The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change
Abstract
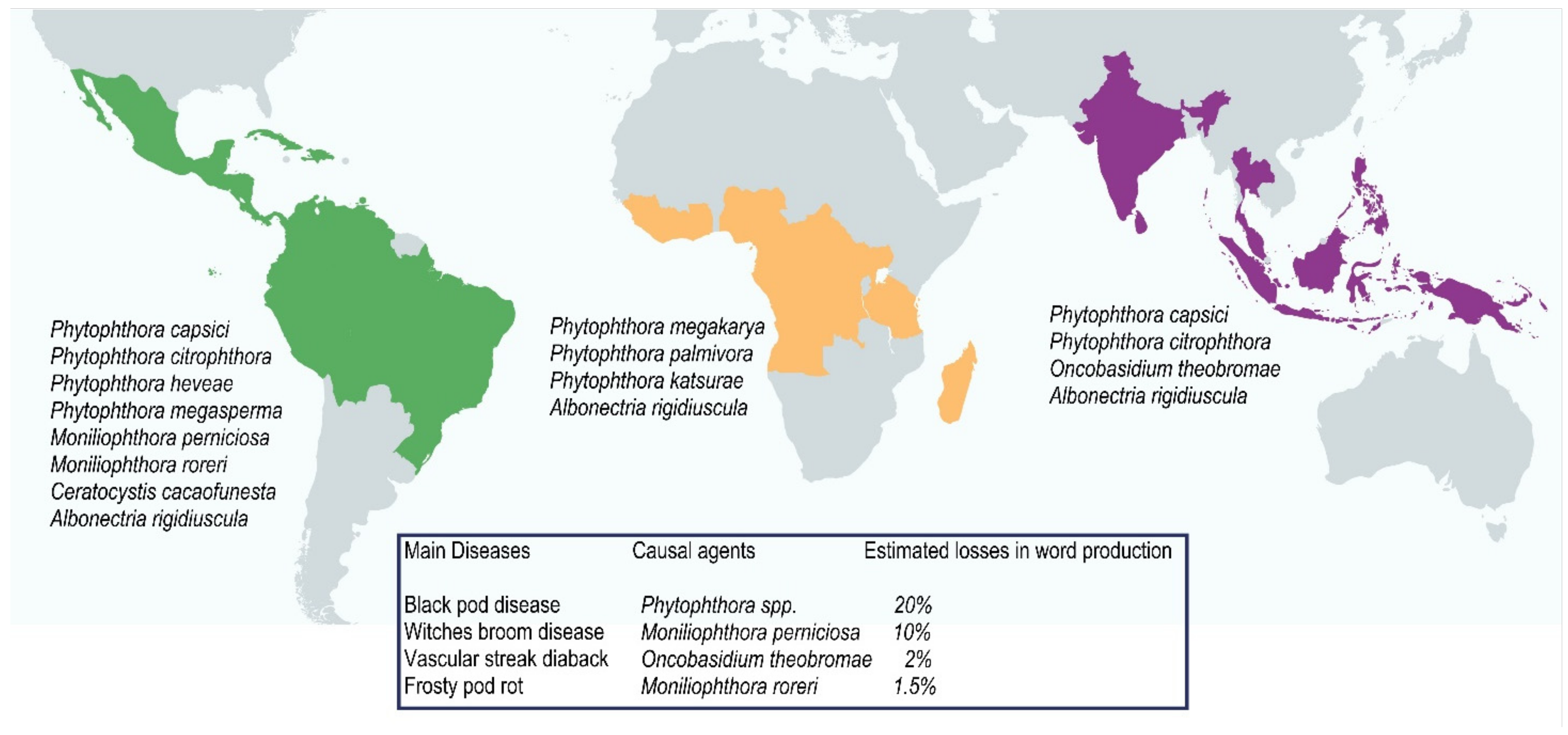
:1. Introduction
2. Preharvest
2.1. Field
2.1.1. Fungi Associated with Diseases of the Aerial Plant Parts
2.1.2. Fungi Associated with Root Diseases
2.2. Epiphytic Fungi
2.3. Endophytic Fungi
2.4. Mycorrhizal Fungi
3. Fermentation
4. Drying
5. Storage
6. Products
7. What about Climate Change?
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Almeida, A.-A.F.; Valle, R.R. Ecophysiology of the cacao tree. Braz. J. Plant Physiol. 2007, 19, 425–448. [Google Scholar] [CrossRef] [Green Version]
- Moreira, I.M.D.V.; Miguel, M.G.D.C.P.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res. Int. 2013, 54, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional Fermented Foods and Beverages from a Microbiological and Nutritional Perspective: The Colombian Heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- International Cocoa Organization ICCO. Quarterly Bulletin of Cocoa Statistics; No. 4, Cocoa Year 2019/20; International Cocoa Organization ICCO: Abidjan, Côte d’Ivoire, 2020; Volume XLVI. [Google Scholar]
- International Trade Centre Trade Map—List of Exporters for the Selected Product in 2019 Product: 18 Cocoa and Cocoa Preparations. Available online: https://www.trademap.org/ (accessed on 11 November 2020).
