Genome Editing Technology and Its Application Potentials in the Industrial Filamentous Fungus Aspergillus oryzae
Abstract
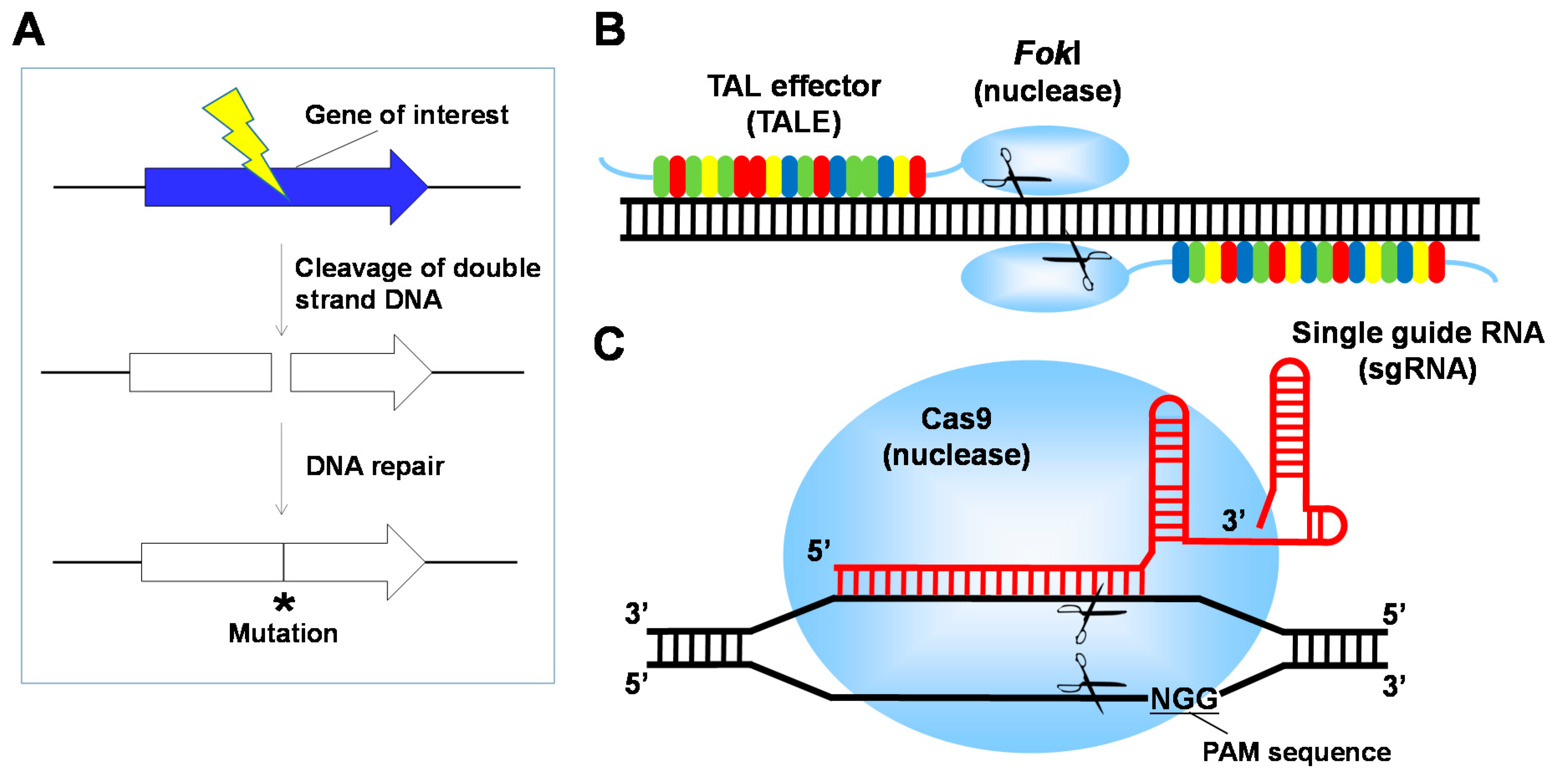
:1. Introduction
2. Genome Editing Using TALENs in A. oryzae
3. Genome Editing Using the CRISPR/Cas9 System in A. oryzae
4. Efficient Multiple-Gene Modification by Recycling the Genome-Editing Plasmid in A. oryzae
5. Efficient Gene Modification Using Genome Editing for Heterologous Production by A. oryzae
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitamoto, K. Molecular biology of the Koji molds. Adv. Appl. Microbiol. 2002, 51, 129–153. [Google Scholar] [PubMed]
- Fleissner, A.; Dersch, P. Expression and export: Recombinant protein production systems for Aspergillus. Appl. Microbiol. Biotechnol. 2010, 87, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, H. Reconstitution of biosynthetic machinery of fungal natural products in heterologous hosts. Biosci. Biotechnol. Biochem. 2020, 84, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.I.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, F.J.; Maruyama, J.; Juvvadi, P.R.; Arioka, M.; Kitamoto, K. Development of a novel quadruple auxotrophic host transformation system by argB gene disruption using adeA gene and exploiting adenine auxotrophy in Aspergillus oryzae. FEMS Microbiol. Lett. 2004, 239, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Masuda, T.; Koyama, Y. Enhanced gene targeting frequency in ku70 and ku80 disruption mutants of Aspergillus sojae and Aspergillus oryzae. Mol. Genet. Genom. 2006, 275, 460–470. [Google Scholar] [CrossRef]
- Mizutani, O.; Kudo, Y.; Saito, A.; Matsuura, T.; Inoue, H.; Abe, K.; Gomi, K. A defect of LigD (human Lig4 homolog) for nonhomologous end joining significantly improves efficiency of gene-targeting in Aspergillus oryzae. Fungal Genet. Biol. 2008, 45, 878–889. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Tada, S.; Gomi, K.; Kitamoto, K.; Kumagai, C.; Jigami, Y.; Tamura, G. High level expression of the synthetic human lysozyme gene in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 1992, 38, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Gomi, K.; Kitamoto, K.; Kumagai, C.; Tamura, G. Secretion of calf chymosin from the filamentous fungus Aspergillus oryzae. Appl. Microbiol. Biotechnol. 1993, 40, 327–332. [Google Scholar] [CrossRef]
- Nakajima, K.; Asakura, T.; Maruyama, J.; Morita, Y.; Oike, H.; Shimizu-Ibuka, A.; Misaka, T.; Sorimachi, H.; Arai, S.; Kitamoto, K.; et al. Extracellular production of neoculin, a sweet-tasting heterodimeric protein with taste-modifying activity, by Aspergillus oryzae. Appl. Environ. Microbiol. 2006, 72, 3716–3723. