Abstract
Histoplasmosis is a frequent fungal opportunistic infection in people living with HIV (PLHIV), associated every year to a total of 5% to 15% of AIDS-related deaths among this population. In 2020, the first global guidelines for diagnosing and managing disseminated histoplasmosis among PLHIV was published. This document recommends (1) detection of circulating Histoplasma antigens as the recommended laboratory assay to diagnose histoplasmosis among PLHIV; (2) the use of liposomal amphotericin for induction therapy in severe or moderately severe disease, followed by a maintenance therapy with itraconazole for 12 months; a shorter maintenance therapy could be considered if the patient is clinically stable and if immune status has improved; (3) antiretroviral therapy initiation as soon as possible among patients with histoplasmosis without involvement of central nervous system; and (4) that for the treatment of co-infection with histoplasmosis and tuberculosis (TB), treatment of TB should be initiated according to the World Health Organization treatment guidelines. Appropriate health education of providers, supportive supervision, and policy guidance for the care of PLHIV are required.
1. Introduction
Opportunistic infections such as tuberculosis (TB) and severe bacterial, fungal, and parasitic infections are the most frequent causes of death among adults with advanced HIV disease [1,2]. Histoplasmosis caused by the fungus Histoplasma capsulatum has been frequently reported in the American continent, where an estimated 15,000 new infections and 9000 deaths occur among people living with HIV (PLHIV); it is also diagnosed in much of the world and presumed to be underdiagnosed in most settings [3,4,5]. Disseminated histoplasmosis (DH), the most frequent clinical presentation of this disease among PLHIV, remains challenging due the non-specificity of symptoms from other infectious diseases, in particular TB, and the limited access to specific diagnostic assays and treatment [6,7,8]. As