Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot
Abstract
:1. Introduction
2. Materials and Methods
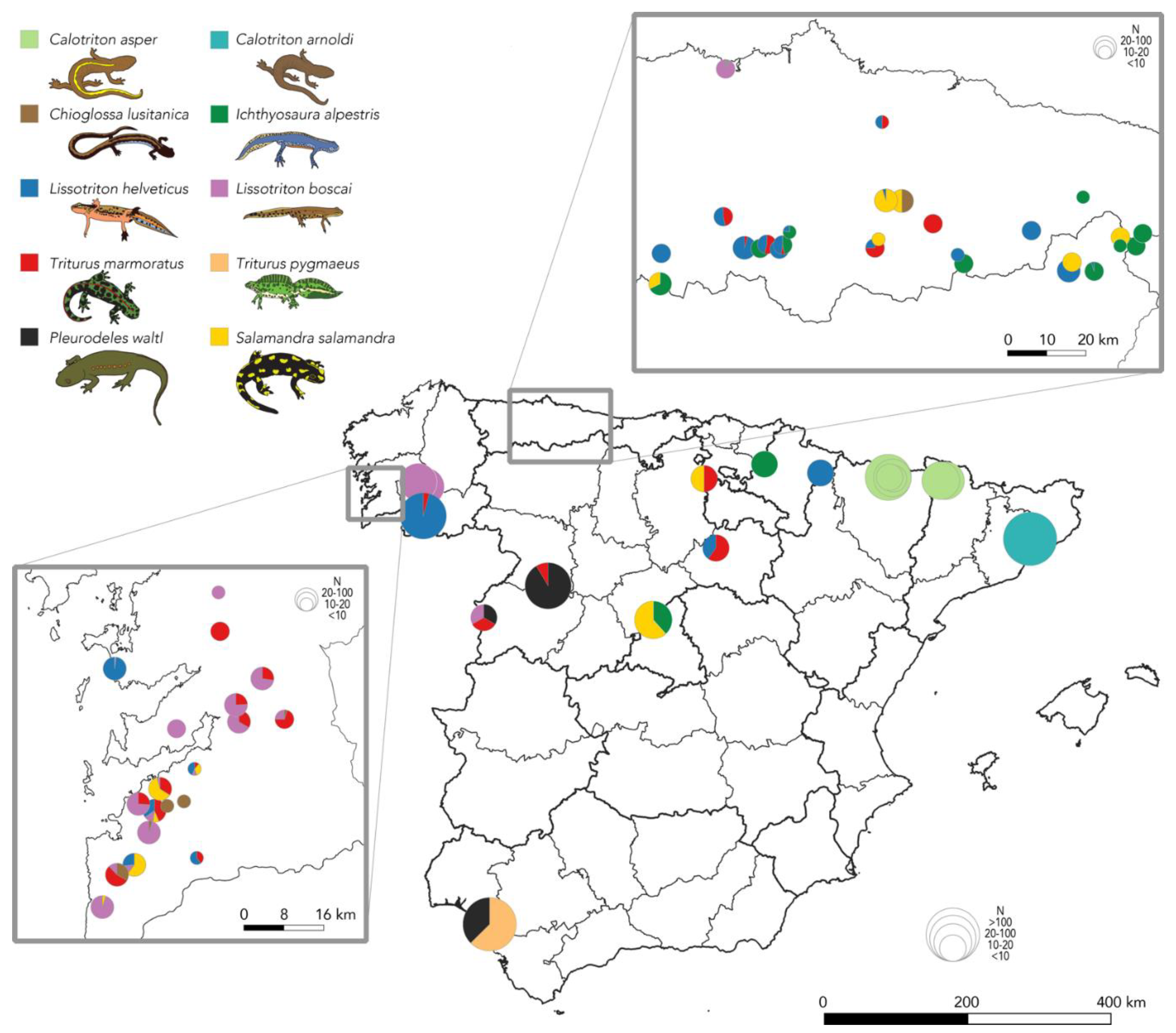
2.1. Distribution of Bsal in the Iberian Peninsula
2.2. Infection Dynamics of Bsal in Iberian Urodeles
2.3. Phylogenetic Influence upon Urodele Susceptibility to Bsal
2.4. Defining Priority Areas and Conservation Units for Iberian Urodeles
3. Results
3.1. Bsal Is Currently Confirmed from a Single Outbreak Site on the Iberian Peninsula
3.2. Infection and Disease Dynamics of Bsal in Iberian Urodeles
3.3. Inferring Bsal Susceptibility within and between Species
3.4. Priority Areas and Lineages for Conserving Iberian Urodele Diversity
4. Discussion
4.1. Distribution of Bsal in the Iberian Peninsula
4.2. Susceptibility of Iberian Urodeles
4.3. Conservation Units for Iberian Urodeles
4.4. Conservation Actions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats Posed by the Fungal Kingdom to Humans, Wildlife, and Agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Bosch, J.; Martínez-Solano, I.; García-París, M. Evidence of a Chytrid Fungus Infection Involved in the Decline of the Common Midwife Toad (Alytes obstetricans) in Protected Areas of Central Spain. Biol. Conserv. 2001, 97, 331–337. [Google Scholar] [CrossRef]
- Rosa, G.M.; Anza, I.; Moreira, P.L.; Conde, J.; Martins, F.; Fisher, M.C.; Bosch, J. Evidence of Chytrid-Mediated Population Declines in Common Midwife Toad in Serra Da Estrela, Portugal. Anim. Conserv. 2013, 16, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Doddington, B.J.; Bosch, J.; Oliver, J.A.; Grassly, N.C.; Garcia, G.; Schmidt, B.R.; Garner, T.W.J.; Fisher, M.C. Context-Dependent Amphibian Host Population Response to an Invading Pathogen. Ecology 2013, 94, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian Origin of Chytrid Fungi Causing Global Amphibian Declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Byrne, A.Q.; Vredenburg, V.T.; Martel, A.; Pasmans, F.; Bell, R.C.; Blackburn, D.C.; Bletz, M.C.; Bosch, J.; Briggs, C.J.; Brown, R.M.; et al. Cryptic Diversity of a Widespread Global Pathogen Reveals Expanded Threats to Amphibian Conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans Sp Nov Causes Lethal Chytridiomycosis in Amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzen-van der Sluijs, A.; Martel, A.; Asselberghs, J.; Bales, E.K.; Beukema, W.; Bletz, M.C.; Dalbeck, L.; Goverse, E.; Kerres, A.; Kinet, T.; et al. Expanding Distribution of Lethal Amphibian Fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 2016, 22, 1286–1288. [Google Scholar] [CrossRef] [Green Version]
- Martel, A.; Blooi, M.; Adriaensen, C.; Van Rooij, P.; Beukema, W.; Fisher, M.C.; Farrer, R.A.; Schmidt, B.R.; Tobler, U.; Goka, K.; et al. Recent Introduction of a Chytrid Fungus Endangers Western Palearctic Salamanders. Science 2014, 346, 630–631. [Google Scholar] [CrossRef] [Green Version]
- Martel, A.; Vila-Escale, M.; Fernandez-Giberteau, D.; Martinez-Silvestre, A.; Canessa, S.; Van Praet, S.; Pannon, P.; Chiers, K.; Ferran, A.; Kelly, M.; et al. Integral Chain Management of Wildlife Diseases. Conserv. Lett. 2020, 13, e12707. [Google Scholar] [CrossRef]