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Asakura, T.; Morita, Y.; Nakajima, K.; Koizumi, A.; Shimizu-Ibuka, A.; Masuda, K.; Ishiguro, M.; Terada, T.; Maruyama, J.; et al. Microbial production of sensory-active miraculin. Biochem. Biophys. Res. Commun. 2007, 360, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.A.; Ishida, N.; Todaka, N.; Nakamura, R.; Maruyama, J. Takahashi, H.; Kitamoto, K. Promotion of efficient saccharification of crystalline cellulose by Aspergillus fumigatus Swo1. Appl. Environ. Microbiol. 2010, 76, 2556–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Aishan, T.; Maruyama, J.; Kitamoto, K. Enhanced production and secretion of heterologous proteins by the filamentous fungus Aspergillus oryzae via disruption of vacuolar protein sorting receptor gene Aovps10. Appl. Environ. Microbiol. 2010, 76, 5718–5727. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Kikuma, T.; Maruyama, J.; Kitamoto, K. Enhanced production of bovine chymosin by autophagy deficiency in the filamentous fungus Aspergillus oryzae. PLoS ONE 2013, 8, e62512. [Google Scholar] [CrossRef] [Green Version]
- Hoang, H.D.; Maruyama, J.; Kitamoto, K. Modulating endoplasmic reticulum-Golgi cargo receptors for improving secretion of carrier-fused heterologous proteins in the filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2015, 81, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Kimura, S.; Maruyama, J.; Kitamoto, K. Construction of quintuple protease gene disruptant for heterologous protein production in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2009, 82, 691–701. [Google Scholar] [CrossRef]
- Yoon, J.; Maruyama, J.; Kitamoto, K. Disruption of ten protease genes in the filamentous fungus Aspergillus oryzae highly improves production of heterologous proteins. Appl. Microbiol. Biotechnol. 2011, 89, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, J.; Kitamoto, K. Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (DeltaligD) in Aspergillus oryzae. Biotechnol. Lett. 2008, 30, 1811–1817. [Google Scholar] [CrossRef]
- Maruyama, J.; Kitamoto, K. Targeted gene disruption in koji mold Aspergillus oryzae. Methods Mol. Biol. 2011, 765, 447–456. [Google Scholar] [PubMed]
- Perez-Pinera, P.; Ousterout, D.G.; Gersbach, C.A. Advances in targeted genome editing. Curr. Opin. Chem. Biol. 2012, 16, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Gaj, T.; Sirk, S.J.; Shui, S.-L.; Liu, J. Genome-editing technologies: Principles and applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754. [Google Scholar] [CrossRef] [Green Version]
- Perisse, I.V.; Fan, Z.; Singina, G.N.; White, K.L.; Polejaeva, I.A. Improvements in gene editing technology boost its applications in livestock. Front. Genet. 2021, 11, 614688. [Google Scholar] [CrossRef]
