Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Definition of IFI
2.3. Diagnosis of IFI
2.4. Antifungal Susceptibility Patterns
2.5. Statistical Analysis
3. Results
3.1. Invasive Candidiasis
3.2. Cryptococcosis
3.3. Invasive Aspergillosis
3.4. Mucormycosis
3.5. Antifungal Susceptibility Testing
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, L.; Lee, D.; Yeh, S.; Bhurani, D.; Khanh, B.; Low, C.; Norasetthada, L.; Chan, T.; Kwong, Y.; Vaid, A.; et al. Epidemiology of invasive fungal diseases among patients with haematological disorders in the Asia-Pacific: A prospective observational study. Clin. Microbiol. Infect. 2015, 21, 594.e7–594.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, M.A.; Chakrabarti, A. Opportunistic fungal infections in the Asia-Pacific region. Med. Mycol. 2012, 50, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoenigl, M. Invasive fungal disease complicating COVID-19: When it rains it pours. Clin. Infect. Dis. 2020, 73, e1645–e1648. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430. [Google Scholar] [CrossRef]
- Nganthavee, V.; Phutthasakda, W.; Atipas, K.; Tanpong, S.; Pungprasert, T.; Dhirachaikulpanich, D.; Krithin, S.; Tanglitanon, S.; Jutidamronphang, W.; Owattanapanich, W.; et al. High incidence of invasive fungal infection during acute myeloid leukemia treatment in a resource-limited country: Clinical risk factors and treatment outcomes. Support. Care Cancer 2019, 27, 3613–3622. [Google Scholar] [CrossRef]
- Tang, J.-L.; Kung, H.-C.; Lei, W.-C.; Yao, M.; Wu, U.-I.; Hsu, S.-C.; Lin, C.-T.; Li, C.-C.; Wu, S.-J.; Hou, H.-A.; et al. High incidences of invasive fungal infections in acute Myeloid Leukemia patients receiving induction chemotherapy without systemic antifungal Prophylaxis: A prospective observational study in Taiwan. PLoS ONE 2015, 10, e0128410. [Google Scholar] [CrossRef]
- Lien, M.-Y.; Chou, C.-H.; Lin, C.-C.; Bai, L.-Y.; Chiu, C.-F.; Yeh, S.-P.; Ho, M.-W. Epidemiology and risk factors for invasive fungal infections during induction chemotherapy for newly diagnosed acute myeloid leukemia: A retrospective cohort study. PLoS ONE 2018, 13, e0197851. [Google Scholar] [CrossRef]
- Gheith, S.; Saghrouni, F.; Bannour, W.; Ben Youssef, Y.; Khelif, A.; Normand, A.-C.; Ben Saïd, M.; Piarroux, R.; Njah, M.; Ranque, S. Characteristics of invasive Aspergillosis in Neutropenic Haematology patients (Sousse, Tunisia). Mycopathologia 2014, 177, 281–289. [Google Scholar] [CrossRef]
- Hammond, S.P.; Marty, F.M.; Bryar, J.M.; DeAngelo, D.J.; Baden, L.R. Invasive fungal disease in patients treated for newly diagnosed acute leukemia. Am. J. Hematol. 2010, 85, 695–699. [Google Scholar] [CrossRef]
- Chen, C.Y.; Sheng, W.H.; Tien, F.M.; Lee, P.C.; Huang, S.Y.; Tang, J.L.; Tsay, W.; Tien, H.F.; Hsueh, P.R. Clinical characteristics and treatment outcomes of pulmonary invasive fungal infection among adult patients with hematological malignancy in a medical centre in Taiwan, 2008–2013. J. Microbiol. Immunol. Infect. 2020, 53, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Singh, A.; Seth, R.; Xess, I.; Jana, M.; Kabra, S.K. Prevalence and predictors of invasive fungal infections in children with persistent febrile neutropenia treated for acute leukemia—A prospective study. Indian J. Pediatrics 2018, 85, 1090–1095. [Google Scholar] [CrossRef]
- Jacobs, S.; Wengenack, N.L.; Walsh, T.J. Non-Aspergillus Hyaline molds: Emerging causes of sino-pulmonary fungal infections and other invasive mycoses. Semin. Respir. Crit. Care Med. 2020, 41, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Niu, X.; Wu, Y.; Du, Y.; Yang, Y.; Qi, R.; Chen, H.; Gao, X.; Song, B.; et al. A 5-year review of invasive fungal infection at an academic medical center. Front. Cell. Infect. Microbiol. 2020, 10, 553648. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendrickson, J.; Hu, C.; Aitken, S.L.; Beyda, N. Antifungal resistance: A concerning trend for the present and future. Curr. Infect. Dis. Rep. 2019, 21, 47. [Google Scholar] [CrossRef]