- Lastra González, D.; Baláž, V.; Vojar, J.; Chajma, P. Dual Detection of the Chytrid Fungi Batrachochytrium spp. with an Enhanced Environmental DNA Approach. J. Fungi 2021, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Lastra González, D.; Baláž, V.; Solský, M.; Thumsová, B.; Kolenda, K.; Najbar, A.; Najbar, B.; Kautman, M.; Chajma, P.; Balogová, M.; et al. Recent Findings of Potentially Lethal Salamander Fungus Batrachochytrium salamandrivorans. Emerg. Infect. Dis. 2019, 25, 1416–1418. [Google Scholar] [CrossRef] [Green Version]
- Stegen, G.; Pasmans, F.; Schmidt, B.R.; Rouffaer, L.O.; Van Praet, S.; Schaub, M.; Canessa, S.; Laudelout, A.; Kinet, T.; Adriaensen, C.; et al. Drivers of Salamander Extirpation Mediated by Batrachochytrium salamandrivorans. Nature 2017, 544, 353–356. [Google Scholar] [CrossRef]
- Carter, E.D.; Bletz, M.C.; Le Sage, M.; LaBumbard, B.; Rollins-Smith, L.A.; Woodhams, D.C.; Miller, D.L.; Gray, M.J. Winter Is Coming. Temperature Affects Immune Defenses and Susceptibility to Batrachochytrium salamandrivorans. PLoS Pathog. 2021, 17, e1009234. [Google Scholar] [CrossRef]
- Beukema, W.; Pasmans, F.; Van Praet, S.; Ferri-Yanez, F.; Kelly, M.; Laking, A.E.; Erens, J.; Speybroeck, J.; Verheyen, K.; Lens, L.; et al. Microclimate Limits Thermal Behaviour Favourable to Disease Control in a Nocturnal Amphibian. Ecol. Lett. 2021, 24, 909–910. [Google Scholar] [CrossRef]
- Kumar, R.; Malagon, D.A.; Carter, E.D.; Miller, D.L.; Bohanon, M.L.; Cusaac, J.P.W.; Peterson, A.C.; Gray, M.J. Experimental Methodologies Can Affect Pathogenicity of Batrachochytrium salamandrivorans Infections. PLoS ONE 2020, 15, e0235370. [Google Scholar] [CrossRef]
- Malagon, D.A.; Melara, L.A.; Prosper, O.F.; Lenhart, S.; Carter, E.D.; Fordyce, J.A.; Peterson, A.C.; Miller, D.L.; Gray, M.J. Host Density and Habitat Structure Influence Host Contact Rates and Batrachochytrium salamandrivorans Transmission. Sci. Rep. 2020, 10, 5584. [Google Scholar] [CrossRef] [PubMed]
- Van Rooij, P.; Martel, A.; Haesebrouck, F.; Pasmans, F. Amphibian Chytridiomycosis: A Review with Focus on Fungus-Host Interactions. Vet. Res. 2015, 46, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canessa, S.; Bozzuto, C.; Pasmans, F.; Martel, A. Quantifying the Burden of Managing Wildlife Diseases in Multiple Host Species. Conserv. Biol. 2019, 33, 1131–1140. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.V.; Ziegler, T.; Pasmans, F.; Martel, A. Trade in Wild Anurans Vectors the Urodelan Pathogen Batrachochytrium salamandrivorans into Europe. Amphib. Reptil. 2017, 38, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Martel, A.; Wu, J.; Van Praet, S.; Canessa, S.; Pasmans, F. Widespread Occurrence of an Emerging Fungal Pathogen in Heavily Traded Chinese Urodelan Species. Conserv. Lett. 2018, 11, e12436. [Google Scholar] [CrossRef] [Green Version]
- Beukema, W.; Martel, A.; Nguyen, T.T.; Goka, K.; Schmeller, D.S.; Yuan, Z.; Laking, A.E.; Nguyen, T.Q.; Lin, C.-F.; Shelton, J.; et al. Environmental Context and Differences between Native and Invasive Observed Niches of Batrachochytrium salamandrivorans Affect Invasion Risk Assessments in the Western Palaearctic. Divers. Distrib. 2018, 24, 1788–1801. [Google Scholar] [CrossRef] [Green Version]
