Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Coffee Corky-Roots Sampling
2.2. Obtention of Fusarium Isolates
2.3. Molecular Characterization of Fusarium Isolates
2.3.1. DNA Extraction
2.3.2. Phylogenetic Analysis
2.3.3. PCR Analysis of SIX Genes
2.3.4. Molecular Detection of Putative Toxigenic Fusarium Isolates
2.4. Pathogenicity Testing in Coffee Seedlings
2.5. Chlorophyll a Fluorescence Measurement in Coffee Seedlings
2.6. Re-Isolation and Identification of Fusarium Isolates by PCR Amplification
2.7. Statistical Data Analysis
3. Results
3.1. Identification of Fusarium Isolates
3.2. Phylogenetic Analysis of Fusarium Isolates
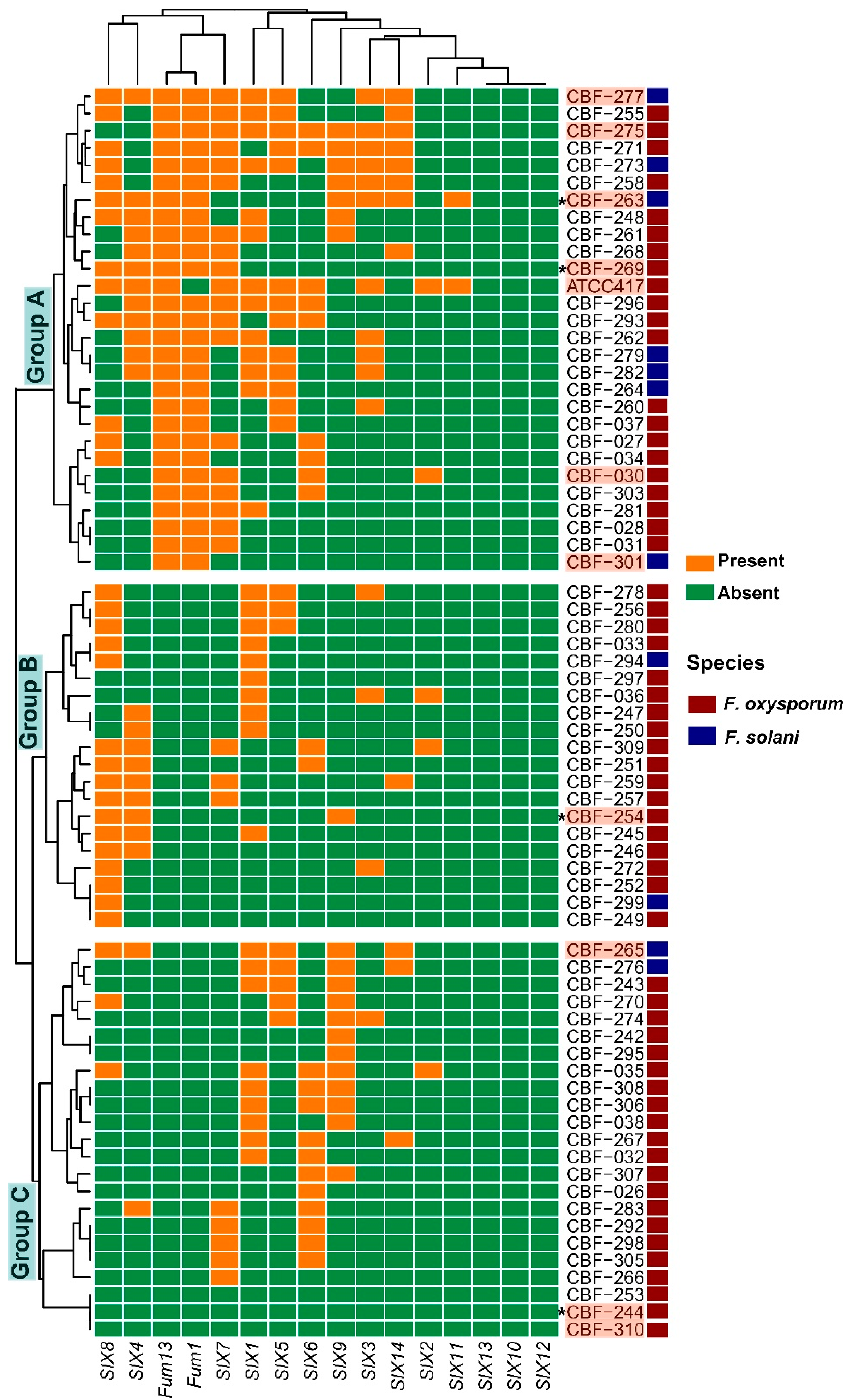
3.3. Pathogenicity and Toxigenic Genes Characterization: SIX1–SIX14 and Fum Genes
3.4. Relationship between Global Disease Damages and the SIX and Fum Gene Repertoires of Fusarium Isolates
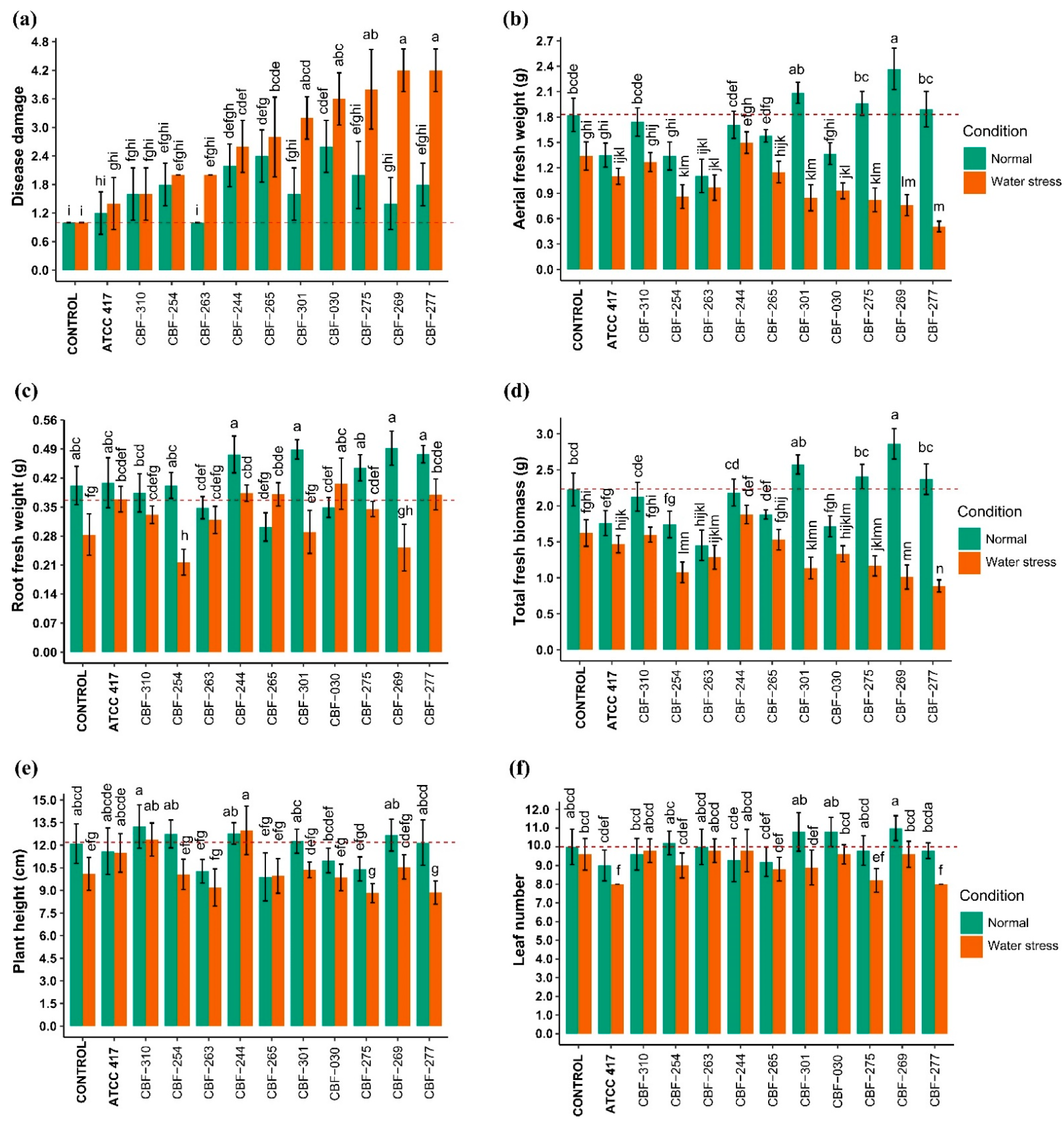
3.5. Effects of Fusarium Isolates Inoculation on Coffee Seedling Growth
3.6. Effects of Fusarium Isolates Inoculation on Photosynthetic Activity of Coffee Seedlings
3.7. Correlation between Disease Damage and the Phenotypic Variables Assessed in Coffee Seedlings
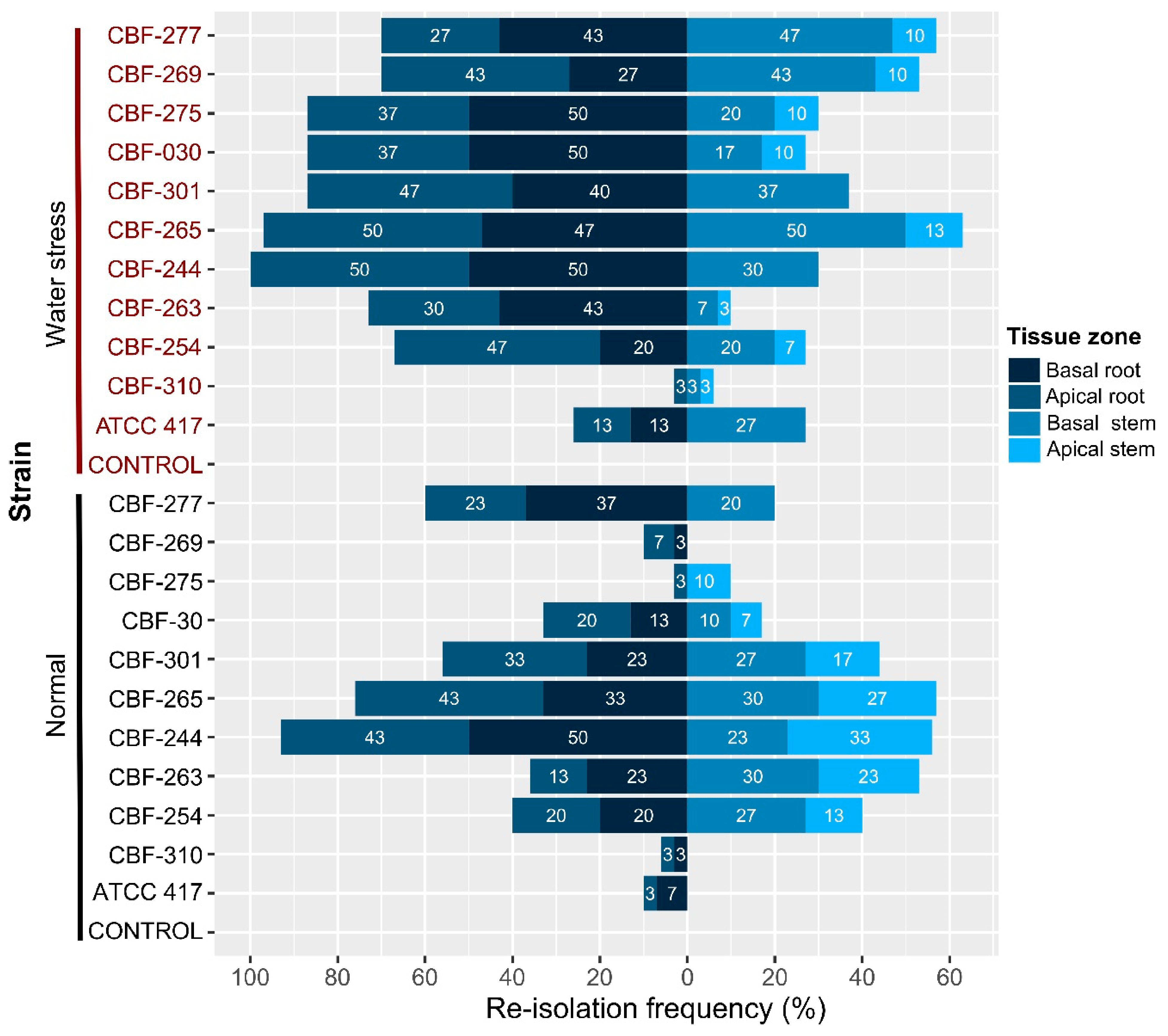
3.8. Vascular Colonization of Fusarium Isolates in Aerial and Root Tissues of Coffee Seedlings
4. Discussion
4.1. Diversity of Fusarium Linked to Coffee Corky-Root Disease
4.2. Isolates of F. oxysporum and F. solani Causing Disease Symptoms in Coffee Seedlings under Water Stress
4.3. The Repertoire of SIX-Fum Genes Is Associated with Pathogenicity of Fusarium Isolates
4.4. Host-Specificity of Fusarium oxysporum f. sp. lycopersici Results in Low Vascular Colonization without Causing Disease Symptoms in Coffee Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bertrand, B.; Nunez, C.; Sarah, J.-L. Disease Complex in Coffee Involving Meloidogyne arabicida and Fusarium oxysporum. Plant Pathol. 2000, 49, 383–388. [Google Scholar] [CrossRef]
- Villain, L.; Lima-Salgada, S.M.; Trinh-Phap, Q. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. In Nematode Parasites of Coffee and Cocoa; Sikora, R.A., Coyne, D., Hallman, J., Timper, P., Eds.; CABI: Wallingford, UK, 2018; pp. 536–583. [Google Scholar]
- Lamelas, A.; Desgarennes, D.; López-Lima, D.; Villain, L.; Alonso-Sánchez, A.; Artacho, A.; Latorre, A.; Moya, A.; Carrión, G. The Bacterial Microbiome of Meloidogyne-Based Disease Complex in Coffee and Tomato. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- López-Lima, D.; Carrión, G.; Sánchez-Nava, P.; Desgarennes, D.; Villain, L. Fungal Diversity and Fusarium oxysporum Pathogenicity Associated with Coffee Corky-Root Disease in Mexico. Rev. Fac. Cienc. Agrar. UNCuyo 2020, 52, 276–292. [Google Scholar]
- Tian, B.-Y.; Cao, Y.; Zhang, K.-Q. Metagenomic Insights into Communities, Functions of Endophytes, and Their Associates with Infection by Root-Knot Nematode, Meloidogyne Incognita, in Tomato Roots. Sci. Rep. 2015, 5, 17087. [Google Scholar] [CrossRef]
- Carneiro, R.M.D.G.; Cofcewicz, E.T. Taxonomy of Coffee-Parasitic Root-Knot Nematodes, Meloidogyne spp. In Plant-Parasitic Nematodes of Coffee; Souza, R.M., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 87–122. ISBN 978-1-4020-8720-2. [Google Scholar]
- Villain, L.; Sarah, J.L.; Hernández, A.; Bertrand, B.; Anthony, F.; Lashermes, P.; Charmetant, P.; Anzueto, F.; Figueroa, P.; Carneiro, R.M.D.G. Diversity of Root-Knot Nematodes Parasitizing Coffee in Central America. Nematropica 2013, 43, 194–206. [Google Scholar]
