The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond
Abstract
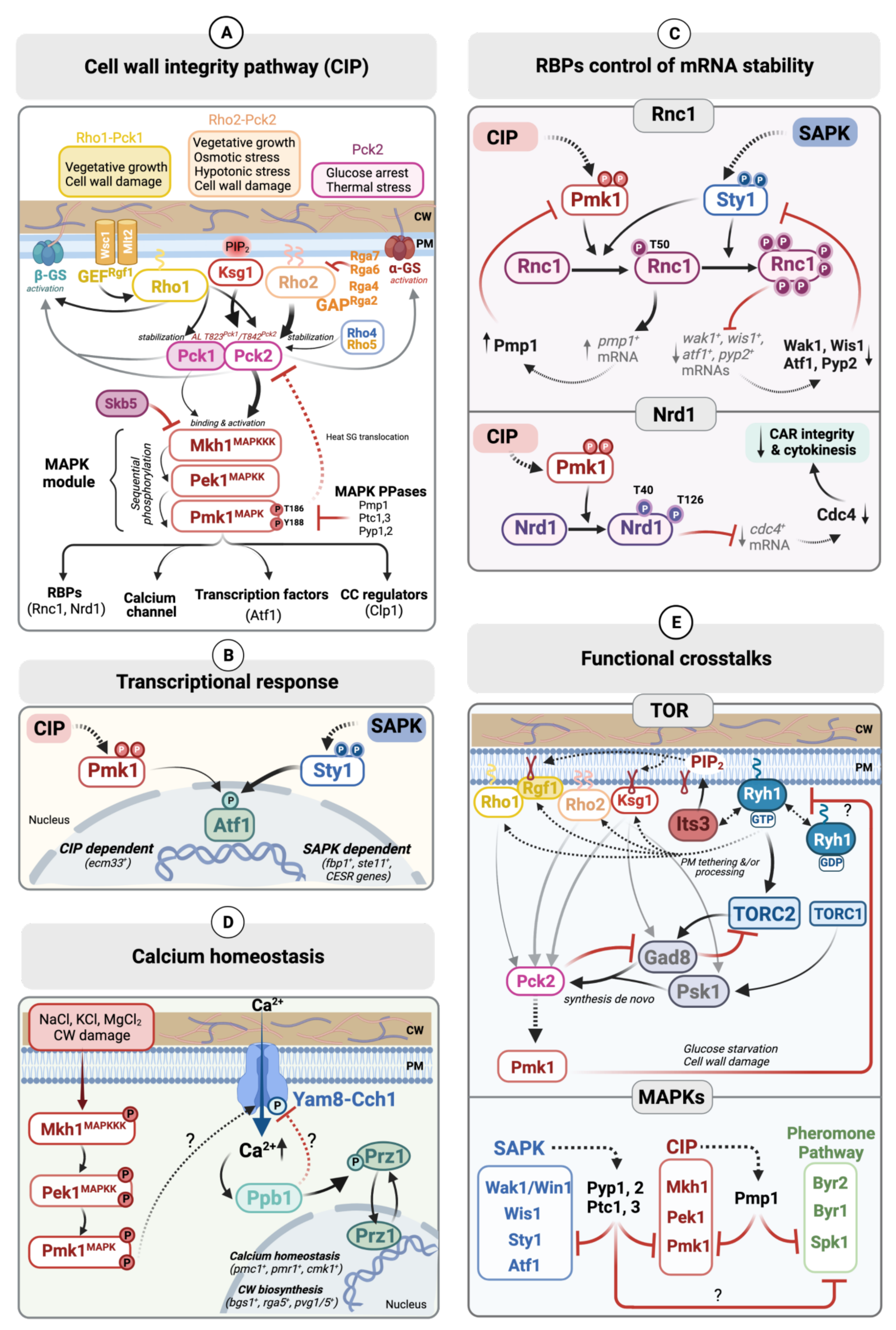
:1. Introduction
2. Architecture and Organization of the Fission Yeast CIP
2.1. Upstream of the CIP MAPK Module
2.1.1. Sensors
2.1.2. Rho-GTPases and Their Regulators
2.1.3. Phospholipid-Dependent Kinase-1 (PDK-1) and Protein Kinase C (PKC) Orthologs
2.2. The CIP MAPK Module
2.2.1. MAPKKK: Mkh1
2.2.2. MAPKK: Pek1
2.2.3. MAPK: Pmk1
2.3. Downstream Targets of Pmk1
2.3.1. Transcription Factor Atf1
2.3.2. RNA Binding Proteins (RBPs) Nrd1 and Rnc1
2.3.3. Other Downstream Targets
2.4. Downregulation of Pmk1 Signaling by MAPK Phosphatases
3. Main Regulatory Functions of the CIP
3.1. Control of Mrna-Stability through Rbps
3.1.1. Nrd1
3.1.2. Rnc1
3.2. Calcium Homeostasis
3.3. Cell-Wall Integrity
3.4. Cytokinesis
4. Functional Crosstalks of the CIP
4.1. Interplay with Other Fission Yeast MAPK-Signaling Cascades
4.2. CIP and TOR Signaling Crosstalk
4.3. Interaction between the CIP and Camp/PKA Pathways
5. Concluding Remarks and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Errede, B.; Levin, D.E. A conserved kinase cascade for MAP kinase activation in yeast. Curr. Opin. Cell Biol. 1993, 5, 254–260. [Google Scholar] [CrossRef]
- Marshall, C.J. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell 1995, 80, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Bluthgen, N.; Legewie, S. Systems analysis of MAPK signal transduction. Essays Biochem. 2008, 45, 95–107. [Google Scholar] [CrossRef]
- Waskiewicz, A.J.; Cooper, J.A. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 1995, 7, 798–805. [Google Scholar] [CrossRef]
- Marshall, C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr. Opin. Genet. Dev. 1994, 4, 82–89. [Google Scholar] [CrossRef]
- Pérez, P.; Cansado, J. Cell integrity signaling and response to stress in fission yeast. Curr. Protein Pept. Sci. 2010, 11, 680–692. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.S.; Treisman, R. Transcriptional Regulation by Extracellular signals: Mechanisms and Specificity. Cell 1995, 80, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Krishna, M.; Narang, H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell. Mol. Life Sci. 2008, 65, 3525–3544. [Google Scholar] [CrossRef]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef]
- Cohen, A.; Kupiec, M.; Weisman, R. Glucose activates TORC2-Gad8 protein via positive regulation of the cAMP/cAMP-dependent protein kinase A (PKA) pathway and negative regulation of the Pmk1 protein-mitogen-activated protein kinase pathway. J. Biol. Chem. 2014, 289, 21727–21737. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer, H.J.; Weber, M.J. Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Mol. Cell. Biol. 1999, 19, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, K.; Russell, P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 1995, 378, 739–743. [Google Scholar] [CrossRef]
- Toda, T.; Shimanuki, M.; Yanagida, M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991, 5, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Zaitsevskaya-Carter, T.; Cooper, J.A. Spm1, a stress-activated MAP kinase that regulates morphogenesis in S.pombe. Embo J. 1997, 16, 1318–1331. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Sato, M.; Takagi, T.; Kamasaki, T.; Ohno, N.; Osumi, M. In situ localization of cell wall alpha-1,3-glucan in the fission yeast Schizosaccharomyces pombe. J. Electron. Microsc. 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Toda, T.; Dhut, S.; Superti-Furga, G.; Gotoh, Y.; Nishida, E.; Sugiura, R.; Kuno, T. The fission yeast pmk1+ gene encodes a novel mitogen-activated protein kinase homolog which regulates cell integrity and functions coordinately with the protein kinase C pathway. Mol. Cell. Biol. 1996, 16, 6752–6764. [Google Scholar] [CrossRef] [Green Version]
- Madrid, M.; Soto, T.; Khong, H.K.; Franco, A.; Vicente, J.; Pérez, P.; Gacto, M.; Cansado, J. Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J. Biol. Chem. 2006, 281, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Loewith, R.; Hubberstey, A.; Young, D. Skh1, the MEK component of the mkh1 signaling pathway in Schizosaccharomyces pombe. J. Cell Sci. 2000, 113 Pt 1, 153–160. [Google Scholar] [CrossRef]
- Madrid, M.; Núñez, A.; Soto, T.; Vicente-Soler, J.; Gacto, M.; Cansado, J. Stress-activated protein kinase-mediated down-regulation of the cell integrity pathway mitogen-activated protein kinase Pmk1p by protein phosphatases. Mol. Biol. Cell 2007, 18, 4405–4419. [Google Scholar] [CrossRef]
- Garcia, P.; Tajadura, V.; Sanchez, Y. The Rho1p exchange factor Rgf1p signals upstream from the Pmk1 mitogen-activated protein kinase pathway in fission yeast. Mol. Biol. Cell 2009, 20, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, R.; Toda, T.; Dhut, S.; Shuntoh, H.; Kuno, T. The MAPK kinase Pek1 acts as a phosphorylation-dependent molecular switch. Nature 1999, 399, 479–483. [Google Scholar] [CrossRef]
- Viana, R.A.; Pinar, M.; Soto, T.; Coll, P.M.; Cansado, J.; Pérez, P. Negative functional interaction between cell integrity MAPK pathway and Rho1 GTPase in fission yeast. Genetics 2013, 195, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Sengar, A.S.; Markley, N.A.; Marini, N.J.; Young, D. Mkh1, a MEK kinase required for cell wall integrity and proper response to osmotic and temperature stress in Schizosaccharomyces pombe. Mol. Cell. Biol. 1997, 17, 3508–3519. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, R.; Toda, T.; Shuntoh, H.; Yanagida, M.; Kuno, T. pmp1+, a suppressor of calcineurin deficiency, encodes a novel MAP kinase phosphatase in fission yeast. EMBO J. 1998, 17, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Kuno, T.; Kita, A.; Asayama, Y.; Sugiura, R. Rho2 is a target of the farnesyltransferase Cpp1 and acts upstream of Pmk1 mitogen-activated protein kinase signaling in fission yeast. Mol. Biol. Cell 2006, 17, 5028–5037. [Google Scholar] [CrossRef]
- Qi, M.; Elion, E.A. MAP kinase pathways. J. Cell Sci. 2005, 118, 3569–3572. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Gil, E.; Franco, A.; Vázquez-Marín, B.; Prieto-Ruiz, F.; Pérez-Díaz, A.; Vicente-Soler, J.; Madrid, M.; Soto, T.; Cansado, J. Specific Functional Features of the Cell Integrity MAP Kinase Pathway in the Dimorphic Fission Yeast. J. Fungi 2021, 7, 482. [Google Scholar] [CrossRef]