- Copetti, M.V.; Iamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Fungi and mycotoxins in cocoa: From farm to chocolate. Int. J. Food Microbiol. 2014, 178, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Marelli, J.-P.; Guest, D.I.; Bailey, B.A.; Evans, H.C.; Brown, J.K.; Junaid, M.; Barreto, R.W.; Lisboa, D.O.; Puig, A.S. Chocolate Under Threat from Old and New Cacao Diseases. Phytopathology 2019, 109, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Schroth, G.; Läderach, P.; Martinez-Valle, A.I.; Bunn, C.; Jassogne, L. Vulnerability to climate change of cocoa in West Africa: Patterns, opportunities and limits to adaptation. Sci. Total Environ. 2016, 556, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Anim-Kwapong, G.; Frimpong, E. Vulnerability and Adaptation Assessment under the Netherlands Climate Change Studies Assistance Programme Phase 2 (NCCSAP2) Vulnerability of Agriculture to Climate Change-Impact of Climate Change on Cocoa Production; Cocoa Research Institute of Ghana: New Tafo-Akim, Ghana, 2010. [Google Scholar]
- Hebbar, P.K. Cacao Diseases: A Global Perspective from an Industry Point of View. Phytopathology 2007, 97, 1658–1663. [Google Scholar] [CrossRef] [Green Version]
- Ploetz, R.C. Cacao Diseases: Important Threats to Chocolate Production Worldwide. Phytopathology 2007, 97, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Ploetz, R. The Impact of Diseases on Cacao Production: A Global Overview. In Cacao Diseases; Springer International Publishing: New York, NY, USA, 2016; pp. 33–59. [Google Scholar]
- Adeniyi, D. Diversity of Cacao Pathogens and Impact on Yield and Global Production. In Theobroma Cacao—Deploying Science for Sustainability of Global Cocoa Economy; IntechOpen: London, UK, 2019. [Google Scholar]
- Ndoumbe-Nkeng, M.; Cilas, C.; Nyemb, E.; Nyassé, S.; Bieysse, D.; Flori, A.; Sache, I. Impact of removing diseased pods on cocoa black pod caused by Phytophthora megakarya and on cocoa production in Cameroon. Crop. Prot. 2004, 23, 415–424. [Google Scholar] [CrossRef]
- Wessel, M.; Quist-Wessel, P.F. Cocoa production in West Africa, a review and analysis of recent developments. NJAS Wagening. J. Life Sci. 2015, 74–75, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.S.; Shao, J.; Lary, D.J.; Kronmiller, B.A.; Shen, D.; Strem, M.D.; Amoako-Attah, I.; Akrofi, A.Y.; Begoude, B.D.; Hoopen, G.M.T.; et al. Phytophthora megakarya and Phytophthora palmivora, Closely Related Causal Agents of Cacao Black Pod Rot, Underwent Increases in Genome Sizes and Gene Numbers by Different Mechanisms. Genome Biol. Evol. 2017, 9, 536–557. [Google Scholar] [CrossRef]
- Akrofi, A.Y.; Amoako-Atta, I.; Assuah, M.; Asare, E.K. Black pod disease on cacao (Theobroma cacao, L.) in Ghana: Spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop. Prot. 2015, 72, 66–75. [Google Scholar] [CrossRef]
- Akrofi, A.; Appiah, A.; Opoku, I. Management of Phytophthora pod rot disease on cocoa farms in Ghana. Crop. Prot. 2003, 22, 469–477. [Google Scholar] [CrossRef]
- Ali, S.S.; Amoako-Attah, I.; Bailey, B.A.; Strem, M.D.; Schmidt, M.; Akrofi, A.Y.; Surujdeo-Maharaj, S.; Kolawole, O.O.; Begoude, B.A.D.; Hoopen, G.M.T.; et al. PCR-based identification of cacao black pod causal agents and identification of biological factors possibly contributing to Phytophthora megakarya’s field dominance in West Africa. Plant Pathol. 2015, 65, 1095–1108. [Google Scholar] [CrossRef]
- Kudjordjie, E.N. Phytophthora Megakarya and P. palmivora on Theobroma Cacao: Aspects of Virulence and the Effects of Temperature on Growth and Resistance to Fungicides; University of Copenhagen: Copenhagen, Denmark, 2015. [Google Scholar]
- Morales-Cruz, A.; Ali, S.S.; Minio, A.; Figueroa-Balderas, R.; García, J.F.; Kasuga, T.; Puig, A.S.; Marelli, J.-P.; Bailey, B.A.; Cantu, D. Independent Whole-Genome Duplications Define the Architecture of the Genomes of the Devastating West African Cacao Black Pod Pathogen Phytophthora megakarya and Its Close Relative Phytophthora palmivora. G3 Genes Genomes Genetics 2020, 10, 2241–2255. [Google Scholar] [CrossRef]
- Bowers, J.H.; Bailey, B.A.; Hebbar, P.K.; Sanogo, S.; Lumsden, R.D. The Impact of Plant Diseases on World Chocolate Production. Plant Health Prog. 2001, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- End, M.J.; Daymond, A.J.; Hadley, P. Technical Guidelines for the Safe Movement of Cacao Germplasm; Revised from the FAO/IPGRI Technical Guidelines No. 20 (Third Update, October 2017); Global Cacao Genetic Resources Network (CacaoNet), Bioversity International: Rome, Italy, 2017; ISBN 9789292550790. [Google Scholar]
- Liyanage, N.I.S.; Wheeler, B.E.J. Phytophthora katsurae from cocoa. Plant Pathol. 1989, 38, 627–629. [Google Scholar] [CrossRef]
- Ndoumbe-Nkeng, M.; Efombagn, M.; Nyassé, S.; Nyemb, E.; Sache, I.; Cilas, C. Relationships between cocoa Phytophthorapod rot disease and climatic variables in Cameroon. Can. J. Plant Pathol. 2009, 31, 309–320. [Google Scholar] [CrossRef]
- Puig, A.S.; Ali, S.; Strem, M.; Sicher, R.; Gutierrez, O.A.; Bailey, B.A. The differential influence of temperature on Phytophthora megakarya and Phytophthora palmivora pod lesion expansion, mycelia growth, gene expression, and metabolite profiles. Physiol. Mol. Plant Pathol. 2018, 102, 95–112. [Google Scholar] [CrossRef]
- Hanada, R.E.; Pomella, A.W.; Soberanis, W.; Loguercio, L.L.; Pereira, J.O. Biocontrol potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora) of cacao. Biol. Control. 2009, 50, 143–149. [Google Scholar] [CrossRef]
- Iwaro, A.D.; Butler, D.R.; Eskes, A.B. Sources of Resistance to Phytophthora Pod Rot at the International Cocoa Genebank, Trinidad. Genet. Resour. Crop. Evol. 2005, 53, 99–109. [Google Scholar] [CrossRef]
