Antifungal Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In Vitro and in Planta Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antifungals
2.2. Verticillium Dahliae Isolates
2.3. Antifungal In Vitro Assays
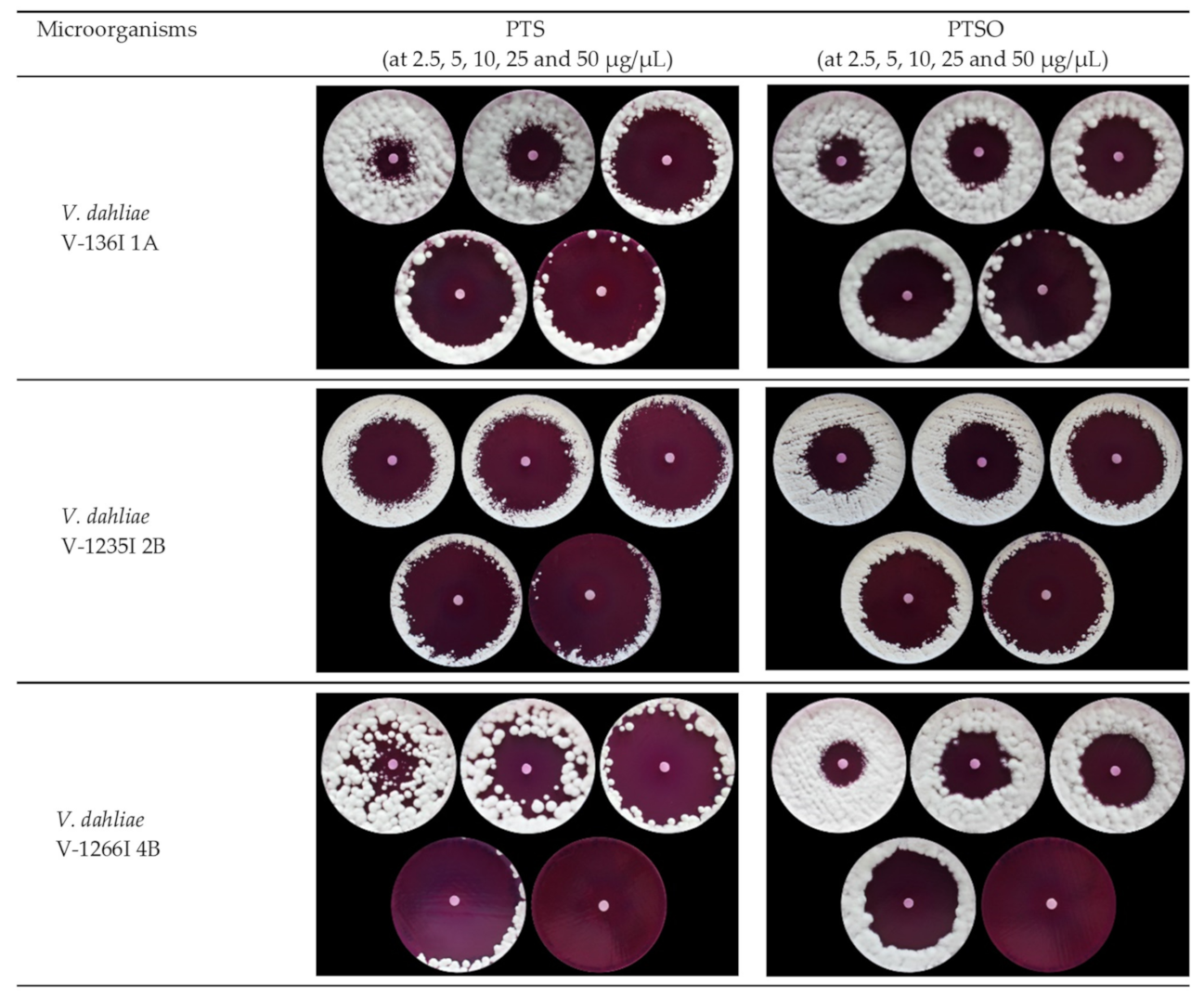
2.3.1. Agar Diffusion Test
2.3.2. Minimum Fungicidal Concentration (MFC)
2.3.3. Antifungal Activity of Gaseous PTS and PTSO
2.4. Effect of PTS and PTSO on Mycelial Growth
2.5. Soil Sanitization
2.6. Effect of PTS and PTSO on Verticillium Wilt Suppression
2.7. Statistical Analyses
3. Results
3.1. In Vitro Antifungal Assays
3.1.1. Antifungal Activity
3.1.2. Antifungal Activity of the Gas Phase
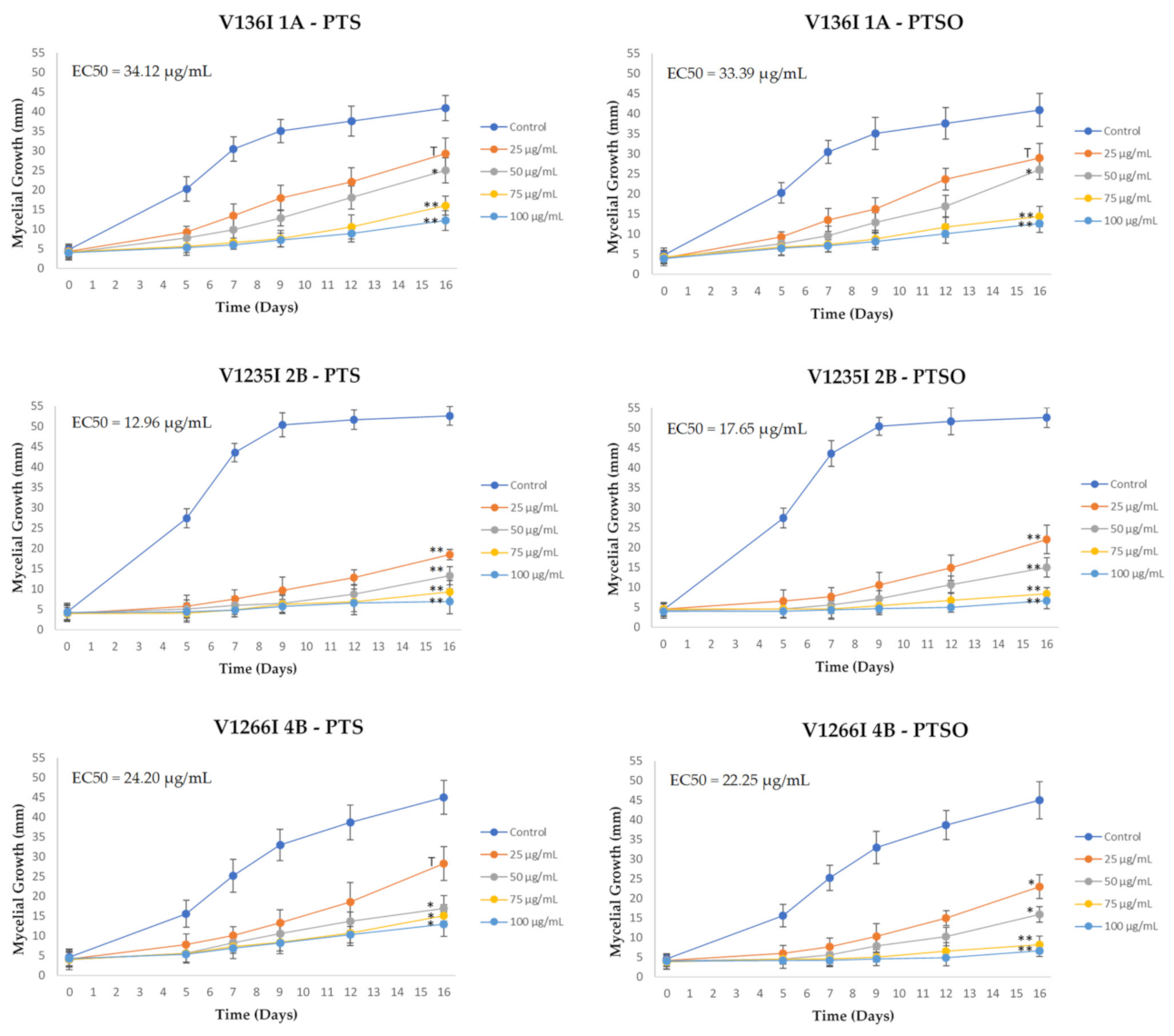
3.2. Effect of PTS and PTSO on Mycelial Growth
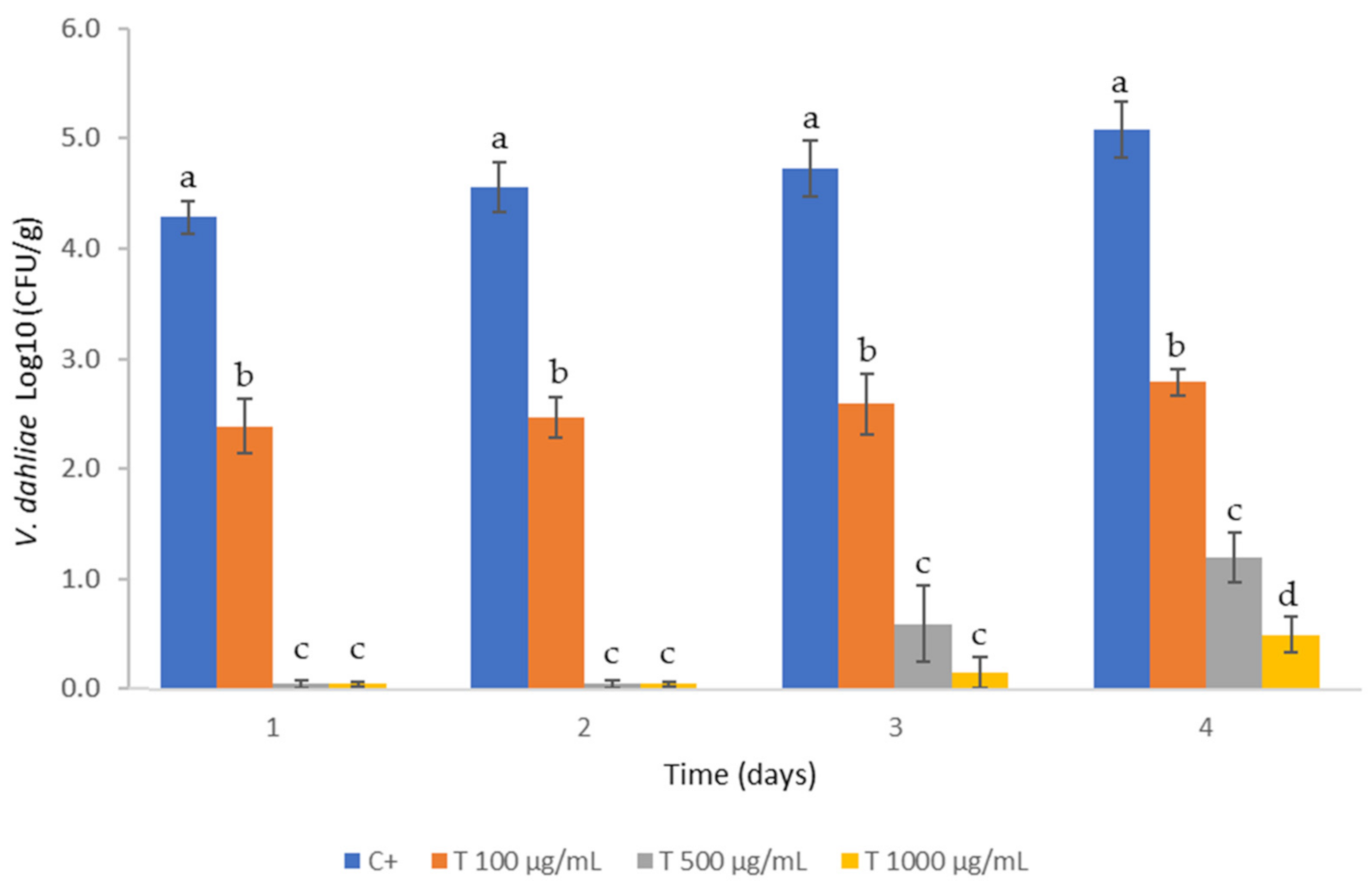
3.3. Application of PTS and PTSO in Soil Sanitization
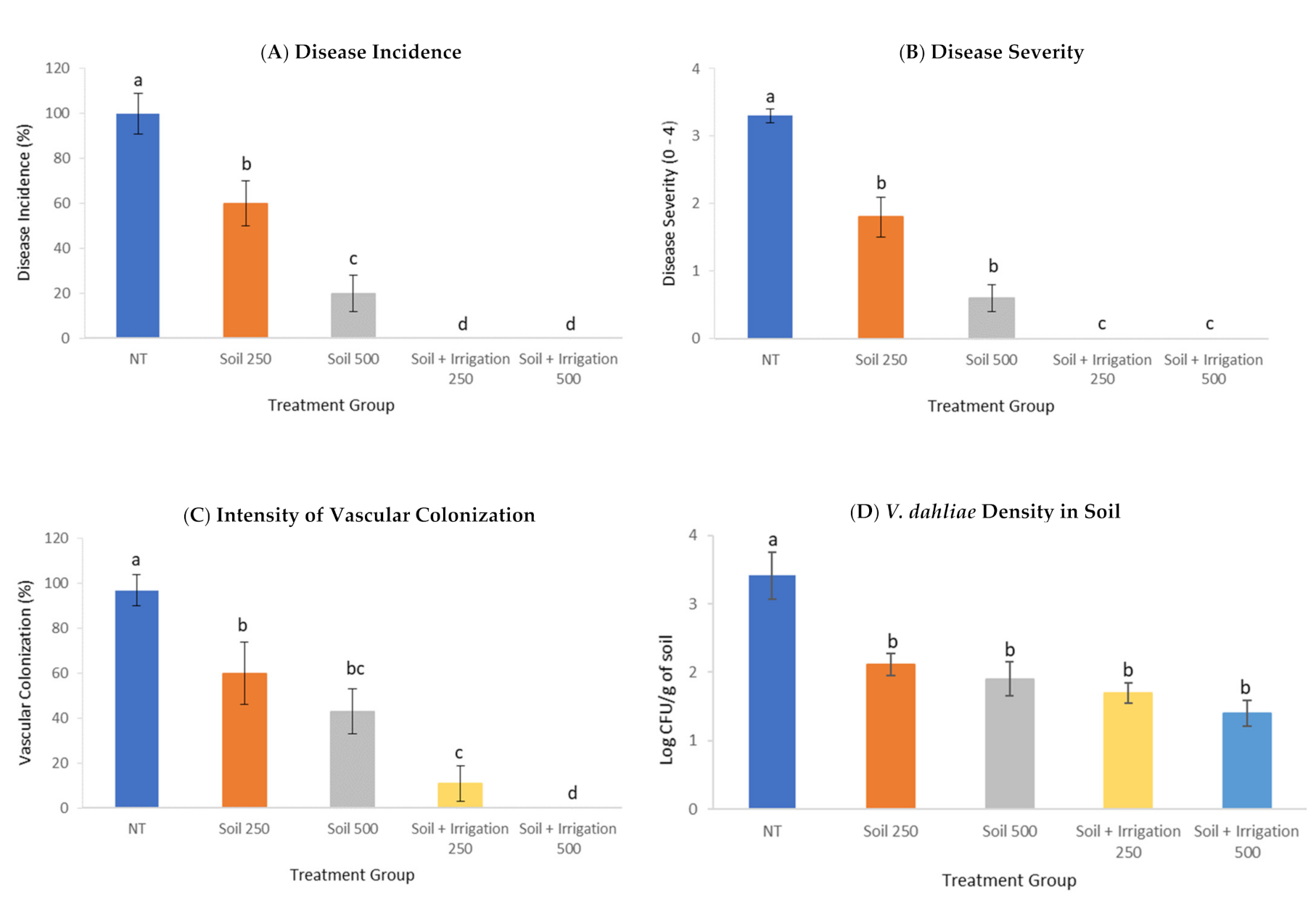
3.4. Effect of PTS and PTSO on Verticillium Wilt Suppression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Landa, B.B.; Pérez, A.G.; Luaces, P.; Montes-Borrego, M.; Navas-Cortés, J.A.; Sanz, C. Insights Into the Effect of Verticillium dahliae Defoliating-Pathotype Infection on the Content of Phenolic and Volatile Compounds Related to the Sensory Properties of Virgin Olive Oil. Front. Plant Sci. 2019, 10, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, D.; Torres, M.; Sampedro, I.; Martínez-Checa, F.; Torres, B.; Béjar, V. Biological Control of Verticillium Wilt on Olive Trees by the Salt-Tolerant Strain Bacillus velezensis XT1. Microorganisms 2020, 8, 1080. [Google Scholar] [CrossRef]