- Blooi, M.; Pasmans, F.; Longcore, J.E.; Spitzen-van der Sluijs, A.; Vercammen, F.; Martel, A. Duplex Real-Time PCR for Rapid Simultaneous Detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in Amphibian Samples. J. Clin. Microbiol. 2013, 51, 4173–4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, C.; Forzán, M.; Pessier, A.; Allender, M.; Ballard, J.; Catenazzi, A.; Fenton, H.; Martel, A.; Pasmans, F.; Miller, D.; et al. Amphibian: A Case Definition and Diagnostic Criteria for Batrachochytrium salamandrivorans Chytridiomycosis. Herpet. Rev. 2016, 47, 207. [Google Scholar]
- Revell, L.J. Phytools: An R Package for Phylogenetic Comparative Biology (and Other Things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Jetz, W.; Pyron, R.A. The Interplay of Past Diversification and Evolutionary Isolation with Present Imperilment across the Amphibian Tree of Life. Nat. Ecol. Evol. 2018, 2, 850–858. [Google Scholar] [CrossRef]
- Hijmans, R.J.; van Etten, J. Raster: Geographic Data Analysis and Modeling; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the Geospacial Data Abstraction Library; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Valbuena-Urena, E.; Amat, F.; Carranza, S. Integrative Phylogeography of Calotriton Newts (Amphibia, Salamandridae), with Special Remarks on the Conservation of the Endangered Montseny Brook Newt (Calotriton arnoldi). PLoS ONE 2013, 8, e62542. [Google Scholar] [CrossRef] [Green Version]
- Valbuena-Urena, E.; Soler-Membrives, A.; Steinfartz, S.; Orozco-terWengel, P.; Carranza, S. No Signs of Inbreeding despite Long-Term Isolation and Habitat Fragmentation in the Critically Endangered Montseny Brook Newt (Calotriton arnoldi). Heredity 2017, 118, 424–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucati, F.; Poignet, M.; Miro, A.; Trochet, A.; Aubret, F.; Barthe, L.; Bertrand, R.; Buchaca, T.; Calvez, O.; Caner, J.; et al. Multiple Glacial Refugia and Contemporary Dispersal Shape the Genetic Structure of an Endemic Amphibian from the Pyrenees. Mol. Ecol. 2020, 29, 2904–2921. [Google Scholar] [CrossRef] [PubMed]
- Valbuena-Urena, E.; Oromi, N.; Soler-Membrives, A.; Carranza, S.; Amat, F.; Camarasa, S.; Denoel, M.; Guillaume, O.; Sanuy, D.; Loyau, A.; et al. Jailed in the Mountains: Genetic Diversity and Structure of an Endemic Newt Species across the Pyrenees. PLoS ONE 2018, 13, e0200214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrino, J.; Froufe, E.; Arntzen, J.W.; Ferrand, N. Genetic Subdivision, Glacial Refugia and Postglacial Recolonization in the Golden-Striped Salamander, Chioglossa lusitanica (Amphibia: Urodela). Mol. Ecol. 2000, 9, 771–781. [Google Scholar] [CrossRef]
- Sequeira, F.; Alexandrino, J.; Rocha, S.; Arntzen, J.W.; Ferrand, N. Genetic Exchange across a Hybrid Zone within the Iberian Endemic Golden-Striped Salamander, Chioglossa lusitanica. Mol. Ecol. 2005, 14, 245–254. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Groenenberg, D.S.J.; Alexandrino, J.; Ferrand, N.; Sequeira, F. Geographical Variation in the Golden-Striped Salamander, Chioglossa lusitanica Bocage, 1864 and the Description of a Newly Recognized Subspecies. J. Nat. Hist. 2007, 41, 925–936. [Google Scholar] [CrossRef]
