Candida albicans and Oral Carcinogenesis. A Brief Review
Abstract
1. Introduction
2. The Main Features of Candida albicans
2.1. Cell Wall Structure and Virulence Factors
2.2. The Genome
2.3. The Immune Response
3. The Role of Oral Dysbiosis
4. The Current Clinical Evidence about C. albicans’ Role in Oral Cancer
4.1. Epidemiological Findings
4.2. C. albicans and Oral Malignant Transformation Condition
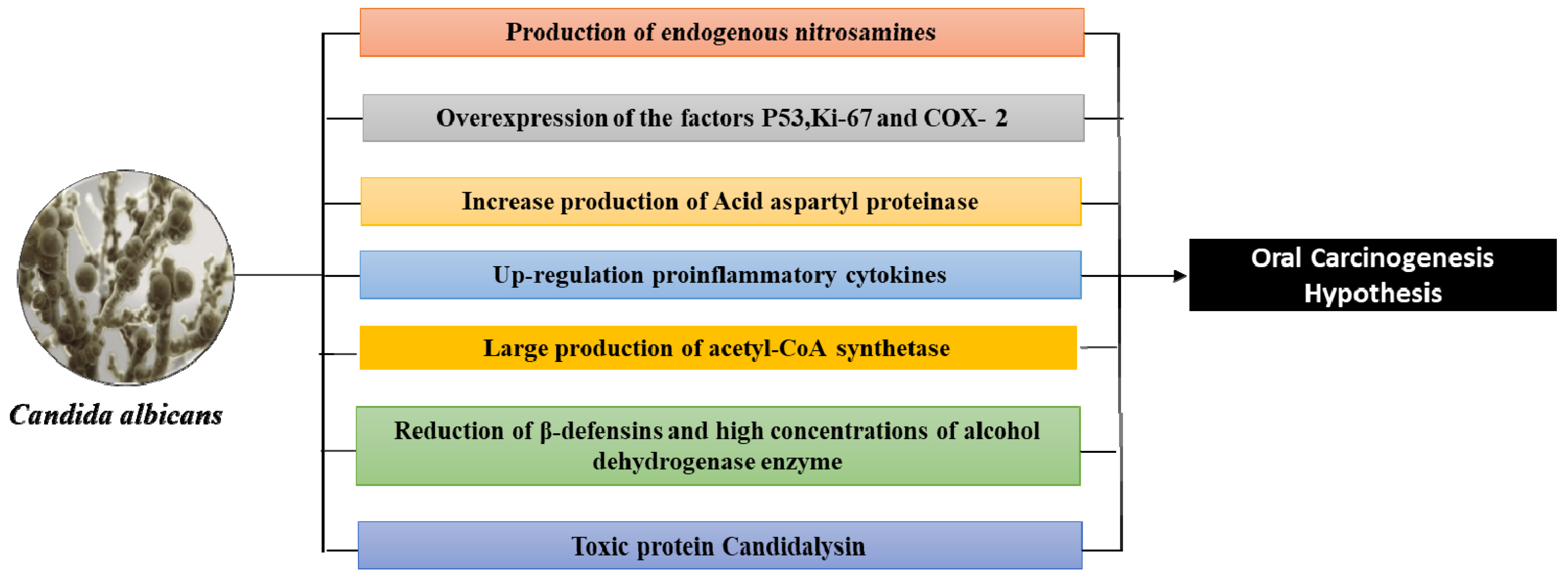
5. The Biomolecular Mechanisms of C. albicans-Induced Oncogenesis
- An over-expression of P53, Ki-67 labeling index, and Prostaglandin-endoperoxide synthase 2 (COX-2) are some of the additional mechanisms by which Candida can affect malignant transformation into oral leukoplakia. P53 and Ki-67, which are markers of cell proliferation, have overexpression that is well established in malignant lesions, and COX-2, which is markedly increased in inflammation states and is associated with the release of prostaglandins, thus influencing cell proliferation, cell death, and tumor invasion [67,84,85,86].
