Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Candida Tropicalis Isolates
2.2. Antifungal Susceptibility Testing and Trailing
2.3. Multilocus Sequence Typing
2.4. ERG11 and UPC2 Sequencing
2.5. Quantitative RT-PCR
2.6. Statistical Analysis
3. Results
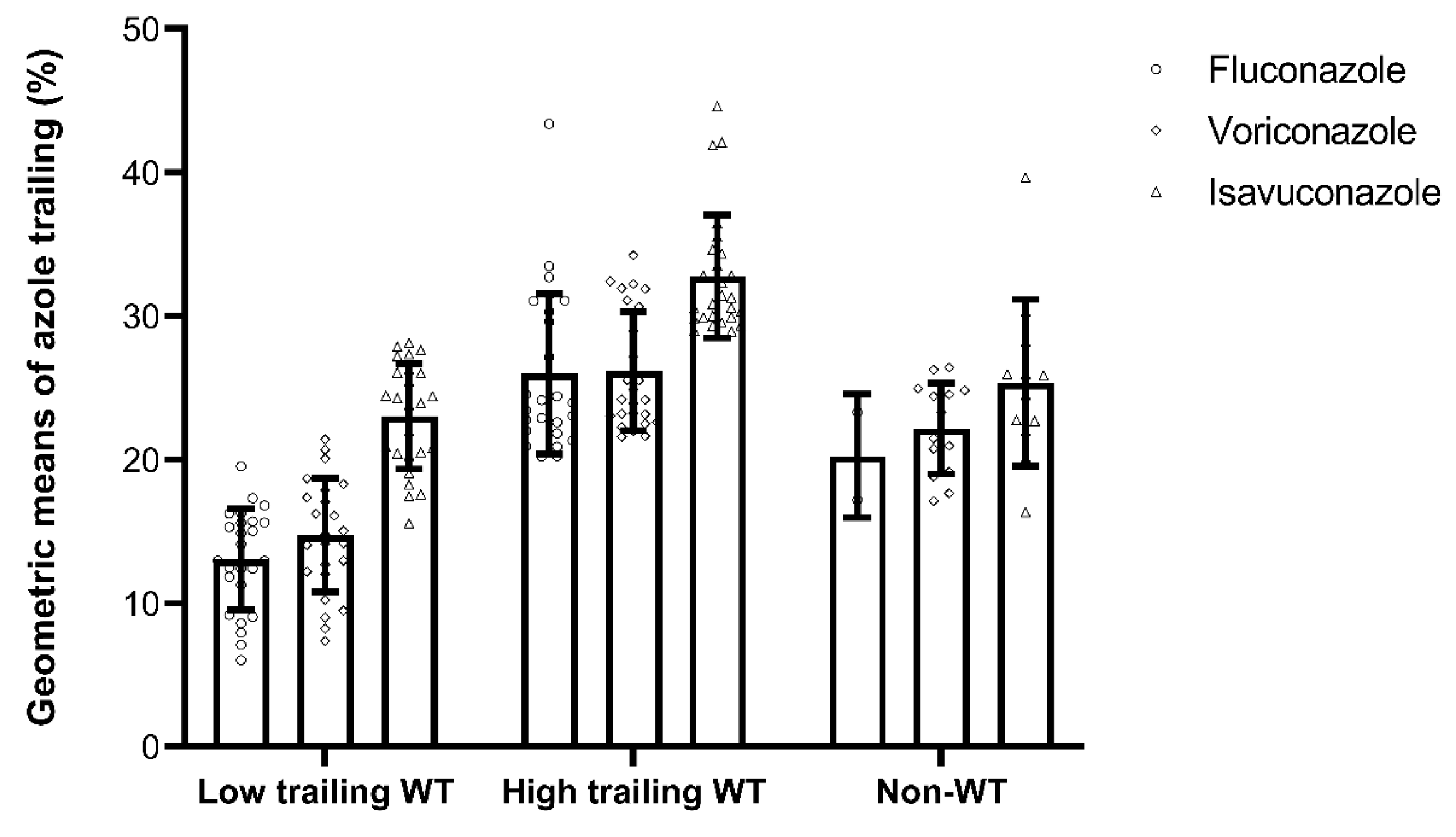
3.1. Azole Minimum Inhibitory Concentrations, Trailing, and Corresponding Genotypes
3.2. ERG11 and UPC2 Sequencing
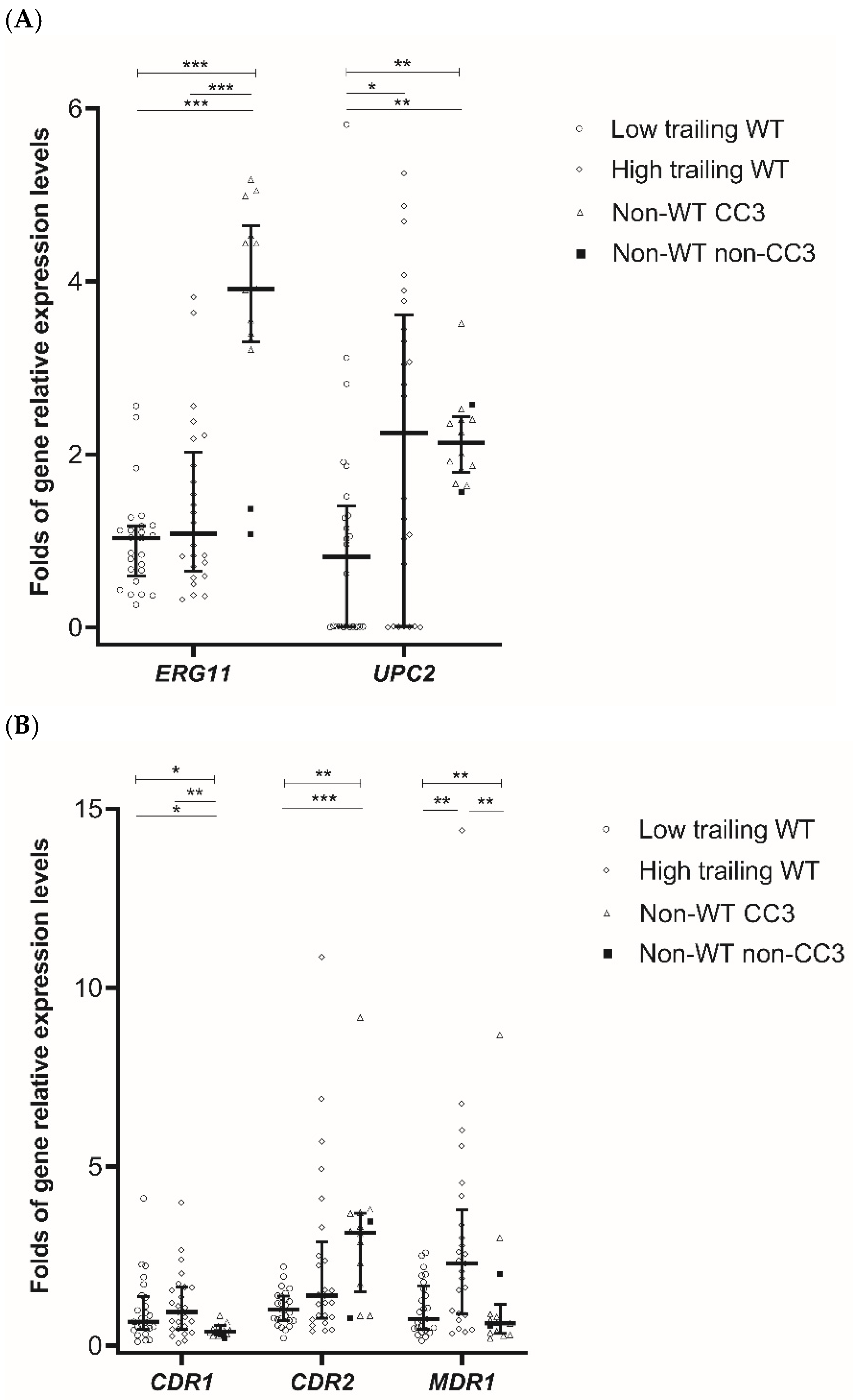
3.3. Expression of ERG11, UPC2, and Genes Encoding Efflux Pumps
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Tan, B.H.; Chakrabarti, A.; Li, R.Y.; Patel, A.K.; Watcharananan, S.P.; Liu, Z.; Chindamporn, A.; Tan, A.L.; Sun, P.L.; Wu, U.I.; et al. Incidence and species distribution of candidaemia in Asia: A laboratory-based surveillance study. Clin. Microbiol. Infect. 2015, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of candidemia in Latin America: A laboratory-based survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef]
- Tan, T.Y.; Hsu, L.Y.; Alejandria, M.M.; Chaiwarith, R.; Chinniah, T.; Chayakulkeeree, M.; Choudhury, S.; Chen, Y.H.; Shin, J.H.; Kiratisin, P.; et al. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med. Mycol. 2016, 54, 471–477. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Liu, J.Y.; Li, W.J.; Zhao, Y.; Xiang, M.J. Multilocus sequence typing of Candida tropicalis shows clonal cluster enrichment in azole-resistant isolates from patients in Shanghai, China. Infect. Genet. Evol. 2016, 44, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Xisto, M.I.; Caramalho, R.D.; Rocha, D.A.; Ferreira-Pereira, A.; Sartori, B.; Barreto-Bergter, E.; Junqueira, M.L.; Lass-Florl, C.; Lackner, M. Pan-azole-resistant Candida tropicalis carrying homozygous erg11 mutations at position K143R: A new emerging superbug? J. Antimicrob. Chemother. 2017, 72, 988–992. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chuang, Y.C.; Wu, U.I.; Sun, H.Y.; Wang, J.T.; Sheng, W.H.; Lo, H.J.; Wang, H.Y.; Chen, Y.C.; Chang, S.C. Clonality of fluconazole-nonsusceptible Candida tropicalis in bloodstream infections, Taiwan, 2011–2017. Emerg. Infect. Dis. 2019, 25, 1660–1667. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hafez, A.; Khodavaisy, S.; Najafzadeh, M.J.; Charsizadeh, A.; Zarrinfar, H.; Salehi, M.; Shahrabadi, Z.Z.; Sasani, E.; et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med. Mycol. 2020, 58, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Hilmioglu-Polat, S.; Daneshnia, F.; Hafez, A.; Salehi, M.; Polat, F.; Yasar, M.; Arslan, N.; Hosbul, T.; Unal, N.; et al. Recent increase in the prevalence of fluconazole-non-susceptible Candida tropicalis blood isolates in Turkey: Clinical implication of azole-non-susceptible and fluconazole tolerant phenotypes and genotyping. Front. Microbiol. 2020, 11, 587278. [Google Scholar] [CrossRef]
- Tulyaprawat, O.; Pharkjaksu, S.; Chongtrakool, P.; Ngamskulrungroj, P. An Association of an eBURST group with triazole resistance of Candida tropicalis blood isolates. Front. Microbiol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, D.; Tang, K.; Guo, J.; Huang, Y.; Li, C. Multilocus Sequence Typing Reveals Clonality of Fluconazole-Nonsusceptible Candida tropicalis: A study from Wuhan to the global. Front. Microbiol. 2020, 11, 554249. [Google Scholar] [CrossRef]
- Jiang, C.; Dong, D.; Yu, B.; Cai, G.; Wang, X.; Ji, Y.; Peng, Y. Mechanisms of azole resistance in 52 clinical isolates of Candida tropicalis in China. J. Antimicrob. Chemother. 2013, 68, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cao, Z.; Wang, Q.; Wang, Y.; Wang, X.; Chen, H.; Wang, H. MDR1 overexpression combined with ERG11 mutations induce high-level fluconazole resistance in Candida tropicalis clinical isolates. BMC Infect. Dis. 2018, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiao, M.; Zhang, D.; Huang, J.J.; Wang, H.; Hou, X.; Zhang, L.; Kong, F.; Chen, S.C.; Tong, Z.H.; et al. Molecular mechanisms of azole resistance in Candida tropicalis isolates causing invasive candidiasis in China. Clin. Microbiol. Infect. 2019, 25, 885–891. [Google Scholar] [CrossRef]
- Teo, J.Q.; Lee, S.J.; Tan, A.L.; Lim, R.S.; Cai, Y.; Lim, T.P.; Kwa, A.L. Molecular mechanisms of azole resistance in Candida bloodstream isolates. BMC Infect. Dis. 2019, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Won, E.J.; Shin, J.H.; Kim, S.H.; Lee, W.G.; Kim, M.N.; Lee, K.; Shin, M.G.; Suh, S.P.; Ryang, D.W.; et al. Resistance mechanisms and clinical features of fluconazole-nonsusceptible Candida tropicalis isolates compared with fluconazole-less-susceptible isolates. Antimicrob. Agents. Chemother. 2016, 60, 3653–3661. [Google Scholar] [CrossRef]
- Munoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Sanchez-Carrillo, C.; Bouza, E.; Guinea, J. Scope and frequency of fluconazole trailing assessed using EUCAST in invasive Candida spp. isolates. Med. Mycol. 2016, 54, 733–739. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Gomez, A.; Sanchez-Carrillo, C.; Bouza, E.; Munoz, P.; Escribano, P.; Guinea, J. Isavuconazole is highly active in vitro against Candida species isolates but shows trailing effect. Clin. Microbiol. Infect. 2018, 24, 1343.e1–1343.e4. [Google Scholar] [CrossRef] [PubMed]
- Astvad, K.M.T.; Sanglard, D.; Delarze, E.; Hare, R.K.; Arendrup, M.C. Implications of the EUCAST trailing phenomenon in Candida tropicalis for the in vivo susceptibility in invertebrate and murine models. Antimicrob. Agents Chemother. 2018, 62, e01624-18. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Subcommittee on antifungal susceptibility testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). In EUCAST Technical Note on Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeast—EUCAST Definitive document E.DEF 7.3.1; EUCAST: Växjö, Sweden, 2017. [Google Scholar]
- Marr, K.A.; Rustad, T.R.; Rex, J.H.; White, T.C. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 1999, 43, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Williams, L.E.; Warnock, D.W.; Arthington-Skaggs, B.A. Drug resistance genes and trailing growth in Candida albicans isolates. J. Antimicrob. Chemother. 2004, 53, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, K.M.; Astvad, K.M.T.; Hare, R.K.; Arendrup, M.C. EUCAST susceptibility testing of isavuconazole: MIC data for contemporary clinical mold and yeast isolates. Antimicrob. Agents Chemother. 2019, 63, e00073-19. [Google Scholar] [CrossRef]
- Francisco, A.P.; Bugalho, M.; Ramirez, M.; Carrico, J.A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinform. 2009, 10, 152. [Google Scholar] [CrossRef]
