Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Plate Assay
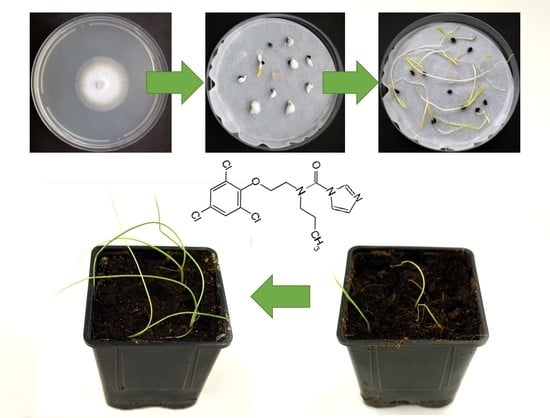
2.3. Seed Germination Pathogenicity Assay
2.4. Pot Pathogenicity Assay
2.5. Statistical Analyses
3. Results
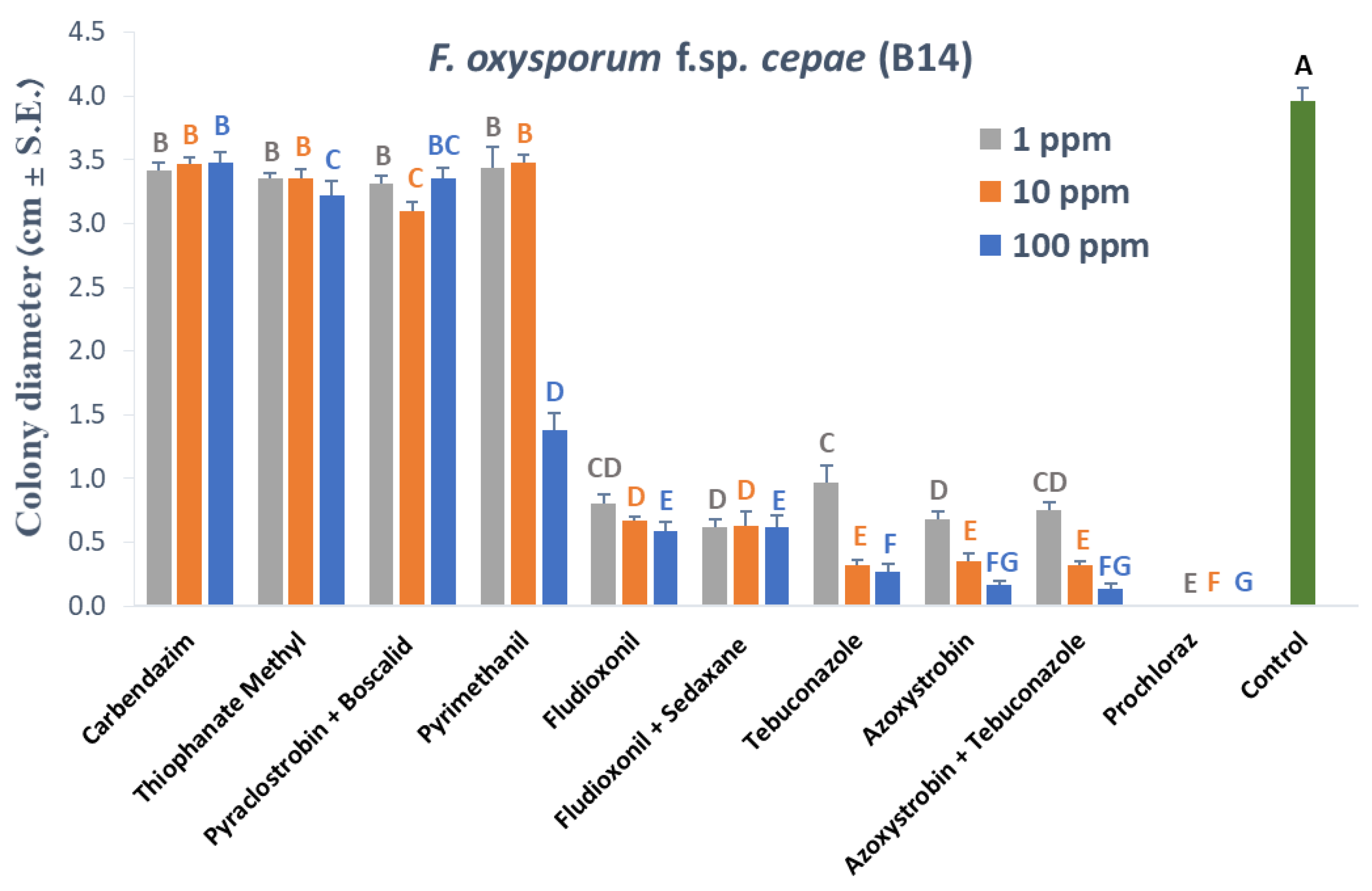
3.1. Plate Assay
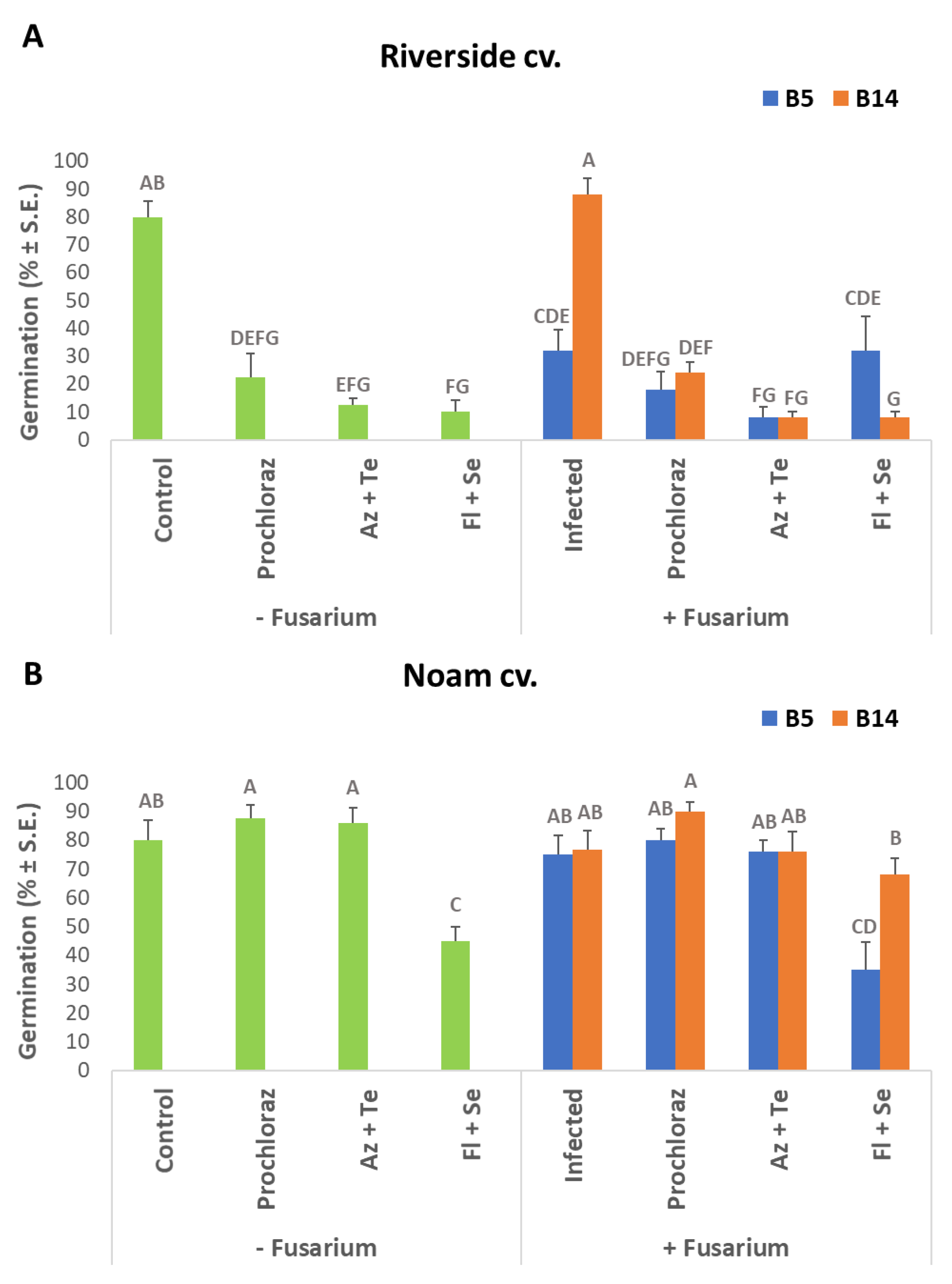
3.2. Seed Pathogenicity Assay
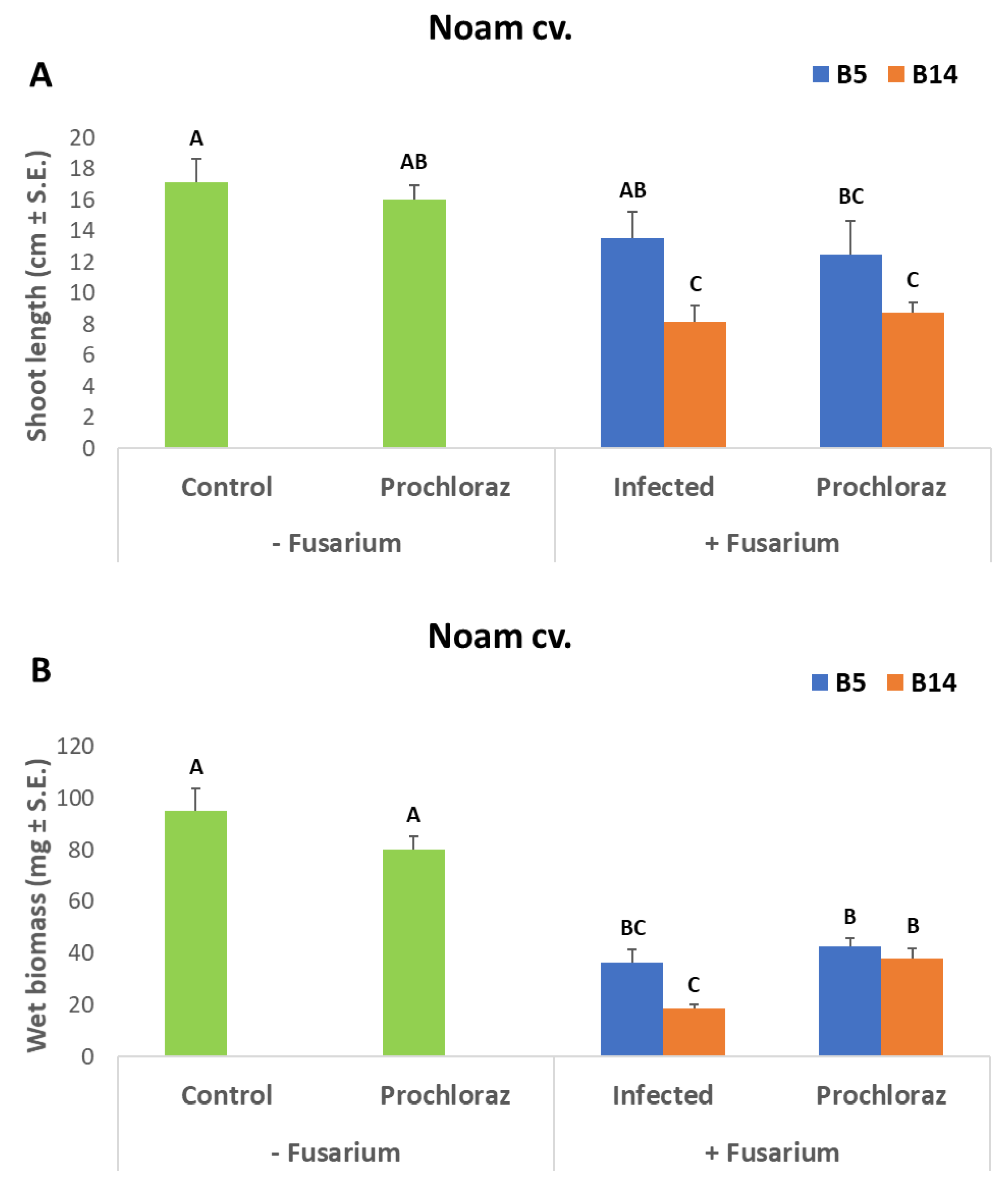
3.3. Onion Seedlings Inoculation Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bektas, I.; Kusek, M. Biological control of onion basal rot disease using phosphate solubilising rhizobacteria. Biocontrol Sci. Technol. 2020, 31, 190–205. [Google Scholar] [CrossRef]
- Rajamohan, K.; Udhayakumar, R.; Sanjaygandhi, S.; Vengadesh Kumar, L.; Thamarai Selvi, M.; Sudhasha, S.; Yuvarani, R. Management of basal rot of onion caused by Fusarium oxysporumi. sp. cepae using bioregulators. J. Biopestic. 2019, 12, 239–247. [Google Scholar]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Meller Harel, Y.; Degani, O. Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Lebiush-Mordechai, S.; Erlich, O.; Maymon, M.; Freeman, S.; Ben-David, T.; Ofek, T.; Palevsky, E.; Tsror, L. Bulb and Root rot in lily (Lilium longiflorum) and onion (Allium cepa) in Israel. J. Phytopathol. 2014, 162, 466–471. [Google Scholar] [CrossRef]
- Nasr Esfahani, M. Genetic and virulence variation in Fusarium oxysporum f. sp. cepae causing root and basal rot of common onion in Iran. J. Phytopathol. 2018, 166, 572–580. [Google Scholar] [CrossRef]
- Le, D.; Ameye, M.; De Boevre, M.; De Saeger, S.; Audenaert, K.; Haesaert, G.J. Population, virulence and mycotoxin profile of Fusarium spp. associated with basal rot of Allium spp. in Vietnam. Plant Disease 2020. [Google Scholar] [CrossRef]
