Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC-MS/MS Analysis
2.2. Antimicrobial Activity
2.3. Mechanisms of Action
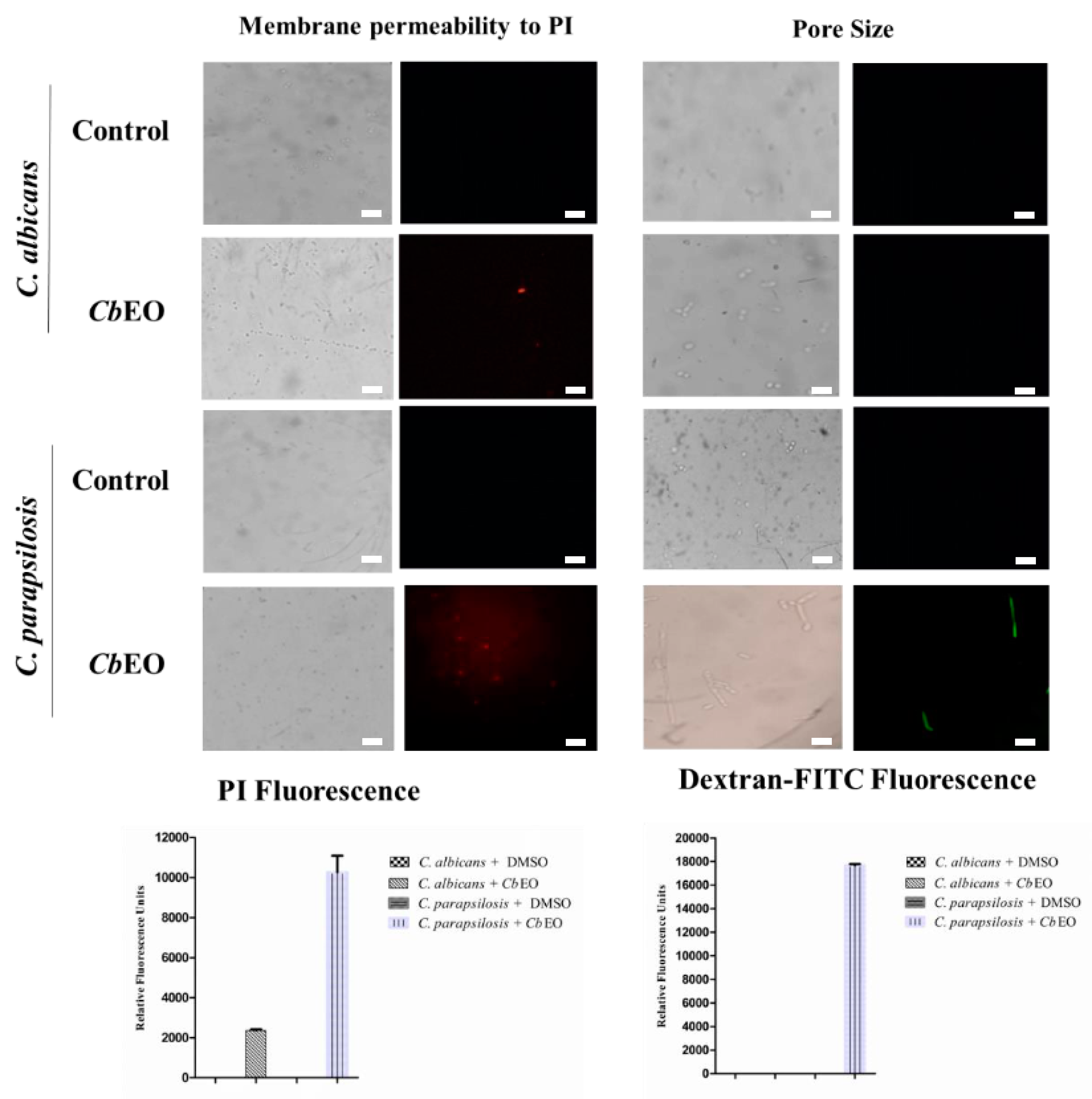
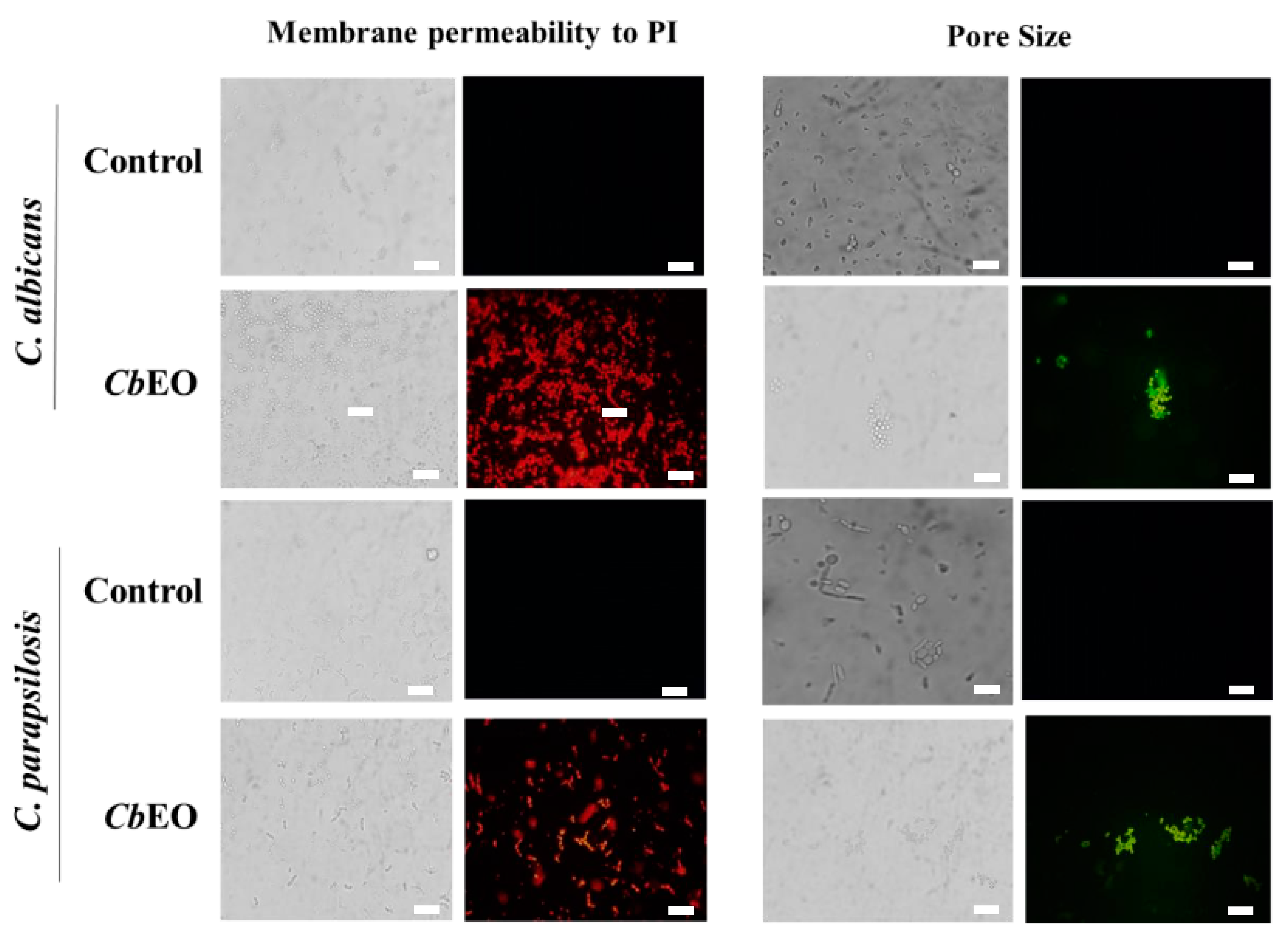
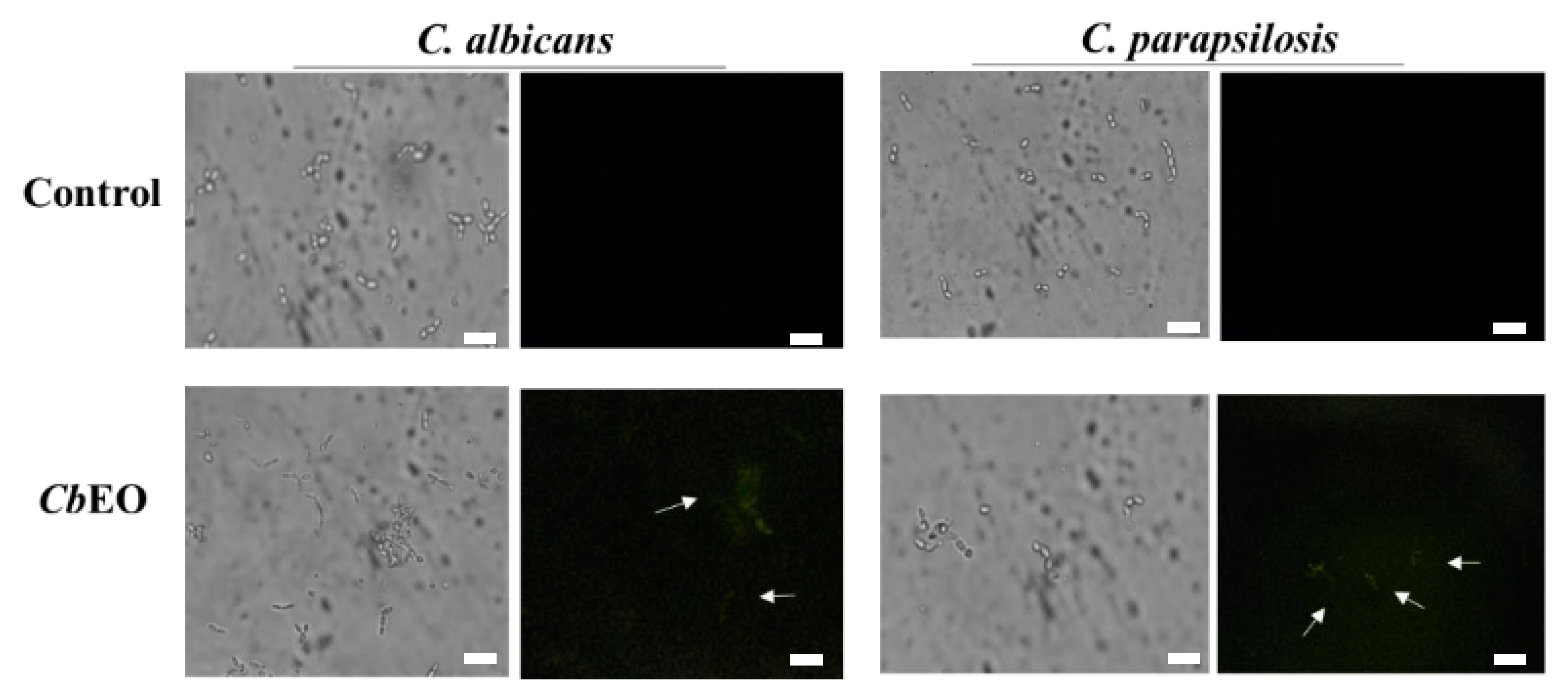
2.3.1. Membrane Damage and Pore Formation
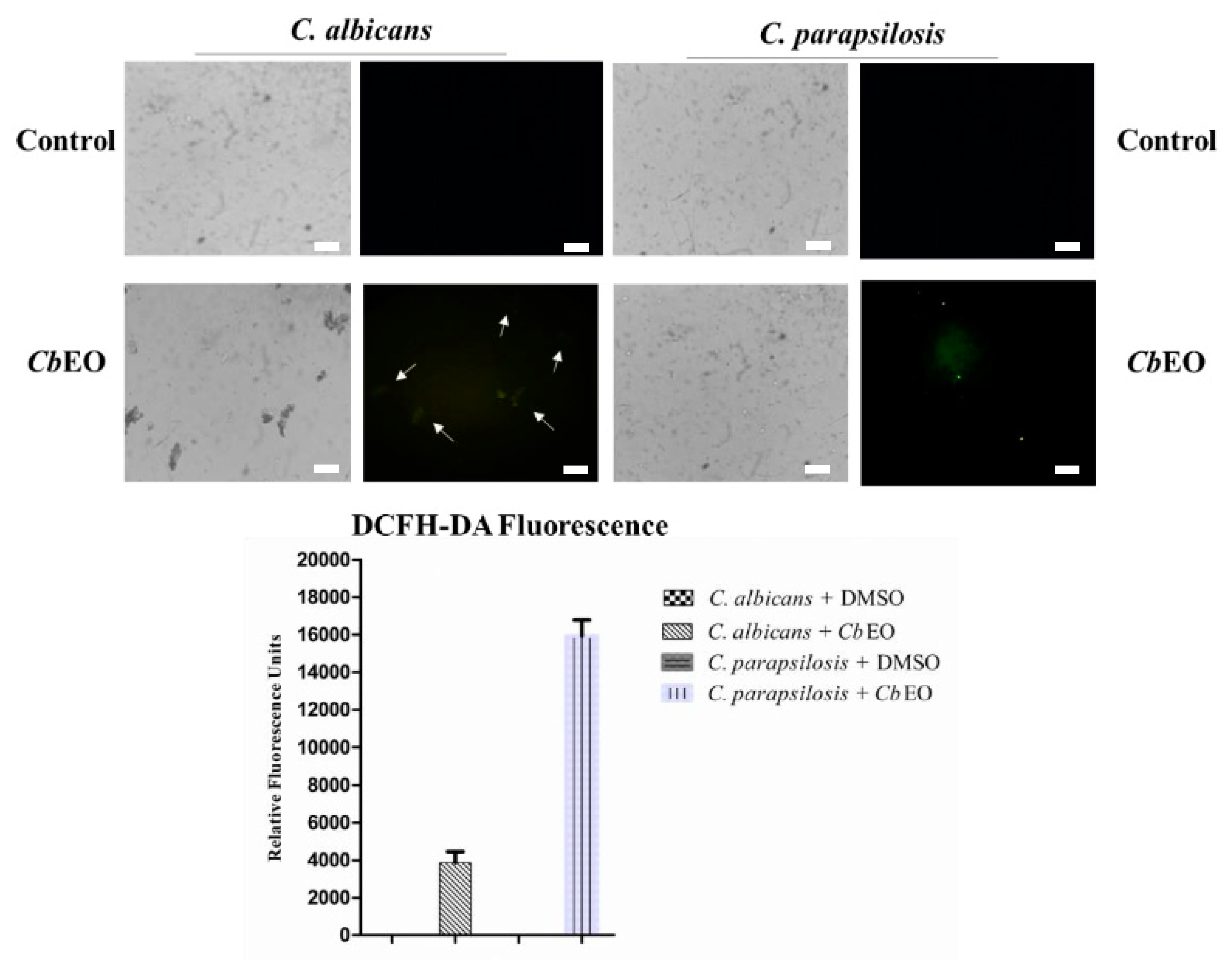
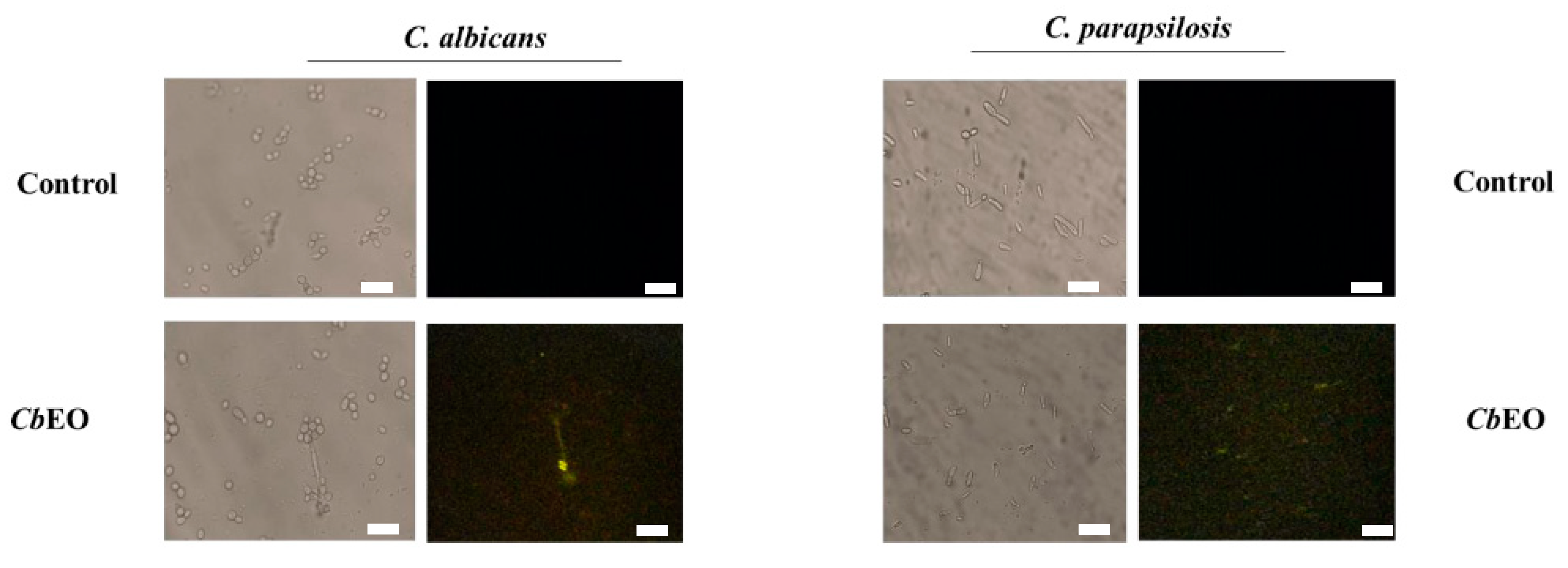
2.3.2. Overproduction of Reactive Oxygen Species (ROS)
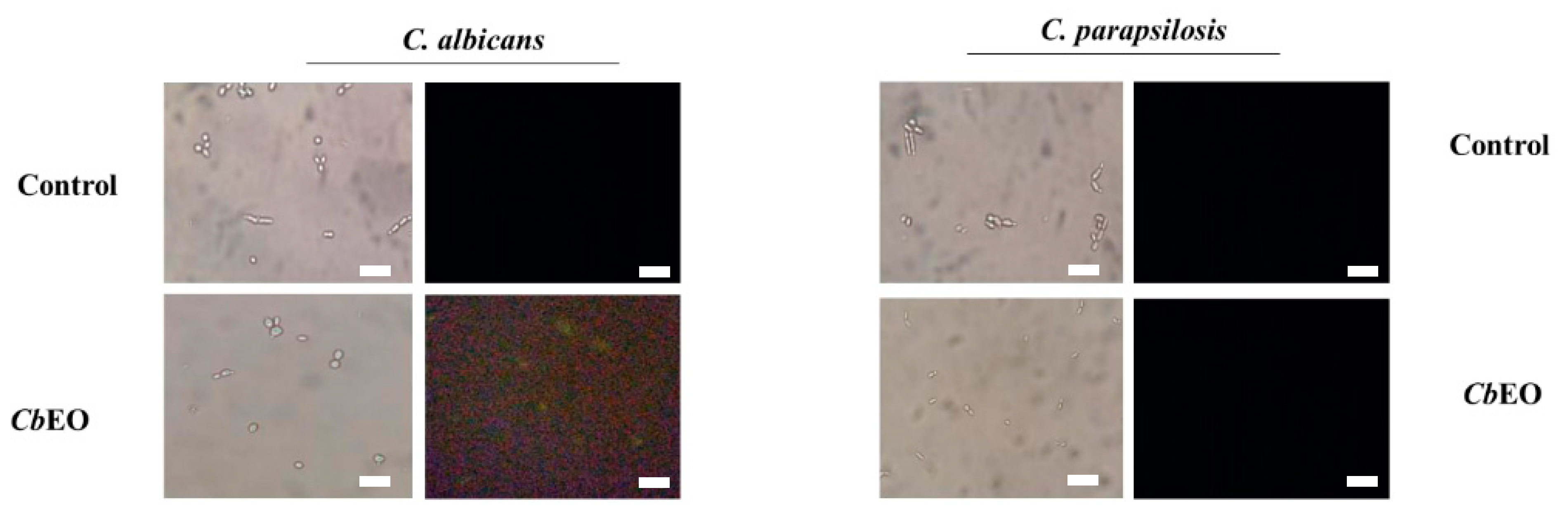
2.3.3. Caspase 3/7-Mediated Apoptosis
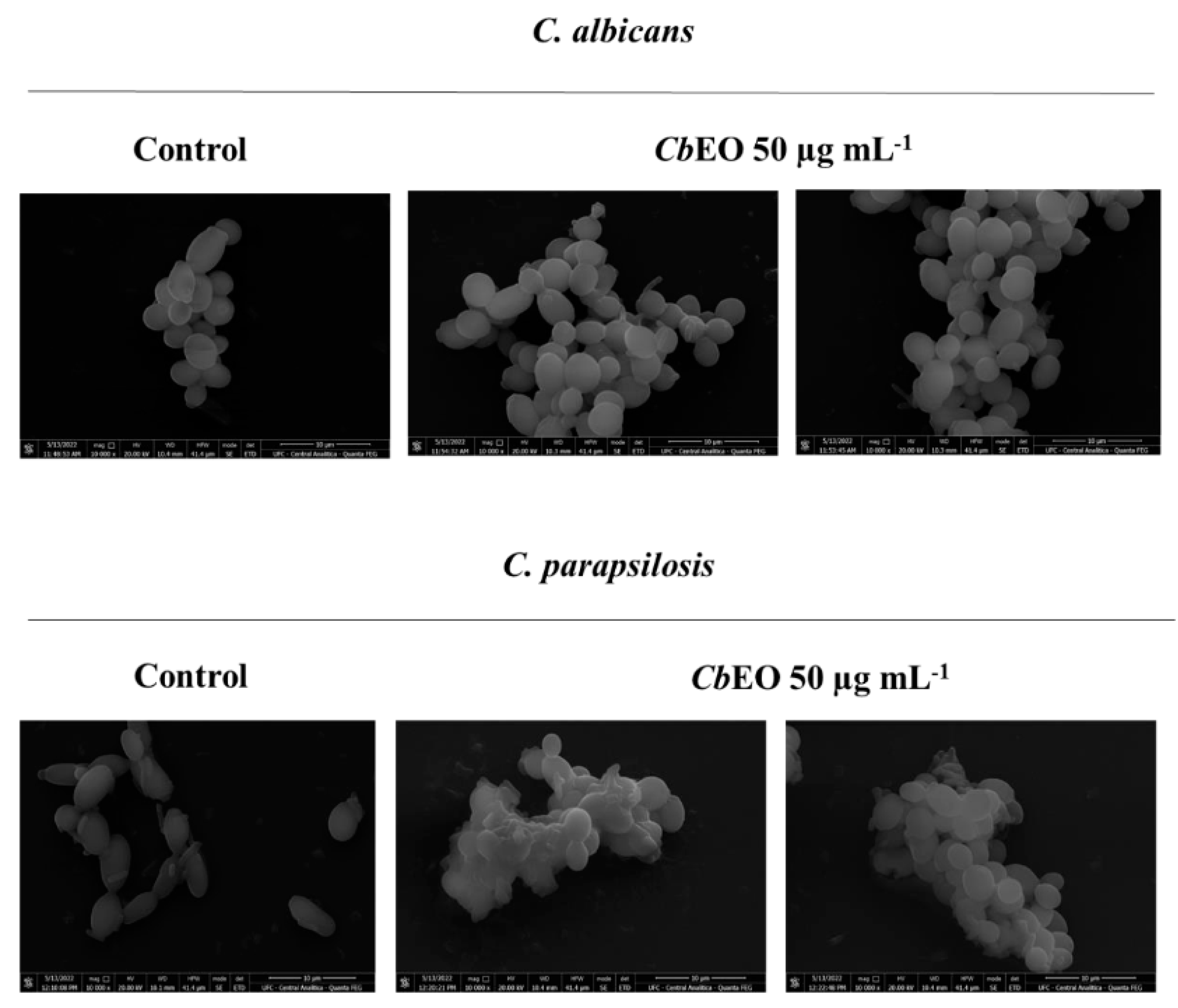
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. Hemolytic Activity
3. Conclusions
4. Experimental Section
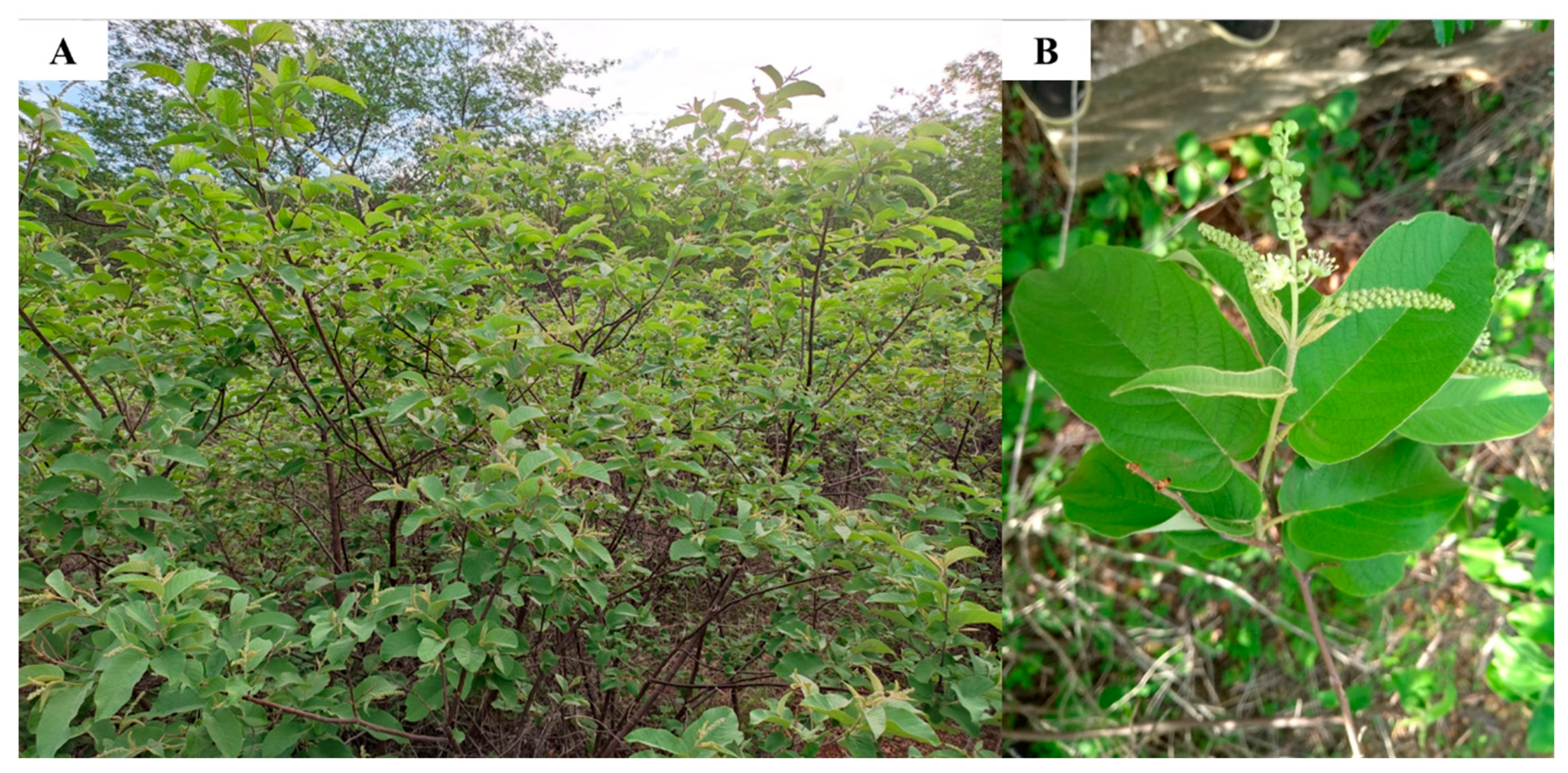