- Acid aspartyl proteinase appears to be more present in oral lesions and therefore also in those with leukoplakia than in healthy subjects [67]. The production of acid aspartyl-proteinase are putative virulence factors in candidiasis, and are why an acidic pH exists, thus degrading the sub endothelial extracellular matrix, as well as laminin 332 and E-cadherin. This induces dysplastic alterations and thus begins the C. albicans dissemination in the systemic circulation and therefore in the organs [48,85,86]. On the other hand, in a model of hyphal invasion (localized or uniform) of Candida, there is no difference between oral potentially precancerous disorders and oral squamous cell carcinoma. These biomolecular mechanisms highlight the ability of Candida to influence malignant and cellular changes in oral leukoplakia [86].
- Oral Candida infection is a cause of up-regulation in proinflammatory cytokines (interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-18, tumor necrosis factor (TNF)-α, IFN-γ, and GM-CSF), that influences the metabolic pathways and induces directly an endothelial dysfunction, playing a role in immune-related mechanisms with cancer development [48,62,63].
- C. albicans can produce acetaldehyde (carcinogen due to mutagenic qualities in DNA) from precursors found in the oral cavity (metabolizing ethanol and glucose in high quantities, especially when associated with smoking and alcohol consumption) [67,72,84]. Thus, Candida can produce large quantities of acetaldehyde and acetyl-CoA synthetase (more in smokers) in cases of potentially malignant disorders and in oral carcinomas (concentrations of acetaldehyde and acetyl-CoA synthetase increase) compared to healthy individuals and those with ectodermal dystrophy and autoimmune polyendocrinopathy (with candidiasis) [72,85,86,87,88,89,90,91]. However, the increase in the mutagenic amounts of acetaldehyde is more marked even in occupationally exposed workers to carcinogen [92] and people with poor oral hygiene, than in healthy subjects, via the oral microbiota (Streptococcus viridans and resident fungi such as Candida) that can convert ethanol into acetaldehyde (possess the enzyme alcohol-dehydrogenase) [93]. Indeed, the levels of acetaldehyde produced by Candida increase in proportion to the increase in alcohol consumption [93,94].
- In oral squamous cell carcinoma, the reduction of β-defensins favors Candida superinfections. In chronic hyperplastic candidiasis, C. albicans is the predominant species and is associated with high concentrations of alcohol dehydrogenase enzyme and P53 that suggests a dysplastic potential factor [95]. In fact, there is evidence that Candida’s epithelial invasion can cause hyperplastic conditions (Figure 2) [67,72,96].
- The candidalysin (or 31-amino acid α-helical amphipathic peptide) is a cytolytic toxin of C. albicans. It is encoded by the ECE1 gene initially associated with fungal filamentation ability (release the toxin from the hypha) and host cell adhesion. Initially, ECE1 encodes 271 amino acid pre-proproteins that are cleaved by Kex8p enzyme into eight smaller peptides (Ece1-I to Ece1- VIII). Ece1-III6-93 is an epithelial immune activator and collaborates with the cytolytic activity of C. albicans [97]. Likewise, candidalysin is an inducer for NF-κB and MAPK pathways. Candidalysin has been reported to excite granulocyte macrophage colony-stimulating factor GM-CSF, an essential molecule in carcinogenesis. After the macrophage death, the C. albicans can escape, survive, and outgrow other macrophages. On the other hand, it induces epithelial damage and elicits host inflammatory processes because it is a trigger for NLR family pyrin domain containing protein 3 (NLRP3) [98].
