature 2001, 411, 937–940. [Google Scholar] [CrossRef]
- Kauff, F.; Lutzoni, F. Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): Among and within order relationships based on nuclear ribosomal RNA small and large subunits. Mol. Phylogenet. Evol. 2002, 25, 138–156. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Schmitt, I.; Palice, Z.; Wiklund, E.; Ekman, S.; Wedin, M. Supraordinal phylogenetic relationships of Lecanoromycetes based on a Bayesian analysis of combined nuclear and mitochondrial sequences. Mol. Phylogenet. Evol. 2004, 31, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Lumbsch, H.T.; Schmitt, I.; Mangold, A.; Wedin, M. Ascus types are phylogenetically misleading in Trapeliaceae and Agyriaceae (Ostropomycetidae, Ascomycota). Mycol. Res. 2007, 111, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Wedin, M.; Doering, H.; Koenberg, K.; Gilenstam, G. Generic delimitations in the family Stictidaceae (Ostropales, Ascomycota): The Stictis–Conotrema problem. Lichenologist 2005, 37, 67–75. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Kauff, F.; Hofstetter, V.; Fraker, E.; Grube, M.; Hafellner, J.; Reeb, V.; Hodkinson, B.P.; Kukwa, M.; Lücking, R.; et al. New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA-and two protein-coding genes. Mycologia 2006, 98, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Miadlikowska, J.; Kauff, F.; Högnabba, F.; Oliver, J.C.; Molnár, K.; Fraker, E.; Gaya, E.; Hafellner, J.; Hofstetter, V.; Gueidan, C.; et al. A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Mol. Phylogenet. Evol. 2014, 79, 132–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Baral, H.-O.; Xu, X.; Liu, Y. Parakarstenia phyllostachydis, a new genus and species of non-lichenized Odontotremataceae (Ostropales, Ascomycota). Mycol. Prog. 2019, 18, 833–845. [Google Scholar] [CrossRef]
- Baloch, E.; Gilenstam, G.; Wedin, M. Phylogeny and classification of Cryptodiscus, with a taxonomic synopsis of the Swedish species. Fungal Divers. 2009, 38, 51–68. [Google Scholar]
- Tehler, A.; Wedin, M. Systematics of lichenised fungi. Lichen Biol. 2008, 336–352. [Google Scholar] [CrossRef]
- Lücking, R. Stop the abuse of time! Strict temporal banding is not the future of rank-based classifications in fungi (including lichens) and other organisms. Crit. Rev. Plant Sci. 2019, 38, 199–253. [Google Scholar] [CrossRef]
- Fernández-Brime, S.; Olariaga, I.; Baral, H.-O.; Friebes, G.; Jaklitsch, W.; Senn-Irlet, B.; Wedin, M. Cryptodiscus muriformis and Schizoxylon gilenstamii, two new species of Stictidaceae (Ascomycota). Mycol. Prog. 2018, 17, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Wedin, M.; Döring, H.; Gilenstam, G. Saprotrophy and lichenization as options for the same fungal species on different substrata: Environmental plasticity and fungal lifestyles in the Stictis–Conotrema complex. New Phytol. 2004, 164, 459–465. [Google Scholar] [CrossRef]
- Sherwood, M.A. The ostropalean fungi. Mycotaxon 1977, 5, 1–277. [Google Scholar]
- Index Fungorum. Available online: http://www.indexfungorum.org/Names/Names.asp (accessed on 15 December 2020).
- Jayasiri, S.; Hyde, K.; Ariyawansa, H.; Bhat, J.; Buyck, B.; Cai, L.; Dai, Y.; Abd-Elsalam, K.; Ertz, D.; Hidayat, I.; et al. The faces of fungi database: Fungal names linked with morphology, molecular and human attributes. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Jones, E.G.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Zoller, S.; Scheidegger, C.; Sperisen, C. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/nuccore/?term= (accessed on 15 December 2020).