4.1. Biological Material
4.2. Oil Extraction
4.3. Characterization of CbEO by GC-MS/MS Analysis
4.4. Antimicrobial and Antibiofilm Activities
4.5. Mechanisms of Action
4.5.1. Membrane Damage
4.5.2. Induction of Reactive Oxygen Species (ROS)
4.5.3. Induction of Apoptosis
4.6. Scanning Electron Microscopy (SEM)
4.7. Hemolytic Activity
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Resistance; Global Report on Surveillance; Bulletin of the World Health Organization: Geneva, Switzerland, 2014; Volume 61, pp. 383–394. [Google Scholar]
- López-Martínez, R. Candidosis, a New Challenge. Clin. Dermatol. 2010, 28, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and Emerged Pathogenic Candida Species: Beyond the Candida albicans Paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Vale, J.P.C.; Ribeiro, L.H.d.F.; de Vasconcelos, M.A.; Sá-Firmino, N.C.; Pereira, A.L.; do Nascimento, M.F.; Rodrigues, T.H.S.; da Silva, P.T.; de Sousa, K.C.; da Silva, R.B.; et al. Chemical Composition, Antioxidant, Antimicrobial and Antibiofilm Activities of Vitex gardneriana Schauer Leaves’s Essential Oil. Microb. Pathog. 2019, 135, 103608. [Google Scholar] [CrossRef] [PubMed]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, Pathogenesis and Prevention—A Journey to Break the Wall: A Review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Mathur, S.; Hoskins, C. Drug Development: Lessons from Nature. Biomed. Rep. 2017, 6, 612–614. [Google Scholar] [CrossRef] [Green Version]