- Guo, J.; Gaj, T.; Barbas, C.F., 3rd. Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J. Mol. Biol. 2010, 400, 96–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, M.; Cermak, T.; Doyle, E.L.; Schmidt, C.; Zhang, F.; Hummel, A.; Bogdanove, A.J.; Voytas, D.F. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 2010, 186, 757–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizutani, O.; Arazoe, T.; Toshida, K.; Hayashi, R.; Ohsato, S.; Sakuma, T.; Yamamoto, T.; Kuwata, S.; Yamada, O. Detailed analysis of targeted gene mutations caused by the Platinum-Fungal TALENs in Aspergillus oryzae RIB40 strain and a ligD disruptant. J. Biosci. Bioeng. 2017, 123, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.F.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Katayama, T.; Tanaka, Y.; Okabe, T.; Nakamura, H.; Fujii, W.; Kitamoto, K.; Maruyama, J. Development of a genome editing technique using the CRISPR/Cas9 system in the industrial filamentous fungus Aspergillus oryzae. Biotechnol. Lett. 2016, 38, 637–642. [Google Scholar] [CrossRef]
- Nødvig, C.S.; Nielsen, J.B.; Kogle, M.E.; Mortensen, U.H. A CRISPR-Cas9 system for engineering of filamentous fungi. PLoS ONE 2015, 10, e0133085. [Google Scholar]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Chutrakul, C.; Panchanawaporn, S.; Jeennor, S.; Anantayanon, J.; Vorapreeda, T.; Vichai, V.; Laoteng, K. Functional characterization of novel U6 RNA polymerase III promoters: Their omplication for CRISPR-Cas9-mediated gene editing in Aspergillus oryzae. Curr. Microbiol. 2019, 76, 1443–1451. [Google Scholar] [CrossRef]
- Song, L.; Ouedraogo, J.P.; Kolbusz, M.; Nguyen, T.T.M.; Tsang, A. Efficient genome editing using tRNA promoter-driven CRISPR/Cas9 gRNA in Aspergillus niger. PLoS ONE 2018, 13, e0202868. [Google Scholar]
- Katayama, T.; Nakamura, H.; Zhang, Y.; Pascal, A.; Fujii, W.; Maruyama, J. Forced recycling of an AMA1-based genome-editing plasmid allows for efficient multiple gene deletion/integration in the industrial filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2019, 85, e01896-18. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Cobine, P.A.; Coleman, J.J. Efficient genome editing in Fusarium oxysporum based on CRISPR/Cas9 ribonucleoprotein complexes. Fungal Genet. Biol. 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Todokoro, T.; Bando, H.; Kotaka, A.; Tsutsumi, H.; Hata, Y.; Ishida, H. Identification of a novel pyrithiamine resistance marker gene thiI for genome co-editing in Aspergillus oryzae. J. Biosci. Bioeng. 2020, 130, 227–232. [Google Scholar] [CrossRef]