- Kock, C.; Dufrêne, Y.F.; Heinisch, J.J. Up against the wall: Is yeast cell wall integrity ensured by mechanosensing in plasma membrane microdomains? Appl. Environ. Microbiol. 2015, 81, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Heinisch, J.J.; Rodicio, R. Protein kinase C in fungi-more than just cell wall integrity. FEMS Microbiol. Rev. 2018, 42, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [Green Version]
- Cruz, S.; Muñoz, S.; Manjón, E.; García, P.; Sanchez, Y. The fission yeast cell wall stress sensor-like proteins Mtl2 and Wsc1 act by turning on the GTPase Rho1p but act independently of the cell wall integrity pathway. Microbiologyopen 2013, 2, 778–794. [Google Scholar] [CrossRef] [Green Version]
- Pérez, P.; Cortés, J.C.G.; Cansado, J.; Ribas, J.C. Fission yeast cell wall biosynthesis and cell integrity signalling. Cell Surf. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Banavar, S.P.; Gomez, C.; Trogdon, M.; Petzold, L.R.; Yi, T.M.; Campàs, O. Mechanical feedback coordinates cell wall expansion and assembly in yeast mating morphogenesis. PLoS Comput. Biol. 2018, 14, e1005940. [Google Scholar] [CrossRef] [Green Version]
- Davì, V.; Tanimoto, H.; Ershov, D.; Haupt, A.; De Belly, H.; Le Borgne, R.; Couturier, E.; Boudaoud, A.; Minc, N. Mechanosensation Dynamically Coordinates Polar Growth and Cell Wall Assembly to Promote Cell Survival. Dev. Cell 2018, 45, 170–182.e177. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; van Drogen, F.; Dechant, R.; Oh, S.; Jeon, N.L.; Lee, S.S.; Peter, M. Protein kinase C and calcineurin cooperatively mediate cell survival under compressive mechanical stress. Proc. Natl. Acad. Sci. USA 2017, 114, 13471–13476. [Google Scholar] [CrossRef] [Green Version]
- Neeli-Venkata, R.; Diaz, C.M.; Celador, R.; Sanchez, Y.; Minc, N. Detection of surface forces by the cell-wall mechanosensor Wsc1 in yeast. Dev. Cell 2021, 56, 2856–2870.e2857. [Google Scholar] [CrossRef]
- Vicente-Soler, J.; Soto, T.; Franco, A.; Cansado, J.; Madrid, M. The Multiple Functions of Rho GTPases in Fission Yeasts. Cells 2021, 10, 1422. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Pérez, P.; Soto, T.; Gómez-Gil, E.; Cansado, J. Functional interaction between Cdc42 and the stress MAPK signaling pathway during the regulation of fission yeast polarized growth. Int. Microbiol. 2020, 23, 31–41. [Google Scholar] [CrossRef]
- Arellano, M.; Durán, A.; Pérez, P. Rho 1 GTPase activates the (1-3)beta-D-glucan synthase and is involved in Schizosaccharomyces pombe morphogenesis. EMBO J. 1996, 15, 4584–4591. [Google Scholar] [CrossRef]
- Miller, P.J.; Johnson, D.I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol. Cell. Biol. 1994, 14, 1075–1083. [Google Scholar] [CrossRef]
- Hirata, D.; Nakano, K.; Fukui, M.; Takenaka, H.; Miyakawa, T.; Mabuchi, I. Genes that cause aberrant cell morphology by overexpression in fission yeast: A role of a small GTP-binding protein Rho2 in cell morphogenesis. J. Cell Sci. 1998, 111 Pt 2, 149–159. [Google Scholar] [CrossRef]
- Arellano, M.; Duran, A.; Perez, P. Localisation of the Schizosaccharomyces pombe rho1p GTPase and its involvement in the organisation of the actin cytoskeleton. J. Cell Sci. 1997, 110 Pt 20, 2547–2555. [Google Scholar] [CrossRef]
- Calonge, T.M.; Nakano, K.; Arellano, M.; Arai, R.; Katayama, S.; Toda, T.; Mabuchi, I.; Perez, P. Schizosaccharomyces pombe rho2p GTPase regulates cell wall alpha-glucan biosynthesis through the protein kinase pck2p. Mol. Biol. Cell 2000, 11, 4393–4401. [Google Scholar] [CrossRef]
- Sánchez-Mir, L.; Franco, A.; Martín-García, R.; Madrid, M.; Vicente-Soler, J.; Soto, T.; Gacto, M.; Pérez, P.; Cansado, J. Rho2 palmitoylation is required for plasma membrane localization and proper signaling to the fission yeast cell integrity mitogen- activated protein kinase pathway. Mol. Cell. Biol. 2014, 34, 2745–2759. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.; Soto, T.; Martín-García, R.; Madrid, M.; Vázquez-Marín, B.; Vicente-Soler, J.; Coll, P.M.; Gacto, M.; Pérez, P.; Cansado, J. Distinct functional relevance of dynamic GTPase cysteine methylation in fission yeast. Sci. Rep. 2017, 7, 6057. [Google Scholar] [CrossRef] [Green Version]
- Kabeche, R.; Madrid, M.; Cansado, J.; Moseley, J.B. Eisosomes Regulate Phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2) Cortical Clusters and Mitogen-activated Protein (MAP) Kinase Signaling upon Osmotic Stress. J. Biol. Chem. 2015, 290, 25960–25973. [Google Scholar] [CrossRef] [Green Version]
- Cansado, J. To finish things well: Cysteine methylation ensures selective GTPase membrane localization and signalling. Curr. Genet. 2018, 64, 341–344. [Google Scholar] [CrossRef]
- Soto, T.; Villar-Tajadura, M.A.; Madrid, M.; Vicente, J.; Gacto, M.; Pérez, P.; Cansado, J. Rga4 modulates the activity of the fission yeast cell integrity MAPK pathway by acting as a Rho2 GTPase-activating protein. J. Biol. Chem. 2010, 285, 11516–11525. [Google Scholar] [CrossRef] [Green Version]
- Cansado, J.; Soto, T.; Gacto, M.; Pérez, P. Rga4, a Rho-GAP from fission yeast: Finding specificity within promiscuity. Commun. Integr. Biol. 2010, 3, 436–439. [Google Scholar] [CrossRef] [Green Version]
- Villar-Tajadura, M.A.; Coll, P.M.; Madrid, M.; Cansado, J.; Santos, B.; Pérez, P. Rga2 is a Rho2 GAP that regulates morphogenesis and cell integrity in S. pombe. Mol. Microbiol. 2008, 70, 867–881. [Google Scholar] [CrossRef]
- Nakano, K.; Arai, R.; Mabuchi, I. The small GTP-binding protein Rho1 is a multifunctional protein that regulates actin localization, cell polarity, and septum formation in the fission yeast Schizosaccharomyces pombe. Genes Cells 1997, 2, 679–694. [Google Scholar] [CrossRef]
- Arellano, M.; Coll, P.M.; Pérez, P. RHO GTPases in the control of cell morphology, cell polarity, and actin localization in fission yeast. Microsc. Res. Technol. 1999, 47, 51–60. [Google Scholar] [CrossRef]
- Arellano, M.; Valdivieso, M.H.; Calonge, T.M.; Coll, P.M.; Duran, A.; Perez, P. Schizosaccharomyces pombe protein kinase C homologues, pck1p and pck2p, are targets of rho1p and rho2p and differentially regulate cell integrity. J. Cell Sci. 1999, 112 Pt 20, 3569–3578. [Google Scholar] [CrossRef]
- Sayers, L.G.; Katayama, S.; Nakano, K.; Mellor, H.; Mabuchi, I.; Toda, T.; Parker, P.J. Rho-dependence of Schizosaccharomyces pombe Pck2. Genes Cells 2000, 5, 17–27. [Google Scholar] [CrossRef]