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irani, S. Pre-Cancerous Lesions in the Oral and Maxillofacial Region: A Literature Review with Special Focus on Etiopathogenesis. Iran. J. Pathol. 2016, 11, 303–322. [Google Scholar]
- Sujir, N.; Ahmed, J.; Pai, K.; Denny, C.; Shenoy, N. Challenges in Early Diagnosis of Oral Cancer: Cases Series. Acta Stomatol. Croat. 2019, 53, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Santacroce, L.; Ballini, A.; Topi, S.; DiPalma, G.; Haxhirexha, K.; Bottalico, L.; Charitos, I.A. Oral Cancer: A Historical Review. Int. J. Environ. Res. Public Health 2020, 17, 3168. [Google Scholar] [CrossRef] [PubMed]
- van der Waal, I.; Schepman, K.P.; van der Meij, E.H.; Smeele, L.I. Oral leukoplakia: A Clinicopathological review. Oral Oncol. 1997, 33, 291–301. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; Van Der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Coronado-Castellote, L.; Jiménez-Soriano, Y. Clinical and microbiological diagnosis of oral candidiasis. J. Clin. Exp. Dent. 2013, 5, e279. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer-systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Cosola, M.; Bottalico, L.; Topi, S.; Charitos, I.; Ballini, A.; Inchingolo, F.; Cazzolla, A.; Dipalma, G. Focus on HPV Infection and the Molecular Mechanisms of Oral Carcinogenesis. Viruses 2021, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Romo, J.A.; Kumamoto, C.A. On Commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef]
- Ballini, A.; Scattarella, A.; Carlaio, R.G.; Papa, F.; Perillo, L.; Romanazzo, T.; Bux, M.V.; Nardi, G.M.; Dituri, A.; Cantore, S.; et al. Surgical treatment of gingival overgrowth with 10 years of follow-up. Head Face Med. 2010, 6, 19. [Google Scholar] [CrossRef]
- Charitos, I.A.; Topi, S.; Castellaneta, F.; D’Agostino, D. Current Issues and Perspectives in Patients with Possible Sepsis at Emergency Departments. Antibiotics 2019, 8, 56. [Google Scholar] [CrossRef]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Arya, N.R.; Rafiq, N.B. Candidiasis. [Updated 2020 Nov 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560624 (accessed on 25 April 2021).
- Tortorano, A.M.; Peman, J.; Bernhardt, H.; Klingspor, L.; Kibbler, C.C.; Faure, O.; Biraghi, E.; Canton, E.; Zimmermann, K.; Seaton, S.; et al. ECMM Working Group on Candidemia. Epidemiology of candidemia in Europe: Results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Ciurea, C.N.; Pasaroiu, D.; Savin, A.-I.; Toma, F.; Sular, F.; Santacroce, L.; Mare, A. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Memórias do Instituto Oswaldo Cruz 2017, 112, 587–592. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Elorza, M.V.; Valentin, E.; Sentandreu, R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res 2006, 6, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Bolard, J.; Prasad, R. Emerging role of lipids of Candida albicans, a pathogenic dimorphic yeast. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1992, 1127, 1–14. [Google Scholar] [CrossRef]
- Cotter, G.; Kavanagh, K. Adherence mechanisms of Candida albicans. Br. J. Biomed. Sci. 2000, 57, 241–249. [Google Scholar]
- Shibata, N.; Okawa, Y. Structure of Fungal Cell Wall Polysaccharides. Nippon. Ishinkin Gakkai Zasshi 2006, 47, 179–184. [Google Scholar] [CrossRef][Green Version]
- Wang, K.; Luo, Y.; Zhang, W.; Xie, S.; Yan, P.; Liu, Y.; Li, Y.; Ma, X.; Xiao, K.; Fu, H.; et al. Diagnostic value of Candida mannan antigen and anti-mannan IgG and IgM antibodies for Candida infection. Mycoses 2019, 63, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Fukazawa, Y.; Kagaya, K. Molecular bases of adhesion of Candida albicans. Med Mycol. 1997, 35, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.D.; Shibata, N.; Podzorski, R.P.; Herron, M.J. Candida mannan: Chemistry, suppression of cell-mediated immunity, and possible mechanisms of action. Clin. Microbiol. Rev. 1991, 4, 1–19. [Google Scholar] [CrossRef]