disseminated histoplasmosis is associated with high mortality rates due to late diagnosis [9,10], rapid detection of histoplasmosis and specific treatment are needed [9,11,12]. The development of a novel histoplasmosis diagnostic assay and its recent inclusion in the WHO list of Essential Diagnostics as well as the inclusion of new effective antifungal agents in the Essential Medicine List, generate new opportunities for improving the management and care of PLHIV having disseminated histoplasmosis [13,14,15]. This review summarizes the recently published World Health Organization (WHO)/Pan American Health Organization (PAHO) guidelines for diagnosing and managing disseminated histoplasmosis among PLHIV [16].
2. Materials and Methods
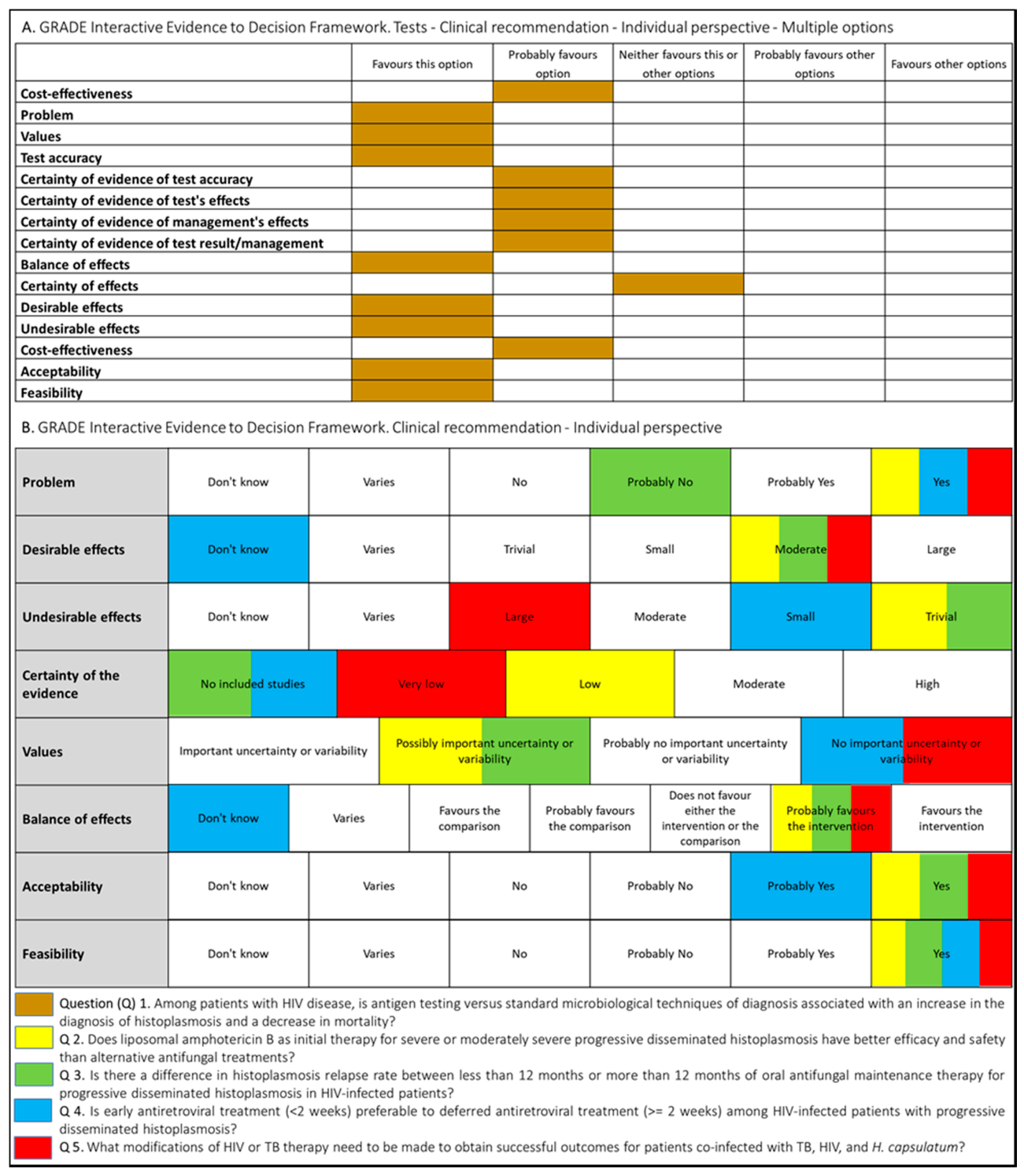
Following WHO processes for guideline development [17], the PAHO/WHO secretariat established a guideline development group (GDG) that included individuals with recognized expertise in the histoplasmosis field. The group was chaired by an expert in the management of histoplasmosis with support from a guideline methodologist. In order to develop systematic reviews and guidelines recommendations, PICO (Patient–Intervention–Comparison–Outcome) questions were proposed (Figure 1).
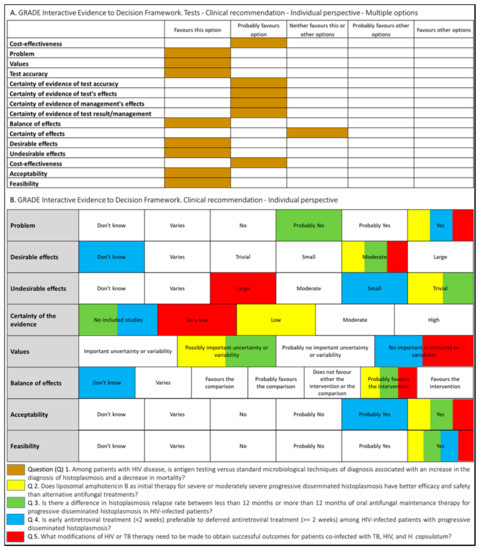
Figure 1.
Summary of judgment of questions proposed for the development of the guidelines for diagnosing and managing disseminated histoplasmosis among people living with HIV.
Systematic reviews were conducted to summarize the evidence relevant to these questions, and the key findings are summarized in the final guidelines [16]. The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method was applied to assess the certainty of the evidence as well as to determine the strength of the recommendations [18]. Additional considerations included feasibility, acceptability, supply use and cost implications of implementing the recommendations, and clinical outcomes (such as drug resistance and drug toxicity).