- Lopez-Lima, D.; Sánchez-Nava, P.; Carrion, G.; de los Monteros, A.E.; Villain, L. Corky-Root Symptoms for Coffee in Central Veracruz Are Linked to the Root-Knot Nematode Meloidogyne paranaensis, a New Report for Mexico. Eur. J. Plant Pathol. 2015, 141, 623–629. [Google Scholar] [CrossRef]
- Hua, G.K.H.; Timper, P.; Ji, P. Meloidogyne incognita Intensifies the Severity of Fusarium Wilt on Watermelon Caused by Fusarium oxysporum f. sp. niveum. Can. J. Plant Pathol. 2019, 41, 261–269. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-Necrotrophy Switch in Pathogen Evoke Differential Response in Resistant and Susceptible Sesame Involving Multiple Signaling Pathways at Different Phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of Pathogenicity-Related Genes in Fusarium oxysporum f. sp. cepae: Pathogenicity in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef]
- Guo, L.; Han, L.; Yang, L.; Zeng, H.; Fan, D.; Zhu, Y.; Feng, Y.; Wang, G.; Peng, C.; Jiang, X.; et al. Genome and Transcriptome Analysis of the Fungal Pathogen Fusarium oxysporum f. sp. cubense Causing Banana Vascular Wilt Disease. PLoS ONE 2014, 9, e95543. [Google Scholar] [CrossRef]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; O’Neill, W.T.; Meldrum, R.A.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Investigation of the Diversity of Effector Genes in the Banana Pathogen, Fusarium oxysporum f. sp. cubense, Reveals Evidence of Horizontal Gene Transfer: Effector Genes in Fusarium oxysporum f. sp. cubense. Mol. Plant Pathol. 2018, 19, 1155–1171. [Google Scholar] [CrossRef]
- Ma, L.; Houterman, P.M.; Gawehns, F.; Cao, L.; Sillo, F.; Richter, H.; Clavijo-Ortiz, M.J.; Schmidt, S.M.; Boeren, S.; Vervoort, J.; et al. The AVR2-SIX5 Gene Pair Is Required to Activate I-2 -Mediated Immunity in Tomato. New Phytol. 2015, 208, 507–518. [Google Scholar] [CrossRef]
- Li, J.; Fokkens, L.; Conneely, L.J.; Rep, M. Partial Pathogenicity Chromosomes in Fusarium oxysporum Are Sufficient to Cause Disease and Can Be Horizontally Transferred. Environ. Microbiol. 2020, 22, 4985–5004. [Google Scholar] [CrossRef] [PubMed]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector Gene Screening Allows Unambiguous Identification of Fusarium oxysporum f. sp. lycopersici Races and Discrimination from other formae speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Rep, M.; Wang, B.; Ashton, A.; Dodds, P.; Ellis, J. Variation in Potential Effector Genes Distinguishing Australian and Non-Australian Isolates of the Cotton Wilt Pathogen Fusarium oxysporum f.sp. vasinfectum. Plant Pathol. 2011, 60, 232–243. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Gardiner, D.M.; Kazan, K.; Manners, J.M. A Highly Conserved Effector in Fusarium oxysporum is Required for Full Virulence on Arabidopsis. Mol. Plant-Microbe Interact. 2011, 25, 180–190. [Google Scholar] [CrossRef]
- Meldrum, R.A.; Fraser-Smith, S.; Tran-Nguyen, L.T.T.; Daly, A.M.; Aitken, E.A.B. Presence of Putative Pathogenicity Genes in Isolates of Fusarium oxysporum f. sp. cubense from Australia. Australas. Plant Pathol. 2012, 41, 551–557. [Google Scholar] [CrossRef]
- Covey, P.A.; Kuwitzky, B.; Hanson, M.; Webb, K.M. Multilocus Analysis Using Putative Fungal Effectors to Describe a Population of Fusarium oxysporum from Sugar Beet. Phytopathology 2014, 104, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Laurence, M.H.; Summerell, B.A.; Liew, E.C.Y. Fusarium oxysporum f. sp. canariensis: Evidence for Horizontal Gene Transfer of Putative Pathogenicity Genes. Plant Pathol. 2015, 64, 1068–1075. [Google Scholar] [CrossRef]