- Hostetter, M.K. Linkage of adhesion, morphogenesis, and virulence in Candida albicans. J. Lab. Clin. Med. 1998, 132, 258–263. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.N.; Hannemann, H.; Sehnal, M.; Biesemeier, A.; Schweizer, A.; Röllinghoff, M.; Schröppel, K. Induction of SAP7 Correlates with Virulence in an Intravenous Infection Model of Candidiasis but Not in a Vaginal Infection Model in Mice. Infect. Immun. 2005, 73, 7061–7063. [Google Scholar] [CrossRef]
- Kolotila, M.P.; Diamond, R.D. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect. Immun. 1990, 58, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicansBiofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Magee, B.B.; Magee, P.T.; Holland, B.R.; Rodrigues, E.; Holmes, A.; Cannon, R.D.; Schmid, J. Selective Advantages of a Parasexual Cycle for the Yeast Candida albicans. Genetics 2015, 200, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- Forche, A.; Alby, K.; Schaefer, D.; Johnson, A.D.; Berman, J.; Bennett, R.J. The Parasexual Cycle in Candida albicans Provides an Alternative Pathway to Meiosis for the Formation of Recombinant Strains. PLoS Biol. 2008, 6, e110. [Google Scholar] [CrossRef]
- Reedy, J.L.; Floyd, A.M.; Heitman, J. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr. Biol. 2009, 19, 891–899. [Google Scholar] [CrossRef]
- Doi, M.; Homma, M.; Chindamporn, A.; Tanaka, K. Estimation of chromosome number and size by pulsed-field gel electrophoresis (PFGE) in medically important Candida species. J. Gen. Microbiol. 1992, 138, 2243–2251. [Google Scholar] [CrossRef]
- Schmid, J.; Herd, S.; Hunter, P.; Cannon, R.; Yasin, M.S.M.; Samad, S.; Carr, M.; Parr, D.; McKinney, W.; Schousboe, M.; et al. Evidence for a general-purpose genotype in Candida albicans, highly prevalent in multiple geographical regions, patient types and types of infection. Microbiology 1999, 145, 2405–2413. [Google Scholar] [CrossRef]
- Zhang, N.; Upritchard, J.E.; Holland, B.R.; Fenton, L.E.; Ferguson, M.M.; Cannon, R.; Schmid, J. Distribution of mutations distinguishing the most prevalent disease-causing Candida albicans genotype from other genotypes. Infect. Genet. Evol. 2009, 9, 493–500. [Google Scholar] [CrossRef]
- Zhang, N.; Harrex, A.L.; Holland, B.R.; Fenton, L.E.; Cannon, R.; Schmid, J. Sixty Alleles of the ALS7 Open Reading Frame in Candida albicans: ALS7 Is a Hypermutable Contingency Locus. Genome Res. 2003, 13, 2005–2017. [Google Scholar] [CrossRef]
- Ropars, J.; Maufrais, C.; Diogo, D.; Marcet-Houben, M.; Perin, A.; Sertour, N.; Mosca, K.; Permal, E.; Laval, G.; Bouchier, C.; et al. Gene flow contributes to diversification of the major fungal pathogen Candida albicans. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Rodrigues, C.F. Microbial interactions and immunity response in oral Candida species. Futur. Microbiol. 2020, 15, 1653–1677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jönsson, F. Expression, Role, and Regulation of Neutrophil Fcγ Receptors. Front. Immunol. 2019, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, M.; Greene, R.R.; Noss, I.; Lowman, D.W.; Williams, D.L.C. albicans increases cell wall mannoprotein, but not mannan, in response to blood, serum and cultivation at physiological temperature. Glycobiology 2011, 21, 1173–1180. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Underhill, D.M. Immunity to Commensal Fungi: Detente and Disease. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Prigitano, A.; Biraghi, E.; Viviani, M.A. The European Confederation of Medical Mycology (ECMM) survey of candidaemia in Italy: In vitro susceptibility of 375 Candida albicans isolates and biofilm production. J. Antimicrob. Chemother. 2005, 56, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog 2010, 6, e1000713. [Google Scholar] [CrossRef]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; DiPalma, G.; Inchingolo, F. The pivotal role of oral microbiota in health and disease. J. Biol. Regul. Homeost. Agents. 2020, 34, 733–737. [Google Scholar]