- Recuero, E.; Buckley, D.; García-París, M.; Arntzen, J.W.; Cogalniceanu, D.; Martínez-Solano, I. Evolutionary History of Ichthyosaura alpestris (Caudata, Salamandridae) Inferred from the Combined Analysis of Nuclear and Mitochondrial Markers. Mol. Phylogenet. Evol. 2014, 81, 207–220. [Google Scholar] [CrossRef]
- Palomar, G.; Vörös, J.; Bosch, J. Tracking the Introduction History of Ichthyosaura alpestris in a Protected Area of Central Spain. Conserv. Genet. 2017, 18, 867–876. [Google Scholar] [CrossRef]
- Martínez-Solano, I.; Teixeira, J.; Buckley, D.; García-París, M. Mitochondrial DNA Phylogeography of Lissotriton boscai (Caudata, Salamandridae): Evidence for Old, Multiple Refugia in an Iberian Endemic. Mol. Ecol. 2006, 15, 3375–3388. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J.; Martínez-Solano, I.; Buckley, D.; Tarroso, P.; García-París, M.; Ferrand, N. Genealogy of the Nuclear β-Fibrinogen Intron 7 in Lissotriton boscai (Caudata, Salamandridae): Concordance with MtDNA and Implications for Phylogeography and Speciation. Contrib. Zool. 2015, 84, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, A.; Sequeira, F.; Buckley, D.; Velo-Antón, G. Role of Colonization History and Species-Specific Traits on Contemporary Genetic Variation of Two Salamander Species in a Holocene Island-Mainland System. J. Biogeogr. 2018, 45, 1054–1066. [Google Scholar] [CrossRef]
- Sequeira, F.; Bessa-Silva, A.; Tarroso, P.; Sousa-Neves, T.; Vallinoto, M.; Goncalves, H.; Martínez-Solano, I. Discordant Patterns of Introgression across a Narrow Hybrid Zone between Two Cryptic Lineages of an Iberian Endemic Newt. J. Evol. Biol. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Recuero, E.; García-París, M. Evolutionary History of Lissotriton helveticus: Multilocus Assessment of Ancestral vs. Recent Colonization of the Iberian Peninsula. Mol. Phylogenet. Evol. 2011, 60, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Carranza, S.; Arnold, E.N. History of West Mediterranean Newts, Pleurodeles (Amphibia: Salamandridae), Inferred from Old and Recent DNA Sequences. Systemat. Biodivers. 2004, 1, 327–337. [Google Scholar] [CrossRef] [Green Version]
- van de Vliet, M.S.; Diekmann, O.E.; Machado, M.; Beebee, T.J.C.; Beja, P.; Serrao, E.A. Genetic Divergence for the Amphibian Pleurodeles waltl in Southwest Portugal: Dispersal Barriers Shaping Geographic Patterns. J. Herpetol. 2014, 48, 38–44. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, J.; Barbosa, A.M.; Martínez-Solano, I. Integrative Inference of Population History in the Ibero-Maghrebian Endemic Pleurodeles waltl (Salamandridae). Mol. Phylogenet. Evol. 2017, 112, 122–137. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.J.; Martínez-Solano, I.; Buckley, D. Hybridization during Altitudinal Range Shifts: Nuclear Introgression Leads to Extensive Cyto-Nuclear Discordance in the Fire Salamander. Mol. Ecol. 2016, 25, 1551–1565. [Google Scholar] [CrossRef] [Green Version]
- Antunes, B.; Velo-Antón, G.; Buckley, D.; Pereira, R.J.; Martínez-Solano, I. Physical and Ecological Isolation Contribute to Maintain Genetic Differentiation between Fire Salamander Subspecies. Heredity 2021, 126, 776–789. [Google Scholar] [CrossRef]