- Williams, A.H.; Sharma, M.; Thatcher, L.F.; Azam, S.; Hane, J.K.; Sperschneider, J.; Kidd, B.N.; Anderson, J.P.; Ghosh, R.; Garg, G.; et al. Comparative Genomics and Prediction of Conditionally Dispensable Sequences in Legume–Infecting Fusarium oxysporum formae speciales Facilitates Identification of Candidate Effectors. BMC Genom. 2016, 17, 191. [Google Scholar] [CrossRef]
- Nicholson, T.P.; Rudd, B.A.; Dawson, M.; Lazarus, C.M.; Simpson, T.J.; Cox, R.J. Design and Utility of Oligonucleotide Gene Probes for Fungal Polyketide Synthases. Chem. Biol. 2001, 8, 157–178. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two Divergent Intragenomic RDNA ITS2 Types within a Monophyletic Lineage of the Fungus Fusarium Are Nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Sarver, B.A.J.; Brandt, M.; Chang, D.C.; Noble-Wang, J.; Park, B.J.; Sutton, D.A.; Benjamin, L.; Lindsley, M.; Padhye, A.; et al. Phylogenetic Diversity and Microsphere Array-Based Genotyping of Human Pathogenic Fusaria, Including Isolates from the Multistate Contact Lens-Associated U.S. Keratitis Outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007, 45, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Ramana, M.V.; Balakrishna, K.; Murali, H.C.S.; Batra, H.V. Multiplex PCR-Based Strategy to Detect Contamination with Mycotoxigenic Fusarium Species in Rice and Fingermillet Collected from Southern India. J. Sci. Food Agric. 2011, 91, 1666–1673. [Google Scholar] [CrossRef]
- Ramana, M.V.; Nayaka, S.C.; Balakrishna, K.; Murali, H.S.; Batra, H.V. A Novel PCR–DNA Probe for the Detection of Fumonisin-Producing Fusarium Species from Major Food Crops Grown in Southern India. Mycology 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Reis, A.; Boiteux, L.S. Outbreak of Fusarium oxysporum f. Sp. lycopersici Race 3 in Commercial Fresh-Market Tomato Fields in Rio de Janeiro State, Brazil. Hortic. Bras. 2007, 25, 451–454. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring Fast Fluorescence Transients to Address Environmental Questions: The JIP-Test. Photosynth. Light Biosphere 1995, 4869–4872. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a Fluorescence Induction: Can Just a One-Second Measurement Be Used to Quantify Abiotic Stress Responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Bertrand, B.; Ramirez, G.; Topart, P.; Anthony, F. Resistance of Cultivated Coffee (Coffea arabica and C. canephora) Trees to Corky-Root Caused by Meloidogyne arabicida and Fusarium oxysporum, under Controlled and Field Conditions. Crop Prot. 2002, 21, 713–719. [Google Scholar] [CrossRef]
- Baker, C.J. Fusarium solani Associated with a Wilt of Coffea arabica in Kenya. East Afr. Agric. For. J. 1972, 38, 137–140. [Google Scholar] [CrossRef]
- Al-Areqi, A.H.N.A.; Chliyeh, M.; Touati, J.; Outcoumit, A.; Sghir, F.; Touhami, A.O.; Benkirane, R.; Douira, A. Effect of Endomycorrhizae on Decline of the Coffee Plants (Coffea arabica) Caused by Fusarium solani. IJAPBC 2015, 4, 397–404. [Google Scholar]