- Gramaje, D.; Pérez-Serrano, V.; Borrego, M.M.; Navas-Cortes, J.; Díaz, R.M.J.; Landa, B.B. A Comparison of Real-Time PCR Protocols for the Quantitative Monitoring of Asymptomatic Olive Infections by Verticillium dahliae Pathotypes. Phytopathology 2013, 103, 1058–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navas-Cortés, J.A.; Landa, B.B.; Mercado-Blanco, J.; Trapero-Casas, J.L.; Jurado, R.; Jiménez-Díaz, R.M. Spatiotemporal Analysis of Spread of Infections by Verticillium dahliae Pathotypes within a High Tree Density Olive Orchard in Southern Spain. Phytopathology 2008, 98, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Verticillium wilt caused by Verticillium dahliae in woody plants with emphasis on olive and shade trees. Eur. J. Plant Pathol. 2018, 150, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Fernández, D.; Trapero-Casas, J.L.; Landa, B.B.; Navas-Cortes, J.; Bubici, G.; Cirulli, M.; Jiménez-Díaz, R.M. Characterization of resistance against the olive-defoliatingVerticillium dahliaepathotype in selected clones of wild olive. Plant Pathol. 2016, 65, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Díaz, R.M.J.; Cirulli, M.; Bubici, G.; Jiménez-Gasco, M.D.M.; Antoniou, P.P.; Tjamos, E.C. Verticillium Wilt, A Major Threat to Olive Production: Current Status and Future Prospects for its Management. Plant Dis. 2012, 96, 304–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varo, A.; Raya-Ortega, M.; Trapero, A. Selection and evaluation of micro-organisms for biocontrol ofVerticillium dahliaein olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef]
- Díaz, R.J.; Casas, J.T.; Boned, J.; del Castillo, B.L.; Cortés, J.N. Uso de Bioten para la protección biológica de plantones de olivo contra la Verticilosis causada por el patotipo defoliante de Verticillium dahliae. Boletín Sanid. Veg. Plagas 2009, 35, 595–615. [Google Scholar]