- Melo, G.F.d.A.; da Costa, A.C.V.; Garino, F., Jr.; Medeiros, R.S.; Madruga, M.S.; Neto, V.Q. The sensitivity of bacterial foodborne pathogens to Croton blanchetianus Baill essential oil. Braz. J. Microbiol. 2013, 44, 1189–1194. [Google Scholar] [CrossRef] [Green Version]
- Aquino, V.V.F.; Costa, J.G.M.; Angélico, E.C.; Medeiros, R.S.; Lucena, M.d.A.; Rodrigues, O.G. Metabólitos secundários e ação antioxidante de Croton heliotripifolius e Croton blanchetianus. Acta Bras. 2017, 1, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Freitas, A.F.S.; Costa, W.K.; Machado, J.C.B.; Ferreira, M.R.A.; Paiva, P.M.G.; Medeiros, P.L.; Soares, L.A.L.; Oliveira, A.M.; Napoleão, T.H. Toxicity Assessment and Antinociceptive Activity of an Ethanolic Extract from Croton blanchetianus (Euphorbiaceae) Leaves. South Afr. J. Bot. 2020, 133, 30–39. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira Sobrinho, A.C.; de Morais, S.M.; Marinho, M.M.; de Souza, N.V.; Lima, D.M. Antiviral Activity on the Zika Virus and Larvicidal Activity on the Aedes Spp. of Lippia alba Essential Oil and β-Caryophyllene. Ind. Crops Prod. 2021, 162, 113281. [Google Scholar] [CrossRef]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity. Pharmaceuticals 2020, 14, 27. [Google Scholar] [CrossRef]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene Inhibits Candida albicans Growth by Inducing Apoptosis. Med. Mycol. 