- Wall, G.; Lopez-Ribot, J.L. Current antimycotics, new prospects, and future approaches to antifungal therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Kontoyiannis, D.P. Role and interpretation of antifungal susceptibility testing for the management of invasive fungal infections. J. Fungi 2020, 7, 17. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [Green Version]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [PubMed]
- Blot, S.I.; Taccone, F.S.; Abeele, A.-M.V.D.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary Aspergillosis in critically Ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Volume M60ed2. [Google Scholar]
- Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume M38-A2. [Google Scholar]
- Xess, I.; Pandey, M.; Dabas, Y.; Agarwal, R.; Das, S.; Srivastava, P.M.V.; Thakur, R.; Sharma, S.; Mani, P.; Biswas, A.; et al. Multilocus sequence typing of clinical isolates of Cryptococcus from India. Mycopathologia 2021, 186, 199–211. [Google Scholar] [CrossRef]
- Dabas, Y.; Xess, I.; Bakshi, S.; Mahapatra, M.; Seth, R. Emergence of Azole-Resistant Aspergillus fumigatus from immunocompromised hosts in India. Antimicrob. Agents Chemother. 2018, 62, 02264-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almyroudis, N.G.; Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G.; Kusne, S. In vitro susceptibilities of 217 clinical isolates of Zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 2007, 51, 2587–2590. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.Y.; Burns, J.; Freyer, C.W.; Hamilton, K.W.; Frey, N.V.; Gill, S.I.; Hexner, E.O.; Luger, S.M.; Mangan, J.K.; Martin, M.E.; et al. Risk of invasive fungal infections in patients with high-risk MDS and AML receiving hypomethylating agents. Am. J. Hematol. 2020, 95, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Chien, S.-H.; Fan, N.-W.; Hu, M.-H.; Gau, J.-P.; Liu, C.-J.; Yu, Y.-B.; Liu, C.-Y.; Hsiao, L.-T.; Liu, J.-H.; et al. Incidence and risk factors of probable and proven invasive fungal infection in adult patients receiving allogeneic hematopoietic stem cell transplantation. J. Microbiol. Immunol. Infect. 2016, 49, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Şular, F.-L.; Szekely, E.; Cristea, V.C.; Dobreanu, M. Invasive fungal infection in Romania: Changing incidence and epidemiology during six years of surveillance in a tertiary hospital. Mycopathologia 2018, 183, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Lien, M.-Y.; Yeh, S.-P.; Gau, J.-P.; Wang, P.-N.; Li, S.-S.; Dai, M.-S.; Chen, T.C.; Hsieh, P.-Y.; Chiou, L.-W.; Ko, B.S.; et al. High rate of invasive fungal infections after non-T cell depleted haploidentical allo-HSCT even under antifungal prophylaxis. Bone Marrow Transplant. 2021, 56, 1–4. [Google Scholar] [CrossRef]
- Harrison, N.; Mitterbauer, M.; Tobudic, S.; Kalhs, P.; Rabitsch, W.; Greinix, H.T.; Burgmann, H.; Willinger, B.; Presterl, E.; Forstner, C. Incidence and characteristics of invasive fungal diseases in allogeneic hematopoietic stem cell transplant recipients: A retrospective cohort study. BMC Infect. Dis. 2015, 15, 584. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, A.J.; Tovar-Torres, M.P.; Hingwe, A.; Cheema, F.; Welch, V.L.; Ford, K.D. The changing epidemiology of invasive Aspergillosis in the non-traditional host: Risk factors and outcomes. Pulm. Crit. Care Med. 2016, 1, 5. [Google Scholar] [CrossRef]