A SWOT (strengths, weaknesses, opportunities, and threats) analysis was conducted. This analysis focused on the following: (1) access to rapid diagnostic assays, (2) access to specific antifungal therapy, and (3) surveillance of HIV-associated histoplasmosis (focusing on morbidity and mortality) [7]. Prior to finalization, the draft guideline was reviewed by an external peer review group.
3. Results
The judgments made by the guideline development group for each intervention are summarized in Figure 1.
3.1. Diagnosis of Histoplasmosis
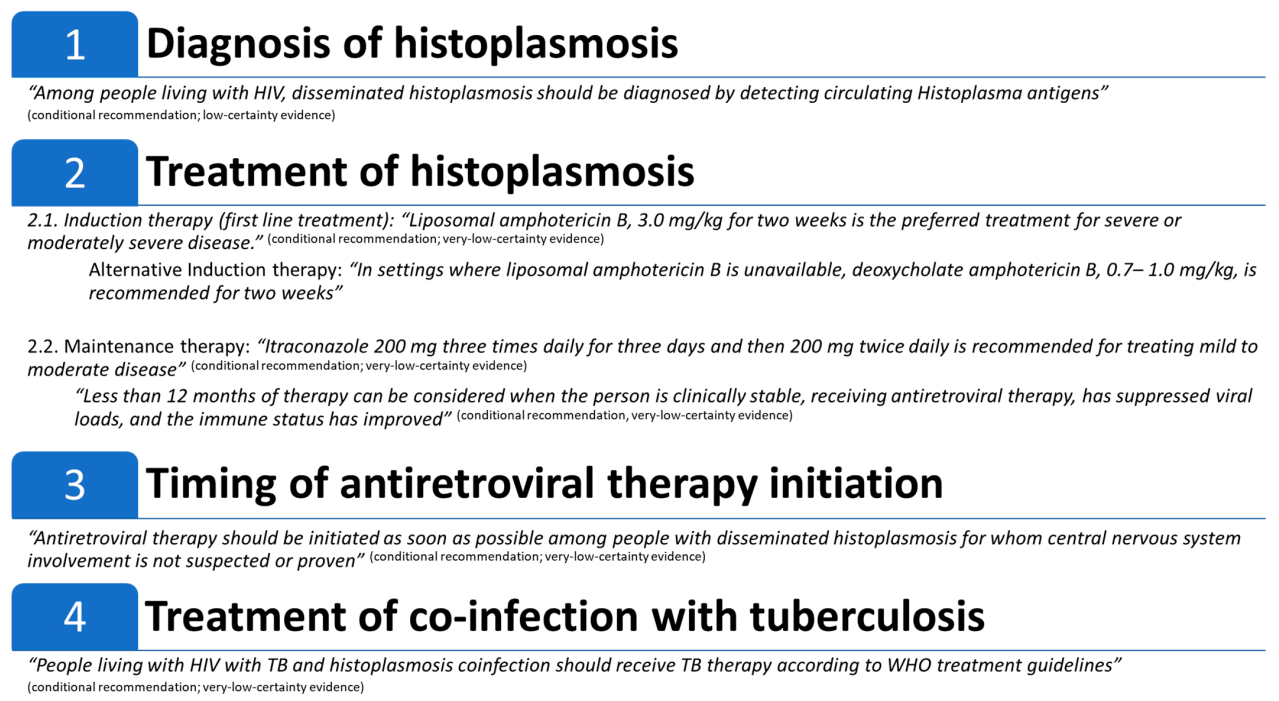
Recommendation 1. “Among people living with HIV, disseminated histoplasmosis should be diagnosed by detecting circulating Histoplasma antigens” (GRADE classified this recommendation as conditional with low-certainty evidence).