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT, Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; pp. 95–98. [Google Scholar]
- Daniel, G.-P.; Daniel, G.-B.; Reboiro-Jato, M.; Fdez-Riverola, F.; Posada, D. ALTER: Program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010, 38, W14–W18. [Google Scholar]
- Nylander, J. MrModeltest (Version 2.2); Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees; Gateway Computing Environments Workshop (GCE): New Orleans, LA, USA, 2010. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTree. Version 1.4.2; University of Edinburgh: Edinburgh, UK, 2014. [Google Scholar]
- Joy, J.B.; Liang, R.H.; McCloskey, R.M.; Nguyen, T.; Poon, A.F. Ancestral reconstruction. PLoS Comput. Biol. 2016, 12, e1004763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Blair, C.; He, X. RASP 4: Ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 2020, 37, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Harris, A.J.; Blair, C.; He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef]
- Nannfeldt, J.A. Studien uber die Morphologie und Systematik der nicht-lichenisierten inoperculaten Discomyceten. Nova Acta Regiae Soc. Sci. Upsal. Ser. IV 1932, 8, 1–368. [Google Scholar]
- Fries, E. Systema Mycologicum. Gryphiswaldiae 1849, 2, 1–275. [Google Scholar]
- Sherwood, M.A. The ostropalean fungi: Schizoxylon, with notes on Stictis, Acarosporina, Cocropezia, and Carestiella. Mycotaxon 1977, 6, 215–260. [Google Scholar]
- Phukhamsakda, C.; McKenzie, E.H.; Phillips, A.J.; Jones, E.G.; Bhat, D.J.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Ekanayaka, A.; Ariyawansa, H.; Hyde, K.; Jones, E.; Daranagama, D.; Phillips, A.; Hongsanan, S.; Jayasiri, S.; Zhao, Q. DISCOMYCETES: The apothecial representatives of the phylum Ascomycota. Fungal Divers. 2017, 87, 237–298. [Google Scholar] [CrossRef]
- Ekanayaka, A.; Hyde, K.; Jones, E.; Zhao, Q.; Bulgakov, T. New and known discolichens from Asia and eastern Europe. Asian J. Mycol. 2019, 2, 48–86. [Google Scholar] [CrossRef]
- Bernardin, J.R. A Morphological and Molecular Reassessment of Robergea albicedrae (Ascomycota). Master’s Thesis, Texas State University, San Marcos, TX, USA, 2019. [Google Scholar]
- Guderley, R.; Lumbsch, H.T.; Feige, G.B. Ingvariella, a new genus in the Thelotremataceae (lichenized Ascomycotina). Nova Hedwig. 1997, 147–154. [Google Scholar] [CrossRef]
- Hayova, V.P. Some new and rare records of ascomycetes in Ukraine. Ukr. Bot. J. 2005, 62, 70–77. [Google Scholar]
- Konoreva, L.A.; Chesnokov, S.V.; Davydov, E.A. Stictis and Schizoxylon (Stictidaceae, Ostropales) in Russia. Herzogia 2016, 29, 706–711. [Google Scholar] [CrossRef]
- Popov, E.S.; Chesnokov, S.V.; Konoreva, L.A.; Ezhkin, A.K.; Stepanchikova, I.S.; Kuznetsova, E.S.; Himelbrant, D.E.; Galanina, I.A.; Tchabanenko, S.I. Stictis sl (Ostropales, Ascomycota) in the Russian Far East. Bot. Pac. J. Plant Sci. Conserv. 2020, 9, 1–8. [Google Scholar]