- Vandeputte, P.; Larcher, G.; Berges, T.; Renier, G.; Chabasse, D.; Bouchara, J.P. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob. Agents Chemother. 2005, 49, 4608–4615. [Google Scholar] [CrossRef]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef]
- Jiang, C.; Ni, Q.; Dong, D.; Zhang, L.; Li, Z.; Tian, Y.; Peng, Y. The Role of UPC2 Gene in Azole-Resistant Candida tropicalis. Mycopathologia 2016, 181, 833–838. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Bretagne, S.; Boullie, A.; Gautier, C.; Dromer, F.; Lortholary, O.; French Mycoses Study Group. Isavuconazole MIC distribution of 29 yeast species responsible for invasive infections (2015–2017). Clin. Microbiol. Infect. 2019, 25, 634. [Google Scholar] [CrossRef]
- Chindamporn, A.; Chakrabarti, A.; Li, R.; Sun, P.L.; Tan, B.H.; Chua, M.; Wahyuningsih, R.; Patel, A.; Liu, Z.; Chen, Y.C.; et al. Survey of laboratory practices for diagnosis of fungal infection in seven Asian countries: An Asia Fungal Working Group (AFWG) initiative. Med. Mycol. 2018, 56, 416–425. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ene, I.V.; Bibi, M.; Zakin, S.; Segal, E.S.; Ziv, N.; Dahan, A.M.; Colombo, A.L.; Bennett, R.J.; Berman, J. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat. Commun. 2018, 9, 2470. [Google Scholar] [CrossRef]
- Chou, H.H.; Lo, H.J.; Chen, K.W.; Liao, M.H.; Li, S.Y. Multilocus sequence typing of Candida tropicalis shows clonal cluster enriched in isolates with resistance or trailing growth of fluconazole. Diagn. Microbiol. Infect. Dis. 2007, 58, 427–433. [Google Scholar] [CrossRef]
- Sanglard, D.; Coste, A.T. Activity of isavuconazole and other azoles against Candida clinical isolates and yeast model systems with known azole resistance mechanisms. Antimicrob. Agents Chemother. 2016, 60, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Aigner, M.; Risslegger, B.; Hortnagl, C.; Lass-Florl, C.; Lackner, M. Minimal Inhibitory Concentration (MIC)-phenomena in Candida albicans and their impact on the diagnosis of antifungal resistance. J. Fungi 2019, 5, 83. [Google Scholar] [CrossRef]