- Lourenco, A.; Alvarez, D.; Wang, I.J.; Velo-Antón, G. Trapped within the City: Integrating Demography, Time since Isolation and Population-Specific Traits to Assess the Genetic Effects of Urbanization. Mol. Ecol. 2017, 26, 1498–1514. [Google Scholar] [CrossRef]
- Lourenco, A.; Goncalves, J.; Carvalho, F.; Wang, I.J.; Velo-Antón, G. Comparative Landscape Genetics Reveals the Evolution of Viviparity Reduces Genetic Connectivity in Fire Salamanders. Mol. Ecol. 2019, 28, 4573–4591. [Google Scholar] [CrossRef]
- Velo-Antón, G.; Lourenco, A.; Galán, P.; Nicieza, A.; Tarroso, P. Landscape Resistance Constrains Hybridization across Contact Zones in a Reproductively and Morphologically Polymorphic Salamander. Sci. Rep. 2021, 11, 9259. [Google Scholar] [CrossRef]
- Figueiredo-Vázquez, C.; Lourenco, A.; Velo-Antón, G. Riverine Barriers to Gene Flow in a Salamander with Both Aquatic and Terrestrial Reproduction. Evol. Ecol. 2021, 35, 483–511. [Google Scholar] [CrossRef]
- Antunes, B.F. Evaluating Gene Flow and Habitat Connectivity between Salamandra salamandra Lineages across Heterogeneous Landscapes; University of Porto: Porto, Portugal, 2016. [Google Scholar]
- García-París, M.; Alcobendas, M.; Buclkey, D.; Wake, D.B. Dispersal of Viviparity across Contact Zones in Iberian Populations of Fire Salamanders (Salamandra) Inferred from Discordance of Genetic and Morphological Traits. Evolution 2003, 57, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Burgon, J.D.; Vences, M.; Steinfartz, S.; Bogaerts, S.; Bonato, L.; Donaire-Barroso, D.; Martínez-Solano, I.; Velo-Antón, G.; Vieites, D.R.; Mable, B.K.; et al. Phylogenomic Inference of Species and Subspecies Diversity in the Palearctic Salamander Genus Salamandra. Mol. Phylogenet. Evol. 2021, 157, 107063. [Google Scholar] [CrossRef]
- Velo-Antón, G.; Zamudio, K.R.; Cordero-Rivera, A. Genetic Drift and Rapid Evolution of Viviparity in Insular Fire Salamanders (Salamandra salamandra). Heredity 2012, 108, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, B.; Lourenco, A.; Caeiro-Dias, G.; Dinis, M.; Goncalves, H.; Martínez-Solano, I.; Tarroso, P.; Velo-Antón, G. Combining Phylogeography and Landscape Genetics to Infer the Evolutionary History of a Short-Range Mediterranean Relict, Salamandra salamandra longirostris. Conserv. Genet. 2018, 19, 1411–1424. [Google Scholar] [CrossRef]
- Wielstra, B.; Crnobrnja-Isailovic, J.; Litvinchuk, S.N.; Reijnen, B.T.; Skidmore, A.K.; Sotiropoulos, K.; Toxopeus, A.G.; Tzankov, N.; Vukov, T.; Arntzen, J.W. Tracing Glacial Refugia of Triturus Newts Based on Mitochondrial DNA Phylogeography and Species Distribution Modeling. Front. Zool. 2013, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Iwanowicz, D.; Schill, W.; Olson, D.; Adams, M.; Densmore, C.; Cornman, R.S.; Adams, C.; Figiel, J.; Chester; Anderson, C.W.; et al. Potential Concerns with Analytical Methods Used for the Detection of Batrachochytrium salamandrivorans from Archived DNA of Amphibian Swab Samples, Oregon, USA. Herpetol. Rev. 2019, 48, 352–355. [Google Scholar]