- Javaid, A.; Niaz, L.; Shoaib, A. Effect of incorporation of leaf biomass of Coronopus didymus on management of basal rot disease of onion and its physiology. Int. J. Agric. Biol. 2017, 19, 445–452. [Google Scholar] [CrossRef]
- Etana, M.B.; Aga, M.C.; Fufa, B.O. Major onion (Allium cepa L.) production challenges in Ethiopia: A review. J. Biol. Agric. Healthc. 2019, 9. [Google Scholar] [CrossRef]
- Cramer, C.S. Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Behrani, G.; Syed, R.; Abro, M.; Jiskani, M.; Khanzada, M.A. Pathogenicity and chemical control of basal rot of onion caused by Fusarium oxysporum f. sp. cepae. Pak. J. Agric. Agric. Eng. Vet. Sci. 2015, 31, 60–70. [Google Scholar]
- Leadbeater, A.J. Plant health management: Fungicides and antibiotics. Engineering 2014, 408–424. [Google Scholar] [CrossRef]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post flowering stalk rot complex of maize-present status and future prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- Degani, O.; Dor, S.; Abraham, D.; Cohen, R. Interactions between Magnaporthiopsis maydis and Macrophomina phaseolina, the causes of wilt diseases in maize and cotton. Microorganisms 2020, 8, 249. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide resistance evolution and detection in plant pathogens: Plasmopara viticola as a case study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cao, Q.; Li, N.; Liu, D.; Yuan, Y. Transcriptome analysis of fungicide-responsive gene expression profiles in two Penicillium italicum strains with different response to the sterol demethylation inhibitor (DMI) fungicide prochloraz. BMC Genom. 2020, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, C.X.; Zhai, X.Y.; Jamieson, P.A.; Zhang, C.Q. Mutation in cyp51b and overexpression of cyp51a and cyp51b confer multiple resistant to DMIs fungicide prochloraz in Fusarium fujikuroi. Pest Manag. Sci. 2021, 77, 824–833. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S.; Chen, A.; Orlov-Levin, V.; Stolov-Yosef, A.; Regev, D.; Rabinovitz, O. Molecular tracking and remote sensing to evaluate new chemical treatments against the maize late wilt disease causal agent, Magnaporthiopsis maydis. J. Fungi 2020, 6, 54. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S.; Movshowitz, D.; Fraidman, E.; Rabinovitz, O. Effective chemical protection against the maize late wilt causal agent, Harpophora maydis, in the field. PLoS ONE 2018, 13, e0208353. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Coşkuntuna, A.; Özer, N. Biological control of onion basal rot disease using Trichoderma harzianum and induction of anti-fungal compounds in onion set following seed treatment. Crop Prot. 2008, 27, 330–336. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Biocontrol measures against onion basal rot incidence under natural field conditions. J. Plant Pathol. 2019, 101, 579–586. [Google Scholar] [CrossRef]
- Degani, O.; Movshowitz, D.; Dor, S.; Meerson, A.; Goldblat, Y.; Rabinovitz, O. Evaluating Azoxystrobin seed coating against maize late wilt disease using a sensitive qPCR-based method. Plant Dis. 2019, 103, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Özer, N.; Köycü, N.D. Evaluation of seed treatments for controlling Aspergillus niger and Fusarium oxysporum on onion seed. Phytopathol. Mediterr. 1998, 37, 33–40. [Google Scholar]






| Species | Isolate | NCBI Accession and Score | Collection Sites b | Onion Cultivar c |
|---|---|---|---|---|
| F. acutatum | B5 | MK507814.1 (100%) | Kibbutz Ortal | Riverside |
| F. oxysporum f. sp. cepae | B14 | KP964881.1 (99.55%) | Moshav Eliad | 565/505 |