A systematic review and meta-analysis compared the accuracy of different diagnostics assays for the detection of histoplasmosis in PLHIV [6]. In this meta-analysis, antigen detection assays showed the highest performance to detect histoplasmosis in PLHIV (pooled sensitivity, 95%, and pooled specificity, 97%) [6,16]. Advantages of this assay include its commercial availability and the ability to be implemented in a laboratory with lower-level biosecurity (levels 1 and 2).
The GDG recommended complementary testing using microscopic analysis of the buffy coat, histopathological analysis, culture, and antibody or DNA detection in patients with clinical forms of histoplasmosis different to the disseminated disease [16,19].
3.2. Treatment of Disseminated Histoplasmosis
Three recommendations address the induction and maintenance therapy for histoplasmosis among PLHIV and the clinical stage of the disease (severe or moderately severe and mild to moderately mild).
Recommendation 2.1A. Induction therapy, for severe or moderately severe disseminated histoplasmosis: “Liposomal amphotericin B, 3.0 mg/kg for two weeks is the preferred treatment for severe or moderately severe disease” (GRADE classified this recommendation as conditional with very-low-certainty evidence).
Within the systematic review, four studies that provided information about relevant outcomes for induction therapies were identified. This included one randomized control trial that compared liposomal and deoxycholate amphotericin B. Compared to deoxycholate amphotericin B, liposomal amphotericin B may have higher clinical success rates (Relative Risk [RR] 1.46, 95% confidence interval [CI] 1.01 to 2.11; low-certainty evidence). Compared to deoxycholate amphotericin B, liposomal amphotericin B has lower rates of nephrotoxicity (RR 0.25, 95% CI 0.09 to 0.67; 1 study, 77 participants; high-certainty evidence) [20,21,22].
The GDG also recommended that in resource-constrained settings where liposomal amphotericin B is not available, deoxycholate amphotericin B (0.7–1.0 mg/kg) should be used as induction therapy for two weeks in severe or moderately severe DH (conditional recommendation, very low certainty of evidence) [16,20]. In patients with central nervous system involvement, extension of induction therapy or increased amphotericin B dosage could be needed [16].
Recommendation 2.1B. Treating mild to moderate histoplasmosis: “Itraconazole 200 mg twice daily after a loading dose of 200 mg three times daily for three days” (GRADE classified this recommendation as conditional with very-low-certainty evidence).
Very-low-certainty evidence was identified to inform comparisons between induction therapy in mild to moderate disease in the systematic review undertaken. Treatment with fluconazole achieved poor results in comparison to other azoles in the single-arm trial [16]. No further trials were done as there was no longer sufficient equipoise to justify this. In conclusion, it was found that triazole drugs present lower efficacy than polyenes among severely ill people. No randomized trial comparing amphotericin B formulations with triazole drugs have been undertaken [20,21,23].
Recommendation 2.2. Maintenance therapy: “Itraconazole 200 mg twice daily for 12 months is recommended” (GRADE classified this recommendation as conditional with very-low-certainty evidence). Less than 12 months of therapy can be considered when the person is clinically stable, receiving antiretroviral therapy, has suppressed viral load, and the immune status has improved (GRADE classified this recommendation as conditional with very-low-certainty evidence).
In regard to maintenance therapy, the systematic review that was undertaken did not find studies comparing less than 12 months of oral itraconazole or 12 months or greater of oral itraconazole. One prospective single-arm cohort study followed a cohort of participants who discontinued antifungal therapy after at least 12 months, providing the participant had received at least six months of antiretroviral therapy (ART) and had achieved a CD4 count of 150 cells/μL or greater [22]. There were no relapses in 32 participants who discontinued therapy after 12 months. A single, retrospective cohort study provided anecdotal evidence that maintenance therapy could be safely discontinued before 12 months but was at critical risk of bias [20,24]. The recommendation was therefore based on expert opinion.
3.3. Timing of Antiretroviral Therapy Initiation
Recommendation 3. “Antiretroviral therapy should be initiated as soon as possible among people with disseminated histoplasmosis for whom central nervous system involvement is not suspected or proven” (GRADE classified this recommendation as conditional with very-low-certainty evidence).