- Fries, E. EM 1821–1832. Syst. Mycol. 1821, 3. [Google Scholar]
- Corda, A. CJ. 1837. Icon. Fung. 1838, 1, 12. [Google Scholar]
- Crous, P.; Wingfield, M.; Schumacher, R.; Akulov, A.; Bulgakov, T.; Carnegie, A.; Jurjević, Ž.; Decock, C.; Denman, S.; Lombard, L.; et al. New and interesting fungi. 3. Fungal Syst. Evol. 2020, 6, 157. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Carnegie, A.J.; Hardy, G.S.J.; Smith, D.; Summerell, B.A.; Cano-Lira, J.F.; Guarro, J.; Houbraken, J.; et al. Fungal Planet description sheets: 625–715. Pers. Mol. Phylogeny Evol. Fungi 2017, 39, 270. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Schumacher, R.; Summerell, B.A.; Giraldo, A.; Gené, J.; Guarro, J.; Wanasinghe, D.; Hyde, K.D.; Camporesi, E.; et al. Fungal Planet description sheets: 281–319. Pers. Mol. Phylogeny Evol. Fungi 2014, 33, 212. [Google Scholar] [CrossRef]
- Fernández-Brime, S.; Llimona, X.; Molnar, K.; Stenroos, S.; Hognabba, F.; Bjork, C.; Lutzoni, F.; Gaya, E. Expansion of the Stictidaceae by the addition of the saxicolous lichen-forming genus Ingvariella. Mycologia 2011, 103, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Gilenstam, G. Studies in the lichen genus Conotrema. Ark. Bot. 1974, 7, 149–179. [Google Scholar]
- Aptroot, A.; de Oliveira Mendonça, C.; Ferraro, L.I.; da Silva Caceres, M.E. A world key to species of the genera Topelia and Thelopsis (Stictidaceae), with the description of three new species from Brazil and Argentina. Lichenologist 2014, 46, 801. [Google Scholar] [CrossRef]
- Bely, P. Absconditella lignicola (Stictidaceae)–lichen species new to Belarus. Botanica 2012, 18, 164–165. [Google Scholar] [CrossRef] [Green Version]
- Lücking, R.; Rivas Plata, E.; Mangold, A.; Sipman, H.; Aptroot, A.; Miranda González, R.; Kalb, K.; Chaves, J.; Ventura, N.; Esmeralda Esquivel, R. Natural history of Nash’s Pore Lichens, Trinathotrema (Ascomycota: Lecanoromycetes: Ostropales: Stictidaceae). Bibl. Lichenol. 2011, 106, 183–206. [Google Scholar]
- Winka, K.; Ahlberg, C.; Eriksson, O.E. Are there lichenized Ostropales? Lichenologist 1998, 30, 455–462. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Jeewon, R.; Hyde, K. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]
- Van Nieuwenhuijzen, E.; Miadlikowska, J.; Houbraken, J.A.; Adan, O.C.; Lutzoni, F.; Samson, R. Wood staining fungi revealed taxonomic novelties in Pezizomycotina: New order Superstratomycetales and new species Cyanodermella oleoligni. Stud. Mycol. 2016, 85, 107–124. [Google Scholar] [CrossRef]
- Saccardo, P.A. Conspectus generum Discomycetum hucuscue cognitorum. Bot. Cent. 1884, 18, 247–256. [Google Scholar]
- Zhu, H. Biogeography of Shangri-la flora in southwestern China. Phytotaxa 2015, 203, 231–244. [Google Scholar]