- Ballini, A.; DiPalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Saini, R.; Cantore, S.; Saini, S.R.; Mastrangelo, F.; Ballini, A.; Santacroce, L. Efficacy of Fluorescence Technology vs. Conventional Oral Examination for the Early Detection of Oral Pre-Malignant Lesions. A Clinical Comparative Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 852–858. [Google Scholar] [CrossRef]
- Yang, S.-F.; Huang, H.-D.; Fan, W.-L.; Jong, Y.-J.; Chen, M.-K.; Huang, C.-N.; Kuo, Y.-L.; Chung, W.-H.; Su, S.-C. Compositional and functional variations of oral microbiota associated with the mutational changes in oral cancer. Oral. Oncol. 2018, 77, 1–8. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Domingue, J.C.; Sears, C.L. Microbiota dysbiosis in select human cancers: Evidence of association and causality. Semin. Immunol. 2017, 32, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.M.; Abdelbary, M.M.H.; Conrads, G. A concerted probiotic activity to inhibit periodontitis-associated bacteria. PLoS ONE 2021, 16, e0248308. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti-infective Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef]
- Fux, C.A.; Stoodley, P.; Hall-Stoodley, L.; Costerton, J.W. Bacterial biofilms: A diagnostic and therapeutic challenge. Expert Rev. Anti-infective Ther. 2003, 1, 667–683. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiology 2015, 6, 1391. [Google Scholar] [CrossRef]
- Kabir, M.A.; Ahmad, Z. Candida Infections and Their Prevention. ISRN Prev. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Hayes, R.B.; Ahn, J. The oral fungal mycobiome: Characteristics and relation to periodontitis in a pilot study. BMC Microbiol. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Mori, G.; Brunetti, G.; Colucci, S.; Ciccolella, F.; Coricciati, M.; Pignataro, P.; Oranger, A.; Ballini, A.; Farronato, D.; Mastrangelo, F.; et al. Alteration of activity and survival of osteoblasts obtained from human periodontitis patients: Role of TRAIL. J. Biol. Regul. Homeost. Agents 2007, 21, 105–114. [Google Scholar]
- Sztukowska, M.N.; Dutton, L.C.; Delaney, C.; Ramsdale, M.; Ramage, G.; Jenkinson, H.F.; Nobbs, A.H.; Lamont, R.J. Community Development between Porphyromonas gingivalis and Candida albicans Mediated by InlJ and Als3. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, L.; Tatullo, M.; Marrelli, M.; Santacroce, L. Lights and shadows of dental implants: Focus on mucositis and perimplantitis and their biological markers. J. Biol. Regul. Homeost. Agents 2016, 30, 859–861. [Google Scholar] [PubMed]
- Kollia, K.; Velegraki, A. Candidemia. Arch. Hell. Med. 2008, 25, 41–59. [Google Scholar]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef]
- Jayachandran, A.L.; Katragadda, R.; Thyagarajan, R.; Vajravelu, L.; Manikesi, S.; Kaliappan, S.; Jayachandran, B. Oral Candidiasis among Cancer Patients Attending a Tertiary Care Hospital in Chennai, South India: An Evaluation of Clinicomycological Association and Antifungal Susceptibility Pattern. Can. J. Infect. Dis. Med Microbiol. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, A.I.; Mäkitie, A.; Meurman, J.H. Candida prevalence in saliva before and after oral cancer treatment. Surgeon 2021. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, A.; Nawaz, A.; Mäkitie, A.; Meurman, J.H. Role of Non-Albicans Candida and Candida Albicans in Oral Squamous Cell Cancer Patients. J. Oral Maxillofac. Surg. 2018, 76, 2564–2571. [Google Scholar] [CrossRef]