- Sánchez-Mir, L.; Soto, T.; Franco, A.; Madrid, M.; Viana, R.A.; Vicente, J.; Gacto, M.; Pérez, P.; Cansado, J. Rho1 GTPase and PKC ortholog Pck1 are upstream activators of the cell integrity MAPK pathway in fission yeast. PLoS ONE 2014, 9, e88020. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Mutoh, T.; Mabuchi, I. Characterization of GTPase-activating proteins for the function of the Rho-family small GTPases in the fission yeast Schizosaccharomyces pombe. Genes Cells 2001, 6, 1031–1042. [Google Scholar] [CrossRef]
- Mutoh, T.; Nakano, K.; Mabuchi, I. Rho1-GEFs Rgf1 and Rgf2 are involved in formation of cell wall and septum, while Rgf3 is involved in cytokinesis in fission yeast. Genes Cells 2005, 10, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- García, P.; Tajadura, V.; García, I.; Sánchez, Y. Role of Rho GTPases and Rho-GEFs in the regulation of cell shape and integrity in fission yeast. Yeast 2006, 23, 1031–1043. [Google Scholar] [CrossRef]
- Tajadura, V.; García, B.; García, I.; García, P.; Sánchez, Y. Schizosaccharomyces pombe Rgf3p is a specific Rho1 GEF that regulates cell wall beta-glucan biosynthesis through the GTPase Rho1p. J. Cell Sci. 2004, 117, 6163–6174. [Google Scholar] [CrossRef] [Green Version]
- Calonge, T.M.; Arellano, M.; Coll, P.M.; Perez, P. Rga5p is a specific Rho1p GTPase-activating protein that regulates cell integrity in Schizosaccharomyces pombe. Mol. Microbiol. 2003, 47, 507–518. [Google Scholar] [CrossRef] [Green Version]
- García, P.; Tajadura, V.; García, I.; Sánchez, Y. Rgf1p is a specific Rho1-GEF that coordinates cell polarization with cell wall biogenesis in fission yeast. Mol. Biol. Cell 2006, 17, 1620–1631. [Google Scholar] [CrossRef] [Green Version]
- Barba, G.; Soto, T.; Madrid, M.; Núñez, A.; Vicente, J.; Gacto, M.; Cansado, J.; Group, Y.P. Activation of the cell integrity pathway is channelled through diverse signalling elements in fission yeast. Cell. Signal. 2008, 20, 748–757. [Google Scholar] [CrossRef]
- Nakano, K.; Mutoh, T.; Arai, R.; Mabuchi, I. The small GTPase Rho4 is involved in controlling cell morphology and septation in fission yeast. Genes Cells 2003, 8, 357–370. [Google Scholar] [CrossRef]
- Santos, B.; Martín-Cuadrado, A.B.; Vázquez de Aldana, C.R.; del Rey, F.; Pérez, P. Rho4 GTPase is involved in secretion of glucanases during fission yeast cytokinesis. Eukaryot. Cell 2005, 4, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Santos, B.; Gutiérrez, J.; Calonge, T.M.; Pérez, P. Novel Rho GTPase involved in cytokinesis and cell wall integrity in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 2003, 2, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Arai, R.; Mabuchi, I. Small GTPase Rho5 is a functional homologue of Rho1, which controls cell shape and septation in fission yeast. FEBS Lett. 2005, 579, 5181–5186. [Google Scholar] [CrossRef] [Green Version]
- Rincón, S.A.; Santos, B.; Pérez, P. Fission yeast Rho5p GTPase is a functional paralogue of Rho1p that plays a role in survival of spores and stationary-phase cells. Eukaryot. Cell 2006, 5, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Doi, A.; Kita, A.; Kanda, Y.; Uno, T.; Asami, K.; Satoh, R.; Nakano, K.; Sugiura, R. Geranylgeranyltransferase Cwg2-Rho4/Rho5 module is implicated in the Pmk1 MAP kinase-mediated cell wall integrity pathway in fission yeast. Genes Cells 2015, 20, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef]
- Madrid, M.; Vazquez-Marin, B.; Franco, A.; Soto, T.; Vicente-Soler, J.; Gacto, M.; Cansado, J. Multiple crosstalk between TOR and the cell integrity MAPK signaling pathway in fission yeast. Sci. Rep. 2016, 6, 37515. [Google Scholar] [CrossRef]
- Freeley, M.; Kelleher, D.; Long, A. Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites. Cell. Signal. 2011, 23, 753–762. [Google Scholar] [CrossRef]
- Madrid, M.; Vázquez-Marín, B.; Soto, T.; Franco, A.; Gómez-Gil, E.; Vicente-Soler, J.; Gacto, M.; Pérez, P.; Cansado, J. Differential functional regulation of protein kinase C (PKC) orthologs in fission yeast. J. Biol. Chem. 2017, 292, 11374–11387. [Google Scholar] [CrossRef] [Green Version]
- Toda, T.; Shimanuki, M.; Yanagida, M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993, 12, 1987–1995. [Google Scholar] [CrossRef]
- Matsuyama, A.; Arai, R.; Yashiroda, Y.; Shirai, A.; Kamata, A.; Sekido, S.; Kobayashi, Y.; Hashimoto, A.; Hamamoto, M.; Hiraoka, Y.; et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 2006, 24, 841–847. [Google Scholar] [CrossRef]
- Magliozzi, J.O.; Sears, J.; Cressey, L.; Brady, M.; Opalko, H.E.; Kettenbach, A.N.; Moseley, J.B. Fission yeast Pak1 phosphorylates anillin-like Mid1 for spatial control of cytokinesis. J. Cell Biol. 2020, 219, e201908017. [Google Scholar] [CrossRef]
- Madrid, M.; Jiménez, R.; Sánchez-Mir, L.; Soto, T.; Franco, A.; Vicente-Soler, J.; Gacto, M.; Pérez, P.; Cansado, J. Multiple layers of regulation influence cell integrity control by the PKC ortholog Pck2 in fission yeast. J. Cell Sci. 2015, 128, 266–280. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y.; Satoh, R.; Takasaki, T.; Tomimoto, N.; Tsuchiya, K.; Tsai, C.A.; Tanaka, T.; Kyomoto, S.; Hamada, K.; Fujiwara, T.; et al. Sequestration of the PKC ortholog Pck2 in stress granules as a feedback mechanism of MAPK signaling in fission yeast. J. Cell Sci. 2021, 134, jcs250191. [Google Scholar] [CrossRef]
- Newton, A.C. Protein kinase C: Poised to signal. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E395–E402. [Google Scholar] [CrossRef] [Green Version]
- Niederberger, C.; Schweingruber, M.E. A Schizosaccharomyces pombe gene, ksg1, that shows structural homology to the human phosphoinositide-dependent protein kinase PDK1, is essential for growth, mating and sporulation. Mol. Gen. Genet. 1999, 261, 177–183. [Google Scholar] [CrossRef]
- Gräub, R.; Hilti, N.; Niederberger, C.; Schweingruber, M.E. Ksg1, a homologue of the phosphoinositide-dependent protein kinase 1, controls cell wall integrity in Schizosaccharomyces pombe. J. Basic Microbiol. 2003, 43, 473–482. [Google Scholar] [CrossRef]
- Madrid, M.; Fernández-Zapata, J.; Sánchez-Mir, L.; Soto, T.; Franco, A.; Vicente-Soler, J.; Gacto, M.; Cansado, J. Role of the fission yeast cell integrity MAPK pathway in response to glucose limitation. BMC Microbiol. 2013, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Kanda, Y.; Satoh, R.; Matsumoto, S.; Ikeda, C.; Inutsuka, N.; Hagihara, K.; Matzno, S.; Tsujimoto, S.; Kita, A.; Sugiura, R. Skb5, an SH3 adaptor protein, regulates Pmk1 MAPK signaling by controlling the intracellular localization of the MAPKKK Mkh1. J. Cell Sci. 2016, 129, 3189–3202. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Pimental, R.; Lai, H.; Marcus, S. Direct activation of the fission yeast PAK Shk1 by the novel SH3 domain protein, Skb5. J. Biol. Chem. 1999, 274, 36052–36057. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, B.; Soto, T.; del Dedo, J.E.; Franco, A.; Vicente, J.; Hidalgo, E.; Gacto, M.; Cansado, J.; Madrid, M. Distinct biological activity of threonine monophosphorylated MAPK isoforms during the stress response in fission yeast. Cell. Signal. 2015, 27, 2534–2542. [Google Scholar] [CrossRef]