- Levinson, T.; Dahan, A.; Novikov, A.; Paran, Y.; Berman, J.; Ben-Ami, R. Impact of tolerance to fluconazole on treatment response in Candida albicans bloodstream infection. Mycoses 2021, 64, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.; Puig-Asensio, M.; Guinea, J.; Almirante, B.; Cuenca-Estrella, M.; Zaragoza, O. Evaluation of the possible influence of trailing and paradoxical effects on the clinical outcome of patients with candidemia—For the members of the CANDIPOP project from GEIH-GEMICOMED and Reipi. Clin. Microbiol. Infect. 2017, 23, 49.E1–49.E8. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sobel, J.D.; White, T.C. A Combination fluorescence assay demonstrates increased efflux pump activity as a resistance mechanism in azole-resistant vaginal Candida albicans isolates. Antimicrob. Agents Chemother. 2016, 60, 5858–5866. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Lass-Florl, C.; Garcia-Rubio, R.; Daneshnia, F.; Ilkit, M.; Boekhout, T.; Gabaldon, T.; Perlin, D.S. The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. J. Fungi 2020, 6, 138. [Google Scholar] [CrossRef]
| Fluconazole (FLC) | Voriconazole (VRC) | Isavuconazole (ISA) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Overall | FLC vs. VRC | FLC vs. ISA | VRC vs. ISA | ||||
| MIC Range a,b | ≤0.125–>64 | ≤0.015–4 | ≤0.002–0.125 | ||||
| MIC50/MIC90 | 0.5/>64 | 0.06/4 | 0.008/0.125 | ||||
| GM MIC | 1.163 | 0.090 | 0.008 | <0.001 | <0.001 | <0.001 | <0.001 |
| % of Non-WT c Isolates(No. of Isolates) | 21.9 (14) | 21.9 (14) | 18.8 (12) | ||||
| GM (±SD) of Trailing (%) | 19.5 (±7.9) d | 20.8 (±6.4) | 27.4 (±6.3) | <0.001 | >0.99 | <0.001 | <0.001 |
| Fluconazole | Voriconazole | Isavuconazole | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low Trailing WT | High Trailing WT | Non-WT | Low Trailing WT | High Trailing WT | Non-WT | Low Trailing WT | High Trailing WT | Non-WT | |
| Clonal complex b (No. of Isolates) | |||||||||
| 1 (4) | 4 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 |
| 2 (11) | 0 | 11 | 0 | 0 | 11 | 0 | 1 | 10 | 0 |
| 3 (14) | 2 | 0 | 12 | 0 | 2 | 12 | 0 | 2 | 12 |
| 4 (10) | 5 | 5 | 0 | 6 | 4 | 0 | 4 | 6 | 0 |
| 6 (2) | 1 | 1 | 0 | 2 | 0 | 0 | 1 | 1 | 0 |
| 8 (2) | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 |
| 9 (2) | 2 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| 11 (1) | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| 20 (2) | 2 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 |