- Sarfraz, M.; Nasim, M.J.; Jacob, C.; Gruhlke, M.C.H. Efficacy of Allicin against Plant Pathogenic Fungi and Unveiling the Underlying Mode of Action Employing Yeast Based Chemogenetic Profiling Approach. Appl. Sci. 2020, 10, 2563. [Google Scholar] [CrossRef] [Green Version]
- Goicoechea, N. To what extent are soil amendments useful to control Verticillium wilt? Pest Manag. Sci. 2009, 65, 831–839. [Google Scholar] [CrossRef]
- Short, D.P.G.; Sandoya, G.; Vallad, G.E.; Koike, S.T.; Xiao, C.-L.; Wu, B.-M.; Gurung, S.; Hayes, R.J.; Subbarao, K. Dynamics of Verticillium Species Microsclerotia in Field Soils in Response to Fumigation, Cropping Patterns, and Flooding. Phytopathology 2015, 105, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Deberdt, P.; Perrin, B.; Coranson-Beaudu, R.; Duyck, P.-F.; Wicker, E. Effect of Allium fistulosum Extract on Ralstonia solanacearum Populations and Tomato Bacterial Wilt. Plant Dis. 2012, 96, 687–692. [Google Scholar] [CrossRef] [Green Version]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2020, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In Vitro Antibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium spp. against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. BioMed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef] [Green Version]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Keusgen, M.; Schulz, H.; Glodek, J.; Krest, I.; Krüger, H.; Herchert, N.; Keller, J. Characterization of Some Allium Hybrids by Aroma Precursors, Aroma Profiles, and Alliinase Activity. J. Agric. Food Chem. 2002, 50, 2884–2890. [Google Scholar] [CrossRef]
- Guillamón, E. Effect of phytochemical compounds of the genus Allium on the immune system and the inflammatory response. Ars Pharm. 2018, 59, 185–196. [Google Scholar]