- Sánchez-Mir, L.; Franco, A.; Madrid, M.; Vicente-Soler, J.; Villar-Tajadura, M.A.; Soto, T.; Pérez, P.; Gacto, M.; Cansado, J. Biological significance of nuclear localization of mitogen-activated protein kinase Pmk1 in fission yeast. J. Biol. Chem. 2012, 287, 26038–26051. [Google Scholar] [CrossRef] [Green Version]
- Soto, T.; Núñez, A.; Madrid, M.; Vicente, J.; Gacto, M.; Cansado, J. Transduction of centrifugation-induced gravity forces through mitogen-activated protein kinase pathways in the fission yeast Schizosaccharomyces pombe. Microbiology 2007, 153, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Kaino, T.; Tonoko, K.; Mochizuki, S.; Takashima, Y.; Kawamukai, M. Schizosaccharomyces japonicus has low levels of CoQ(10) synthesis, respiration deficiency, and efficient ethanol production. Biosci. Biotechnol. Biochem. 2018, 82, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Degols, G.; Shiozaki, K.; Russell, P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 1996, 16, 2870–2877. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, K.; Russell, P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996, 10, 2276–2288. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, M.G.; Samuels, M.; Takeda, T.; Toone, W.M.; Shieh, J.C.; Toda, T.; Millar, J.B.; Jones, N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996, 10, 2289–2301. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, C.L.; Maekawa, H.; Worthington, J.L.; Reiter, W.; Wilkinson, C.R.; Jones, N. Regulation of Schizosaccharomyces pombe Atf1 protein levels by Sty1-mediated phosphorylation and heterodimerization with Pcr1. J. Biol. Chem. 2007, 282, 5160–5170. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wilkinson, C.R.; Watt, S.; Penkett, C.J.; Toone, W.M.; Jones, N.; Bähler, J. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol. Biol. Cell 2008, 19, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Reiter, W.; Watt, S.; Dawson, K.; Lawrence, C.L.; Bahler, J.; Jones, N.; Wilkinson, C.R. Fission yeast MAP kinase Sty1 is recruited to stress-induced genes. J. Biol. Chem. 2008, 283, 9945–9956. [Google Scholar] [CrossRef] [Green Version]
- Takada, H.; Nishimura, M.; Asayama, Y.; Mannse, Y.; Ishiwata, S.; Kita, A.; Doi, A.; Nishida, A.; Kai, N.; Moriuchi, S.; et al. Atf1 is a target of the mitogen-activated protein kinase Pmk1 and regulates cell integrity in fission yeast. Mol. Biol. Cell 2007, 18, 4794–4802. [Google Scholar] [CrossRef] [Green Version]
- Takada, H.; Nishida, A.; Domae, M.; Kita, A.; Yamano, Y.; Uchida, A.; Ishiwata, S.; Fang, Y.; Zhou, X.; Masuko, T.; et al. The cell surface protein gene ecm33+ is a target of the two transcription factors Atf1 and Mbx1 and negatively regulates Pmk1 MAPK cell integrity signaling in fission yeast. Mol. Biol. Cell 2010, 21, 674–685. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, K.; Ng, S.S.; Ohkura, H.; Geymonat, M.; Sedgwick, S.G.; McInerny, C.J. Regulation of gene expression during M-G1-phase in fission yeast through Plo1p and forkhead transcription factors. J. Cell Sci. 2008, 121, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Faoro, H.; Alves, L.R.; Goldenberg, S. RNA-binding proteins and their role in the regulation of gene expression in Trypanosoma cruzi and Saccharomyces cerevisiae. Genet. Mol. Biol. 2017, 40, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, R.; Satoh, R.; Ishiwata, S.; Umeda, N.; Kita, A. Role of RNA-Binding Proteins in MAPK Signal Transduction Pathway. J. Signal Transduct. 2011, 2011, 109746. [Google Scholar] [CrossRef] [Green Version]
- Venigalla, R.K.; Turner, M. RNA-binding proteins as a point of convergence of the PI3K and p38 MAPK pathways. Front. Immunol. 2012, 3, 398. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Cotobal, C.; Duncan, C.D.; Mata, J. Systematic analysis of the role of RNA-binding proteins in the regulation of RNA stability. PLoS Genet. 2014, 10, e1004684. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Cuervo, H.; Bueno, A. Cds1 controls the release of Cdc14-like phosphatase Flp1 from the nucleolus to drive full activation of the checkpoint response to replication stress in fission yeast. Mol. Biol. Cell 2008, 19, 2488–2499. [Google Scholar] [CrossRef] [Green Version]
- Broadus, M.R.; Gould, K.L. Multiple protein kinases influence the redistribution of fission yeast Clp1/Cdc14 phosphatase upon genotoxic stress. Mol. Biol. Cell 2012, 23, 4118–4128. [Google Scholar] [CrossRef]
- Sugiura, R.; Kita, A.; Shimizu, Y.; Shuntoh, H.; Sio, S.O.; Kuno, T. Feedback regulation of MAPK signalling by an RNA-binding protein. Nature 2003, 424, 961–965. [Google Scholar] [CrossRef]
- Tsukahara, K.; Yamamoto, H.; Okayama, H. An RNA binding protein negatively controlling differentiation in fission yeast. Mol. Cell. Biol. 1998, 18, 4488–4498. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.T.; Ozoe, F.; Tanaka, K.; Nakagawa, T.; Matsuda, H.; Kawamukai, M. A novel gene, msa1, inhibits sexual differentiation in Schizosaccharomyces pombe. Genetics 2004, 167, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.T.; Oowatari, Y.; Abe, M.; Tanaka, K.; Matsuda, H.; Kawamukai, M. Interaction between a negative regulator (Msa2/Nrd1) and a positive regulator (Cpc2) of sexual differentiation in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2004, 68, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Mata, J.; Bähler, J. Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast. Proc. Natl. Acad. Sci. USA 2006, 103, 15517–15522. [Google Scholar] [CrossRef] [Green Version]
- Satoh, R.; Morita, T.; Takada, H.; Kita, A.; Ishiwata, S.; Doi, A.; Hagihara, K.; Taga, A.; Matsumura, Y.; Tohda, H.; et al. Role of the RNA-binding Protein Nrd1 and Pmk1 Mitogen-activated Protein Kinase in the Regulation of Myosin mRNA Stability in Fission Yeast. Mol. Biol. Cell 2009, 20, 2473–2485. [Google Scholar] [CrossRef] [Green Version]