- Másmela-Mendoza, J.E. Distribución potencial y nicho fundamental de Moniliophthora spp. en cacao de América y África. Agron. Mesoam. 2019, 30, 659–679. [Google Scholar] [CrossRef]
- De Oliveira, B.V.; Teixeira, G.S.; Reis, O.; Barau, J.G.; Teixeira, P.J.P.; Rio, M.C.S.D.; Domingues, R.R.; Meinhardt, L.W.; Leme, A.F.P.; Rincones, J.; et al. A potential role for an extracellular methanol oxidase secreted by Moniliophthora perniciosa in Witches’ broom disease in cacao. Fungal Genet. Biol. 2012, 49, 922–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, F.C.; Gianfagna, T.J. Necrotrophic phase of Moniliophthora perniciosa causes salicylic acid accumulation in infected stems of cacao. Physiol. Mol. Plant Pathol. 2006, 69, 104–108. [Google Scholar] [CrossRef]
- Fiorin, G.L.; Sanchéz-Vallet, A.; Thomazella, D.P.D.T.; Prado, P.F.V.D.; Nascimento, L.C.D.; Figueira, A.V.D.O.; Thomma, B.P.; Pereira, G.A.G.; Teixeira, P.J.P.L. Suppression of Plant Immunity by Fungal Chitinase-like Effectors. Curr. Biol. 2018, 28, 3023–3030.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, W.B.; Júnior, W.C.D.J.; Peixoto, L.D.A.; Moraes, W.B.; Furtado, E.L.; Da Silva, L.G.; Cecílio, R.A.; Alves, F.R. An analysis of the risk of cocoa moniliasis occurrence in Brazil as the result of climate change. Summa Phytopathol. 2012, 38, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Rocha, H.M.; Wheeler, B.E.J. Factors influencing the production of basidiocarps and the deposition and germination of basidiospores of Crinipellis perniciosa, the causal fungus of witches’ broom on cocoa (Theobroma cacao). Plant Pathol. 1985, 34, 319–328. [Google Scholar] [CrossRef]
- Garcés, C. La Escoba de Bruja del Cacao. Rev. Fac. Nac. Agron. 1946, 6, 329–369. [Google Scholar]
- Cubillos, G. Frosty Pod Rot, disease that affects the cocoa (Theobroma cacao) crops in Colombia. Crop. Prot. 2017, 96, 77–82. [Google Scholar] [CrossRef]
- Phillips-Mora, W.; Coutiño, A.; Ortiz, C.F.; Lopez, A.P.; Hernandez, J.; Aime, M.C. First report of Moniliophthora roreri causing frosty pod rot (moniliasis disease) of cocoa in Mexico. Plant Pathol. 2006, 55, 584. [Google Scholar] [CrossRef] [Green Version]
- Krauss, U.; Hoopen, G.M.T.; Rees, A.R.; Stirrup, T.; Argyle, T.; George, A.; Arroyo, C.; Corrales, E.; Casanoves, F. Mycoparasitism by Clonostachys byssicola and Clonostachys rosea on Trichoderma spp. from cocoa (Theobroma cacao) and implication for the design of mixed biocontrol agents. Biol. Control 2013, 67, 317–327. [Google Scholar] [CrossRef]
- Bailey, B.A.; Crozier, J.; Sicher, R.C.; Strem, M.D.; Melnick, R.L.; Carazzolle, M.F.; Costa, G.G.; Pereira, G.A.G.; Zhang, D.; Maximova, S.N.; et al. Dynamic changes in pod and fungal physiology associated with the shift from biotrophy to necrotrophy during the infection of Theobroma cacao by Moniliophthora roreri. Physiol. Mol. Plant Pathol. 2013, 81, 84–96. [Google Scholar] [CrossRef]
- Meinhardt, L.W.; Costa, G.G.L.; Thomazella, D.P.T.; Teixeira, P.J.P.L.; Carazzolle, M.F.; Schuster, S.C.; Carlson, J.E.; Guiltinan, M.J.; Mieczkowski, P.; Farmer, A.; et al. Genome and secretome analysis of the hemibiotrophic fungal pathogen, Moniliophthora roreri, which causes frosty pod rot disease of cacao: Mechanisms of the biotrophic and necrotrophic phases. BMC Genom. 2014, 15, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, B.A.; Evans, H.C.; Phillips-Mora, W.; Ali, S.S.; Meinhardt, L.W. Moniliophthora roreri, causal agent of cacao frosty pod rot. Mol. Plant Pathol. 2018, 19, 1580–1594. [Google Scholar] [CrossRef] [Green Version]
- Torres-Pal, C.; Ramirez-Le, M. Expression of Hydrolytic Enzymes During Interaction of Moniliophthora roreri, Causal Agent of Frosty Pod Rot and Theobroma cacao Pods. Plant Pathol. J. 2016, 15, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Evans, H.C. A reassessment of Moniliophthora (Monilia) pod rot of cocoa. Cocoa Grow. Bull. 1986, 37, 34–43. [Google Scholar]
- Munoz, M.E.L.; Tixier, P.; Germon, A.; Rakotobe, V.; Phillips-Mora, W.; Maximova, S.N.; Avelino, J. Effects of microclimatic variables on the symptoms and signs onset of Moniliophthora roreri, causal agent of Moniliophthora pod rot in cacao. PLoS ONE 2017, 12, e0184638. [Google Scholar] [CrossRef] [Green Version]
- Engelbrecht, C.J.B.; Harrington, T.C. Intersterility, morphology and taxonomy of Ceratocystis fimbriata on sweet potato, cacao and sycamore. Mycology 2005, 97, 57–69. [Google Scholar] [CrossRef]
- Engelbrecht, C.J.B.; Harrington, T.C.; Alfenas, A.C.; Suarez, C. Genetic variation in populations of the cacao wilt pathogen, Ceratocystis cacaofunesta. Plant Pathol. 2007, 56, 923–933. [Google Scholar] [CrossRef]
- Engelbrecht, C.J.; Harrington, T.C.; Alfenas, A. Ceratocystis Wilt of Cacao—A Disease of Increasing Importance. Phytopathology 2007, 97, 1648–1649. [Google Scholar] [CrossRef]
- Santos, R.M.F.; Silva, S.D.V.M.; Sena, K.; Micheli, F.; Gramacho, K.P. Kinetics and Histopathology of the Cacao-Ceratocystis cacaofunesta Interaction. Trop. Plant Biol. 2013, 6, 37–45. [Google Scholar] [CrossRef]
- Molano, E.P.L.; Cabrera, O.G.; Jose, J.; Nascimento, L.C.D.; Carazzolle, M.F.; Teixeira, P.J.P.L.; Alvarez, J.C.; Tiburcio, R.A.; Filho, P.M.T.; De Lima, G.M.A.; et al. Ceratocystis cacaofunesta genome analysis reveals a large expansion of extracellular phosphatidylinositol-specific phospholipase-C genes (PI-PLC). BMC Genom. 2018, 19, 58. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A.; Rosmana, A.; Junaid, M.; Guest, D.; McMahon, P.; Keane, P.; Purwantara, A.; Lambert, S.; Rodriguez-Carres, M.; et al. Vascular Streak Dieback of cacao in Southeast Asia and Melanesia: In planta detection of the pathogen and a new taxonomy. Fungal Biol. 2012, 116, 11–23. [Google Scholar] [CrossRef]