- Vezza, T.; Algieri, F.; Garrido-Mesa, J.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Baños, A.; Guillamón, E.; García, F.; Rodríguez-Nogales, A.; Galvez, J. The Immunomodulatory Properties of Propyl-Propane Thiosulfonate Contribute to its Intestinal Anti-Inflammatory Effect in Experimental Colitis. Mol. Nutr. Food Res. 2019, 63, e1800653. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.J.; Ruiz, R.; Echávarri, A.; Rubio, L.A. Garlic derivative propyl propane thiosulfonate is effective against broiler enteropathogens in vivo. Poult. Sci. 2012, 91, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Lillehoj, E.P.; Bravo, D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br. J. Nutr. 2012, 109, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Rabelo-Ruiz, M.; Teso-Pérez, C.; Peralta-Sánchez, J.; Ariza, J.; Martín-Platero, A.; Casabuena-Rincón, Ó.; Vázquez-Chas, P.; Guillamón, E.; Aguinaga-Casañas, M.; Maqueda, M.; et al. Allium Extract Implements Weaned Piglet’s Productive Parameters by Modulating Distal Gut Microbiota. Antibiotics 2021, 10, 269. [Google Scholar] [CrossRef]
- Rabelo-Ruiz, M.; Ariza-Romero, J.; Zurita-González, M.; Martín-Platero, A.; Baños, A.; Maqueda, M.; Valdivia, E.; Martínez-Bueno, M.; Peralta-Sánchez, J. Allium-Based Phytobiotic Enhances Egg Production in Laying Hens through Microbial Composition Changes in Ileum and Cecum. Animals 2021, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Cascajosa-Lira, A.; Puerto, M.; Prieto, A.; Pichardo, S.; Jiménez, L.D.-Q.; Baños, A.; Guillamón, E.; Moyano, R.; Molina-Hernández, V.; Jos, Á.; et al. Genotoxicity Evaluation of Propyl-Propane-Thiosulfinate (PTS) from Allium genus Essential Oils by a Combination of Micronucleus and Comet Assays in Rats. Foods 2021, 10, 989. [Google Scholar] [CrossRef]