- Ma, L.-J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.-J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative Genomics Reveals Mobile Pathogenicity Chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Yadeta, K.A.; Thomma, B.P.H.J. The Xylem as Battleground for Plant Hosts and Vascular Wilt Pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Houterman, P.M.; Ma, L.; van Ooijen, G.; de Vroomen, M.J.; Cornelissen, B.J.C.; Takken, F.L.W.; Rep, M. The Effector Protein Avr2 of the Xylem-Colonizing Fungus Fusarium oxysporum Activates the Tomato Resistance Protein I-2 Intracellularly. Plant J. Cell Mol. Biol. 2009, 58, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Farahani-Kofoet, R.D.; Witzel, K.; Graefe, J.; Grosch, R.; Zrenner, R. Species-Specific Impact of Fusarium Infection on the Root and Shoot Characteristics of Asparagus. Pathogens 2020, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Warman, N.M.; Aitken, E.A.B. The Movement of Fusarium oxysporum f.sp. cubense (Sub-Tropical Race 4) in Susceptible Cultivars of Banana. Front. Plant Sci. 2018, 9, 1748. [Google Scholar] [CrossRef]
- Bertrand, R.; Riofrío-Dillon, G.; Lenoir, J.; Drapier, J.; de Ruffray, P.; Gégout, J.-C.; Loreau, M. Ecological Constraints Increase the Climatic Debt in Forests. Nat. Commun. 2016, 7, 12643. [Google Scholar] [CrossRef]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; De Lucia, E.H. Biotic Stress Globally Downregulates Photosynthesis Genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous Application of Heat, Drought, and Virus to Arabidopsis Plants Reveals Significant Shifts in Signaling Networks. Plant Physiol. 2013, 162, 1849–1866. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K. Regulation of Primary Plant Metabolism during Plant-Pathogen Interactions and Its Contribution to Plant Defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Sinha, R.; Mysore, K.S.; Senthil-Kumar, M. Impact of Concurrent Drought Stress and Pathogen Infection on Plants. In Combined Stresses in Plants: Physiological, Molecular, and Biochemical Aspects; Mahalingam, R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 203–222. ISBN 978-3-319-07899-1. [Google Scholar]
- Sinha, R.; Irulappan, V.; Mohan-Raju, B.; Suganthi, A.; Senthil-Kumar, M. Impact of Drought Stress on Simultaneously Occurring Pathogen Infection in Field-Grown Chickpea. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- De Torres Zabala, M.; Littlejohn, G.; Jayaraman, S.; Studholme, D.; Bailey, T.; Lawson, T.; Tillich, M.; Licht, D.; Bölter, B.; Delfino, L.; et al. Chloroplasts Play a Central Role in Plant Defence and Are Targeted by Pathogen Effectors. Nat. Plants 2015, 1, 15074. [Google Scholar] [CrossRef]
- Qi, J.; Song, C.-P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.-K.; Gong, Z. Reactive Oxygen Species Signaling and Stomatal Movement in Plant Responses to Drought Stress and Pathogen Attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Pshibytko, N.L.; Zenevich, L.A.; Kabashnikova, L.F. Changes in the Photosynthetic Apparatus during Fusarium Wilt of Tomato. Russ. J. Plant Physiol. 2006, 53, 25–31. [Google Scholar] [CrossRef]
- Prokopová, J.; Spundová, M.; Sedlárová, M.; Husicková, A.; Novotný, R.; Dolezal, K.; Naus, J.; Lebeda, A. Photosynthetic Responses of Lettuce to Downy Mildew Infection and Cytokinin Treatment. Plant Physiol. Biochem. PPB 2010, 48, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Han, G.; Ren, C.; Zhao, S.; Wu, X.; Bian, T. Fusarium solani Infection Depressed Photosystem Performance by Inducing Foliage Wilting in Apple Seedlings. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Jedmowski, C.; Bayramov, S.; Brüggemann, W. Comparative Analysis of Drought Stress Effects on Photosynthesis of Eurasian and North African Genotypes of Wild Barley. Photosynthetica 2014, 52, 564–573. [Google Scholar] [CrossRef]