Only one randomized clinical trial with 282 participants met the inclusion criteria of a systematic review of studies comparing outcomes of early versus delayed initiation of antiretroviral therapy in patients with disseminated histoplasmosis [25]. Of the total number of participants, 10 had HIV and a presumptive or confirmed diagnosis of disseminated histoplasmosis. By day 30 of follow-up, one of the seven patients in the early antiretroviral therapy group and none of the three in the late group died.
Based on this restrictive evidence, the efficacy and safety outcomes of early versus late initiation of antiretroviral therapy are unknown [16,20,26]. Recommendation is based on the panel clinical expertise and field reports with regard to the balance between the substantial risk of dying from another opportunistic infection when delaying ART and the low incidence of histoplasmosis-associated immune reconstitution inflammatory syndrome (IRIS) versus morbidity and mortality associated with histoplasmosis-associated IRIS among PLHIV receiving ART [26]. However, WHO HIV/AIDS guidelines recommend initiating ART as soon as possible after the diagnosis of HIV (within 7 days) [27]. The panel also recalled that PLHIV presenting for the first time or those returning to care must undergo evaluation for opportunistic infections (TB and cryptococcosis), particularly those affecting the central nervous system, before ART initiation. Immediate ART initiation is not recommended in PLHIV who have cryptococcal meningitis because of the increased mortality presumably related with IRIS [27,28].
3.4. Treatment of Co-Infection with Tuberculosis
Recommendation 4. “People living with HIV with TB and histoplasmosis coinfection should receive TB therapy according to WHO treatment guidelines” (GRADE classified this recommendation as conditional with very-low-certainty evidence).
Two studies, including one case report describing treatment outcomes among patients coinfected with HIV, tuberculosis, and disseminated histoplasmosis were identified through a systematic review and showed very limited evidence [29,30]. Risks of Mycobacterium tuberculosis resistance and drug–drug interactions of a simultaneous rifampicin–itraconazole regimen (leading to sub-therapeutic and ineffective itraconazole blood levels and histoplasmosis treatment failure) were balanced in the context of existing strong WHO’s guidance on managing HIV and TB coinfection [16,20]. The recommendation relies on WHO guidance regarding prompt TB therapy initiation and on the panel’s clinical expertise regarding alternative options to concomitant antifungal therapy with extending the duration of amphotericin B induction therapy, or increasing itraconazole daily dosage as well as monitoring itraconazole blood levels if available, or considering other azole drugs (posaconazole or voriconazole) or replacing rifampicin with rifabutin [16]. Strong consideration was also given to a critical review of potential ART regimen options in the context of concomitant antifungal and anti-TB therapies in order to make necessary adjustments as recommended in WHO’s consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection.
Figure 2 summarizes the guidelines’ recommendations.

Figure 2.
Summary of: Pan American Health Organization (PAHO)/World Health Organization (WHO) guidelines for diagnosing and managing disseminated histoplasmosis among people living with HIV.
4. Discussion
This review summarizes the evidence and other considerations, supporting the formulation of WHO/PAHO global recommendations for the diagnosis, treatment, and management of disseminated histoplasmosis and disseminated histoplasmosis–tuberculosis co-infection among PLHIV. These recommendations have been developed through a public health approach taking into consideration contexts with limited resources and a high burden of disease. They are intended for healthcare providers, HIV program managers, policymakers, and other professionals involved in caring for people who either have or may be at risk of developing disseminated histoplasmosis. While the highest burden of histoplasmosis is concentrated in the Americas [4], the recommendations contained within these guidelines are applicable globally and adaptable according to the resources and logistical capabilities.