- Lira, A.C.; Prieto, A.I.; Baños, A.; Guillamón, E.; Moyano, R.; Jos, A.; Cameán, A.M. Safety assessment of propyl-propane-thiosulfonate (PTSO): 90-days oral subchronic toxicity study in rats. Food Chem. Toxicol. 2020, 144, 111612. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Moreno, F.J.; Baños, A.; Nuñez, C.; Guillamón, E.; Cameán, A.M. Acute toxicological studies of the main organosulfur compound derived from Allium sp. intended to be used in active food packaging. Food Chem. Toxicol. 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Focke, M.; Feld, A.; Lichtenthaler, H.K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett. 1990, 261, 106–108. [Google Scholar] [CrossRef] [Green Version]
- Gruhlke, M.C.; Portz, D.; Stitz, M.; Anwar, A.; Schneider, T.; Jacob, C.; Schlaich, N.L.; Slusarenko, A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free. Radic. Biol. Med. 2010, 49, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.J.; Theato, P. Thiol–thiosulfonate chemistry in polymer science: Simple functionalization of polymers via disulfide linkages. In Thiol-X Chemistries in Polymer and Materials Science; Lowe, A., Bowman, C., Eds.; Abingdon: Nashville, TN, USA, 2013; pp. 76–94. [Google Scholar]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutawa, M.D.D.A.B. Antifungal Activity of Garlic (Allium sativum) Extract on Some Selected Fungi. J. Med. Herbs Ethnomed. 2018, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Daniel, C.; Lennox, C.; Vries, F. In-vitro effects of garlic extracts on pathogenic fungi Botrytis cinerea, Penicillium expansum and Neofabraea alba. S. Afr. J. Sci. 2015, 111, 8. [Google Scholar] [CrossRef] [Green Version]