- Toniutti, L.; Breitler, J.-C.; Etienne, H.; Campa, C.; Doulbeau, S.; Urban, L.; Lambot, C.; Pinilla, J.-C.H.; Bertrand, B. Influence of Environmental Conditions and Genetic Background of Arabica Coffee (C. arabica L) on Leaf Rust (Hemileia vastatrix) Pathogenesis. Front. Plant Sci. 2017, 8, 2025. [Google Scholar] [CrossRef] [PubMed]
- Rep, M.; van der Does, H.C.; Meijer, M.; van Wijk, R.; Houterman, P.M.; Dekker, H.L.; de Koster, C.G.; Cornelissen, B.J.C. A Small, Cysteine-Rich Protein Secreted by Fusarium oxysporum during Colonization of Xylem Vessels Is Required for I-3-Mediated Resistance in Tomato. Mol. Microbiol. 2004, 53, 1373–1383. [Google Scholar] [CrossRef]
- Maldonado-Bonilla, L.D.; Calderón-Oropeza, M.A.; Villarruel-Ordaz, J.L.; Sánchez-Espinosa, A.C. Identification of Novel Potential Causal Agents of Fusarium Wilt of Musa sp. AAB in Southern Mexico. J. Plant Pathol. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Vlaardingerbroek, I.; Beerens, B.; Rose, L.; Fokkens, L.; Cornelissen, B.J.C.; Rep, M. Exchange of Core Chromosomes and Horizontal Transfer of Lineage-Specific Chromosomes in Fusarium oxysporum: Chromosome Transfer and Exchange in F. oxysporum. Environ. Microbiol. 2016, 18, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Borah, N.; Albarouki, E.; Schirawski, J. Comparative Methods for Molecular Determination of Host-Specificity Factors in Plant-Pathogenic Fungi. Int. J. Mol. Sci. 2018, 19, 863. [Google Scholar] [CrossRef] [PubMed]
- Bertazzoni, S.; Williams, A.H.; Jones, D.A.; Syme, R.A.; Tan, K.-C.; Hane, J.K. Accessories Make the Outfit: Accessory Chromosomes and Other Dispensable DNA Regions in Plant-Pathogenic Fungi. Mol. Plant Microbe Interact. MPMI 2018, 31, 779–788. [Google Scholar] [CrossRef]
- Brown, D.W.; Cheung, F.; Proctor, R.H.; Butchko, R.A.E.; Zheng, L.; Lee, Y.; Utterback, T.; Smith, S.; Feldblyum, T.; Glenn, A.E.; et al. Comparative Analysis of 87,000 Expressed Sequence Tags from the Fumonisin-Producing Fungus Fusarium verticillioides. Fungal Genet. Biol. 2005, 42, 848–861. [Google Scholar] [CrossRef]
- Proctor, R.H.; Busman, M.; Seo, J.-A.; Lee, Y.W.; Plattner, R.D. A Fumonisin Biosynthetic Gene Cluster in Fusarium oxysporum Strain O-1890 and the Genetic Basis for B versus C Fumonisin Production. Fungal Genet. Biol. 2008, 45, 1016–1026. [Google Scholar] [CrossRef]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. FUM Cluster Divergence in Fumonisins-Producing Fusarium Species. Fungal Biol. 2011, 115, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Irzykowska, L.; Bocianowski, J.; Waśkiewicz, A.; Weber, Z.; Karolewski, Z.; Goliński, P.; Kostecki, M.; Irzykowski, W. Genetic Variation of Fusarium oxysporum Isolates Forming Fumonisin B(1) and Moniliformin. J. Appl. Genet. 2012, 53, 237–247. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Polyphasic Approach for Detecting Toxigenic Fusarium Species Collected from Imported Grain and Seed Commodities. Plant Pathol. Quar. 2016, 6, 81–99. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakahara, K.; Tanaka, S.; Shigyo, M.; Ito, S. Genetic and Pathogenic Variability of Fusarium oxysporum f. sp. cepae Isolated from Onion and Welsh Onion in Japan. Phytopathology 2015, 105, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Qu, W.; Chang, S.; Li, C.; Xu, F.; Ju, M.; Zhao, R.; Wang, H.; Zhang, H.; Miao, H. Identification of Pathogenicity Groups and Pathogenic Molecular Characterization of Fusarium oxysporum f. sp. sesami in China. Phytopathology 2020, 110. [Google Scholar] [CrossRef]