- Guest, D.; Keane, P. Vascular-Streak Dieback: A New Encounter Disease of Cacao in Papua New Guinea and Southeast Asia Caused by the Obligate Basidiomycete Oncobasidium theobromae. Phytopathology 2007, 97, 1654–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhakaran Nair, K.P. Cocoa (Theobroma cacao L.). In The Agronomy and Economy of Important Tree Crops of the Developing World; Elsevier: Amsterdam, Netherlands, 2010; pp. 131–180. ISBN 978-0-12-384677-8. [Google Scholar]
- McMahon, P.J.; Susilo, A.W.; Parawansa, A.K.; Bryceson, S.R.; Mulia, S.; Saftar, A.; Purwantara, A.; bin Purung, H.; Lambert, S.; et al. Testing local cacao selections in Sulawesi for resistance to vascular streak dieback. Crop. Prot. 2018, 109, 24–32. [Google Scholar] [CrossRef]
- McMahon, P.; Purwantara, A.; Susilo, A.W.; Sukamto, S.; Wahab, A.; Bin Purung, H.; Hidayat, M.; Ismail, D.; Taproni, T.; Lambert, S.; et al. On-farm selection for quality and resistance to pest/diseases of cocoa in Sulawesi: (ii) quality and performance of selections against Phytophthora pod rot and vascular-streak dieback. Int. J. Pest Manag. 2010, 56, 351–361. [Google Scholar] [CrossRef]
- Ali, S.S.; Asman, A.; Shao, J.; Firmansyah, A.P.; Susilo, A.W.; Rosmana, A.; McMahon, P.; Junaid, M.; Guest, D.; Kheng, T.Y.; et al. Draft genome sequence of fastidious pathogen Ceratobasidium theobromae, which causes vascular-streak dieback in Theobroma cacao. Fungal Biol. Biotechnol. 2019, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.A.; Wharton, A.L. Transmission of a Gall Disease of Cocoa. Nat. Cell Biol. 1960, 187, 80–81. [Google Scholar] [CrossRef]
- Vicente, L.P.; Martínez De La Parte, E.; Cantillo Pérez, T. First report in Cuba of green point gall of cocoa cushion caused by Albonectria rigidiuscula (Fusarium decemcellulare). Fitosanidad 2012, 16, 19–25. [Google Scholar]
- Owen, H. Further observations on the pathogenicity of calonectria rigidiuscula (Berk. & BR.) SACC. to Theobroma cacao L. Ann. Appl. Biol. 1956, 44, 307–321. [Google Scholar] [CrossRef]
- Agrios, G.N. Insect Involvement in the Transmission of Fungal Pathogens. Vectors Plant Pathog. 1980, 293–324. [Google Scholar] [CrossRef]
- Akrofi, A.Y. Pink Disease Caused by Erythricium salmonicolor (Berk. & Broome) Burdsall: An Epidemiological Assessment of its Potential Effect on Cocoa Production in Ghana. J. Plant Pathol. Microbiol. 2014, 5, 1000215. [Google Scholar] [CrossRef] [Green Version]
- Singh, G. Evaluation of fungicides against vascular streak dieback, white thread blight and pink disease of cocoa. J. Plant Prot. Trop. 1989, 6, 19–24. [Google Scholar]
- Schneider-Christians, J.; Fliege, F.H.; Schlosser, E.; Tamani, R. Pink disease of cocoa caused by Corticium salmonicolor Berk. & Br. in Western Samoa. On the release of basidiospores of Corticium salmonicolor. Berk. Br. Alafua Agric. Bull. 1983, 8, 9–19. [Google Scholar]
- Polanía Sánchez, R. Enfermedades del cacao (Theobroma cacao L.) en Colombia. Acta Agron. 1957, 7, 1–70. [Google Scholar]
- Ann, P.-J.; Chang, T.-T.; Ko, W.-H. Phellinus noxius Brown Root Rot of Fruit and Ornamental Trees in Taiwan. Plant Dis. 2002, 86, 820–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarze, F.W.; Jauss, F.; Spencer, C.; Hallam, C.; Schubert, M. Evaluation of an antagonistic Trichoderma strain for reducing the rate of wood decomposition by the white rot fungus Phellinus noxius. Biol. Control 2012, 61, 160–168. [Google Scholar] [CrossRef]
- Mohd Farid, A.; Lee, S.S.; Maziah, A.; Rosli, H.; Norwati, M. Basal Root Rot, a new Disease of Teak (Tectona grandis) in Malaysia caused by Phellinus noxius. Malays. J. Microbiol. 2005, 1, 40–45. [Google Scholar] [CrossRef]
- Ogbebor, N.O.; Adekunle, A.T.; Eghafona, O.N.; Ogboghodo, A.I. Biological control of Rigidoporus lignosus in Hevea brasiliensis in Nigeria. Fungal Biol. 2015, 119, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aranzazu, H.F.; Cárdenas, L.J.; Mujica, J.J.; Gómez, Q.R. Manejo de las llagas radicales (Rosellinia sp.). In Boletin de Sanidad Vegetal 23; Instituto Colombiano Agropecuario (ICA) and Corpoica, Produmedios: Bogotá, Colombia, 1999; p. 35. [Google Scholar]
- García, R.A.M.; Hoopen, G.M.T.; Kass, D.C.; Garita, V.A.S.; Krauss, U. Evaluation of mycoparasites as biocontrol agents of Rosellinia root rot in cocoa. Biol. Control. 2003, 27, 210–227. [Google Scholar] [CrossRef]
- García-Córdoba, J.; George, A.; Argyle, T.; ten Hoopen, G.M.; Krauss, U. ¿Existe la tolerancia genética del cacao (Theobroma cacao) a Rosellinia bunodes y Rosellinia pepo? Manejo Integr. Plagas y Agroecol. Costa Rica 2005, 75, 21–31. [Google Scholar]
- Hoopen, G.M.T.; Krauss, U. Biology and control of Rosellinia bunodes, Rosellinia necatrix and Rosellinia pepo: A review. Crop. Prot. 2006, 25, 89–107. [Google Scholar] [CrossRef]
- Baumgartner, K.; Coetzee, M.P.A.; Hoffmeister, D. Secrets of the subterranean pathosystem of Armillaria. Mol. Plant Pathol. 2011, 12, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Isaac, S.; Frankland, J.C.; Watling, R.; Whalley, A.J.S. (Eds.) Aspects of Tropical Mycology; Symposium of the British Mycological Society held at the University of Liverpool, April 1992; British Mycological Society by Cambridge University Press: Cambridge, UK, 1993; ISBN 9780521450508. [Google Scholar]