- Neofytos, D.; Fishman, J.; Horn, D.; Anaissie, E.; Chang, C.-H.; Olyaei, A.; Pfaller, M.; Steinbach, W.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl. Infect. Dis. 2010, 12, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, A. Emerging invasive fungal infections: Clinical features and controversies in diagnosis and treatment processes. Infect. Drug Resist. 2020, 13, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Shariati, A.; Moradabadi, A.; Chegini, Z.; Khoshbayan, A.; Didehdar, M. An overview of the management of the most important invasive fungal infections in patients with blood malignancies. Infect. Drug Resist. 2020, 13, 2329–2354. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.H.; Patel, R.D.; Vanikar, A.V.; Kanodia, K.V.; Suthar, K.S.; Nigam, L.K.; Patel, H.V.; Patel, A.H.; Kute, V.B.; Trivedi, H.L. Invasive fungal infections in renal transplant patients: A single center study. Ren. Fail. 2017, 39, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.; Musk, M.; Lavender, M.; Wrobel, J.; Yaw, M.; Lawrence, S.; Chirayath, S.; Boan, P. Epidemiology of invasive fungal infections in lung transplant recipients in Western Australia. Transpl. Infect. Dis. 2019, 21, e13085. [Google Scholar] [CrossRef]
- Boroujeni, Z.B.; Shamsaei, S.; Yarahmadi, M.; Getso, M.I.; Khorashad, A.S.; Haghighi, L.; Raissi, V.; Zareei, M.; Mohammadzade, A.S.; Moqarabzadeh, V.; et al. Distribution of invasive fungal infections: Molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: A 3-year experience with 490 patients under intensive care. Microb. Pathog. 2021, 152, 104616. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Khan, I.; Shukla, S.; Kumar, P.; Rather, I.A.; Park, Y.-H.; Huh, Y.S.; Han, Y.-K. Invasive fungal infections and their epidemiology: Measures in the clinical scenario. Biotechnol. Bioprocess. Eng. 2019, 24, 436–444. [Google Scholar] [CrossRef]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. In Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1508, pp. 17–65. [Google Scholar]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive fungal infections in the ICU: How to approach, how to treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, M.T.; Caggiano, G.; Lovero, G.; De Giglio, O.; Coretti, C.; Cuna, T.; Iatta, R.; Giglio, M.; Dalfino, L.; Bruno, F.; et al. Epidemiology of invasive fungal infections in the intensive care unit: Results of a multicenter Italian survey (AURORA Project). Infection 2013, 41, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xess, I.; Jain, N.; Hasan, F.; Mandal, P.; Banerjee, U. Epidemiology of Candidemia in a Tertiary Care Centre of North India: 5-year study. Infection 2007, 35, 256–259. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sood, P.; Rudramurthy, S.; Chen, S.; Kaur, H.; Capoor, M.; Chhina, D.; Rao, R.; Eshwara, V.K.; Xess, I.; et al. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensiv. Care Med. 2014, 41, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, diagnosis, antifungal resistance, and management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.; Wickes, B.L.; Keller, S.M.; Fu, J.; Casadevall, A.; Jain, P.; Ragan, M.A.; Banerjee, U.; Fries, B.C. Molecular epidemiology of clinical Cryptococcus neoformans strains from India. J. Clin. Microbiol. 2005, 43, 5733–5742. [Google Scholar] [CrossRef] [Green Version]
- Coste, A.T.; Kritikos, A.; Li, J.; Khanna, N.; Goldenberger, D.; Garzoni, C.; Zehnder, C.; Boggian, K.; Neofytos, D.; Riat, A.; et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection 2020, 48, 761–766. [Google Scholar] [CrossRef]
- Beyda, N.D.; John, J.; Kilic, A.; Alam, M.J.; Lasco, T.M.; Garey, K.W. FKS mutant Candida glabrata: Risk factors and outcomes in patients with Candidemia. Clin. Infect. Dis. 2014, 59, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Cumbie, R.; Driscoll, E.; Pasculle, A.W.; Clancy, C.J. Rate of FKS Mutations among consecutive Candida isolates causing bloodstream infection. Antimicrob. Agents Chemother. 2016, 60, 1954. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Li, C.; Cao, J.; Wu, X.; Zhang, L. Clinical characteristics and predictors of mortality in patients with candidemia: A six-year retrospective study. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1717–1724. [Google Scholar] [CrossRef]