- Chung, L.-M.; Liang, J.-A.; Lin, C.-L.; Sun, L.-M.; Kao, C.-H. Cancer risk in patients with candidiasis: A nationwide population-based cohort study. Oncotarget 2017, 8, 63562–63573. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Ariyawardana, A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J. Oral Pathol. Med. 2016, 45, 155–166. [Google Scholar] [CrossRef]
- Bánóczy, J.; Sugár, L. Progressive and regressive changes in Hungarian oral leukoplakias in the course of longitudinal studies. Community Dent. Oral Epidemiology 1975, 3, 194–197. [Google Scholar] [CrossRef]
- Silverman, S., Jr.; Gorsky, M.; Lozada, F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer 1984, 53, 563–568. [Google Scholar]
- Abdulrahim, M.H.; McManus, B.A.; Flint, S.R.; Coleman, D.C. Genotyping Candida albicans from Candida Leukoplakia and Non-Candida Leukoplakia Shows No Enrichment of Multilocus Sequence Typing Clades but Enrichment of ABC Genotype C in Candida Leukoplakia. PLoS ONE 2013, 8, e73738. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA A Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Bombeccaria, G.P.; Spadaria, F.; Rossia, M.; Porrinia, M.; Bosottia, M.; Giannì, A.B. Biology of Candida spp. in potentially malignant disorders and carcinoma of the oral cavity. Dental Cadmos 2016, 84, 624–634. [Google Scholar] [CrossRef]
- Hongal, B.P.; Kulkarni, V.V.; Joshi, P.S.; Karande, P.P.; Shroff, A.S.; Deshmukh, R.S. Prevalence of fungal hyphae in potentially malignant lesions and conditions-does its occurrence play a role in epithelial dysplasia? J. Oral Maxillofac. Pathol. 2015, 19, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T.; Waguri, N.; Nozawa, A.; Wakui, I.; Kobayashi, I. A case of multiple gastric ulcers associated with Candida infection. Nihon Shokakibyo Gakkai Zasshi 1997, 94, 480–486. [Google Scholar] [PubMed]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef]
- Birman, E.G.; Kignel, S.; Da Silveira, F.R.; Paula, C.R. Candida albicans: Frequency and characterization in oral cancer (Stage I) from smokers and drinkers. Rev. Iberoam. Micol. 1997, 14, 101–103. [Google Scholar] [PubMed]
- Rosa, D.D.; Pasqualotto, A.C.; Denning, D.W. Chronic mucocutaneous candidiasis and oesophageal cancer. Med Mycol. 2008, 46, 85–91. [Google Scholar] [CrossRef]
- Astekar, M.; Roy, S.K.; Sapra, G.; Chitlangia, R.K.; Raj, N. Evaluation of candidal species among individuals with oral potentially malignant disorders and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019, 23, 302. [Google Scholar] [CrossRef]
- Walsh, T.; Liu, J.L.; Brocklehurst, P.; Glenny, A.-M.; Lingen, M.; Kerr, A.R.; Ogden, G.; Warnakulasuriya, S.; Scully, C. Clinical assessment to screen for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Carlaio, R.G.; Bottalico, L. Does it make sense that diabetes is reciprocally associated with periodontal disease? Endocr. Metab. Immune Disord. Drug Targets 2010, 10, 57–70. [Google Scholar] [CrossRef]
- Krogh, P.; Hald, B.; Holmstrup, P. Possible mycological etiology of oral mucosal cancer: Catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis 1987, 8, 1543–1548. [Google Scholar] [CrossRef]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Nguyen, K.C.D.; Cantore, S.; Bottalico, L.; Quagliuolo, L.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; et al. Rebalancing the Oral Microbiota as an Efficient Tool in Endocrine, Metabolic and Immune Disorders. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 777–784. [Google Scholar] [CrossRef]
- Gayathri, K.; Balachander, N.; Malathi, L.; Sankari, S.L. Candida in potentially malignant oral disorders. J. Pharm. Bioallied Sci. 2015, 7, 164–174. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Ramdzan, A.N.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Kolev, S.D.; Reynolds, E.C.; McCullough, M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral. Dis. 2016, 22, 805–814. [Google Scholar] [CrossRef]