- Johnston, P.; Park, D.; Renner, M. Sphaeropezia leucocheila sp. nov. (Stictidaceae): A liverwort pathogen from New Zealand. Phytotaxa 2019, 409, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Da Silva Cáceres, M.E.; Lücking, R.; Schumm, F.; Aptroot, A. A lichenized family yields another renegade lineage: Papilionovela albothallina is the first non-lichenized, saprobic member of Graphidaceae subfam. Graphidoideae. Bryologist 2020, 123, 144–154. [Google Scholar] [CrossRef]
- Pino-Bodas, R.; Zhurbenko, M.; Stenroos, S. Phylogenetic placement within Lecanoromycetes of lichenicolous fungi associated with Cladonia and some other genera. Pers. Mol. Phylogeny Evol. Fungi 2017, 39, 91. [Google Scholar] [CrossRef] [PubMed]
- Aptroot, A. Aspects of the integration of the taxonomy of lichenized and non-lichenized pyrenocarpous ascomycetes. Lichenologist 1998, 30, 501–514. [Google Scholar] [CrossRef]
- Jahn, L.; Schafhauser, T.; Pan, S.; Weber, T.; Wohlleben, W.; Fewer, D.P.; Sivonen, K.; Flor, L.; van Pée, K.H.; Caradec, T. Cyanodermella asteris sp. nov. (Ostropales) from the inflorescence axis of Aster tataricus. Mycotaxon 2017, 132, 107–123. [Google Scholar] [CrossRef]
- Stenroos, S.; Laukka, T.; Huhtinen, S.; Döbbeler, P.; Myllys, L.; Syrjänen, K.; Hyvönen, J. Multiple origins of symbioses between ascomycetes and bryophytes suggested by a five-gene phylogeny. Cladistics 2010, 26, 281–300. [Google Scholar] [CrossRef]
- Lücking, R.; Tehler, A.; Bungartz, F.; Rivas Plata, E.; Lumbsch, H.T. Journey from the West: Did tropical Graphidaceae (lichenized Ascomycota: Ostropales) evolve from a saxicolous ancestor along the American Pacific coast? Am. J. Bot. 2013, 100, 844–856. [Google Scholar] [CrossRef]
- Mangold, A.; Martín, M.P.; Lücking, R.; Thorsten Lumbsch, H. Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota: Ostropales). Taxon 2008, 57, 476–486. [Google Scholar]
- Parnmen, S.; Cáceres, M.E.; Lücking, R.; Lumbsch, H.T. Myriochapsa and Nitidochapsa, two new genera in Graphidaceae (Ascomycota: Ostropales) for chroodiscoid species in the Ocellularia clade. Bryologyst 2013, 116, 127–133. [Google Scholar] [CrossRef]
- Diederich, P.; Zhurbenko, M.; Etayo, J. The lichenicolous species of Odontotrema (syn. Lethariicola) (Ascomycota, Ostropales). Lichenologist 2002, 34, 479–501. [Google Scholar] [CrossRef] [Green Version]


| GenBank Accession Numbers | ||||
|---|---|---|---|---|
| Species | Strains | mtSSU | LSU | ITS |
| Absconditella sphagnorum 1 | T. Laukka 52 (TUR) | EU940247 | EU940095 | – |
| Absconditella sphagnorum 2 | 17 Feb 02 Palice (HB Palice) | AY300872 | AY300824 | – |
| Acarosporina microspora | AFTOL-ID 78 | AY584612 | AY584643 | DQ782834 |
| Carestiella socia 1 | GG2410 | AY661677 | AY661687 | AY661687 |
| Carestiella socia 2 | GG2437a | AY661678 | AY661682 | AY661682 |