- Pollard, T.D.; Wu, J.Q. Understanding cytokinesis: Lessons from fission yeast. Nat. Rev. Mol. Cell Biol. 2010, 11, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, A.; Kanaba, T.; Satoh, R.; Fujiwara, T.; Ito, Y.; Sugiura, R.; Mishima, M. Structure of the second RRM domain of Nrd1, a fission yeast MAPK target RNA binding protein, and implication for its RNA recognition and regulation. Biochem. Biophys. Res. Commun. 2013, 437, 12–17. [Google Scholar] [CrossRef]
- Satoh, R.; Tanaka, A.; Kita, A.; Morita, T.; Matsumura, Y.; Umeda, N.; Takada, M.; Hayashi, S.; Tani, T.; Shinmyozu, K.; et al. Role of the RNA-Binding Protein Nrd1 in Stress Granule Formation and Its Implication in the Stress Response in Fission Yeast. PLoS ONE 2012, 7, e29683. [Google Scholar] [CrossRef] [Green Version]
- Oowatari, Y.; Jeong, H.; Tanae, K.; Nakagawa, T.; Kawamukai, M. Regulation and role of an RNA-binding protein Msa2 in controlling the sexual differentiation of fission yeast. Curr. Genet. 2011, 57, 191–200. [Google Scholar] [CrossRef]
- Kettenbach, A.N.; Deng, L.; Wu, Y.; Baldissard, S.; Adamo, M.E.; Gerber, S.A.; Moseley, J.B. Quantitative phosphoproteomics reveals pathways for coordination of cell growth and division by the conserved fission yeast kinase pom1. Mol. Cell. Proteomics 2015, 14, 1275–1287. [Google Scholar] [CrossRef] [Green Version]
- Swaffer, M.P.; Jones, A.W.; Flynn, H.R.; Snijders, A.P.; Nurse, P. Quantitative Phosphoproteomics Reveals the Signaling Dynamics of Cell-Cycle Kinases in the Fission Yeast Schizosaccharomyces pombe. Cell Rep. 2018, 24, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Tay, Y.D.; Leda, M.; Spanos, C.; Rappsilber, J.; Goryachev, A.B.; Sawin, K.E. Fission Yeast NDR/LATS Kinase Orb6 Regulates Exocytosis via Phosphorylation of the Exocyst Complex. Cell Rep. 2019, 26, 1654–1667.e1657. [Google Scholar] [CrossRef] [Green Version]
- Nunez, A.; Franco, A.; Madrid, M.; Soto, T.; Vicente, J.; Gacto, M.; Cansado, J. Role for RACK1 Orthologue Cpc2 in the Modulation of Stress Response in Fission Yeast. Mol. Biol. Cell 2009, 20, 3996–4009. [Google Scholar] [CrossRef] [Green Version]
- Hollingworth, D.; Candel, A.M.; Nicastro, G.; Martin, S.R.; Briata, P.; Gherzi, R.; Ramos, A. KH domains with impaired nucleic acid binding as a tool for functional analysis. Nucleic Acids Res. 2012, 40, 6873–6886. [Google Scholar] [CrossRef]
- Grishin, N.V. KH domain: One motif, two folds. Nucleic Acids Res. 2001, 29, 638–643. [Google Scholar] [CrossRef]
- Sugiura, R.; Kita, A.; Kuno, T. Upregulation of mRNA in MAPK signaling—Transcriptional activation or mRNA stabilization? Cell Cycle 2004, 3, 286–288. [Google Scholar] [CrossRef] [Green Version]
- Satoh, R.; Matsumura, Y.; Tanaka, A.; Takada, M.; Ito, Y.; Hagihara, K.; Inari, M.; Kita, A.; Fukao, A.; Fujiwara, T.; et al. Spatial regulation of the KH domain RNA-binding protein Rnc1 mediated by a Crm1-independent nuclear export system in Schizosaccharomyces pombe. Mol. Microbiol. 2017, 104, 428–448. [Google Scholar] [CrossRef] [Green Version]
- Satoh, R.; Hagihara, K.; Sugiura, R. Rae1-mediated nuclear export of Rnc1 is an important determinant in controlling MAPK signaling. Curr. Genet. 2018, 64, 103–108. [Google Scholar] [CrossRef]
- Satoh, R.; Hara, N.; Kawasaki, A.; Takasaki, T.; Sugiura, R. Distinct modes of stress granule assembly mediated by the KH-type RNA-binding protein Rnc1. Genes Cells 2018, 23, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Ruiz, F.; Vicente-Soler, J.; Franco, A.; Gomez-Gil, E.; Sanchez-Marinas, M.; Vazquez-Marin, B.; Aligue, R.; Madrid, M.; Moreno, S.; Soto, T.; et al. RNA-Binding Protein Rnc1 Regulates Cell Length at Division and Acute Stress Response in Fission Yeast through Negative Feedback Modulation of the Stress-Activated Mitogen-Activated Protein Kinase Pathway. mBio 2020, 11, e02815-19. [Google Scholar] [CrossRef] [Green Version]
- Hirayama, S.; Sugiura, R.; Lu, Y.; Maeda, T.; Kawagishi, K.; Yokoyama, M.; Tohda, H.; Giga-Hama, Y.; Shuntoh, H.; Kuno, T. Zinc finger protein Prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J. Biol. Chem. 2003, 278, 18078–18084. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Sugiura, R.; Takeuchi, M.; Suzuki, M.; Ebina, H.; Takami, T.; Koike, A.; Iba, S.; Kuno, T. Real-time monitoring of calcineurin activity in living cells: Evidence for two distinct Ca2+-dependent pathways in fission yeast. Mol. Biol. Cell 2006, 17, 4790–4800. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki-Katagiri, N.; Ames, J.B. Neuronal calcium sensor-1 (Ncs1p) is up-regulated by calcineurin to promote Ca2+ tolerance in fission yeast. J. Biol. Chem. 2010, 285, 4405–4414. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Sugiura, R.; Kita, A.; Saito, M.; Deng, L.; He, Y.; Yabin, L.; Fujita, Y.; Takegawa, K.; Shuntoh, H.; et al. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: The importance of Mn2+ homeostasis. Genes Cells 2004, 9, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Cisneros-Barroso, E.; Yance-Chávez, T.; Kito, A.; Sugiura, R.; Gómez-Hierro, A.; Giménez-Zaragoza, D.; Aligue, R. Negative feedback regulation of calcineurin-dependent Prz1 transcription factor by the CaMKK-CaMK1 axis in fission yeast. Nucleic Acids Res. 2014, 42, 9573–9587. [Google Scholar] [CrossRef] [Green Version]
- Chatfield-Reed, K.; Vachon, L.; Kwon, E.J.; Chua, G. Conserved and Diverged Functions of the Calcineurin-Activated Prz1 Transcription Factor in Fission Yeast. Genetics 2016, 202, 1365–1375. [Google Scholar] [CrossRef]
- Yoshida, T.; Toda, T.; Yanagida, M. A calcineurin-like gene ppb1+ in fission yeast: Mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J. Cell Sci. 1994, 107 Pt 7, 1725–1735. [Google Scholar] [CrossRef]
- Aydar, E.; Palmer, C.P. Polycystic kidney disease channel and synaptotagmin homologues play roles in schizosaccharomyces pombe cell wall synthesis/repair and membrane protein trafficking. J. Membr. Biol. 2009, 229, 141–152. [Google Scholar] [CrossRef]
- Carnero, E.; Ribas, J.C.; García, B.; Durán, A.; Sánchez, Y. Schizosaccharomyces pombe ehs1p is involved in maintaining cell wall integrity and in calcium uptake. Mol. Gen. Genet. 2000, 264, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Sugiura, R.; Koike, A.; Ebina, H.; Sio, S.O.; Kuno, T. Transient receptor potential (TRP) and Cch1-Yam8 channels play key roles in the regulation of cytoplasmic Ca2+ in fission yeast. PLoS ONE 2011, 6, e22421. [Google Scholar] [CrossRef] [Green Version]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, FUNK-0035-2016. [Google Scholar] [CrossRef] [Green Version]