- Van Dam, P.; Rep, M. The Distribution of Miniature Impala Elements and SIX Genes in the Fusarium Genus Is Suggestive of Horizontal Gene Transfer. J. Mol. Evol. 2017, 85, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Kashiwa, T.; Kozaki, T.; Ishii, K.; Turgeon, B.G.; Teraoka, T.; Komatsu, K.; Arie, T. Sequencing of Individual Chromosomes of Plant Pathogenic Fusarium oxysporum. Fungal Genet. Biol. 2017, 98, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Suleman, P.; Tohamy, A.M.; Saleh, A.A.; Madkour, M.A.; Straney, D.C. Variation in Sensitivity to Tomatine and Rishitin among Isolates Of Fusarium oxysporum f. sp. lycopersici, and Strains Not Pathogenic on Tomato. Physiol. Mol. Plant Pathol. 1996, 48, 131–144. [Google Scholar] [CrossRef]
- Takken, F.; Rep, M. The Arms Race between Tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef]



| SIX Genes | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fusarium Species | Isolate Code | Accession | Municipality | Locality | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Fum1 | Fum13 |
| F. oxysporum | CBF-297 | CP053267.1 | Atzalan | Chachalacas | + | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| F. oxysporum f. sp. vasinfectum | CBF-298 | KT323848.1 | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | ||
| F. solani | CBF-299 | JF740846.1 | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-032 | KX822794.1 | Napoala | + | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | |
| F. oxysporum | CBF-033 | KP964880.1 | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-296 | KP964859.1 | + | - | - | + | + | + | + | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum | CBF-027 | KP964859.1 | Cosautlán | La Lagunilla | - | - | - | - | - | + | + | + | - | - | - | - | - | - | + | + |
| F. oxysporum | CBF-038 | KP964880.1 | + | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-242 | KP964900.1 | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-243 | KP964880.1 | + | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-244 * | KP964880.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-308 | CP053267.1 | + | - | - | - | - | + | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-309 | KP964859.1 | - | + | - | + | - | + | + | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-245 | KP964880.1 | Ixhuatlán del café | Ocotitlán | + | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - |
| F. oxysporum | CBF-246 | KP964878.1 | - | - | - | + | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-026 | KP964880.1 | Moctezuma | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | - | |
| F. oxysporum | CBF-247 | KP964880.1 | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-248 | KP964900.1 | + | - | - | + | - | - | - | + | + | - | - | - | - | - | + | + | ||
| F. oxysporum f. sp. vasinfectum | CBF-249 | KT323856.1 | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-310 | KP964880.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |||
| F. oxysporum f. sp. vasinfectum | CBF-035 | KT323856.1 | Nevería | + | + | - | - | - | + | - | + | + | - | - | - | - | - | - | - | |