- Xiao, M.; Chen, S.C.; Kong, F.; Xu, X.L.; Yan, L.; Kong, H.S.; Fan, X.; Hou, X.; Cheng, J.W.; Zhou, M.L. Dis-tribution and antifungal susceptibility of Candida species causing candidemia in China: An update from the CHIF-NET study. J. Infect. Dis. 2020, 221, S139–S147. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Updike, C.L.; Clancy, C.J. Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive Candidiasis and prior Echinocandin exposure. Antimicrob. Agents Chemother. 2013, 57, 3528–3535. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.I.; Xess, I.; Mathur, P.; Behera, B.; Gupta, B.; Misra, M.C. Epidemiology of candidaemia in critically ill trauma patients: Experiences of a level I trauma centre in North India. J. Med. Microbiol. 2010, 60, 342–348. [Google Scholar] [CrossRef]
- Donadu, M.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian essential oil of Ruta graveolens against Nosocomial Antifungal Resistant Candida strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Donadu, M.; Usai, D.; Marchetti, M.; Usai, M.; Mazzarello, V.; Molicotti, P.; Montesu, M.; Delogu, G.; Zanetti, S. Antifungal activity of oils macerates of North Sardinia plants against Candida species isolated from clinical patients with candidiasis. Nat. Prod. Res. 2020, 34, 3280–3284. [Google Scholar] [CrossRef] [PubMed]
- Nyazika, T.K.; Tatuene, J.K.; Kenfak-Foguena, A.; Verweij, P.; Meis, J.F.; Robertson, V.J.; Hagen, F. Epidemiology and aetiologies of cryptococcal meningitis in Africa, 1950–2017: Protocol for a systematic review. BMJ Open 2018, 8, e020654. [Google Scholar] [CrossRef] [Green Version]
- Datta, K.; Jain, N.; Sethi, S.; Rattan, A.; Casadevall, A.; Banerjee, U. Fluconazole and itraconazole susceptibility of clinical isolates of Cryptococcus neoformans at a tertiary care centre in India: A need for care. J. Antimicrob. Chemo-Ther. 2003, 52, 683–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capoor, M.R.; Mandal, P.; Deb, M.; Aggarwal, P.; Banerjee, U. Current scenario of cryptococcosis and antifungal susceptibility pattern in India: A cause for reappraisal. Mycoses 2008, 51, 258–265. [Google Scholar] [CrossRef]
- Chowdhary, A.; Randhawa, H.S.; Sundar, G.; Kathuria, S.; Prakash, A.; Khan, Z.; Sun, S.; Xu, J. In vitro antifungal susceptibility profiles and genotypes of 308 clinical and environmental isolates of Cryptococcus neoformans var. grubii and Cryptococcus gattii serotype B from north-western. India. J. Med. Microbiol. 2011, 60, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Lestrade, P.P.; Van Der Velden, W.J.F.M.; Bouwman, F.; Stoop, F.J.; Blijlevens, A.N.M.; Melchers, W.J.G.; Verweij, P.E.; Donnelly, J.P. Epidemiology of invasive aspergillosis and triazole-resistant Aspergillus fumigatus in patients with haematological malignancies: A single-centre retrospective cohort study. J. Antimicrob. Chemother. 2018, 73, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Beer, K.; Toda, M. Azole-Resistant Aspergillus fumigatus: What you need to know. Clin. Microbiol. Newsl. 2020, 42, 1–6. [Google Scholar] [CrossRef]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of Aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Cuenca-Estrella, M.; Mellado, E. Triazole resistance in Aspergillus species: An emerging problem. Drugs 2017, 77, 599–613. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Mellado, E.; Cuenca-Estrella, M. Triazole Resistance in Aspergillus spp.: A Worldwide Problem? J. Fungi 2016, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Alanio, A.; Sitterlé, E.; Liance, M.; Farrugia, C.; Foulet, F.; Botterel, F.; Hicheri, Y.; Cordonnier, C.; Costa, J.M.; Bretagne, S. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J. Antimicrob. Chemother. 2011, 66, 371–374. [Google Scholar] [CrossRef]
- Bader, O.; Weig, M.; Reichard, U.; Lugert, R.; Kuhns, M.; Christner, M.; Held, J.; Peter, S.; Schumacher, U.; Buchheidt, D.; et al. cyp51A-Based Mechanisms of Aspergillus fumigatus Azole Drug Resistance Present in Clinical Samples from Germany. Antimicrob. Agents Chemother. 2013, 57, 3513–3517. [Google Scholar] [CrossRef] [Green Version]