| Fungicide Commercial Name | Manufacturer Supplier | Active Ingredient (Common Name and Chemical Structure) | Group Name | Chemical Group | Target Site of Action | Section Mode of Action | Active Ingredient (g/l) |
|---|---|---|---|---|---|---|---|
| Sportak | Bayer CropScience (Monheim am Rhein, Germany) Gadot Agro (Kidron, Israel) | Prochloraz | DMI-fungicides (demethylation inhibitors) DMI-fungicides (demethylation inhibitors(SBI: Class I) | Imidazoles | C14- demethylase in sterol biosynthesis (erg11/cyp51) | Sterol biosynthesis in membranes | 450 |
| Celest 100FS | Syngenta (Basel, Switzerland), Gadot Agro (Kidron, Israel) | Fludioxonil | PP-fungicides (phenyl pyrroles) | Phenylpyrroles | MAP/histidine kinase in osmotic signal transduction (os-2, HOG1) | Signal transduction | 100 |
| Signum W.G. (Bc + Ps) | BASF (Ludwigshafen, Germany), Agan (Ashdod, Israel) | Boscalid 26.7% | SDHI (succinate dehydrogenase inhibitors) | Pyridine-carboxamides | Succinate dehydro- genase | Respiration | 267 |
Pyraclostrobin 6.7% | QoI-fungicides (quinone outside inhibitors) | Methoxy-carbamates | Cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 67 | |||
| Topaz W.P. | Nippon Soda (Japan), Adama Makhteshim (Be’er Sheva, Israel) | Thiophanate-Methyl | Fungicides (methyl benzimidazole carbamates) | Thiophanates | ß-tubulin assembly in mitosis | Cytoskeleton and motor protein | 700 |
| Mythos 300 SC | Buyer (Cyprus) Lidor Chemicals (Israel) | Pyrimethanil | AP-fungicides (anilino pyrimidines) | Anilino-pyrimidines | Methionine biosynthesis (proposed) (cgs gene) | Amino acids and protein synthesis | 300 |
| Orius 25 | Adama Irvita (Netherlands) Adama Makhteshim (Be’er Sheva, Israel) | Tebuconazole | DMI-fungicides (demethylation inhibitors) (SBI: Class I) | Triazoles | C14- demethylase in sterol biosynthesis (erg11/cyp51) | Sterol biosynthesis in membranes | 250 |