- Nagamine, S.; Liu, C.; Nishishita, J.; Kozaki, T.; Sogahata, K.; Sato, Y.; Minami, A.; Ozaki, T.; Schmidt-Dannert, C.; Maruyama, J.; et al. Ascomycete Aspergillus oryzae is an efficient expression host for production of basidiomycete terpenes by using genomic DNA sequences. Appl. Environ. Microbiol. 2019, 85, e00409-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Minami, A.; Ozaki, T.; Wu, J.; Kawagishi, H.; Maruyama, J.; Oikawa, H. Efficient reconstitution of basidiomycota diterpene erinacine gene cluster in ascomycota host Aspergillus oryzae based on genomic DNA sequences. J. Am. Chem. Soc. 2019, 141, 15519–15523. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, Z.; Long, C.; He, B.; Hu, Z.; Jiang, C.; Zeng, B. Identification and functional characterization of glycerol dehydrogenase reveal the role in kojic acid synthesis in Aspergillus oryzae. World, J. Microbiol. Biotechnol. 2020, 36, 136. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.H.; Morita, N.; Sakamoto, T.; Katayama, T.; Miyakawa, T.; Tanokura, M.; Chiba, Y.; Shinkura, R.; Maruyama, J. Functional production of human antibody by the filamentous fungus Aspergillus oryzae. Fungal Biol. Biotechnol. 2020, 7, 7. [Google Scholar] [CrossRef]
- Nakamura, H.; Katayama, T.; Okabe, T.; Iwashita, K.; Fujii, W.; Kitamoto, K.; Maruyama, J. Highly efficient gene targeting in Aspergillus oryzae industrial strains under ligD mutation introduced by genome editing: Strain-specific differences in the effects of deleting EcdR, the negative regulator of sclerotia formation. J. Gen. Appl. Microbiol. 2017, 63, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Okabe, T.; Katayama, T.; Mo, T.; Mori, N.; Jin, F.J.; Fujii, I.; Iwashita, K.; Kitamoto, K.; Maruyama, J. BiFC-based visualisation system reveals cell fusion morphology and heterokaryon incompatibility in the filamentous fungus Aspergillus oryzae. Sci. Rep. 2018, 8, 2922. [Google Scholar] [CrossRef] [Green Version]
- Food Hygiene Handling Procedures for Foods and Additives Derived from Genome Editing Technology in the Website of Ministry of Health, Labour and Welfare of Japan. Available online: https://www.mhlw.go.jp/content/000550824.pdf (accessed on 23 December 2020).





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, J.-i. Genome Editing Technology and Its Application Potentials in the Industrial Filamentous Fungus Aspergillus oryzae. J. Fungi 2021, 7, 638. https://doi.org/10.3390/jof7080638
Maruyama J-i. Genome Editing Technology and Its Application Potentials in the Industrial Filamentous Fungus Aspergillus oryzae. Journal of Fungi. 2021; 7(8):638. https://doi.org/10.3390/jof7080638
Chicago/Turabian StyleMaruyama, Jun-ichi. 2021. "Genome Editing Technology and Its Application Potentials in the Industrial Filamentous Fungus Aspergillus oryzae" Journal of Fungi 7, no. 8: 638. https://doi.org/10.3390/jof7080638
APA StyleMaruyama, J.-i. (2021). Genome Editing Technology and Its Application Potentials in the Industrial Filamentous Fungus Aspergillus oryzae. Journal of Fungi, 7(8), 638. https://doi.org/10.3390/jof7080638