Early diagnosis and opportune treatment initiation are key to improving mortality from histoplasmosis; for this reason, it is necessary for countries to give priority access to high-quality in vitro diagnostics for the rapid detection of Histoplasma antigens [7,11]. Implementing this recommendation infers taking appropriate steps to assure adequate availability of antigen testing in health services managing advanced HIV disease. There is a need to apply price negotiation and pooled procurement mechanisms to improve access, especially where histoplasmosis is highly endemic. Access to optimal antifungal drugs is of great importance. Optimal antifungal medicines for treating people with histoplasmosis (conventional and liposomal amphotericin B and itraconazole) are part of the WHO Model List of Essential Medicines [14]. The cost of treatment is still high, and these drugs are not widely available in many of the countries with a high burden of histoplasmosis. Among the interventions to consider improving the access to antifungal medications for treating histoplasmosis are increased advocacy for drug price reduction and promoting generic production, national registration of all antifungal drugs, applying joint regional procurement mechanisms, and developing proper drug forecasting and monitoring systems. Furthermore, appropriate health education for providers, supportive supervision, and policy guidance at the national level on managing histoplasmosis among people living with HIV are required.
The systematic reviews highlighted that overall, the certainty of the evidence for most outcomes was low or very low in this field. Non-randomized study designs predominate in this area of research, many of which were compromised by uncontrolled confounding. This led to the development of a number of priority research recommendations [7]. The process has offered an opportunity to set a research agenda and thereby accelerate the development of new and improved diagnostics and optimize program implementation.
Author Contributions
Conceptualization, F.P., D.H.C., N.F., and G.R.; methodology, F.P., D.H.C., N.F., and M.N.; writing—original draft preparation, F.P. and D.H.C.; writing—review and editing, F.P., D.H.C., N.F., G.R., B.L.G., A.C.P., P.H., A.A.A., M.N., T.C., and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
The development of the PAHO/WHO guidelines for diagnosing and managing disseminated histoplasmosis among people living with HIV was supported by funding to PAHO from the cooperative agreement with the United States Centers for Disease Control and Prevention (PAHO-CK18-1801-03 Grant NU50CK000494).
Institutional Review Board Statement
This World Health Organization (WHO)/Pan American Health Organization (PAHO) guidelines was approved by the WHO Guidelines Review Committee, approval number: EMID:c7e5a1d963dc7878.
Informed Consent Statement
Declarations of interest were collected for the development of the guideline that this manuscript is based on and managed according to WHO policy. No conflict of interest was identified that prevented participation or hindered the objectivity of the co-authors in the processes and decision-making leading to the development of the WHO guideline and this manuscript.
Acknowledgments
PAHO/WHO is grateful to the many peer reviewers who provided valuable comments in support of the development of these guidelines as well as Ludovic Reveiz, Paul Garner, and Marylou Murray. The following people participated in the PAHO/WHO GDG: Ana Belen Arauz, Cristina Elena Canteros, Eduardo Arathoon, Flavio Queiroz Telles, and Nataly Garcia. The following people participated as external reviewers: Alexandro Bonifaz, Ana Alastruey, Angela Tobon, Arnaldo Colombo, Blanca Samayoa, Carol Kauffman, Juan Luis Rodriguez-Tudela, Mohamed Chakroun, Nathan Bahr, Nelesh P. Govender, Stephen Vreden, and Thuy Le. We also want to thank the members of the International Histoplasmosis Advocacy Group (iHAG): https://docs.google.com/spreadsheets/d/189W6gMfVb2zW5StAW2U2AbwVwGhls8bkAc7npZgNdC8/edit?ts=5d6d4758#gid=474498568.
Conflicts of Interest
A.C.P. has received research grants and speaker honoraria from Gilead, IMMY, and Teva, both companies producing diagnostic or treatment products for histoplasmosis. The other authors declare no competing interests. The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- UNAIDS. Fact Sheet-Latest Global and Regional Statistics on the Status of the AIDS Epidemic. 2018. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 31 December 2020).