- Perelló, A.; Gruhlke, M.; Slusarenko, A.J. Effect of garlic extract on seed germination, seedling health, and vigour of pathogen-infested wheat. J. Plant Prot. Res. 2013, 53, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Lević, J.; Tanackov, I.; Tepić, A.; Vujičić, B.; Gvozdanović-Varga, J. Effects of Onion (Allium cepa L.) and Garlic (Allium sativum L.) Essential Oils on the Aspergillus versicolor Growth and Sterigmatocystin Production. J. Food Sci. 2012, 77, M278–M284. [Google Scholar] [CrossRef]
- Shams-Ghahfarokhi, M.; Shokoohamiri, M.-R.; Amirrajab, N.; Moghadasi, B.; Ghajari, A.; Zeini, F.; Sadeghi, G.; Razzaghi-Abyaneh, M. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia 2006, 77, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Genatrika, E.; Sundhani, E.; Oktaviana, M.I. Gel potential of red onion (Allium cepa L.) ethanol extract as antifungal cause tinea pedis. J. Pharm. Bioallied Sci. 2020, 12, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Díaz, R.M.; Olivares-García, C.; Landa, B.B.; Jiménez-Gasco, M.D.M.; Navas-Cortes, J. Region-Wide Analysis of Genetic Diversity in Verticillium dahliae Populations Infecting Olive in Southern Spain and Agricultural Factors Influencing the Distribution and Prevalence of Vegetative Compatibility Groups and Pathotypes. Phytopathology 2011, 101, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Calvo, M.A.; Asensio, J.J. Evaluación de la eficacia de productos antimicrobianos en la alimentación animal. Anaporc 1999, 192, 142–146. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd Edition. 2018. Available online: https://clsi.org/standards/products/microbiology/documents/m38/ (accessed on 1 August 2021).