- Desjardin, D.E.; Oliveira, A.G.; Stevani, C.V. Fungi bioluminescence revisited. Photochem. Photobiol. Sci. 2008, 7, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-O.; Chang, K.-C.; Lee, Y.-S.; Park, C.-H.; Kim, H.-Y.; Lee, U.-Y.; Lee, T.-S.; Lee, M.-W. The Fruiting Body Formation of Armillaria mellea on Oak Sawdust Medium Covered with Ground Raw Carrots. Mycobiology 2006, 34, 206–208. [Google Scholar] [CrossRef] [Green Version]
- De Mattos-Shipley, K.M.J.; Ford, K.L.; Alberti, F.; Banks, A.; Bailey, A.M.; Foster, G. The good, the bad and the tasty: The many roles of mushrooms. Stud. Mycol. 2016, 85, 125–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoopen, G.M.T.; Rees, R.; Aisa, P.; Stirrup, T.; Krauss, U. Population dynamics of epiphytic mycoparasites of the genera Clonostachys and Fusarium for the biocontrol of black pod (Phytophthora palmivora) and moniliasis (Moniliophthora roreri) on cocoa (Theobroma cacao). Mycol. Res. 2003, 107, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Van Bael, S.; Mejía, L.; Bischoff, J.; Arnold, A.; Rojas, E.; Robbins, N.; Herre, E.; Kyllo, D.; Maynard, Z. Emerging Perspectives on the Ecological Roles of Endophytic Fungi in Tropical Plants. In Mycology; CRC Press: Boca Raton, FL, USA, 2005; pp. 181–191. [Google Scholar]
- Arnold, A.E.; Herre, E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao(Malvaceae). Mycology 2003, 95, 388–398. [Google Scholar] [CrossRef]
- Hanada, R.E.; Pomella, A.W.V.; Costa, H.S.; Bezerra, J.L.; Loguercio, L.L.; Pereira, J.O. Endophytic fungal diversity in Theobroma cacao (cacao) and T. grandiflorum (cupuaçu) trees and their potential for growth promotion and biocontrol of black-pod disease. Fungal Biol. 2010, 114, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Crozier, J.; Thomas, S.E.; Aime, M.C.; Evans, H.C.; Holmes, K.A. Molecular characterization of fungal endophytic morphospecies isolated from stems and pods of Theobroma cacao. Plant Pathol. 2006, 55, 783–791. [Google Scholar] [CrossRef]
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Thomas, S.E.; Crozier, J.; Aime, M.C.; Evans, H.C.; Holmes, K.A. Molecular characterisation of fungal endophytic morphospecies associated with the indigenous forest tree, Theobroma gileri, in Ecuador. Mycol. Res. 2008, 112, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Chafla, A.L.; Rodríguez, Z.; Boucourt, R.; Espín, J.; Silva, L. Isolation, selection and characterization of cellulolytic fungi from cocoa (Theobroma cacao L.) hull. Cuba. J. Agric. Sci. 2016, 50, 411–420. [Google Scholar]
- Wemheuer, F.; Berkelmann, D.; Wemheuer, B.; Daniel, R.; Vidal, S.; Daghela, H.B.B. Agroforestry Management Systems Drive the Composition, Diversity, and Function of Fungal and Bacterial Endophyte Communities in Theobroma Cacao Leaves. Microorganisms 2020, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Rojas, E.I.; Rehner, S.A.; Samuels, G.J.; Van Bael, S.A.; Herre, E.A.; Cannon, P.; Chen, R.; Pang, J.; Wang, R.; Zhang, Y.; et al. Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panama: Multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 2010, 102, 1318–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosmana, A.; Nasaruddin, N.; Hendarto, H.; Hakkar, A.A.; Agriansyah, N. Endophytic Association of Trichoderma asperellum within Theobroma cacao Suppresses Vascular Streak Dieback Incidence and Promotes Side Graft Growth. Mycobiology 2016, 44, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriwati, R.; Melnick, R.L.; Muarif, R.; Strem, M.D.; Samuels, G.J.; Bailey, B.A. Trichoderma from Aceh Sumatra reduce Phytophthora lesions on pods and cacao seedlings. Biol. Control 2015, 89, 33–41. [Google Scholar] [CrossRef]
- Rosmana, A.; Taufik, M.; Asman, A.; Jayanti, N.J.; Hakkar, A.A. Dynamic of Vascular Streak Dieback Disease Incidence on Susceptible Cacao Treated with Composted Plant Residues and Trichoderma asperellum in Field. Agronomy 2019, 9, 650. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, M.V.; Lozano, R.E.; Del Castillo, D.S.; Martínez, S.P. Hongos endófitos foliares como candidatos a biocontroladores contra Moniliophthora spp. de Theobroma cacao (Malvaceae) en Ecuador. Acta Biológica Colomb. 2018, 23, 235–241. [Google Scholar] [CrossRef]
- Tondje, P.; Roberts, D.; Bon, M.; Widmer, T.; Samuels, G.; Ismaiel, A.; Begoude, A.; Tchana, T.; Nyemb-Tshomb, E.; Ndoumbe-Nkeng, M.; et al. Isolation and identification of mycoparasitic isolates of Trichoderma asperellum with potential for suppression of black pod disease of cacao in Cameroon. Biol. Control 2007, 43, 202–212. [Google Scholar] [CrossRef]
- Mbarga, J.; Begoude, B.; Ambang, Z.; Meboma, M.; Kuaté, J.; Schiffers, B.; Ewbank, W.; Dedieu, L.; Hoopen, G.T. A new oil-based formulation of Trichoderma asperellum for the biological control of cacao black pod disease caused by Phytophthora megakarya. Biol. Control 2014, 77, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.A.; Schroers, H.-J.; Thomas, S.E.; Evans, H.C.; Samuels, G.J. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Prog. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Villamizar-Gallardo, R.A.; Ortíz-Rodriguez, O.O.; Escobar, J.W. Symbiotic and endophytic fungi as biocontrols against cocoa (Theobroma cacao L.) phytopathogens. Summa Phytopathol. 2017, 43, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Tchameni, S.; Ngonkeu, M.; Begoude, B.; Nana, L.W.; Fokom, R.; Owona, A.; Mbarga, J.; Tchana, T.; Tondje, P.; Etoa, F.; et al. Effect of Trichoderma asperellum and arbuscular mycorrhizal fungi on cacao growth and resistance against black pod disease. Crop. Prot. 2011, 30, 1321–1327. [Google Scholar] [CrossRef]