| Cryptodiscus cladoniicola 1 | RP160 | KY661675 | KY661653 | KY661620 |
| Cryptodiscus cladoniicola 2 | RP159 | KY661674 | KY661652 | KY661619 |
| Cryptodiscus epicladonia | RP208 | KY661680 | – | KY661628 |
| Cryptodiscus foveolaris 1 | EB155 | FJ904695 | – | FJ904673 |
| Cryptodiscus foveolaris 2 | EB86 | FJ904692 | – | FJ904670 |
| Cryptodiscus foveolaris 3 | EB147 | FJ904694 | – | FJ904672 |
| Cryptodiscus galaninae | RP314 | – | – | KY661636 |
| Cryptodiscus gloeocapsa | EB93 | FJ904696 | – | FJ904674 |
| Cryptodiscus incolor | EB164 | FJ904697 | – | FJ904675 |
| Cryptodiscus muriformis 1 | UPS F-647154 | MG281972 | MG281962 | MG281962 |
| Cryptodiscus muriformis 2 | H.B. 6773 | MG281973 | MG281963 | MG281963 |
| Cryptodiscus pallidus 1 | EB60 | FJ904700 | FJ904678 | FJ904678 |
| Cryptodiscus pallidus 2 | EB173 | FJ904702 | FJ904680 | FJ904680 |
| Cryptodiscus pini 1 | EB82 | FJ904704 | FJ904682 | FJ904682 |
| Cryptodiscus pini 2 | EB178 | FJ904705 | FJ904683 | FJ904683 |
| Cryptodiscus pini 3 | EB181 | FJ904706 | FJ904684 | FJ904684 |
| Cryptodiscus tabularum 1 | CO205 | FJ904712 | FJ904690 | FJ904690 |
| Cryptodiscus tabularum 2 | EB169 | FJ904711 | FJ904689 | FJ904689 |
| Cryptodiscus tabularum 3 | EB77 | FJ904709 | FJ904687 | FJ904687 |
| Cyanodermella asteris | 03HOR06-2-4 | – | KT758843 | KT758843 |
| Cyanodermella banksiae | CPC:32105 | – | NG_064548 | NR_159835 |
| Cyanodermella oleoligni | DTO 301-G1 | KX999144 | KX950461 | KX950434 |
| Cyanodermella viridula | EB146 | – | MG281964 | MG281964 |
| Diploschistes scruposus | SFB 95 | KC167052 | – | KC167001 |
| Eriospora leucostoma 1 | CPC:35594 | – | MT223890 | MT223795 |
| Eriospora leucostoma 2 | CPC:35598 | – | MT223891 | MT223796 |
| Fitzroyomyces cyperacearum 1 | CPC:32209 | – | NG_058513 | NR_156387 |
| Fitzroyomyces cyperacearum 2 | MFLU 18-0695b | – | MK499361 | MK499349 |
| Fitzroyomyces cyperacearum 3 | MFLU 18-0695a | – | MK499363 | – |
| Geisleria sychnogonoides 1 | Caceres & Aptroot 13560 (ABL) | KC689751 | KC689752 | – |
| Geisleria sychnogonoides 2 | GESY7510 | KF220306 | KF220304 | – |
| Geisleria sychnogonoides 3 | GESY7509 | KF220305 | – | – |
| Glomerobolus gelineus 1 | AFTOL-ID 1349 | DQ247784 | DQ247803 | DQ247782 |
| Glomerobolus gelineus 2 | JK 5584C | DQ247783 | DQ247798 | – |
| Hormodochis aggregata 1 | CBS:145904 | – | – | NR_166307 |
| Hormodochis aggregate 2 | CPC:37499 | – | MN317288 | MN313807 |
| Hormodochis aggregata 3 | CPC:35475 | – | MN317287 | MN313806 |
| Ingvariella bispora 1 | DUKE 1444446 | HQ659175 | – | – |
| Ingvariella bispora 2 | MALich 15288 | HQ659173 | HQ659184 | – |
| Ingvariella bispora 3 | BCNLich 17183 | HQ659174 | HQ659185 | – |
| Myriotrema olivaceum | Kalb 39107 | KJ435181 | KJ435111 | – |
| Neofitzroyomyces nerii | CBS:145088 | – | MK047504 | MK047454 |