- Hopke, A.; Brown, A.J.P.; Hall, R.A.; Wheeler, R.T. Dynamic Fungal Cell Wall Architecture in Stress Adaptation and Immune Evasion. Trends Microbiol. 2018, 26, 284–295. [Google Scholar] [CrossRef]
- Mazur, P.; Baginsky, W. In vitro activity of 1,3-beta-D-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 1996, 271, 14604–14609. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Shen, X.; Yan, G.; Ma, D.; Bai, X.; Li, S.; Jiang, Y. A MAP Kinase Dependent Feedback Mechanism Controls Rho1 GTPase and Actin Distribution in Yeast. PLoS ONE 2009, 4, e6089. [Google Scholar] [CrossRef] [Green Version]
- Kampmeyer, C.; Johansen, J.V.; Holmberg, C.; Karlson, M.; Gersing, S.K.; Bordallo, H.N.; Kragelund, B.B.; Lerche, M.H.; Jourdain, I.; Winther, J.R.; et al. Mutations in a Single Signaling Pathway Allow Cell Growth in Heavy Water. ACS Synth. Biol. 2020, 9, 733–748. [Google Scholar] [CrossRef]
- Imai, Y.; Shimasaki, T.; Enokimura, C.; Ohtsuka, H.; Tsubouchi, S.; Ihara, K.; Aiba, H. gas1 mutation extends chronological lifespan via Pmk1 and Sty1 MAPKs in Schizosaccharomyces pombe. Biosci. Biotechnol. Biochem. 2020, 84, 330–337. [Google Scholar] [CrossRef]
- Merla, A.; Johnson, D.I. The Schizosaccharomyces pombe Cdc42p GTPase signals through Pak2p and the Mkh1p-Pek1p-Spm1p MAP kinase pathway. Curr. Genet. 2001, 39, 205–209. [Google Scholar] [CrossRef]
- Kono, K.; Saeki, Y.; Yoshida, S.; Tanaka, K.; Pellman, D. Proteasomal degradation resolves competition between cell polarization and cellular wound healing. Cell 2012, 150, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Oliferenko, S. Comparative biology of cell division in the fission yeast clade. Curr. Opin. Microbiol. 2015, 28, 18–25. [Google Scholar] [CrossRef]
- Rincon, S.A.; Paoletti, A. Molecular control of fission yeast cytokinesis. Semin. Cell Dev. Biol. 2016, 53, 28–38. [Google Scholar] [CrossRef]
- Garcia Cortes, J.C.; Ramos, M.; Osumi, M.; Perez, P.; Ribas, J.C. The Cell Biology of Fission Yeast Septation. Microbiol. Mol. Biol. Rev. 2016, 80, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Tolliday, N.; VerPlank, L.; Li, R. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 2002, 12, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Chin, C.F.; Bennett, A.M.; Ma, W.K.; Hall, M.C.; Yeong, F.M. Dependence of Chs2 ER export on dephosphorylation by cytoplasmic Cdc14 ensures that septum formation follows mitosis. Mol. Biol. Cell 2012, 23, 45–58. [Google Scholar] [CrossRef]
- Yoshida, S.; Bartolini, S.; Pellman, D. Mechanisms for concentrating Rho1 during cytokinesis. Genes Dev. 2009, 23, 810–823. [Google Scholar] [CrossRef] [Green Version]
- García, R.; Bermejo, C.; Grau, C.; Pérez, R.; Rodríguez-Peña, J.M.; Francois, J.; Nombela, C.; Arroyo, J. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 2004, 279, 15183–15195. [Google Scholar] [CrossRef] [Green Version]
- Cabib, E.; Arroyo, J. How carbohydrates sculpt cells: Chemical control of morphogenesis in the yeast cell wall. Nat. Rev. Microbiol. 2013, 11, 648–655. [Google Scholar] [CrossRef]
- Piña, F.J.; Niwa, M. The ER Stress Surveillance (ERSU) pathway regulates daughter cell ER protein aggregate inheritance. Elife 2015, 4, e06970. [Google Scholar] [CrossRef]
- Babour, A.; Bicknell, A.A.; Tourtellotte, J.; Niwa, M. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell 2010, 142, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Edreira, T.; Celador, R.; Manjón, E.; Sánchez, Y. A novel checkpoint pathway controls actomyosin ring constriction trigger in fission yeast. eLife 2020, 9, e59333. [Google Scholar] [CrossRef]
- Jin, Q.-W.; Zhou, M.; Bimbo, A.; Balasubramanian, M.K.; McCollum, D. A role for the septation initiation network in septum assembly revealed by genetic analysis of sid2-250 suppressors. Genetics 2006, 172, 2101–2112. [Google Scholar] [CrossRef] [Green Version]
- Davidson, R.; Laporte, D.; Wu, J.-Q. Regulation of Rho-GEF Rgf3 by the arrestin Art1 in fission yeast cytokinesis. Mol. Biol. Cell 2014, 26, 453–466. [Google Scholar] [CrossRef]
- Martín-García, R.; Arribas, V.; Coll, P.M.; Pinar, M.; Viana, R.A.; Rincón, S.A.; Correa-Bordes, J.; Ribas, J.C.; Pérez, P. Paxillin-Mediated Recruitment of Calcineurin to the Contractile Ring Is Required for the Correct Progression of Cytokinesis in Fission Yeast. Cell Rep. 2018, 25, 772–783.e774. [Google Scholar] [CrossRef] [Green Version]
- Mascaraque, V.; Hernáez, M.L.; Jiménez-Sánchez, M.; Hansen, R.; Gil, C.; Martín, H.; Cid, V.J.; Molina, M. Phosphoproteomic analysis of protein kinase C signaling in Saccharomyces cerevisiae reveals Slt2 mitogen-activated protein kinase (MAPK)-dependent phosphorylation of eisosome core components. Mol. Cell Proteom. 2013, 12, 557–574. [Google Scholar] [CrossRef] [Green Version]
- Dodgson, J.; Chessel, A.; Vaggi, F.; Giordan, M.; Yamamoto, M.; Arai, K.; Madrid, M.; Geymonat, M.; Abenza, J.F.; Cansado, J.; et al. Reconstructing regulatory pathways by systematically mapping protein localization interdependency networks. bioRxiv 2017. [Google Scholar] [CrossRef] [Green Version]
- Cortés, J.C.; Konomi, M.; Martins, I.M.; Muñoz, J.; Moreno, M.B.; Osumi, M.; Durán, A.; Ribas, J.C. The (1,3)beta-D-glucan synthase subunit Bgs1p is responsible for the fission yeast primary septum formation. Mol. Microbiol. 2007, 65, 201–217. [Google Scholar] [CrossRef]
- Gotoh, Y.; Nishida, E.; Shimanuki, M.; Toda, T.; Imai, Y.; Yamamoto, M. Schizosaccharomyces pombe Spk1 is a tyrosine-phosphorylated protein functionally related to Xenopus mitogen-activated protein kinase. Mol. Cell. Biol. 1993, 13, 6427–6434. [Google Scholar] [CrossRef]
- Millar, J.B.; Buck, V.; Wilkinson, M.G. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995, 9, 2117–2130. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, K.; Russell, P. Counteractive roles of protein phosphatase 2C (PP2C) and a MAP kinase kinase homolog in the osmoregulation of fission yeast. EMBO J. 1995, 14, 492–502. [Google Scholar] [CrossRef]
- Didmon, M.; Davis, K.; Watson, P.; Ladds, G.; Broad, P.; Davey, J. Identifying regulators of pheromone signalling in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 2002, 41, 241–253. [Google Scholar] [CrossRef]
- Degols, G.; Russell, P. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 1997, 17, 3356–3363. [Google Scholar] [CrossRef] [Green Version]
- Soto, T.; Beltrán, F.F.; Paredes, V.; Madrid, M.; Millar, J.B.; Vicente-Soler, J.; Cansado, J.; Gacto, M. Cold induces stress-activated protein kinase-mediated response in the fission yeast Schizosaccharomyces pombe. Eur. J. Biochem. 2002, 269, 5056–5065. [Google Scholar] [CrossRef]