- Tudela, J.L.R.; Denning, D.W. Recovery from serious fungal infections should be realisable for everyone. Lancet Infect. Dis. 2017, 17, 1111–1113. [Google Scholar] [CrossRef]
- Bahr, N.C.; Antinori, S.; Wheat, L.J.; Sarosi, G.A. Histoplasmosis infections worldwide: Thinking outside of the Ohio River valley. Curr. Trop Med. Rep. 2015, 2, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppie, P.; Chiller, T.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis 2018, 18, 1150–1159. [Google Scholar] [CrossRef]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [CrossRef]
- Caceres, D.H.; Knuth, M.; Derado, G.; Lindsley, M.D. Diagnosis of Progressive Disseminated Histoplasmosis in Advanced HIV: A Meta-Analysis of Assay Analytical Performance. J. Fungi 2019, 5, 76. [Google Scholar] [CrossRef]
- Caceres, D.H.; Adenis, A.; De Souza, J.V.B.; Gomez, B.L.; Cruz, K.S.; Pasqualotto, A.C.; Ravasi, G.; Perez, F.; Chiller, T.; de Lacerda, M.V.G.; et al. The Manaus Declaration: Current Situation of Histoplasmosis in the Americas, Report of the II Regional Meeting of the International Histoplasmosis Advocacy Group. Curr. Fungal Infect. Rep. 2019, 13, 244–249. [Google Scholar] [CrossRef]
- Adenis, A.; Nacher, M.; Hanf, M.; Basurko, C.; Dufour, J.; Huber, F.; Aznar, C.; Carme, B.; Couppie, P. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: A comparative study. Am. J. Trop Med. Hyg. 2014, 90, 216–223. [Google Scholar] [CrossRef]
- Samayoa, B.; Aguirre, L.; Bonilla, O.; Medina, N.; Lau-Bonilla, D.; Mercado, D.; Moller, A.; Perez, J.C.; Alastruey-Izquierdo, A.; Arathoon, E.; et al. The Diagnostic Laboratory Hub: A New Health Care System Reveals the Incidence and Mortality of Tuberculosis, Histoplasmosis, and Cryptococcosis of PWH in Guatemala. Open Forum Infect. Dis. 2020, 7, ofz534. [Google Scholar] [CrossRef]
- Samayoa, B.; Roy, M.; Cleveland, A.A.; Medina, N.; Lau-Bonilla, D.; Scheel, C.M.; Gomez, B.L.; Chiller, T.; Arathoon, E. High Mortality and Coinfection in a Prospective Cohort of Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome Patients with Histoplasmosis in Guatemala. Am. J. Trop. Med. Hyg. 2017, 97, 42–48. [Google Scholar] [CrossRef]
- Nacher, M.; Leitao, T.S.; Gomez, B.L.; Couppie, P.; Adenis, A.; Damasceno, L.; Demar, M.; Samayoa, B.; Caceres, D.H.; Pradinaud, R.; et al. The Fight against HIV-Associated Disseminated Histoplasmosis in the Americas: Unfolding the Different Stories of Four Centers. J. Fungi. 2019, 5, 51. [Google Scholar] [CrossRef]
- Falci, D.R.; Monteiro, A.A.; Braz Caurio, C.F.; Magalhaes, T.C.O.; Xavier, M.O.; Basso, R.P.; Melo, M.; Schwarzbold, A.V.; Ferreira, P.R.A.; Vidal, J.E.; et al. Histoplasmosis, An Underdiagnosed Disease Affecting People Living With HIV/AIDS in Brazil: Results of a Multicenter Prospective Cohort Study Using Both Classical Mycology Tests and Histoplasma Urine Antigen Detection. Open Forum. Infect. Dis. 2019, 6, ofz073. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Second WHO Model List of Essential In Vitro Diagnostics. Available online: https://www.who.int/medical_devices/publications/Standalone_document_v8.pdf?ua=1 (accessed on 30 September 2019).
- World Health Organization (WHO). WHO Model List of Essential Medicines. Available online: https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?ua=1 (accessed on 30 September 2019).