| Neostictis nigricans | MFLU 18-1380 | – | MT214610 | MT310654 |
| Ostropa barbara 1 | S F302817 | MG281974 | MG281965 | MG281965 |
| Ostropa barbara 2 | EB85 | HM244752 | HM244773 | HM244773 |
| Ostropa barbara 3 | G. M. 2015-04-28.1 | – | KY608095 | KY608095 |
| Ostropomyces pruinosellus | MFLU 20-0538 | MW400963 | MW400966 | MW400964 |
| Ostropomyces thailandicus | MFLU 20-0539 | – | MW397060 | MW400967 |
| Phacidiella eucalypti | CBS 120255 | – | MT373344 | MT373361 |
| Phacidiella podocarpi | CBS 138904 | – | NG_058118 | NR_137934 |
| Phaeographis spondaica | Lumbsch 19633 | JX421280 | – | – |
| Porina nucula | Lücking 17007-c | KJ449310 | – | – |
| Robergea cubicularis 1 | G.M. 2013-05-09.1 | – | KY611899 | KY611899 |
| Robergea cubicularis 2 | G.M. 2017-10-12.1 | – | MN833317 | MN833317 |
| Schizoxylon albescens 1 | GG236 | AY661680 | AY661689 | AY661689 |
| Schizoxylon albescens 2 | GG2696a | DQ401142 | DQ401144 | DQ401144 |
| Schizoxylon albescens 3 | Wedin 8365 (S) | – | – | HQ287353 |
| Schizoxylon albescens 4 | Wedin 8364 (S) | – | – | HQ287352 |
| Schizoxylon albescens 5 | Wedin 8356 b (S) | – | – | HQ287350 |
| Schizoxylon albescens 6 | Wedin 8359 (S) | – | – | HQ287351 |
| Schizoxylon albescens 7 | Wedin 8327 (S) | – | – | HQ287349 |
| Schizoxylon albescens 8 | Wedin 8324 (S) | – | – | HQ287348 |
| Schizoxylon albescens 9 | Wedin 8254 (S) | – | – | HQ287347 |
| Schizoxylon berkeleyanum | F209682 | MG281975 | MG281966 | MG281966 |
| Schizoxylon gilenstamii 1 | MW9490 | MG281977 | MG281968 | MG281968 |
| Schizoxylon gilenstamii 2 | MW9496 | MG281978 | MG281969 | MG281969 |
| Sphaeropezia arctoalpina | Baloch SW057 | HM244736 | HM244760 | – |
| Sphaeropezia capreae 1 | GG2560 | AY661674 | AY661684 | – |
| Sphaeropezia capreae 2 | UPS (Gilenstam 2633a) | HM244751 | HM244772 | – |
| Sphaeropezia cassiopes | Baloch s.n. (S) | HM244746 | – | – |
| Sphaeropezia diffindens | Baloch SW020 (S) | HM244747 | – | – |
| Sphaeropezia leucocheila | PDD 98299 | MK547101 | MK547099 | MK547090 |
| Sphaeropezia lyckselensis 1 | Gilenstam 2651 (S) | JX266156 | JX266158 | – |
| Sphaeropezia lyckselensis 2 | Gilenstam 2659 | HM244750 | HM244771 | – |
| Sphaeropezia mycoblasti | Wedin 8509 & Westberg (S) | JX266157 | JX266159 | – |
| Sphaeropezia ochrolechiae | Wedin 6729 (UPS) | – | JX266160 | – |
| Sphaeropezia shangrilaensis | MFLU 20-0537 | MW400962 | MW400965 | MW400955 |
| Stictis brunnescens 1 | EB84 | MG281979 | – | – |
| Stictis brunnescens 2 | Gilenstam 2359 (UPS) | AY661679 | – | AY661688 |
| Stictis brunnescens 3 | SFB1100 | MG281981 | – | MG281970 |
| Stictis brunnescens 4 | MW8571 | MG281980 | – | – |
| Stictis brunnescens 5 | SFB1105 | MG281982 | – | MG281971 |
| Stictis confusa 1 | Wedin 7070 (UPS) | DQ401141 | – | DQ401143 |
| Stictis confusa 2 | AN3222 | AY527365 | – | AY527336 |