- Van Ingen, J.; Van Der Lee, H.A.L.; Rijs, A.J.M.M.; Snelders, E.; Melchers, W.; Verweij, P.E. High-level pan-azole-resistant Aspergillosis: TABLE 1. J. Clin. Microbiol. 2015, 53, 2343–2345. [Google Scholar] [CrossRef] [Green Version]
- Fuhren, J.; Voskuil, W.S.; Boel, C.H.E.; Haas, P.J.A.; Hagen, F.; Meis, J.F.; Kusters, J.G. High prevalence of azole resistance in Aspergillus fumigatus isolates from high-risk patients. J. Antimicrob. Chemother. 2015, 70, 2894–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Sharma, C.; Sundar, G.; Singh, P.K.; Gaur, S.N.; Hagen, F.; Klaassen, C.H.; Meis, J.F. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp 51A gene in India. PLoS ONE 2012, 7, e52871. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J.F. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J. Antimicrob. Chemother. 2014, 69, 555–557. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front. Microbiol. 2015, 6, 428. [Google Scholar] [CrossRef] [PubMed]
- Axell-House, D.B.; Wurster, S.; Jiang, Y.; Kyvernitakis, A.; Lewis, R.E.; Tarrand, J.J.; Raad, I.I.; Kontoyiannis, D.P. Breakthrough mucormycosis developing on mucorales-active antifungals portrays a poor prognosis in patients with hematologic cancer. J. Fungi 2021, 7, 217. [Google Scholar] [CrossRef] [PubMed]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiology and diagnosis of mucormycosis: An update. J. Fungi 2020, 6, 265. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Cornely, O.A.; Busca, A.; Caira, M.; Cesaro, S.; Gasbarrino, C.; Girmenia, C.; Heinz, W.J.; Herbrecht, R.; Lass-Flörl, C. Combined antifungal approach for the treatment of invasive mucormycosis in patients with hematologic diseases: A report from the SEIFEM and FUNGISCOPE registries. Haematologica 2013, 98, e127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, D.; Alanio, A.; Lortholary, O.; Dromer, F. Agents of systemic and subcutaneous Mucormycosis and Entomophthoromycosis. In Clinical Microbiology Procedures Handbook; American Society for Microbiology: Washington, DC, USA, 2015; pp. 2087–2108. [Google Scholar]
- Chen, S.C.-A.; Playford, E.G.; Sorrell, T.C. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol. 2010, 10, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Brunet, K.; Rammaert, B. Mucormycosis treatment: Recommendations, latest advances, and perspectives. J. Med. Mycol. 2020, 30, 101007. [Google Scholar] [CrossRef] [PubMed]
- Bala, K.; Chander, J.; Handa, U.; Punia, R.S.; Attri, A.K. A prospective study of mucormycosis in north India: Experience from a tertiary care hospital. Med. Mycol. 2015, 53, 248–257. [Google Scholar] [CrossRef] [Green Version]
- Chowdhary, A.; Kathuria, S.; Singh, P.K.; Sharma, B.; Dolatabadi, S.; Hagen, F.; Meis, J.F. Molecular characterization and in vitro antifungal susceptibility of 80 clinical isolates of mucormycetes in Delhi, India. Mycoses 2014, 57, 97–107. [Google Scholar] [CrossRef]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef]




| Infection Site | Invasive Candidiasis (n = 53) | Cryptococcosis (n = 34) | Invasive Aspergillosis (n = 103) | Mucormycosis (n = 62) | Others (n = 1) | Total (n = 253) |
|---|---|---|---|---|---|---|
| Pulmonary (%) | 0 | 2 (5.6) | 94 (91.3) | 12 (19.3) | 0 | 108 (42.7) |
| Sinus (%) | 0 | 0 | 6 (5.8) | 40 (64.5) | 0 | 46 (18.2) |
| Blood (%) | 53 (100) | 0 | 0 | 0 | 0 | 53 (20.9) |
| Cerebral (%) | 0 | 32 (94.1) | 0 | 2 (3.2) | 0 | 34 (13.4) |
| Others (%) | 0 | 0 | 2 (1.9) | 2 (3.2) | 1 (100) | 5 (2) |
| Disseminated (%) | 0 | 0 | 1 (0.01) | 6 (9.7) | 0 | 7 (2.8) |
| Variables | Total Patients (n= 253) | Proven IFI n = 134 | Probable/Putative IFI n = 119 | p-Value | Univariate OR (95% CI) | Multivariate OR (95% CI) |
|---|---|---|---|---|---|---|
| Age (years) Median, IQR (range) | 40, 33 (0.06–87) | 35, 34 (0.06–83) | 43, 31 (1–87) | 0.003 | 0.98 (0.97–0.99) | |
| Males, n (%) | 167 | 86 (51.5) | 81 (48.5) | 0.51 | 0.84 (0.49–1.41) | |