- Hashim, A.C.; Ragu, P. Growth response of Theobroma cacao L. seeldings to inoculation with vesicular-arbuscular mycorrhizal fungi. Plant Soil 1986, 96, 279–285. [Google Scholar] [CrossRef]
- Edy, N.; Zakaria, E.K.; Lakani, I.; Hasriyanti. Forest conversion into cacao agroforestry and cacao plantation change the diversity of arbuscular mycorrhizal fungi. In Proceedings of the IOP Conference Series: Earth and Environmental Science, The 1st Biennial Conference on Tropical Biodiversity, Makassar, Indonesia, 20–21 September 2018; Volume 270. [Google Scholar]
- Chulan, H.A.; Martin, K. The vesicular-arbuscular (VA) mycorrhiza and its effects on growth of vegetatively propagated Theobroma cacao L. Plant Soil 1992, 144, 227–233. [Google Scholar] [CrossRef]
- Ramirez, J.G.; Osorno, L.; Osorio, N.W. Presence of mycorrhizal fungi and a fluorescent Pseudomonas sp. in the rhizosphere of cacao in two agroecosystems and their effects on cacao seedling growth. Agron. Colomb. 2016, 34, 385–392. [Google Scholar] [CrossRef]
- Oladele, S.O. Mycorrhizal fungus (glomus mossae) inoculation effects on performance and root biomass development of cacao seedlings in the nursery. J. Agric. For. 2015, 61, 69–76. [Google Scholar] [CrossRef]
- Cuenca, G.; Meneses, E. Diversity patterns of arbuscular mycorrhizal fungi associated with cacao in Venezuela. Plant Soil 1996, 183, 315–322. [Google Scholar] [CrossRef]
- Moncada, U.A.P.; Gómez, M.M.R.; Ordoñez, D.P.S.; Rolón, A.M.P.; Ortiz, W.A.W.; Ramírez, L.; Estrada, G.A.R. Hongos formadores de micorrizas arbusculares (HFMA) como estrategia para reducir la absorción de cadmio en plantas de cacao (Theobroma cacao). Rev. Terra Latinoam. 2019, 37, 121–130. [Google Scholar] [CrossRef]
- Djocgoue, P.F.; Simo, C.; Minyaka, E.; Tassong Saah, D.; Njonzo-Nzo, S.A.; Taffouo, V.D. Influence of Gigaspora margarita and Acaulospora tuberculata on tolerance to Phytophthora megakarya in Theobroma cacao under plant nursery conditions. Int. J. Adv. Agric. Res. 2019, 7, 21–31. [Google Scholar] [CrossRef]
- Schwan, R.F.; Wheals, A.E. The Microbiology of Cocoa Fermentation and its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Copetti, M.V.; Iamanaka, B.T.; Nester, M.A.; Efraim, P.; Taniwaki, M.H. Occurrence of ochratoxin A in cocoa by-products and determination of its reduction during chocolate manufacture. Food Chem. 2013, 136, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Romanens, E.; Leischtfeld, S.F.; Volland, A.; Stevens, M.J.; Krähenmann, U.; Isele, D.; Fischer, B.; Meile, L.; Schwenninger, S.M. Screening of lactic acid bacteria and yeast strains to select adapted anti-fungal co-cultures for cocoa bean fermentation. Int. J. Food Microbiol. 2019, 290, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ardhana, M.M. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef]
- Mounjouenpou, P.; Gueule, D.; Fontana-Tachon, A.; Guyot, B.; Tondje, P.R.; Guiraud, J.-P. Filamentous fungi producing ochratoxin a during cocoa processing in Cameroon. Int. J. Food Microbiol. 2008, 121, 234–241. [Google Scholar] [CrossRef]
- Ludlow, C.L.; Cromie, G.A.; Garmendia-Torres, C.; Sirr, A.; Hays, M.; Field, C.; Jeffery, E.W.; Fay, J.C.; Dudley, A.M. Independent Origins of Yeast Associated with Coffee and Cacao Fermentation. Curr. Biol. 2016, 26, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Serra, J.L.; Moura, F.G.; Pereira, G.V.D.M.; Soccol, C.R.; Rogez, H.; Darnet, S. Determination of the microbial community in Amazonian cocoa bean fermentation by Illumina-based metagenomic sequencing. LWT 2019, 106, 229–239. [Google Scholar] [CrossRef]
- Copetti, M.V.; Pereira, J.L.; Iamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Ochratoxigenic fungi and ochratoxin A in cocoa during farm processing. Int. J. Food Microbiol. 2010, 143, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Copetti, M.V.; Iamanaka, B.T.; Pereira, J.L.; Fungaro, M.H.; Taniwaki, M.H. Aflatoxigenic fungi and aflatoxin in cocoa. Int. J. Food Microbiol. 2011, 148, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Ogundero, V.W. Thermophilic fungi and fermenting cocoa beans in Nigeria. Mycopathology 1983, 82, 159–165. [Google Scholar] [CrossRef]
- Souza, J.V.; Silva, É.S.; Maia, M.L.; Teixeira, M.F. Screening of fungal strains for pectinolytic activity: Endopolygalacturonase production by Peacilomyces clavisporus 2A.UMIDA.1. Process. Biochem. 2003, 39, 455–458. [Google Scholar] [CrossRef]
- Lopez, A.S.; Dimick, P.S. Cocoa fermentation. In Enzymes, Biomass, Food and Feed; Reed, G., Nagodawithana, T.W., Eds.; Wiley: Hoboken ,NJ, USA, 1995; pp. 561–577. [Google Scholar]
- Hatmi, R.U.; Kobarsih, M.; Cahyaningrum, N. Fungi Level Analysis of Cocoa Beans Based on Fermentation Box Type and Duration. Procedia Food Sci. 2015, 3, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Kamelé, K.Y.; Martial-Didie, A.K.; Daouda, N.; Carole, K.A.; Tano, K. Improvement of the quality of the cocoa during postharvest process in Côte d’Ivoire. J. Glob. Biosci. 2019, 8, 6404–6423. [Google Scholar]
- Maciel, L.F.; Felício, A.L.D.S.M.; Miranda, L.C.R.; Pires, T.C.; Bispo, E.D.S.; Hirooka, E.Y. Aflatoxins and ochratoxin A in different cocoa clones (Theobroma cacao L.) developed in the southern region of Bahia, Brazil. Food Addit. Contam. Part A 2017, 35, 134–143. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.D.N.D.C.; Chisté, R.C.; Pena, R.D.S.; Gloria, M.B.A.; Lopes, A.S. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-López, C. Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Molina-Hernandez, J.B.; Delgado-Ospina, J.; Sciarra, P.; Fernández-Daza, F.F.; Chaves-López, C. Enzymatic activities and mycotoxin production of fungi isolated on cocoa beans. Manuscript in preparation.