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Liu, X.; Yan, D.; Ouyang, C.; Yang, D.; Wang, Q.; Li, Y.; Guo, M.; Cao, A. Oils extracted from Eupatorium adenophorum leaves show potential to control Phythium myriotylum in commercially-grown ginger. PLoS ONE 2017, 12, e0176126. [Google Scholar] [CrossRef] [Green Version]
- Falade, T.D.; Mohdhamdan, S.H.S.; Sultanbawa, Y.; Fletcher, M.; Harvey, J.J.; Chaliha, M.; Fox, G. In vitro experimental environments lacking or containing soil disparately affect competition experiments of Aspergillus flavus and co-occurring fungi in maize grains. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.-T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Arnault, I.; Fleurance, C.; Vey, F.; Du Fretay, G.; Auger, J. Use of Alliaceae residues to control soil-borne pathogens. Ind. Crops Prod. 2013, 49, 265–272. [Google Scholar] [CrossRef]
- Bayar, Y.; Onaran, A.; Yilar, M.; Gul, F. Determination of the Essential Oil Composition and the Antifungal Activities of Bilberry (Vaccinium myrtillus L.) and Bay Laurel (Laurus nobilis L.). J. Essent. Oil Bear. Plants 2018, 21, 548–555. [Google Scholar] [CrossRef]
- Üstüner, T.; Kordali, Ş.; Bozhüyük, A.U.; Kesdek, M. Investigation of Pesticidal Activities of Essential Oil of Eucalyptus camaldulensis Dehnh. Rec. Nat. Prod. 2018, 12, 557–568. [Google Scholar] [CrossRef]
- Erdogan, O.; Celik, A.; Zeybek, A. In Vitro antifungal activity of mint, thyme, lavender extracts and essential oils on verticillium dahliae kleb. Fresenius Environ. Bull. 2016, 25, 4856–4862. [Google Scholar]
- Varo, A.; Aparicio, A.M.; Adem, M.; Roca, L.; Raya-Ortega, M.; López-Escudero, F.; Trapero, A. Screening water extracts and essential oils from Mediterranean plants against Verticillium dahliae in olive. Crop. Prot. 2017, 92, 168–175. [Google Scholar] [CrossRef]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Distribution and persistence of Verticillium dahliae in the xylem of Norway maple and European ash trees. Eur. J. Plant Pathol. 2018, 150, 323–339. [Google Scholar] [CrossRef]
- Hayat, S.; Cheng, Z.; Ahmad, H.; Ali, M.; Chen, X.; Wang, M. Garlic, from Remedy to Stimulant: Evaluation of Antifungal Potential Reveals Diversity in Phytoalexin Allicin Content among Garlic Cultivars; Allicin Containing Aqueous Garlic Extracts Trigger Antioxidants in Cucumber. Front. Plant Sci. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Ahmad, H.; Hayat, S.; Ghani, M.I.; Amin, B.; Atif, M.J.; Wali, K.; Cheng, Z. Application of garlic allelochemicals improves growth and induces defense responses in eggplant (Solanum melongena) against Verticillium dahliae. Ecotoxicol. Environ. Saf. 2021, 215, 112132. [Google Scholar] [CrossRef]


| Reference Code | VCG 1 | Pathotype 2 |
|---|---|---|
| V136I | 1A | D |
| V1235I | 2B | ND |
| V1266I | 4B | ND |
| Compound | Concentration (µg/µL) | V136I 1A | V1235I 2B | V1266I 4B |
|---|---|---|---|---|
| PTS | 2.5 | 32.0 ± 3.16 | 47.3 ± 2.86 | 37.8 ± 3.49 |
| 5 | 44.0 ± 3.16 | 56.0 ± 1.87 | 48.8 ± 2.38 | |