| Delsene | Agro-Chemie (Hungary) Gadot Agro (Kidron, Israel) | Carbendazim | MBC-fungicides (methyl benzimidazole carbamates) | Benzimidazoles | ß-tubulin assembly in mitosis | Cytoskeleton and motor protein | 500 |
| Azimut | Adama Makhteshim (Be’er Sheva, Israel) | Azoxystrobin 12% | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | Respiration | 120 |
Tebuconazole 20% | DMI-fungicides (demethylation inhibitors) (SBI: Class I) | Triazoles | C14- demethylase in sterol biosynthesis (erg11/cyp51) | Sterol biosynthesis in membranes | 200 | ||
| Amistar | Syngenta (Basel, Switzerland), Adama Makhteshim (Airport City, Israel) | Azoxystrobin | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | Respiration | 250 |
| Vibrance | Syngenta (Basel, Switzerland), Gadot Agro (Kidron, Israel) | Fludioxonil 2.5% | PP-fungicides (phenyl pyrroles) | Phenylpyrroles | MAP/histidine kinase in osmotic signal transduction (os-2, HOG1) | Signal transduction | 25 |
Sedaxane 2.5% | SDHI (succinate dehydrogenase inhibitors) | Pyrazole-4- carboxamides | Complex II: succinate-dehydrogenase | Respiration | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degani, O.; Kalman, B. Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum. J. Fungi 2021, 7, 235. https://doi.org/10.3390/jof7030235
Degani O, Kalman B. Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum. Journal of Fungi. 2021; 7(3):235. https://doi.org/10.3390/jof7030235
Chicago/Turabian StyleDegani, Ofir, and Ben Kalman. 2021. "Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum" Journal of Fungi 7, no. 3: 235. https://doi.org/10.3390/jof7030235
APA StyleDegani, O., & Kalman, B. (2021). Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum. Journal of Fungi, 7(3), 235. https://doi.org/10.3390/jof7030235