| Stictis mollis 1 | GG2440b | AY527342 | – | AY527313 |
| Stictis mollis 2 | GG2445a | AY527347 | – | AY527318 |
| Stictis mollis 3 | GG2370 | AY527339 | – | AY527310 |
| Stictis mollis 4 | GG2458b | AY527345 | – | AY527316 |
| Stictis populorum 1 | GG2618 | AY527360 | – | AY527331 |
| Stictis populorum 2 | GG2610a | AY527356 | – | AY527327 |
| Stictis populorum 3 | MW7301 | AY527363 | – | AY527334 |
| Stictis radiata 1 | MW6493 | AY527338 | – | AY527309 |
| Stictis radiata 2 | GG2449a | AY340532 | – | AY527308 |
| Stictis radiata 3 | AFTOL-ID 398 | AY584727 | – | DQ782846 |
| Stictis urceolata 1 | MFLU 19–2695 | – | MN989186 | – |
| Stictis urceolata 2 | LT21500 | AY661676 | AY661686 | AY661686 |
| Stictis urceolata 3 | AFTOL-ID 96 | – | – | HQ650601 |
| Trichothelium epiphyllum | Baloch CR-127 | AY648901 | – | – |
| Trinathotrema stictideum 1 | F:Luecking 17541b | GU380288 | – | – |
| Trinathotrema stictideum 2 | F:Luecking 28093 | GU380287 | – | – |
| Wirthiotrema glaucopallens | DNA1336 | JF828972 | – | – |
| Xyloschistes platytropa | H:Bjork 05-242 | KJ766517 | KJ766680 | – |
| Species Name | Position of Ascoma | Shape of Ascoma | Size of Ascoma (μm) | Size of Ascoma Pore Opening (μm) | Size of Asci (μm) | Spore Size (μm) | Ascospore Shape | Number of Septate | Known Distribution | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Sphaeropezia santessonii | Immersed, partly erumpent, finally sessile | - | (225–) 280–380 (–440) | (20–) 55–125 (–190) | 40–50 (–55) × 8–13 | (12·5–) 15·4–20·4 (–23·5) × (3–) 3·6–4·6(–5) | Fusiform, often asymmetrical | trans-septate (3–) 6–8 (–9) to submuriform | Russian Arctic, Iceland and Peru, widespread and common in Arctic regions | [79] |
| S. bryoriae | Superficial | Roundish to subspherical | (275–) 310–410 (–440) | (0–) 10–70 (–120) | 40–60 × 5–6 | (7·4–) 7·6–8·8 (–9·2) × (2·8–)3· 1–3·5(–4·0) | Ellipsoid | 1-septate (exceptionally 2-septate) | USA (Washington) | [79] |
| S. capreae | Fully erumpent - | (280–) 350–450 | (60–) 100–150 (–200) | 55–65 × 8–10 | (4–)5–7(–8) × 1–1.3(–1.5) | Bacilliform | - | Sweden | [18] | |
| S. leucocheila | Superficial | Globose-urceolate | Up to 300 | 80 | 50–55 × 6–8 | 8–11.5 × 2–3 | Oblong-elliptic | (0–) 1-septate | New Zealand | [78] |
| S. lyckselensis | Erumpent | - | (175–) 250–350 (–425) | (25–) 40–75 (–125) | 35–60 × 5–6.5 | - | Cylindrical oblong | 3-septate | Northern Sweden | [18] |
| S. melaneliae | Immersed | Roundish | 170–350 | 0–20 | 60–85 × 6·5–8·5 | (12–)12·8–14·4 (–15·5) × (5·4–) 5·5–6·1 (–6·3) | Ellipsoid | (1–)3-septate, exceptionally with one longitudinal septum | Sweden and Alaska | [79] |
| S. mycoblasti | Erumpent | - | (140–) 190–280 (–320) | (0–) 20–50 (–70) | 50–70 × 7–9 | (12.3–) 14.0–15.9 (–17) × (4.0–) 4.7–5.3 (–5.7) | Ellipsoid to narrowly ellipsoid | 3-septate, (exceptionally 4-septate) | USA (Oregon) and northern Sweden | [18,70] |