- Quinn, J.; Findlay, V.J.; Dawson, K.; Millar, J.B.; Jones, N.; Morgan, B.A.; Toone, W.M. Distinct regulatory proteins control the graded transcriptional response to increasing H(2)O(2) levels in fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 2002, 13, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Gabriel, M.A.; Russell, P. Distinct signaling pathways respond to arsenite and reactive oxygen species in Schizosaccharomyces pombe. Eukaryot. Cell 2005, 4, 1396–1402. [Google Scholar] [CrossRef] [Green Version]
- George, V.T.; Brooks, G.; Humphrey, T.C. Regulation of cell cycle and stress responses to hydrostatic pressure in fission yeast. Mol. Biol. Cell 2007, 18, 4168–4179. [Google Scholar] [CrossRef]
- Buck, V.; Quinn, J.; Soto Pino, T.; Martin, H.; Saldanha, J.; Makino, K.; Morgan, B.A.; Millar, J.B. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 2001, 12, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. MMBR 2002, 66, 300–372. [Google Scholar] [CrossRef] [Green Version]
- Samejima, I.; Mackie, S.; Fantes, P.A. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997, 16, 6162–6170. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.N.; Shiozaki, K. Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 1999, 13, 1653–1663. [Google Scholar] [CrossRef]
- Gaits, F.; Russell, P. Active nucleocytoplasmic shuttling required for function and regulation of stress-activated kinase Spc1/StyI in fission yeast. Mol. Biol. Cell 1999, 10, 1395–1407. [Google Scholar] [CrossRef] [Green Version]
- Doi, K.; Gartner, A.; Ammerer, G.; Errede, B.; Shinkawa, H.; Sugimoto, K.; Matsumoto, K. MSG5, a novel protein phosphatase promotes adaptation to pheromone response in S. cerevisiae. EMBO J. 1994, 13, 61–70. [Google Scholar] [CrossRef]
- Watanabe, Y.; Irie, K.; Matsumoto, K. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1995, 15, 5740–5749. [Google Scholar] [CrossRef] [Green Version]
- Davenport, K.D.; Williams, K.E.; Ullmann, B.D.; Gustin, M.C. Activation of the Saccharomyces cerevisiae filamentation/invasion pathway by osmotic stress in high-osmolarity glycogen pathway mutants. Genetics 1999, 153, 1091–1103. [Google Scholar] [CrossRef]
- Martín, H.; Rodríguez-Pachón, J.M.; Ruiz, C.; Nombela, C.; Molina, M. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Cybulski, N.; Hall, M.N. TOR complex 2: A signaling pathway of its own. Trends Biochem. Sci. 2009, 34, 620–627. [Google Scholar] [CrossRef]
- Loewith, R. A brief history of TOR. Biochem. Soc. Trans. 2011, 39, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, B.; Moreno, S. Fission yeast Tor2 promotes cell growth and represses cell differentiation. J. Cell Sci. 2006, 119, 4475–4485. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, A.; Otsubo, Y.; Yamashita, A.; Sato, T.; Yamamoto, M.; Tamanoi, F. Psk1, an AGC kinase family member in fission yeast, is directly phosphorylated and controlled by TORC1 and functions as S6 kinase. J. Cell Sci. 2012, 125, 5840–5849. [Google Scholar] [CrossRef] [Green Version]
- Valbuena, N.; Rozalén, A.E.; Moreno, S. Fission yeast TORC1 prevents eIF2α phosphorylation in response to nitrogen and amino acids via Gcn2 kinase. J. Cell Sci. 2012, 125, 5955–5959. [Google Scholar] [CrossRef] [Green Version]
- Weisman, R.; Choder, M. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 2001, 276, 7027–7032. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Kubo, Y.; Watanabe, Y.; Yamamoto, M. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 2003, 22, 3073–3083. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Morigasaki, S.; Tatebe, H.; Tamanoi, F.; Shiozaki, K. Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle 2008, 7, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Duran, R.V.; Hall, M.N. Regulation of TOR by small GTPases. EMBO Rep. 2012, 13, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Morigasaki, S.; Chin, L.C.; Hatano, T.; Emori, M.; Iwamoto, M.; Tatebe, H.; Shiozaki, K. Modulation of TOR complex 2 signaling by the stress-activated MAPK pathway in fission yeast. J. Cell Sci. 2019, 132, jcs236133. [Google Scholar] [CrossRef]
- Petersen, J.; Hagan, I.M. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature 2005, 435, 507–512. [Google Scholar] [CrossRef]
- Torres, J.; Di Como, C.J.; Herrero, E.; De La Torre-Ruiz, M.A. Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J. Biol. Chem. 2002, 277, 43495–43504. [Google Scholar] [CrossRef] [Green Version]
- Hatano, T.; Morigasaki, S.; Tatebe, H.; Ikeda, K.; Shiozaki, K. Fission yeast Ryh1 GTPase activates TOR Complex 2 in response to glucose. Cell Cycle 2015, 14, 848–856. [Google Scholar] [CrossRef] [Green Version]
- Zhan, K.; Narasimhan, J.; Wek, R.C. Differential activation of eIF2 kinases in response to cellular stresses in Schizosaccharomyces pombe. Genetics 2004, 168, 1867–1875. [Google Scholar] [CrossRef] [Green Version]
- Berlanga, J.J.; Rivero, D.; Martín, R.; Herrero, S.; Moreno, S.; de Haro, C. Role of mitogen-activated protein kinase Sty1 in regulation of eukaryotic initiation factor 2alpha kinases in response to environmental stress in Schizosaccharomyces pombe. Eukaryot. Cell 2010, 9, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Asano, K. Origin of translational control by eIF2α phosphorylation: Insights from genome-wide translational profiling studies in fission yeast. Curr. Genet. 2021, 67, 359–368. [Google Scholar] [CrossRef]
- Nakashima, A.; Sato, T.; Tamanoi, F. Fission yeast TORC1 regulates phosphorylation of ribosomal S6 proteins in response to nutrients and its activity is inhibited by rapamycin. J. Cell Sci. 2010, 123, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Hálová, L.; Kirkham, S.; Atkin, J.; Petersen, J. TORC2 and the AGC kinase Gad8 regulate phosphorylation of the ribosomal protein S6 in fission yeast. Biol. Open 2012, 1, 884–888. [Google Scholar] [CrossRef] [Green Version]
- Omnus, D.J.; Manford, A.G.; Bader, J.M.; Emr, S.D.; Stefan, C.J. Phosphoinositide kinase signaling controls ER-PM cross-talk. Mol. Biol. Cell 2016, 27, 1170–1180. [Google Scholar] [CrossRef]
- Du, W.; Forte, G.M.; Smith, D.; Petersen, J. Phosphorylation of the amino-terminus of the AGC kinase Gad8 prevents its interaction with TORC2. Open Biol. 2016, 6, 150189. [Google Scholar] [CrossRef] [Green Version]
- Tatebe, H.; Murayama, S.; Yonekura, T.; Hatano, T.; Richter, D.; Furuya, T.; Kataoka, S.; Furuita, K.; Kojima, C.; Shiozaki, K. Substrate specificity of TOR complex 2 is determined by a ubiquitin-fold domain of the Sin1 subunit. eLife 2017, 6, e19594. [Google Scholar] [CrossRef] [Green Version]