| Hospitalization (days) Median, IQR (range) | 19, 15 (1–171) | 21, 15 (1–137) | 17, 16 (1–171) | 0.86 | 0.99 (0.98–1) | |
| General ward, n (%) | 141 | 71 (50.3) | 70 (49.6) | 0.41 | ||
| High-efficiency particulate air-filtered room, n (%) | 56 | 34 (60.7) | 22 (39.3) | 1.52 (0.81–2.86) | 1.65 (0.8–3.4) | |
| Intensive care unit (ICU), n (%) | 56 | 29 (51.8) | 27 (48.2) | 1.05 (0.56–1.96) | 1.2 (0.6–2.4) | |
| Chronic granulomatous diseases, n (%) | 50 | 23 (46) | 27 (54) | 0.34 | 0.7 (0.37–1.31) | |
| Long-term corticosteroids, n (%) | 62 | 40 (64.5) | 22 (35.5) | 0.04 | 1.87 (1.03–3.39) | 2.7 (1.34–5.45) |
| Diabetes mellitus, n (%) | 60 | 36 (60) | 24 (40) | 0.2 | 1.45 (0.8–2.61) | 1.3 (0.6–2.8) |
| Hematological malignancy, n (%) | 50 | 12 (24) | 38 (76) | 0.00 | 0.2 (0.1–0.42) | |
| Other cancers, n (%) | 8 | 4 (50) | 4 (50) | 1 | 0.88 (0.21–3.61) | |
| Acquired immunodeficiency syndrome, n (%) | 14 | 10 (71.4) | 4 (28.6) | 0.16 | 2.31 (0.7–7.59) | 4.6 (1.3–16.6) |
| Chronic liver disease, n (%) | 40 | 33 (82.5) | 7 (17.5) | 0.00 | 5.22 (2.21–12.33) | 6.9 (2.8–17.2) |
| Pulmonary manifestations, n (%) | 78 | 18 (23.08) | 60 (76.9) | 0.00 | 0.15 (0.08–0.28) | |
| Chronic kidney disease, n (%) | 87 | 40 (46) | 47 (54) | 0.11 | 0.65 (0.35–1.09) | |
| Coronary artery disease, n (%) | 9 | 8 (89) | 1 (11) | 0.03 | 7.49 (0.92–60.8) | 17 (2–142) |
| Multiorgan involvement, n (%) | 8 | 8 (100) | 0 | 0.00 | 1 | |
| Trauma, n (%) | 15 | 11 (73.3) | 4 (26.7) | 0.11 | 2.57 (0.79–8.3) | 3.9 (1–14) |
| Antifungal administration days median, IQR (range) | 14, 10 (2–138) | 14, 7 (3–87) | 21, 20 (2–138) | 0.00 | 0.95 (0.93–0.97) | |
| Fungal etiology | 0.00 | 0.31 (0.22–0.43) | ||||
| Candidemia, n (%) | 53 | 53 | 0 | |||
| Cryptococcosis, n (%) | 34 | 34 | 0 | |||
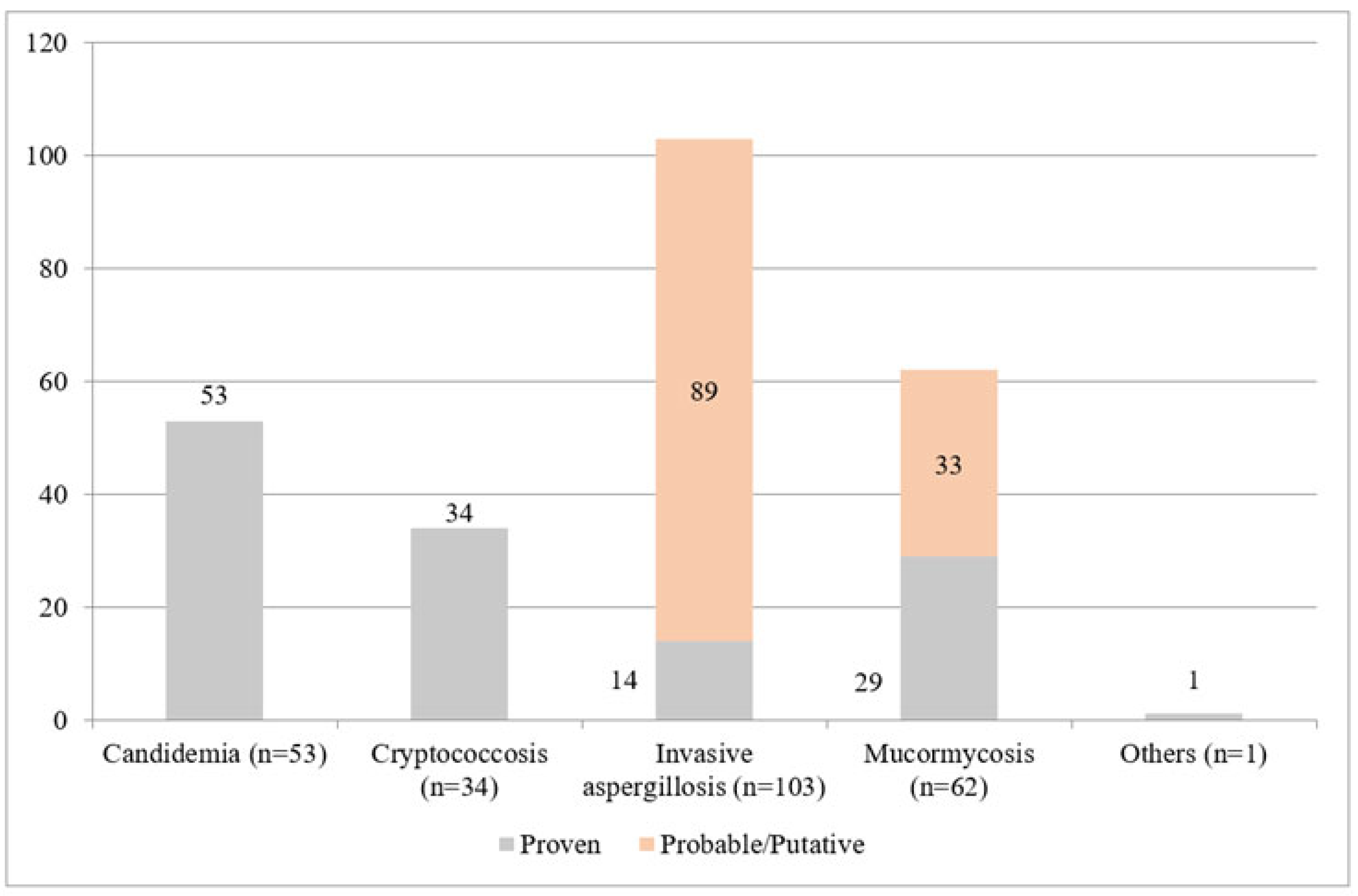
| Invasive aspergillosis, n (%) | 103 | 14 (13.6) | 89 (86.4) | |||
| Mucormycosis, n (%) | 62 | 33 (53.2) | 29 (46.7) | |||
| Taleromycosis, n (%) | 1 | 0 | 1 (100) | |||
| 30-day outcome, n (%) | 0.4 | 1.19 (0.72–1.96) | ||||
| Survived, n (%) | 143 | 73 (51) | 70 (49) | |||
| Expired, n (%) | 110 | 61 (55.4) | 49 (44.5) |
| Variables | Invasive Candidiasis OR (95% CI) | Cryptococcosis OR (95% CI) | Invasive Aspergillosis OR (95% CI) | Mucormycosis OR (95% CI) |
|---|---|---|---|---|
| Age | 1.07 (1.01–1.14) | 1.07 (1.03–1.11) | 1.01 (0.96–1.06) | |
| Sex | 3.27 (0.52–20.46) | 1.39 (0.29–6.6) | ||
| Hospitalization duration | 1.04 (0.85–1.27) | |||
| High-efficiency particulate air-filtered room | 31.14 (1.72–560) | 5.3 (0.75–37.38) | ||
| Intensive care unit (ICU) | 10.49 (0.99–110.3) | 3.15 (0.84–11.75) | ||
| Chronic granulomatous diseases | 1.08 (0.09–12.55) | |||
| Long-term corticosteroids | 2.63 (0.6–11.9) | |||
| Diabetes mellitus | 3.77 (0.19–74.48) | |||
| Hematological malignancy | 15 (2.9–77.9) | |||
| Acquired immunodeficiency syndrome | 3.4 (0.23–48.94) | |||
| Pulmonary manifestations | 2.95 (0.33–26.06) | 1.35 (0.4–4.6) | ||
| Chronic kidney disease | 1.07 (0.15–7.42) | 3.9 (1.1–13.7) | ||
| Multiorgan involvement | 9.5 (0.94–95.7) | |||
| Mechanical ventilation | 2.99 (0.76–11.63) | |||
| Sepsis | 5.8 (0.79–42.3) | |||
| Symptom duration | 1.08 (0.83–1.4) | |||
| Ketoacidosis | 1.13 (0.12–10.68) | |||
| Radiological finding | ||||
| Lung collapse | 1.5 (0.05–40.6) | |||
| Nodules/ground glass opacities | 1.5 (0.22–9.96) | 100.5 (1.44–7006) | ||
| Consolidation | 9.69 (0.09–997) | |||
| Sinus thickening | 1.02 (0.13–7.57) | |||
| Galactomannan antigen index ≥1 | 2.72 (0.76–9.65) | |||
| Species isolation | ||||
| Candida tropicalis: 12.9 (1.11–150) | Rhizopus microsporus: 1.2 (0.09–15.5) | |||
| C. parapsilosis: 1.2 (0.18–7.9) | Mucor circinelloides: 2.06 (0.01–328.9) | |||
| C. pelliculosa: 1.2 (0.03–43.5) |
| Fungal Isolate | N | Amphotericin B | Fluconazole | Voriconazole | Itraconazole | Posaconazole | Caspofungin | Micafungin | Flucytosine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | MIC50/MIC90; MIC Range | Mode, GM | ||