- García-Alamilla, P.; Salgado-Cervantes, M.; Barel, M.; Berthomieu, G.; Rodríguez-Jimenes, G.; García-Alvarado, M. Moisture, acidity and temperature evolution during cacao drying. J. Food Eng. 2007, 79, 1159–1165. [Google Scholar] [CrossRef]
- Zahouli, G.I.B.; Guehi, S.T.; Fae, A.M.; Ban-Koffi, L.; Nemlin, J.G. Effect of drying methods on the chemical quality traits of cocoa raw material. Adv. J. Food Sci. Technol. 2010, 2, 184–190. [Google Scholar]
- Guehi, T.S.; Zahouli, I.B.; Ban-Koffi, L.; Fae, M.A.; Nemlin, J.G. Performance of different drying methods and their effects on the chemical quality attributes of raw cocoa material. Int. J. Food Sci. Technol. 2010, 45, 1564–1571. [Google Scholar] [CrossRef]
- Yao, K.M.; Kambiré, O.; Kouassi, K.C.; Koffi-Névry, R.; Guéhi, T.S. Risk Prevention of Fungal Contamination of Raw Cocoa Beans in Côte d’Ivoire: Case of Polyhexamethylene Guanidine Hydrochloride (PHMGH). Food Public Health 2017, 7, 40–50. [Google Scholar] [CrossRef]
- Sánchez-Hervás, M.; Gil, J.; Bisbal, F.; Ramón, D.; Martínez-Culebras, P. Mycobiota and mycotoxin producing fungi from cocoa beans. Int. J. Food Microbiol. 2008, 125, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Akinfala, T.O.; Houbraken, J.; Sulyok, M.; Adedeji, A.R.; Odebode, A.C.; Krska, R.; Ezekiel, C.N. Moulds and their secondary metabolites associated with the fermentation and storage of two cocoa bean hybrids in Nigeria. Int. J. Food Microbiol. 2020, 316, 108490. [Google Scholar] [CrossRef]
- Delgado-Ospina, N.; Cuervo-Mulet, R.A.; Valencia, M.; González, I.A.; Fernández, F.F. Hongos y sus Aplicaciones en Agroindustria: Casos de Investigación, 1st ed.; Editorial Bonaventuriana: Cali, Colombia, 2020; ISBN 978-958-5415-60-7. [Google Scholar]
- Copetti, M.V.; Iamanaka, B.T.; Frisvad, J.C.; Pereira, J.L.; Taniwaki, M.H. Mycobiota of cocoa: From farm to chocolate. Food Microbiol. 2011, 28, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Dano, S.D.; Manda, P.; Dembélé, A.; Abla, A.M.-J.K.; Bibaud, J.H.; Gouet, J.Z.; Sika, C.B.Z.M. Influence of Fermentation and Drying Materials on the Contamination of Cocoa Beans by Ochratoxin A. Toxins 2013, 5, 2310–2323. [Google Scholar] [CrossRef]
- Thompson, S.S.; Miller, K.B.; Lopez, A.S.; Camu, N. Cocoa and Coffee. In Food Microbiology: Fundamentals and Frontiers, 4th ed.; Doyle, M.P., Buchanan, R.L., Eds.; Wiley: Hoboken ,NJ, USA, 2012; pp. 881–899. [Google Scholar]
- Saltini, R.; Akkerman, R.; Frosch, S. Optimizing chocolate production through traceability: A review of the influence of farming practices on cocoa bean quality. Food Control 2013, 29, 167–187. [Google Scholar] [CrossRef] [Green Version]
- Barreiro, J.A.; Sandoval, A.J. Kinetics of moisture adsorption during simulated storage of whole dry cocoa beans at various relative humidities. J. Food Eng. 2020, 273, 109869. [Google Scholar] [CrossRef]
- Amézqueta, S.; González-Peñas, E.; Dachoupakan, C.; Murillo-Arbizu, M.; De Cerain, A.L.; Guiraud, J. OTA-producing fungi isolated from stored cocoa beans. Lett. Appl. Microbiol. 2008, 47, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Guehi, T.S.; Konan, Y.M.; Koffi-Nevry, R.; Yao, N.D.; Manizan, N.P. Enumeration and identification of main fungal isolates and evaluation of fermentation’s degree of Ivorian raw cocoa beans. Aust. J. Basic Appl. Sci. 2007, 1, 479–486. [Google Scholar]
- Ahima, J.; Zhang, H.; Apaliya, M.T.; Zhang, X.; Yang, Q.; Zhao, L. The effect of Rhodotorula mucilaginosa on degradation of citrinin production by Penicillium digitatum and its toxin in vitro. J. Food Meas. Charact. 2019, 13, 2998–3004. [Google Scholar] [CrossRef]
- Fahrurrozi, F.; Bahmann, C.; Niemenak, N.; Lieberei, R.; Bisping, B. Antifungal protein of seed coat extracts of Theobroma cacao L. during fermentation. New Biotechnol. 2014, 31, S178. [Google Scholar] [CrossRef]
- Brera, C.; Debegnach, F.; De Santis, B.; Iafrate, E.; Pannunzi, E.; Berdini, C.; Prantera, E.; Gregori, E.; Miraglia, M. Ochratoxin A in cocoa and chocolate products from the Italian market: Occurrence and exposure assessment. Food Control 2011, 22, 1663–1667. [Google Scholar] [CrossRef]
- Oyetunji, T.O. Mycological evaluation of a ground cocoa- based beverage. Afr. J. Biotechnol. 2006, 5, 2073–2076. [Google Scholar]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Luck, J.; Spackman, M.; Freeman, A.; Griffiths, W.; Finlay, K.; Chakraborty, S. Climate change and diseases of food crops. Plant Pathol. 2011, 60, 113–121. [Google Scholar] [CrossRef]
- Field, C.B.; Barros, V.R.; Dokken, D.J.; Mach, K.J.; Mastrandrea, M.D.; Bilir, T.E.; Chatterjee, M.; Ebi, K.L.; Estrada, Y.O.; Genova, R.C.; et al. (Eds.) IPCC Cambio Climático 2014: Impactos, Adaptación y Bulnerabilidad. Resúmenes, Preguntas Frecuentes y Recuadros Multicapítulos; Contribución del Grupo de trabajo II al Quinto Informe de Evaluación del Grupo Intergubernamental de Expertos sobre el Cambio Climáti; Organización Meteorológica Mundial: Ginebra, Switzerland, 2014; p. 200. [Google Scholar]
- Lahive, F.; Hadley, P.; Daymond, A.J. The physiological responses of cacao to the environment and the implications for climate change resilience. A review. Agron. Sustain. Dev. 2019, 39, 5. [Google Scholar] [CrossRef] [Green Version]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villamizar-Gallardo, R.; Osma, J.F.; Ortíz-Rodriguez, O.O. Regional Evaluation of Fungal Pathogen Incidence in Colombian Cocoa Crops. Agriculture 2019, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Gateau-Rey, L.; Tanner, E.V.J.; Rapidel, B.; Marelli, J.-P.; Royaert, S. Climate change could threaten cocoa production: Effects of 2015-16 El Niño-related drought on cocoa agroforests in Bahia, Brazil. PLoS ONE 2018, 13, e0200454. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.; Tamargo, A.; Bailey, C.; Kim, Y. Assessment of Climate Change Impacts on Cocoa Production and Approaches to Adaptation and Mitigation: A Contextual View of Ghana and Costa Rica. Available online: https://elliott.gwu.edu/sites/g/files/zaxdzs2141/f/World%20Cocoa%20Foundation.pdf (accessed on 21 September 2020).