- Bakri, M.M.; Hussaini, H.M.; Holmes, A.; Cannon, R.; Rich, A.M. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J. Oral Microbiol. 2010, 2, 2. [Google Scholar] [CrossRef]
- Sitheeque, M.; Samaranayake, L. Chronic Hyperplastic Candidosis/Candidiasis (Candidal Leukoplakia). Crit. Rev. Oral Biol. Med. 2003, 14, 253–267. [Google Scholar] [CrossRef]
- Muzio, L.L.; Bucci, P.; Canfora, M.; Pannone, G.; Santacroce, L.; Bucci, T.; Staibano, S. [Physiopathology of beta and gamma catenin expression in the oral epithelium]. Minerva Stomatol. 1998, 47, 583–588. [Google Scholar]
- Santacroce, L.; Monea, A.; Marrelli, M.; Man, A. Oral Candidiasis and Inflammatory Response: A Potential Synergic Contribution to the Onset of Type-2 Diabetes Mellitus. Australas. Med. J. 2017, 10, 550–556. [Google Scholar] [CrossRef]
- Meurman, J.H.; Uittamo, J. Oral micro-organisms in the etiology of cancer. Acta Odontol. Scand. 2008, 66, 321–326. [Google Scholar] [CrossRef]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma overview. Oral Oncol. 2009, 45, 301–308. [Google Scholar] [CrossRef]
- Marttila, E.; Uittamo, J.; Rusanen, P.; Lindqvist, C.; Salaspuro, M.; Rautemaa, R. Acetaldehyde production and microbial colonization in oral squamous cell carcinoma and oral lichenoid disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 61–68. [Google Scholar] [CrossRef]
- Lovreglio, P.; Bukvic, N.; Fustinoni, S.; Ballini, A.; Drago, I.; Foà, V.; Guanti, G.; Soleo, L. Lack of genotoxic effect in workers exposed to very low doses of 1,3-butadiene. Arch. Toxicol. 2006, 80, 378–381. [Google Scholar] [CrossRef]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral. Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- O’Grady, I.; Anderson, A.; O’Sullivan, J. The interplay of the oral microbiome and alcohol consumption in oral squamous cell carcinomas. Oral Oncol. 2020, 110, 105011. [Google Scholar] [CrossRef]
- Phan, Q.T.; Belanger, P.H.; Filler, S.G. Role of Hyphal Formation in Interactions of Candida albicans with Endothelial Cells. Infect. Immun. 2000, 68, 3426–3430. [Google Scholar] [CrossRef]
- Gupta, B.; Johnson, N.W. Systematic Review and Meta-Analysis of Association of Smokeless Tobacco and of Betel Quid without Tobacco with Incidence of Oral Cancer in South Asia and the Pacific. PLoS ONE 2014, 9, e113385. [Google Scholar] [CrossRef]
- Moyes, D.; Wilson, D.; Richardson, J.P.; Mogavero, S.; Tang, S.X.; Wernecke, J.; Höfs, S.; Gratacap, R.L.; Robbins, J.; Runglall, M.; et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nat. Cell Biol. 2016, 532, 64–68. [Google Scholar] [CrossRef]
- Satiman, E.A.F.E.N.; Ahmad, H.; Ramzi, A.B.; Wahab, R.A.; Kaderi, M.A.; Harun, W.H.A.W.; Dashper, S.; McCullough, M.; Arzmi, M.H. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef]

| Oral Microbiome and Associated Diseases | |
|---|---|
| Oral | Systemic |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. https://doi.org/10.3390/jof7060476
Di Cosola M, Cazzolla AP, Charitos IA, Ballini A, Inchingolo F, Santacroce L. Candida albicans and Oral Carcinogenesis. A Brief Review. Journal of Fungi. 2021; 7(6):476. https://doi.org/10.3390/jof7060476
Chicago/Turabian StyleDi Cosola, Michele, Angela Pia Cazzolla, Ioannis Alexandros Charitos, Andrea Ballini, Francesco Inchingolo, and Luigi Santacroce. 2021. "Candida albicans and Oral Carcinogenesis. A Brief Review" Journal of Fungi 7, no. 6: 476. https://doi.org/10.3390/jof7060476
APA StyleDi Cosola, M., Cazzolla, A. P., Charitos, I. A., Ballini, A., Inchingolo, F., & Santacroce, L. (2021). Candida albicans and Oral Carcinogenesis. A Brief Review. Journal of Fungi, 7(6), 476. https://doi.org/10.3390/jof7060476