- Thomas, V.; Blooi, M.; Van Rooij, P.; Van Praet, S.; Verbrugghe, E.; Grasselli, E.; Lukac, M.; Smith, S.; Pasmans, F.; Martel, A. Recommendations on Diagnostic Tools for Batrachochytrium salamandrivorans. Transbound. Emerg. Dis. 2018, 65, e478–e488. [Google Scholar] [CrossRef]
- Hill, A.J.; Hardman, R.H.; Sutton, W.B.; Grisnik, M.S.; Gunderson, J.H.; Walker, D.M. Absence of Batrachochytrium salamandrivorans in a Global Hotspot for Salamander Biodiversity. J. Wildl. Dis. 2021, 57, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Pinto, J.; Bletz, M.; Hendrix, R.; Perl, R.G.B.; Martel, A.; Pasmans, F.; Loetters, S.; Mutschmann, F.; Schmeller, D.S.; Schmidt, B.R.; et al. First Detection of the Emerging Fungal Pathogen Batrachochytrium salamandrivorans in Germany. Amphib. Reptil. 2015, 36, 411–416. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, L.D.; Pasmans, F.; Martel, A.; Cunningham, A.A. Epidemiological Tracing of Batrachochytrium salamandrivorans Identifies Widespread Infection and Associated Mortalities in Private Amphibian Collections. Sci. Rep. 2018, 8, 13845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Martel, A.; Bogaerts, S.; Gocmen, B.; Pafilis, P.; Lymberakis, P.; Woeltjes, T.; Veith, M.; Pasmans, F. Landscape Connectivity Limits the Predicted Impact of Fungal Pathogen Invasion. J. Fungi 2020, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Blooi, M.; Pasmans, F.; Rouffaer, L.; Haesebrouck, F.; Vercammen, F.; Martel, A. Successful Treatment of Batrachochytrium salamandrivorans Infections in Salamanders Requires Synergy between Voriconazole, Polymyxin E and Temperature. Sci. Rep. 2015, 5, 11788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzen-van der Sluijs, A.; Stegen, G.; Bogaerts, S.; Canessa, S.; Steinfartz, S.; Janssen, N.; Bosman, W.; Pasmans, F.; Martel, A. Post-Epizootic Salamander Persistence in a Disease-Free Refugium Suggests Poor Dispersal Ability of Batrachochytrium salamandrivorans. Sci. Rep. 2018, 8, 3800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thein, J.; Reck, U.; Dittrich, C.; Martel, A.; Schulz, V.; Hansbauer, G. Preliminary Report on the Occurrence of Batrachochytrium salamandrivorans in the Steigerwald, Bavaria, Germany. Salamandra 2020, 56, 227–229. [Google Scholar]
- Schmeller, D.S.; Utzel, R.; Pasmans, F.; Martel, A. Batrachochytrium salamandrivorans Kills Alpine Newts (Ichthyosaura alpestris) in Southernmost Germany. Salamandra 2020, 56, 230–232. [Google Scholar]
- Sandvoss, M.; Wagner, N.; Loetters, S.; Feldmeier, S.; Schulz, V.; Steinfartz, S.; Veith, M. Spread of the Pathogen Batrachochytrium salamandrivorans and Large-Scale Absence of Larvae Suggest Unnoticed Declines of the European Fire Salamander in the Southern Eifel Mountains. Salamandra 2020, 56, 215–226. [Google Scholar]
- Loetters, S.; Wagner, N.; Albaladejo, G.; Boening, P.; Dalbeck, L.; Duessel, H.; Feldmeier, S.; Guschal, M.; Kirst, K.; Ohlhoff, D.; et al. The Amphibian Pathogen Batrachochytrium salamandrivorans in the Hotspot of Its European Invasive Range: Past–Present–Future. Salamandra 2020, 56, 173–188. [Google Scholar]
- Loetters, S.; Veith, M.; Wagner, N.; Martel, A.; Pasmans, F. Bsal-Driven Salamander Mortality Pre-Dates the European Index Outbreak. Salamandra 2020, 56, 239–242. [Google Scholar]