| F. oxysporum | CBF-250 | KP964900.1 | + | - | - | + | - | - | - | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-251 | KP964880.1 | - | - | - | + | - | + | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-252 | KP964880.1 | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-253 | KP964900.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. vasinfectum | CBF-254 * | KT323838.1 | - | - | - | + | - | - | - | + | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-305 | KP964880.1 | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. vasinfectum | CBF-306 | KT323856.1 | + | - | - | - | - | + | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-307 | CP053267.1 | - | - | - | - | - | + | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-255 | CP053267.1 | Emiliano Zapata | Pacho Nuevo | + | - | - | - | + | - | + | + | - | - | - | - | - | + | + | + |
| F. oxysporum | CBF-256 | KP964880.1 | + | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. cepae | CBF-257 | KP964904.1 | - | - | - | + | - | - | + | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. vasinfectum | CBF-258 | KT323846.1 | - | - | + | - | - | - | + | + | + | - | - | - | - | + | + | + | ||
| F. oxysporum | CBF-028 | KP964880.1 | Jilotepec | Paso San Juan | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + | + |
| F. oxysporum | CBF-030 | CP053267.1 | - | + | - | - | - | + | + | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum | CBF-031 | KP964880.1 | - | - | - | - | - | - | + | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum f. sp. dianthi | CBF-259 | LT841231.1 | - | - | - | + | - | - | + | + | - | - | - | - | - | + | - | - | ||
| F. oxysporum f. sp. vasinfectum | CBF-260 | KT323848.1 | - | - | + | - | + | - | - | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum f. sp. dianthi | CBF-261 | LT841231.1 | + | - | - | + | - | - | + | - | + | - | - | - | - | - | + | + | ||
| F. oxysporum | CBF-262 | KP964878.1 | + | - | + | + | - | - | + | - | - | - | - | - | - | - | + | + | ||
| F. solani | CBF-263 * | JF740784.1 | - | - | + | + | - | - | - | + | + | - | + | - | - | + | + | + | ||
| F. oxysporum | CBF-036 | KP964880.1 | Sochiapa | Sochiapa | + | + | + | - | - | - | - | - | - | - | - | - | - | - | - | - |
| F. oxysporum | CBF-037 | KP964859.1 | - | - | - | - | + | - | - | + | - | - | - | - | - | - | + | + | ||
| F. solani | CBF-264 | JF740846.1 | + | - | - | - | + | - | - | - | - | - | - | - | - | - | + | + | ||
| F. solani | CBF-265 | JF740846.1 | + | - | - | + | + | - | - | + | + | - | - | - | - | + | - | - | ||
| F. oxysporum | CBF-266 | KP964880.1 | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. phaseoli | CBF-267 | KP964890.1 | + | - | - | - | - | + | - | - | - | - | - | - | - | + | - | - | ||
| F. oxysporum | CBF-268 | KP964880.1 | - | - | - | + | - | - | + | - | - | - | - | - | - | + | + | + | ||
| F. oxysporum | CBF-269 * | KP964859.1 | - | - | - | + | - | - | + | + | - | - | - | - | - | - | + | + | ||
| F. oxysporum f. sp. dianthi | CBF-292 | LT841231.1 | - | - | - | - | - | + | + | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-293 | KP964859.1 | - | - | - | + | + | + | + | + | - | - | - | - | - | - | + | + | ||
| F. solani | CBF-294 | JF740846.1 | + | - | - | - | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. vasinfectum | CBF-295 | KT323856.1 | - | - | - | - | - | - | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum f. sp. vasinfectum | CBF-270 | KT323869.1 | Totutla | Totutla | - | - | - | - | + | - | - | + | + | - | - | - | - | - | - | - |