- Caceres, D.H.; Fernandez, N.B.; Lockhart, S.R. Innovative Approaches for Histoplasma Detection. Curr. Fungal Infect. Rep. 2020, 14, 310–316. [Google Scholar] [CrossRef]
- PAHO/WHO. Guidelines for Diagnosing and Managing Disseminated Histoplasmosis among People Living with HIV. Available online: https://iris.paho.org/bitstream/handle/10665.2/52304/9789275122495_eng.pdf?sequence=1&isAllowed=y (accessed on 30 April 2020).
- WHO. Handbook for Guideline Development. Available online: https://www.who.int/publications/guidelines/handbook_2nd_ed.pdf?ua=1 (accessed on 31 December 2014).
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Leitão, T.; Oliveira Filho, A.M.P.; Sousa Filho, J.E.P.; Tavares, B.M.; Mesquita, J.R.L.; Farias, L.; Mota, R.S.; Nacher, M.; Damasceno, L.S. Accuracy of Buffy Coat in the Diagnosis of Disseminated Histoplasmosis in AIDS-Patients in an Endemic Area of Brazil. J. Fungi. 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Hine, P. Treating progressive disseminated histoplasmosis in people living with HIV. Cochrane Database Syst. Rev. 2020, 4, Cd013594. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.J.; Freifeld, A.G.; Kleiman, M.B.; Baddley, J.W.; McKinsey, D.S.; Loyd, J.E.; Kauffman, C.A. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis 2007, 45, 807–825. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Wheat, L.J.; Cloud, G.A.; Goldman, M.; Lancaster, D.; Bamberger, D.M.; Powderly, W.G.; Hafner, R.; Kauffman, C.A.; Dismukes, W.E. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann. Intern. Med. 2002, 137, 105–109. [Google Scholar] [CrossRef]
- Wheat, L.J.; Connolly-Stringfield, P.A.; Baker, R.L.; Curfman, M.F.; Eads, M.E.; Israel, K.S.; Norris, S.A.; Webb, D.H.; Zeckel, M.L. Disseminated histoplasmosis in the acquired immune deficiency syndrome: Clinical findings, diagnosis and treatment, and review of the literature. Medicine 1990, 69, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Myint, T.; Anderson, A.M.; Sanchez, A.; Farabi, A.; Hage, C.; Baddley, J.W.; Jhaveri, M.; Greenberg, R.N.; Bamberger, D.M.; Rodgers, M.; et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): Multicenter study of outcomes and factors associated with relapse. Medicine 2014, 93, 11–18. [Google Scholar] [CrossRef]
- Zolopa, A.; Andersen, J.; Powderly, W.; Sanchez, A.; Sanne, I.; Suckow, C.; Hogg, E.; Komarow, L. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: A multicenter randomized strategy trial. PLoS ONE 2009, 4, e5575. [Google Scholar] [CrossRef]
- Melzani, A.; De Reynal De Saint Michel, R.; Ntab, B.; Djossou, F.; Epelboin, L.; Nacher, M.; Blanchet, D.; Demar, M.; Couppie, P.; Adenis, A. Incidence and trends in immune reconstitution inflammatory syndrome associated with Histoplasma capsulatum among people living with HIV: A 20-year case series and literature review. Clin. Infect. Dis 2019. [Google Scholar] [CrossRef] [PubMed]
- WHO | Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Available online: http://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/ (accessed on 31 July 2017).
- World Health Organization (WHO). Guidelines for the Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-Infected Adults, Adolescents and Children. Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Available online: https://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/ (accessed on 31 March 2018).
- Agudelo, C.A.; Restrepo, C.A.; Molina, D.A.; Tobon, A.M.; Kauffman, C.A.; Murillo, C.; Restrepo, A. Tuberculosis and histoplasmosis co-infection in AIDS patients. Am. J. Trop Med. Hyg 2012, 87, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Drayton, J.; Dickinson, G.; Rinaldi, M.G. Coadministration of rifampin and itraconazole leads to undetectable levels of serum itraconazole. Clin. Infect. Dis 1994, 18, 266. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).