- Schulz, V.; Schulz, A.; Klamke, M.; Preissler, K.; Sabino-Pinto, J.; Muesken, M.; Schluepmann, M.; Heldt, L.; Kamprad, F.; Enss, J.; et al. Batrachochytrium salamandrivorans in the Ruhr District, Germany: History, Distribution, Decline Dynamics and Disease Symptoms of the Salamander Plague. Salamandra 2020, 56, 189–214. [Google Scholar]
- Gilbert, M.J.; Spitzen-van der Sluijs, A.M.; Canessa, S.; Bosch, J.; Cunningham, A.A.; Grasselli, E.; Laudelout, A.; Lötters, S.; Miaud, C.; Salvidio, S.; et al. Mitigating Batrachochytrium salamandrivorans in Europe; Batrachochytrium salamandrivorans Action Plan for European Urodeles; European Commision: Nijmegen, The Netherlands, 2020. [Google Scholar]
- Gómez, A.; Lunt, D.H. Refugia within refugia: Patterns of phylogeographic concordance in the Iberian Peninsula. In Phylogeography of Southern European Refugia; Springer: Dordrecht, The Netherlands, 2007; pp. 155–188. [Google Scholar]
- Bozzuto, C.; Schmidt, B.R.; Canessa, S. Active Responses to Outbreaks of Infectious Wildlife Diseases: Objectives, Strategies and Constraints Determine Feasibility and Success. Proc. Biol. Sci. 2020, 287, 20202475. [Google Scholar] [CrossRef]
- Canessa, S.; Bozzuto, C.; Grant, E.H.C.; Cruickshank, S.S.; Fisher, M.C.; Koella, J.C.; Loetters, S.; Martel, A.; Pasmans, F.; Scheele, B.C.; et al. Decision-Making for Mitigating Wildlife Diseases: From Theory to Practice for an Emerging Fungal Pathogen of Amphibians. J. Appl. Ecol. 2018, 55, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Thomas, V.; Wang, Y.; Van Rooij, P.; Verbrugghe, E.; Balaz, V.; Bosch, J.; Cunningham, A.A.; Fisher, M.C.; Garner, T.W.J.; Gilbert, M.J.; et al. Mitigating Batrachochytrium salamandrivorans in Europe. Amphib. Reptil. 2019, 40, 265–290. [Google Scholar] [CrossRef] [Green Version]
- Sabino-Pinto, J.; Veith, M.; Vences, M.; Steinfartz, S. Asymptomatic Infection of the Fungal Pathogen Batrachochytrium salamandrivorans in Captivity. Sci. Rep. 2018, 8, 11767. [Google Scholar] [CrossRef] [PubMed]
- Valbuena-Urena, E.; Soler-Membrives, A.; Steinfartz, S.; Alonso, M.; Carbonell, F.; Larios-Martín, R.; Obón, E.; Carranza, S. Getting off to a Good Start? Genetic Evaluation of the Ex Situ Conservation Project of the Critically Endangered Montseny Brook Newt (Calotriton arnoldi). PeerJ 2017, 5, e3447. [Google Scholar] [CrossRef]
- Olson, D.H.; Haman, K.H.; Gray, M.; Harris, R.; Thompson, T.; Iredale, M.; Christman, M.; Williams, J.; Adams, M.J.; Ballard, J. Enhanced Between-Site Biosecurity to Minimize Herpetofaunal Disease-Causing Pathogen Transmission. Herpetol. Rev. 2021, 52, 29–39. [Google Scholar]
- van Riemsdijk, I.; van Nieuwenhuize, L.; Martínez-Solano, I.; Arntzen, J.W.; Wielstra, B. Molecular Data Reveal the Hybrid Nature of an Introduced Population of Banded Newts (Ommatotriton) in Spain. Conserv. Genet. 2018, 19, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, F.F.; Madeira, F.M.; Crespo, E.; Rebelo, R. Rediscovery of the Golden-Striped Salamander Chioglossa lusitanica of Sintra, Portugal. Herpetolog. J. 2018, 28, 148–154. [Google Scholar]
- Tompros, A.; Dean, A.D.; Fenton, A.; Wilber, M.Q.; Carter, E.D.; Gray, M.J. Frequency-Dependent Transmission of Batrachochytrium salamandrivorans in Eastern Newts. Transbound. Emerg. Dis. 2021, 1–11. [Google Scholar] [CrossRef]