| 10 | 61.3 ± 2.38 | 64.5 ± 2.69 | 68.3 ± 3.34 | |
| 25 | 69.0 ± 1.41 | 72.3 ± 2.59 | 80.8 ± 1.92 | |
| 50 | 75.3 ± 1.92 | 80.5 ± 2.18 | 86.0 ± 3.16 | |
| EC50 (µg/µL) | 7.7 ± 0.03 | 10.0 ± 0.56 | 8.3 ± 0.17 | |
| PTSO | 2.5 | 28.3 ± 2.38 | 40.0 ± 3.16 | 28.8 ± 3.34 |
| 5 | 38.8 ± 2.38 | 48.8 ± 0.83 | 42.3 ± 2.59 | |
| 10 | 50.8 ± 2.38 | 56.5 ± 1.12 | 65.0 ± 3.24 | |
| 25 | 58.5 ± 1.12 | 65.0 ± 3.39 | 76.3 ± 1.64 | |
| 50 | 71.5 ± 3.04 | 75.8 ± 3.03 | 87.3 ± 0.83 | |
| EC50 (µg/µL) | 10.8 ± 0.35 | 11.6 ± 0.42 | 8.8 ± 0.24 |
| MFC (µg/mL) | ||
|---|---|---|
| Isolates | PTS | PTSO |
| V. dahliae V136I 1A | 78.13 | 19.53 |
| V. dahliae V1235I 2B | 78.13 | 39.06 |
| V. dahliae V1266I 4B | 78.13 | 39.06 |
| Compound | Concentration (µg/µL) | V136I 1A | V1235I 2B | V1266I 4B |
|---|---|---|---|---|
| PTS | 2.5 | 48.5 ± 1.12 | 55.0 ± 1.41 | 45.0 ± 1.41 |
| 5 | 53.8 ± 3.49 | 71.5 ± 2.06 | 58.0 ± 2.24 | |
| 10 | 63.8 ± 3.49 | 79.3 ± 1.30 | 72.0 ± 1.41 | |
| 25 | 69.0 ± 2.12 | 87.0 ± 1.58 | 84.3 ± 1.92 | |
| 50 | 75.3 ± 2.86 | 92.5 ± 2.06 | 97.3 ± 1.92 | |
| EC50 (µg/µL) | 9.9 ± 0.29 | 7.0 ± 0.15 | 10.3 ± 0.09 | |
| PTSO | 2.5 | 38.5 ± 1.12 | 40.5 ± 2.06 | 39.0 ± 1.58 |
| 5 | 46.0 ± 1.41 | 52.3 ± 1.48 | 48.5 ± 1.12 | |
| 10 | 54.5 ± 2.96 | 62.3 ± 1.92 | 54.3 ± 1.30 | |
| 25 | 62.3 ± 1.79 | 69.0 ± 1.58 | 66.0 ± 1.87 | |
| 50 | 66.3 ± 2.28 | 73.0 ± 2.24 | 71.5 ± 1.66 | |
| EC50 (µg/µL) | 8.8 ± 0.22 | 7.2 ± 0.08 | 9.9 ± 0.11 |
| Compound | Concentration (µg/µL) | V136I 1A | V1235I 2B | V1266I 4B |
|---|---|---|---|---|
| PTS | 25 | 28.39 | 64.87 | 37.16 |
| 50 | 38.85 | 74.74 | 62.40 | |
| 75 | 60.86 | 82.26 | 66.43 | |
| 100 | 70.09 | 86.71 | 71.27 | |
| PTSO | 25 | 29.22 | 58.13 | 48.93 |
| 50 | 36.32 | 71.48 | 64.62 | |
| 75 | 64.84 | 84.09 | 81.77 | |
| 100 | 69.17 | 87.38 | 85.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcón-Piñeiro, A.; Remesal, E.; Noguera, M.; Ariza, J.J.; Guillamón, E.; Baños, A.; Navas-Cortes, J.A. Antifungal Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In Vitro and in Planta Assays. J. Fungi 2021, 7, 736. https://doi.org/10.3390/jof7090736
Falcón-Piñeiro A, Remesal E, Noguera M, Ariza JJ, Guillamón E, Baños A, Navas-Cortes JA. Antifungal Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In Vitro and in Planta Assays. Journal of Fungi. 2021; 7(9):736. https://doi.org/10.3390/jof7090736
Chicago/Turabian StyleFalcón-Piñeiro, Ana, Efrén Remesal, Miguel Noguera, Juan José Ariza, Enrique Guillamón, Alberto Baños, and Juan Antonio Navas-Cortes. 2021. "Antifungal Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In Vitro and in Planta Assays" Journal of Fungi 7, no. 9: 736. https://doi.org/10.3390/jof7090736
APA StyleFalcón-Piñeiro, A., Remesal, E., Noguera, M., Ariza, J. J., Guillamón, E., Baños, A., & Navas-Cortes, J. A. (2021). Antifungal Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Verticillium dahliae: In Vitro and in Planta Assays. Journal of Fungi, 7(9), 736. https://doi.org/10.3390/jof7090736