| Candida sp. | 53 | 0.25/0.5; 0.03–1 | 0.5, 0.217 | 0.5/8; 0.125–64 | 0.25, 0.812 | 0.03/0.25; 0.03–0.5 | 0.03, 0.063 | 0.06/0.5; 0.03–1 | 0.03, 0.081 | 0.03/0.5; 0.03–1 | 0.03, 0.054 | 0.125/0.5; 0.015–1 | 0.015, 0.097 | 0.015/0.5; 0.015–2 | 0.015, 0.047 | ||
| Candida albicans | 14 | 0.06–0.5 | 0.5, 0.26 | 0.125–64 | 0.25, 0.82 | 0.03–0.5 | 0.03, 0.07 | 0.03–1 | 0.03, 0.11 | 0.03–0.5 | 0.03, 0.05 | 0.015–0.5 | 0.25, 0.08 | 0.015–1 | 0.015, 0.035 | ||
| C. tropicalis | 10 | 0.03–0.5 | 0.03, 0.107 | 0.25–2 | 0.5, 0.466 | 0.03–0.125 | 0.03, 0.039 | 0.03–0.125 | 0.03, 0.045 | 0.03–0.5 | 0.03, 0.056 | 0.015–0.5 | 0.125, 0.107 | 0.015–1 | 0.015, 0.065 | ||
| C. parapsilosis | 14 | 0.125–1 | 0.5, 0.304 | 0.125–64 | 0.5, 1.034 | 0.03–0.5 | 0.03, 0.063 | 0.03–1 | 0.03, 0.081 | 0.03–1 | 0.03, 0.054 | 0.015–1 | 0.25, 0.105 | 0.015–0.5 | 0.015, 0.042 | ||
| C. guillermondii | 2 | 0.03–0.25 | 0.5–32 | 0.03–0.06 | 0.03–0.25 | 0.125–0.5 | 0.125–1 | 0.5–2 | |||||||||
| C. pelliculosa | 2 | 0.125–0.25 | 0.25–2 | 0.03 | 0.03 | 0.03 | 0.015–0.06 | 0.015–0.5 | |||||||||
| C. auris | 2 | 0.125 | 0.125–0.25 | 0.03 | 0.03 | 0.03 | 0.03–0.06 | 0.015 | |||||||||
| C. glabrata | 8 | 0.125–0.5 | 0.5, 0.272 | 0.25–8 | 1, 1 | 0.03–0.5 | 0.25, 0.123 | 0.06–0.5 | 0.06, 0.122 | 0.03–2 | 0.03, 0.06 | 0.015–1 | 0.125, 0.16 | 0.015–0.5 | 0.015, 0.072 | ||
| Lodderomyces longisporus | 1 | 0.125 | 0.5 | 0.03 | 0.03 | 0.03 | 0.015 | 0.015 | |||||||||
| Cryptococcus neoformans | 24 | 0.25/1; 0.03–1 | 0.25, 0.342 | 2/4; 0.5–8 | 2, 2.181 | 0.03/0.06; 0.03–0.06 | 0.03, 0.033 | 2/2 0.25–4 | 2, 1.414 | ||||||||
| Aspergillus sp. | 103 | 1/2; 0.03–4 | 1, 0.831 | 0.5/1; 0.03–2 | 0.5, 0.285 | 0.5/1; 0.03–2 | 0.5, 0.259 | 0.06/0.25; 0.03–0.25 | 0.03, 0.081 | 0.06/0.125; 0.015–1 | 0.015, 0.046 | 0.015/ 0.015; 0.015–0.03 | 0.015, 0.016 | ||||
| Aspergillus flavus | 42 | 2/4; 0.06–4 | 2, 1.559 | 0.5/1; 0.03–1 | 0.5, 0.363 | 0.25/1; 0.03–2 | 0.5, 0.255 | 0.06/0.25; 0.03–0.5 | 0.03, 0.085 | 0.06/0.125; 0.015–0.25 | 0.06, 0.045 | 0.015/0.015; 0.015–0.03 | 0.015, 0.016 | ||||
| A. fumigatus | 43 | 0.5/2; 0.03–2 | 1, 0.539 | 0.25/1; 0.03–1 | 0.5, 0.24 | 0.25/1; 0.03–2 | 0.5, 0.251 | 0.06/0.25; 0.03–0.5 | 0.03, 0.067 | 0.06/0.125; 0.015–1 | 0.015, 0.049 | 0.015/0.015; 0.015–0.03 | 0.015, 0.015 | ||||
| A. terreus | 7 | 0.5–1 | 1, 0.905 | 0.06–1 | 0.5, 0.272 | 0.125–0.5 | 0.5, 0.25 | 0.03–0.5 | 0.125, 0.124 | 0.03–0.25 | 0.06, 0.044 | 0.015–0.03 | 0.015, 0.016 | ||||
| A. nidulans | 3 | 0.06–1 | 1, 0.391 | 0.06–0.5 | -, 0.155 | 0.03–0.5 | 0.5, 0.195 | 0.03–0.125 | 0.125, 0.077 | 0.03–0.125 | -, 0.06 | 0.015–0.03 | 0.015, 0.018 | ||||
| A. niger | 8 | 0.06–2 | 0.25, 0.383 | 0.06–2 | 0.06, 0.268 | 0.06–2 | 0.5, 0.383 | 0.03–0.5 | 0.25, 0.134 | 0.015–0.25 | 0.015, 0.030 | 0.015 | 0.015, 0.015 | ||||
| Mucorales | 54 | 0.125/0.5; 0.06–1 | 0.06, 0.138 | 0.5/1; 0.06–1 | 0.5, 0.450 | 0.25/1; 0.03–2 | 0.25, 0.361 | ||||||||||
| Rhizopus arrhizus | 30 | 0.125/0.25; 0.06–1 | 0.125, 0.135 | 0.5/1; 0.06–1 | 1, 0.499 | 0.25/2; 0.03–2 | 0.25, 0.475 | ||||||||||
| R. microsporus | 13 | 0.06–0.5 | 0.06, 0.115 | 0.25–1 | 0.5, 0.5 | 0.03–1 | 1, 0.360 | ||||||||||
| R. pusillus | 1 | 0.125 | 0.125 | 0.25 | |||||||||||||
| Lichtheimia corymbifera | 4 | 0.25 | 0.25, 0.25 | 0.125–0.25 | 0.25, 0.21 | 0.125 | 0.125, 0.125 | ||||||||||
| L. ramosa | 1 | 0.25 | 0.5 | 0.25 | |||||||||||||
| Apophysomyces variabilis | 1 | 0.06 | 0.25 | 0.03 | |||||||||||||
| Mucor circinelloides | 3 | 0.25 | 0.25, 0.25 | 0.5–1 | 0.5, 0.629 | 0.25–0.5 | 0.25, 0.314 | ||||||||||
| Conidiobolus coronatus | 1 | 0.06 | 0.25 | 0.25 | |||||||||||||
| Taleromyces marneffi | 1 | 0.125 | 0.06 | 0.125 | 0.03 | 0.015 | 0.015 | ||||||||||
| Fungal Isolate | N | Amphotericin B | Fluconazole | Voriconazole | Itraconazole | Posaconazole | Caspofungin | Micafungin | Flucytosine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | I/R | S | I/R | S | I/R | S | R | S | I/R | S | I/R | S | ||