| (Sub-)Species | Bsal Risk | Number of 10 km × 10 km Squares | Pleistocene Lineages | Remark | Reference(s) |
|---|---|---|---|---|---|
| Calotriton arnoldi | 3 | 2 | 1 | Population genetic structure | [32,33] |
| Calotriton asper | 2 | >100 | 1 | Population genetic structure | [34,35] |
| Chioglossa l. longipes | 3 | >100 | 1 | [36,37,38] | |
| Chioglossa l. lusitanica | 3 | <70 | 1 | [36,37,38] | |
| Ichthyosaura alpestris cyreni | 2 | >100 | 1 | Population genetic structure | [39,40] |
| Lissotriton boscai | 2 | >100 for all clades | 4 | Population genetic structure | [41,42,43] |
| Lissotriton maltzani | 2 | >100 | 1 | [41,42,44] | |
| Lissotriton helveticus | 1 | >100 for all clades | 4 | [45] | |
| Pleurodeles waltl | 2 | <30 for Algarve clade >100 for other clades | 4 | Small range population in Algarve somewhat differentiated but nuclear DNA shows admixed group covering a large area | [46,47,48] |
| Salamandra salamandra almanzoris | 3 | <30 for both clades | 2 | Population genetic structure | [49,50] |
| Salamandra s. bejarae | 3 | >100 | 2 | Range of lineage not well known | [50] GV-A unpublished |
| Salamandra s. bernardezi | 3 | approx. 100 for the subspecies | 2–7 | High diversity in a small region; population genetic structure | [51,52,53,54] GV-A unpublished |
| Salamandra s. crespoi | 3 | <70 | 1 | [55] GV-A unpublished | |
| Salamandra s. fastuosa | 3 | >100 | 1 | [53,56,57] GV-A unpublished | |
| Salamandra s. gallaica | 3 | >100 for the subspecies | 4 | Range of lineage not well known; population genetic structure | [43,54,57,58] GV-A unpublished |
| Salamandra s. hispanica | 3 | >100 | 1 | [57] | |
| Salamandra s. longirostris | 3 | <100 | 3 | Shallow lineages, considered one conservation unit; population genetic structure | [59] |
| Salamandra s. morenica | 3 | >100 | 1 | [55] GV-A unpublished | |
| Triturus marmoratus | 3 | >100 for the species | 2 | Range of lineage not well known | [60] |
| Triturus pygmaeus | 3 | >100 for the species | 2 | Range of lineage not well known | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.d |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch, J.; Martel, A.; Sopniewski, J.; Thumsová, B.; Ayres, C.; Scheele, B.C.; Velo-Antón, G.; Pasmans, F. Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot. J. Fungi 2021, 7, 644. https://doi.org/10.3390/jof7080644
Bosch J, Martel A, Sopniewski J, Thumsová B, Ayres C, Scheele BC, Velo-Antón G, Pasmans F. Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot. Journal of Fungi. 2021; 7(8):644. https://doi.org/10.3390/jof7080644
Chicago/Turabian StyleBosch, Jaime, An Martel, Jarrod Sopniewski, Barbora Thumsová, Cesar Ayres, Ben C. Scheele, Guillermo Velo-Antón, and Frank Pasmans. 2021. "Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot" Journal of Fungi 7, no. 8: 644. https://doi.org/10.3390/jof7080644
APA StyleBosch, J., Martel, A., Sopniewski, J., Thumsová, B., Ayres, C., Scheele, B. C., Velo-Antón, G., & Pasmans, F. (2021). Batrachochytrium salamandrivorans Threat to the Iberian Urodele Hotspot. Journal of Fungi, 7(8), 644. https://doi.org/10.3390/jof7080644