- Paterson, R.R.M.; Lima, N. Thermophilic Fungi to Dominate Aflatoxigenic/Mycotoxigenic Fungi on Food under Global Warming. Int. J. Environ. Res. Public Health 2017, 14, 199. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, K.; Żółciak, A.; Damszel, M.; Lech, P.; Sierota, Z. Armillaria Pathogenesis under Climate Changes. Forests 2017, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Andrade, S.O.; Páez, G.T.; Feria, T.P.; Muñoz, J. Climate change and the risk of spread of the fungus from the high mortality of Theobroma cocoa in Latin America. Neotrop. Biodivers. 2017, 3, 30–40. [Google Scholar] [CrossRef]
- De Oliveira, T.B.; De Lucas, R.C.; Scarcella, A.S.D.A.; Contato, A.G.; Pasin, T.M.; Martinez, C.A.; Polizeli, M.D.L.T.D.M. Fungal communities differentially respond to warming and drought in tropical grassland soil. Mol. Ecol. 2020, 29, 1550–1559. [Google Scholar] [CrossRef]
- Suryanarayanan, T.; Shaanker, R.U. Can fungal endophytes fast-track plant adaptations to climate change? Fungal Ecol. 2021, 50, 101039. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Bennett, A.E.; Classen, A.T. Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 2020, 101, e02978. [Google Scholar] [CrossRef] [PubMed]
- Kivlin, S.N.; Emery, S.M.; Rudgers, J.A. Fungal symbionts alter plant responses to global change. Am. J. Bot. 2013, 100, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.; Lima, N. Further mycotoxin effects from climate change. Food Res. Int. 2011, 44, 2555–2566. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Cabrera, H.; Taniwaki, M.H.; Hashimoto, J.M.; De Menezes, H.C. Growth of Aspergillus ochraceus, A. carbonarius and A. niger on culture media at different water activities and temperatures. Braz. J. Microbiol. 2005, 36, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Moretti, A.; Logrieco, A.F. 5 Climate change effects on the biodiversity of mycotoxigenic fungi and their mycotoxins in preharvest conditions in Europe. In Climate Change and Mycotoxins; Walter de Gruyter GmbH: Berlin, Germany, 2015; pp. 91–108. [Google Scholar]


| Country (Region) | Fungi | Reference |
|---|---|---|
| Brazil (eastern Amazon region) | Thielaviopsis, Fusarium, Aspergillus, Colletotrichum, Penicillium, Nigrospora, Hyphopichia, Trichosporon, Cophinforma, Cladosporium, Trichoderma, Agaricus, Talaromyces, Porobeltraniella, Neopestalotiopsis, Paecilomyces, Clonostachys, Lasiodiplodia, Purpureocillium, Cylindrocladiella, Wallemia, Nectria, Arthrinium, Curvularia, and Rhizomucor | [111] |
| Brazil (Igrapiúna, Bahia) | A. heteromorphus | [2] |
| Brazil (Bahia) | A. carbonarius and A. niger aggregate | [112] |
| Brazil (Bahia) | A. flavus and A. parasiticus | [113] |
| Cameroon | A. versicolor, Mucor spp., A. niger, Geotrichum spp., A. fumigatus, Fusarium spp., Rhizopus nigricans, A. tamarii, Syncephalastrum racemosum, P. sclerotiorum, A. flavus, Trichoderma spp., A. versicolor, Scopulariopsis spp., and P. crustosum | [109] |
| Indonesia (East Java) | P. citrinum, A. versicolor, A. wentii, and P. purpurogenum | [108] |
| Nigeria | Thermoascus aurantiacus (thermophilic), Mucor pusillus, and A. fumigatus (thermotolerant) | [114] |
| Crops | Fermentation | Drying | Storage | Product | |||||
|---|---|---|---|---|---|---|---|---|---|
| Diseases of Aerial Plant Parts | Root Diseases | Endophytic Fungi | Epiphytic Fungi | Mycorrhizal Fungi | |||||
| Fungi reported in several stages | Aspergillus spp. | Aspergillus spp. | Aspergillus spp. | Aspergillus spp. | Aspergillus spp. | ||||
| Cladosporium spp. | Cladosporium spp. | ||||||||
| Clonostachys spp. | Clonostachys spp. | Clonostachys spp. | |||||||
| Colletotrichum spp. | Colletotrichum spp. | Colletotrichum spp. | |||||||
| Fusarium spp. | Fusarium spp. | Fusarium spp. | Fusarium spp. | ||||||
| Curvularia spp. | Curvularia spp. | ||||||||
| Paecilomyces spp. | Paecilomyces variotii | ||||||||
| Penicillium spp. | Penicillium spp. | Penicillium spp. | Penicillium spp. | Penicillium spp. | |||||
| Trichoderma spp. | Trichoderma spp. | Trichoderma spp. | |||||||
| Lasiodiplodia spp. | Lasiodiplodia spp. | Lasiodiplodia spp. | |||||||
| Geotrichum spp. | Geotrichum spp. | ||||||||
| Mucor spp. | Mucor spp. | Mucor spp. | Mucor spp. | ||||||
| Nectria spp. | Nectria spp. | ||||||||
| Rhizopus spp. | Rhizopus spp. | Rhizopus spp. | Rhizopus spp. | ||||||
| Scopulariopsis spp. | Scopulariopsis spp. | ||||||||
| Talaromyces spp. | Talaromyces atroroseus | ||||||||
| Wallemia spp. | Wallemia spp. | ||||||||
| Absidia spp. | Absidia spp. | ||||||||
| Eurotium spp. | Eurotium spp. | ||||||||
| Fungi reported in a single stage | Albonectria rigidiuscula | Armillaria mellea | Acremonium sp. | Acaulospora spp. | Agaricus spp. | Pseudopithomyces palmicola | Chaetomium globosum | ||
| Ceratocystis cacaofunesta | Phellinus noxius | Botryosphaeria spp. | Gigaspora spp. | Arthrinium spp. | Simplicillium spp. | Emericella spp. | |||
| Erythricium spp. | Rigidoporus lignosus | Chrysosporium spp. | Glomus mosseae | Cophinforma spp. | Eupenicillium spp. | ||||
| Moniliophthora spp. | Rosellinia spp. | Pestalotiopsis spp. | Scutellospora calospora | Cylindrocladiella spp. | Phoma spp. | ||||
| Oncobasidium theobromae | Phomopsis sp. | Hyphopichia spp. | |||||||
| Phytophthora spp. | Tolypocladium spp. | Neopestalotiopsis spp. | |||||||
| Xylaria sp. | Nigrospora spp. | ||||||||
| Porobeltraniella spp. | |||||||||
| Purpureocillium spp. | |||||||||
| Rhizomucor spp. | |||||||||
| Syncephalastrum racemosum | |||||||||
| Thermoascus aurantiacus | |||||||||
| Thielaviopsis spp. | |||||||||
| Trichosporon spp. | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Ospina, J.; Molina-Hernández, J.B.; Chaves-López, C.; Romanazzi, G.; Paparella, A. The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. J. Fungi 2021, 7, 202. https://doi.org/10.3390/jof7030202
Delgado-Ospina J, Molina-Hernández JB, Chaves-López C, Romanazzi G, Paparella A. The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. Journal of Fungi. 2021; 7(3):202. https://doi.org/10.3390/jof7030202
Chicago/Turabian StyleDelgado-Ospina, Johannes, Junior Bernardo Molina-Hernández, Clemencia Chaves-López, Gianfranco Romanazzi, and Antonello Paparella. 2021. "The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change" Journal of Fungi 7, no. 3: 202. https://doi.org/10.3390/jof7030202
APA StyleDelgado-Ospina, J., Molina-Hernández, J. B., Chaves-López, C., Romanazzi, G., & Paparella, A. (2021). The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. Journal of Fungi, 7(3), 202. https://doi.org/10.3390/jof7030202