| Candida albicans, n (%) | 14 | 14 (100) | 0 | 11 (78.6) | I: 2 (14.3); R: 1 (7.1) | 11 (78.6) | I: 3 (21.4) | 10 (71.4) | I: 3 (21.4); R: 1 (7.1) | 13 (92.9) | I: 1 (7.1) | 14 (100) | 0 | |||
| C. tropicalis, n (%) | 10 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 8 (80) | I: 2 (20) | 7 (70) | I: 2 (20); R: 1 (10) | |||
| C. parapsilosis, n (%) | 14 | 14 (100) | 0 | 10 (71.4) | I: 1 (7.1); R: 3 (21.4) | 11 (78.6) | I: 3 (21.4) | 10 (71.4) | I: 3 (21.4); R: 1 (7.1) | 14 (100) | 0 | 14 (100) | 0 | |||
| C. guillermondii, n (%) | 2 | 2 (100) | 0 | 1 (50) | 1 (50) | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | I: 1 (50); R: 1 (50) | ||||
| C. pelliculosa, n (%) | 2 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | |||
| C. auris, n (%) | 2 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | 2 (100) | 0 | |||
| C. glabrata, n (%) | 8 | 8 (100) | 0 | 8 (100) | 0 | 3 (37.5) | I: 5 (62.5) | 5 (62.5) | I: 3 (37.5) | 4 (50) | I: 1 (12.5); R:3 (37.5) | 4 (50) | I: 1 (12.5); R: 3 (37.5) | |||
| Lodderomyces longisporus, n (%) | 1 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | |||
| Cryptococcus neoformans, n (%) | 24 | 24 (100) | 0 | 24 (100) | 0 | 24 (100) | 0 | 24 (100) | ||||||||
| Aspergillus flavus, n (%) | 42 | 42 (100) | 0 | 42 (100) | 0 | 41 (97.6) | 1 (2.3) | 42 (100) | 0 | 42 (100) | 0 | 42 (100) | 0 | |||
| A. fumigatus, n (%) | 43 | 43 (100) | 0 | 43 (100) | 0 | 42 (97.6) | 1 (2.3) | 43 (100) | 0 | 41 | 2 (4.7) | 43 (100) | 0 | |||
| A. terreus, n (%) | 7 | 7 (100) | 0 | 7 (100) | 0 | 7 (100) | 0 | 7 (100) | 0 | 7 (100) | 0 | 7 (100) | 0 | |||
| A. nidulans, n (%) | 3 | 3 (100) | 0 | 3 (100) | 0 | 3 (100) | 0 | 3 (100) | 0 | 3 (100) | 0 | 3 (100) | 0 | |||
| A. niger, n (%) | 8 | 8 (100) | 0 | 8 (100) | 0 | 7 (87.5) | 1 (12.5) | 8 (100) | 0 | 8 (100) | 0 | 8 (100) | 0 | |||
| Rhizopus arrhizus, n (%) | 54 | 54 (100) | 0 | 54 (100) | 0 | 42 (77.7) | 12 (22.2) | 39 (72.2) | 15 (27.8) | |||||||
| R. microsporus, n (%) | 30 | 30 (100) | 0 | 30 (100) | 0 | 27 (90) | 3 (10) | 23 (76.6) | 7 (23.3) | |||||||
| R. pusillus, n (%) | 13 | 13 (100) | 0 | 13 (100) | 0 | 13 (100) | 0 | 13 (100) | 0 | |||||||
| Lichtheimia corymbifera, n (%) | 1 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | |||||||
| L. ramosa, n (%) | 4 | 4 (100) | 0 | 4 (100) | 0 | 4 (100) | 0 | 4 (100) | 0 | |||||||
| Apophysomyces variabilis, n (%) | 1 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | |||||||
| Mucor circinelloides, n (%) | 1 | 1 (100) | 0 | 1 (100) | 0 | 0 | 1 (100) | 1 (100) | 0 | |||||||
| Conidiobolus coronatus, n (%) | 3 | 3 (100) | 0 | 3 (100) | 0 | 3 (100) | 0 | 3 (100) | 0 | |||||||
| Taleromyces marneffi, n (%) | 1 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabas, Y.; Xess, I.; Pandey, M.; Ahmed, J.; Sachdev, J.; Iram, A.; Singh, G.; Mahapatra, M.; Seth, R.; Bakhshi, S.; et al. Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study. J. Fungi 2022, 8, 33. https://doi.org/10.3390/jof8010033
Dabas Y, Xess I, Pandey M, Ahmed J, Sachdev J, Iram A, Singh G, Mahapatra M, Seth R, Bakhshi S, et al. Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study. Journal of Fungi. 2022; 8(1):33. https://doi.org/10.3390/jof8010033
Chicago/Turabian StyleDabas, Yubhisha, Immaculata Xess, Mragnayani Pandey, Jaweed Ahmed, Janya Sachdev, Azka Iram, Gagandeep Singh, Manoranjan Mahapatra, Rachna Seth, Sameer Bakhshi, and et al. 2022. "Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study" Journal of Fungi 8, no. 1: 33. https://doi.org/10.3390/jof8010033
APA StyleDabas, Y., Xess, I., Pandey, M., Ahmed, J., Sachdev, J., Iram, A., Singh, G., Mahapatra, M., Seth, R., Bakhshi, S., Kumar, R., Jyotsna, V. P., & Mathur, S. (2022). Epidemiology and Antifungal Susceptibility Patterns of Invasive Fungal Infections (IFIs) in India: A Prospective Observational Study. Journal of Fungi, 8(1), 33. https://doi.org/10.3390/jof8010033