| F. oxysporum f. sp. vasinfectum | CBF-271 | KT323869.1 | - | - | + | - | + | + | + | + | + | - | - | - | - | + | + | + | ||
| F. oxysporum | CBF-272 | KP964859.1 | - | - | + | - | - | - | - | + | - | - | - | - | - | - | - | - | ||
| F. solani | CBF-273 | JF740784.1 | Coatepec | Tuzamapan | + | - | + | - | + | - | + | + | + | - | - | - | - | + | + | + |
| F. oxysporum | CBF-274 | KP964880.1 | - | - | + | - | + | - | - | - | + | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-275 | KP964880.1 | + | - | + | - | + | + | + | - | + | - | - | - | - | + | + | + | ||
| F. solani | CBF-276 | JF740846.1 | + | - | - | - | + | - | - | - | + | - | - | - | - | + | - | - | ||
| F. solani | CBF-277 | JF740846.1 | + | - | + | + | + | - | + | + | - | - | - | - | - | + | + | + | ||
| F. oxysporum f. sp. vasinfectum | CBF-278 | KT323848.1 | + | - | + | - | + | - | - | + | - | - | - | - | - | - | - | - | ||
| F. solani | CBF-279 | JF740727.1 | + | - | + | + | + | - | - | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum | CBF-280 | KP964878.1 | + | - | - | - | + | - | - | + | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-281 | KP964880.1 | Zimpizahua | + | - | - | - | - | - | + | - | - | - | - | - | - | - | + | + | |
| F. solani | CBF-282 | JF740846.1 | + | - | + | + | + | - | - | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum | CBF-283 | KP964878.1 | - | - | - | + | - | + | + | - | - | - | - | - | - | - | - | - | ||
| F. oxysporum | CBF-034 | KP964859.1 | Yecuatla | La Victoria | - | - | - | - | - | + | - | + | - | - | - | - | - | - | + | + |
| F. solani | CBF-301 | JF740786.1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum f. sp. phaseoli | CBF-303 | KP964890.1 | - | - | - | - | - | + | + | - | - | - | - | - | - | - | + | + | ||
| F. oxysporum f. sp. lycopersici | ATCC 417 | + | + | + | + | + | + | + | + | - | - | + | - | - | - | - | + | |||
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| PItotal | −0.90 | 0.21 | 0.31 | −0.15 |
| PIabs | −0.94 | 0.26 | −0.01 | −0.06 |
| Fv/Fm | −0.93 | −0.13 | −0.31 | 0.01 |
| ABS/RC | 0.98 | 0.11 | 0.05 | 0.12 |
| Di0/RC | 0.94 | 0.26 | 0.06 | 0.18 |
| Tr0/RC | −0.31 | −0.93 | 0.17 | 0.10 |
| Et0/RC | −0.94 | 0.09 | −0.04 | 0.29 |
| phi(Eo) | −1.00 | −0.01 | −0.01 | −0.02 |
| ψ0/Vj | −0.91 | 0.28 | 0.13 | 0.20 |
| Eigenvalues | 7.20 | 1.16 | 0.25 | 0.21 |
| Variance (%) | 79.95 | 12.89 | 2.73 | 2.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamboa-Becerra, R.; López-Lima, D.; Villain, L.; Breitler, J.-C.; Carrión, G.; Desgarennes, D. Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease. J. Fungi 2021, 7, 253. https://doi.org/10.3390/jof7040253
Gamboa-Becerra R, López-Lima D, Villain L, Breitler J-C, Carrión G, Desgarennes D. Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease. Journal of Fungi. 2021; 7(4):253. https://doi.org/10.3390/jof7040253
Chicago/Turabian StyleGamboa-Becerra, Roberto, Daniel López-Lima, Luc Villain, Jean-Christophe Breitler, Gloria Carrión, and Damaris Desgarennes. 2021. "Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease" Journal of Fungi 7, no. 4: 253. https://doi.org/10.3390/jof7040253
APA StyleGamboa-Becerra, R., López-Lima, D., Villain, L., Breitler, J.-C., Carrión, G., & Desgarennes, D. (2021). Molecular and Environmental Triggering Factors of Pathogenicity of Fusarium oxysporum and F. solani Isolates Involved in the Coffee Corky-Root Disease. Journal of Fungi, 7(4), 253. https://doi.org/10.3390/jof7040253