| S. ochrolechiae | Immersed and become erumpent | - | (180–) 230–330 (–400) | (0–) 5–50 (–150) | 50–75 × 9–14 | (10·8–) 12·1–14·4 (–16·0) × (4·3–) 4·8–5·5 (–6) | Ellipsoid to narrowly ellipsoid | 3-septate | Norway, Sweden and the USA (Alaska) | [79] |
| S. pertusariae | Immersed to erumpent | - | (140–) 170–260 (–310) | (20–) 40–110 (–150) | - | (11·5–)12·5–15·4 (–16·0) × (4·5–) 4·7 –5·5 (–6·0) | Ellipsoid | 1–3-septate | Great Britain (Scotland) | [79] |
| S. rhizocarpicola | Immersed and occasionally erumpent | Roundish | (140–) 155–245 (–300) | (30–) 30–60 (–70) | 50–70 × 6·5–13 | (8·0–)9·3–11·1 (–13·5) × (4·5–) 4·8–5·6(–6·5) | - | (1–)3-septate | Russia, Kola and Peninsula | [79] |
| S. santessonii | Immersed, -finally sessile partly erumpent | - | (225–) 280–380 (–440) | (20–) 55–125 (–190) | 40–50 (–55) × 8–13 | (12·5–)15·4–20·4 (–23·5) × (3–) 3· 6–4·6 (–5) | Fusiform, often asymmetrical | Trans-septate (3–) 6–8 (–9) to submuriform | Widespread and common in Arctic regions | [79] |
| S. sipei | Immersed, soon erumpent | Sub-spherical | (350–) 360–480 (–590) | (0–) 0–40 (–105) | 55–65 × 5–7 | (11·0–)12·2–13·8 (–14·5) × (4·2–) 4·5–5·0 (–5·0) | Ellipsoid to narrowly ellipsoid | 3-septate | USA (Oregon) and Canada (British Columbia) | [79] |
| S. thamnoliae | Immersed and occasionally sessile | Roundish or slightly ellipsoid | (140–) 150–200 (–290) | (0–) 20–60 (–85) | 30–45 × 7–10 | (9·0–)11·0–14·9 (–18·0) × (2·5–) 2·5–3·2 (–3·5) | Fusiform | 1(–2)-septate | Russian and Swedish Arctic | [79] |
| S. shangrilaensis | Slightly erumpent to superficial | Roundish | 345–446 | 273–283 | 21–24 × 4–5.5 | 4–6 × 0.7–1.0 | Fusoid to obvoid | (0–) 1-septate | China | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiyagaraja, V.; Lücking, R.; Ertz, D.; Karunarathna, S.C.; Wanasinghe, D.N.; Lumyong, S.; Hyde, K.D. The Evolution of Life Modes in Stictidaceae, with Three Novel Taxa. J. Fungi 2021, 7, 105. https://doi.org/10.3390/jof7020105
Thiyagaraja V, Lücking R, Ertz D, Karunarathna SC, Wanasinghe DN, Lumyong S, Hyde KD. The Evolution of Life Modes in Stictidaceae, with Three Novel Taxa. Journal of Fungi. 2021; 7(2):105. https://doi.org/10.3390/jof7020105
Chicago/Turabian StyleThiyagaraja, Vinodhini, Robert Lücking, Damien Ertz, Samantha C. Karunarathna, Dhanushka N. Wanasinghe, Saisamorn Lumyong, and Kevin D. Hyde. 2021. "The Evolution of Life Modes in Stictidaceae, with Three Novel Taxa" Journal of Fungi 7, no. 2: 105. https://doi.org/10.3390/jof7020105
APA StyleThiyagaraja, V., Lücking, R., Ertz, D., Karunarathna, S. C., Wanasinghe, D. N., Lumyong, S., & Hyde, K. D. (2021). The Evolution of Life Modes in Stictidaceae, with Three Novel Taxa. Journal of Fungi, 7(2), 105. https://doi.org/10.3390/jof7020105