- García, R.; Bravo, E.; Diez-Muñiz, S.; Nombela, C.; Rodríguez-Peña, J.M.; Arroyo, J. A novel connection between the Cell Wall Integrity and the PKA pathways regulates cell wall stress response in yeast. Sci. Rep. 2017, 7, 5703. [Google Scholar] [CrossRef]
- Gerik, K.J.; Donlin, M.J.; Soto, C.E.; Banks, A.M.; Banks, I.R.; Maligie, M.A.; Selitrennikoff, C.P.; Lodge, J.K. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol. Microbiol. 2005, 58, 393–408. [Google Scholar] [CrossRef]
- Donlin, M.J.; Upadhya, R.; Gerik, K.J.; Lam, W.; VanArendonk, L.G.; Specht, C.A.; Sharma, N.K.; Lodge, J.K. Cross talk between the cell wall integrity and cyclic AMP/protein kinase A pathways in Cryptococcus neoformans. mBio 2014, 5, e01573-14. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Watanabe, Y.; Yamamoto, M. Protein kinase A regulates sexual development and gluconeogenesis through phosphorylation of the Zn finger transcriptional activator Rst2p in fission yeast. Mol. Cell. Biol. 2002, 22, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, C.S. Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem. Soc. Trans. 2005, 33, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, C.S. Except in every detail: Comparing and contrasting G-protein signaling in Saccharomyces cerevisiae and Schizosaccharomyces pombe. Eukaryot. Cell 2005, 4, 495–503. [Google Scholar] [CrossRef] [Green Version]
| Protein | Function | S. cerevisiae Ortholog | |
|---|---|---|---|
| Cell-surface sensors | Wsc1 | - Plasma membrane-associated serine-rich cell wall mechanosensor located at active growth sites and the division septum - Detects perturbations at the CW and plasma membrane and transmits signals through Rgf1 (Rho1 GEF) | WSC1 |
| Mtl2 | - Plasma membrane-associated serine-rich cell wall mechanosensor located at cellular periphery - Detects perturbations at the CW and plasma membrane and transmits signals through Rgf1 | MLT1 | |
| Regulators of GTPases | Rgf1 | - Guanine nucleotide exchange factor (GEF) for Rho1 GTPase - Activates the CIP via Rho1 and Pck2 | ROM1 |
| Rga2 | - GTPase activating protein (GAP) for Rho1 and Rho2. - Negatively regulates the Rho2–Pck2 interaction with the CIP | BEM3 | |
| Rga4 | Rho2 GAP that negatively regulates the activity of the CIP | RGA2 | |
| Rga6 | Rho2 GAP | ||
| Rga7 | Rho2 GAP that negatively regulates the activity of the CIP | RGD1 | |
| Rho GTPases | Rho1 | - Regulation of cell wall biosynthesis by β-glucan synthases Bgs1-4 - Stabilizes Pck1 and Pck2 - Regulates the CIP in response to cell wall damage, independently of Wsc1 and Mlt2 - Cytokinesis checkpoint to cell wall damage through Pmk1 | RHO1 |
| Rho2 | - Regulation of cell wall biosynthesis by α-glucan synthase Mok1/Ags1 - Major CIP regulator together with Pck2 | RHO2 | |
| Rho4 | - Minor role in CIP signaling | RHO4 | |
| Rho5 | - Functional paralogue of Rho1 - Minor role in CIP signaling | RHO1 | |
| Phosphoinositide metabolism | Ksg1 | Serine/threonine protein kinase PDK-1ortholog involved in CIP signaling through the activation of Pck1 and Pck2 | PKH1/PKH2 |
| PKCs and the CIP MAPK cascade | Pck1 | Rho1 target involved in CIP signaling in response to CW damage | PKC1 |
| Pck2 | -Rho1 and Rho2 target - Main upstream activator of the CIP MAPK module during growth and stress | PKC2 | |
| Mkh1 | MAPK kinase kinase | BCK1 | |
| Pek1 | MAPK kinase | MKK1/MKK2 | |
| Pmk1 | MAPK | MPK1/SLT2 | |
| Negative regulators | Skb5 | -Shk1 kinase binding protein - Inhibits Pmk1 by downregulating Mkh1 localization to cell tips | NBP2 |
| Pmp1 | - Dual-specificity MAP kinase phosphatase - Binds and dephosphorylates Pmk1 during vegetative growth | SDP1/MSG5 | |
| Pyp1 | Tyrosine phosphatase: - Transcriptionally regulated by the SAPK pathway (Sty1-Atf1) - Binds and dephosphorylates both Sty1 and Pmk1 during vegetative growth | PTP3, PTP2 | |
| Pyp2 | Tyrosine phosphatase: - Transcriptionally regulated by the SAPK pathway (Sty1-Atf1). - Binds and dephosphorylates both Sty1 and Pmk1 during stress | PTP3, PTP2 | |
| Ptc1 | Serine/threonine phosphatase: - Transcriptionally regulated by the SAPK pathway (Sty1-Atf1). - Binds and dephosphorylates both Sty1 and Pmk1 during vegetative growth | PTC1 | |
| Ptc3 | Serine/threonine phosphatase: - Transcriptionally regulated by the SAPK pathway (Sty1-Atf1). - Binds and dephosphorylates both Sty1 and Pmk1 during stress | PTC3, PTC2 | |
| Downstream targets | Atf1 | - Atf-CREB family bZIP domain transcription factor - Downstream effector of Sty1 MAPK(SAPK pathway) in response to environmental cues and Pmk1 in response to CW damage | --- |
| Nrd1 | RNA-binding protein (RBP): - Phosphorylated by Pmk1 during growth and stress - Negatively modules myosin II essential light chain mRNA stability - Regulation of actomyosin ring integrity during cytokinesis | MRN1 | |
| Rnc1 | KH domain RBP: - Phosphorylated by Pmk1 during growth and stress - Phosphorylated by Sty1 during growth and stress - Stabilizes mRNA encoding Pmk1 dual specificity phosphatase Pmp1 - Negative regulation of Pmk1 activity | PBP2 | |
| Clp1 | Cdc14-related serine/threonine protein phosphatase: -Phosphorylated by Pmk1 - Pmk1-dependent phosphorylation promotes its nucleoplasmic accumulation during genotoxic stress | CDC14 | |
| Cch1- Yam8 | Plasma-membrane channel complex: - Putative substrate for Pmk1 phosphorylation - Putative substrate for dephosphorylation by Calcineurin (Ppb1) - Positively regulates import of calcium ions in response to salt stress | CCH1-MID1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cansado, J.; Soto, T.; Franco, A.; Vicente-Soler, J.; Madrid, M. The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond. J. Fungi 2022, 8, 32. https://doi.org/10.3390/jof8010032
Cansado J, Soto T, Franco A, Vicente-Soler J, Madrid M. The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond. Journal of Fungi. 2022; 8(1):32. https://doi.org/10.3390/jof8010032
Chicago/Turabian StyleCansado, José, Teresa Soto, Alejandro Franco, Jero Vicente-Soler, and Marisa Madrid. 2022. "The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond" Journal of Fungi 8, no. 1: 32. https://doi.org/10.3390/jof8010032
APA StyleCansado, J., Soto, T., Franco, A., Vicente-Soler, J., & Madrid, M. (2022). The Fission Yeast Cell Integrity Pathway: A Functional Hub for Cell Survival upon Stress and Beyond. Journal of Fungi, 8(1), 32. https://doi.org/10.3390/jof8010032