| 22 (3) | 2 | 1 | 0 | 2 | 1 | 0 | 1 | 2 | 0 |
| 28 (1) | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 35 (1) | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 41 (1) | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
| 49 (1) | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| 63 (1) | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Singletons (8) | 4 | 4 | 0 | 5 | 3 | 0 | 6 | 2 | 0 |
| Total (64) | 25 | 25 | 14 | 25 | 25 | 14 | 26 | 26 | 12 |
| MIC c | |||||||||
| GM | 0.330 | 0.379 | >64 | 0.269 | 0.418 | 3.123 | 0.004 | 0.006 | 0.104 |
| MIC50 | 0.25 | 0.5 | >64 | 0.03 | 0.06 | 4 | 0.006 | 0.008 | 0.125 |
| MIC90 | 0.5 | 0.5 | >64 | 0.06 | 0.06 | 4 | 0.015 | 0.015 | 0.125 |
| Range | ≤0.125–0.5 | ≤0.125–0.5 | 8– >64 | ≤0.015–0.06 | ≤0.015–0.06 | 0.5–4 | ≤0.002–0.015 | ≤0.002–0.015 | 0.06–0.125 |
| ERG11 Gene a | UPC2 Gene a | Clonal Complex (No. of Isolates) | MIC Range (mg/L) d | ||||
|---|---|---|---|---|---|---|---|
| Nucleotide Position | 395 | 461 | 503 | FLC | VRC | ISA | |
| A | C | T | Reference b | ||||
| W | Y | Y | CC3 c (11) | >64 | 4 | 0.06–0.125 | |
| W | Y | C | CC3 c (1) | 64 | 4 | 0.125 | |
| T | T | T | CC41 c (1) | 16 | 1 | 0.015 | |
| A | T | T | CC11 c (1) | 8 | 0.5 | 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Chuang, Y.-C.; Wu, U.-I.; Sun, H.-Y.; Wang, J.-T.; Sheng, W.-H.; Chen, Y.-C.; Chang, S.-C. Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates. J. Fungi 2021, 7, 612. https://doi.org/10.3390/jof7080612
Chen P-Y, Chuang Y-C, Wu U-I, Sun H-Y, Wang J-T, Sheng W-H, Chen Y-C, Chang S-C. Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates. Journal of Fungi. 2021; 7(8):612. https://doi.org/10.3390/jof7080612
Chicago/Turabian StyleChen, Pao-Yu, Yu-Chung Chuang, Un-In Wu, Hsin-Yun Sun, Jann-Tay Wang, Wang-Huei Sheng, Yee-Chun Chen, and Shan-Chwen Chang. 2021. "Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates" Journal of Fungi 7, no. 8: 612. https://doi.org/10.3390/jof7080612
APA StyleChen, P.-Y., Chuang, Y.-C., Wu, U.-I., Sun, H.-Y., Wang, J.-T., Sheng, W.-H., Chen, Y.-C., & Chang, S.-C. (2021). Mechanisms of Azole Resistance and Trailing in Candida tropicalis Bloodstream Isolates. Journal of Fungi, 7(8), 612. https://doi.org/10.3390/jof7080612