2018, 56, 565–578. [Google Scholar] [CrossRef]
- Ai, Y.; Fang, F.; Zhang, L.; Liao, H. Antimicrobial Activity of Oregano Essential Oil and Resveratrol Emulsions Co-Encapsulated by Sodium Caseinate with Polysaccharides. Food Control 2022, 137, 108925. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Ren, X.; Yang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Antimicrobial and Anti-dust Mite Efficacy of Cinnamomum Camphora Chvar. Borneol Essential Oil Using Pilot-plant Neutral Cellulase-assisted Steam Distillation. Lett. Appl. Microbiol. 2022, 74, 258–267. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal Activity of Citral, Octanal and α-Terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic Properties of the Terpenoids Aromadendrene and 1,8-Cineole from the Essential Oil of Eucalyptus globulus against Antibiotic-Susceptible and Antibiotic-Resistant Pathogens. Phytomedicine 2010, 17, 1061–1066. [Google Scholar] [CrossRef]
- Keymaram, M.; Falahati, M.; Farahyar, S.; Lotfali, E.; Abolghasemi, S.; Mahmoudi, S.; Sadeghi, F.; Khalandi, H.; Ghasemi, R.; Shamsaei, S.; et al. Anti-Biofilm Properties of Eucalyptol in Combination with Antifungals against Candida albicans Isolates in Patients with Hematological Malignancy. Arch. Microbiol. 2022, 204. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A. Phytochemical Analysis of Cymbopogon flexuosus (Lemongrass) Oil, Its Antifungal Activity, and Role in Inhibiting Biofilm Formation in Candida albicans MTCC854. J. King Saud Univ. Sci. 2022, 34, 102072. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Bisignano, C.; Irrera, P.; Smeriglio, A.; Zagami, R.; Trombetta, D.; Romeo, O.; Mandalari, G. In Vitro Evaluation of the Activity of an Essential Oil from Pistacia vera L. Variety Bronte Hull against Candida Sp. BMC Complement. Altern. Med. 2019, 19, 6. [Google Scholar] [CrossRef]
- Sahal, G.; Woerdenbag, H.J.; Hinrichs, W.L.J.; Visser, A.; Tepper, P.G.; Quax, W.J.; van der Mei, H.C.; Bilkay, I.S. Antifungal and Biofilm Inhibitory Effect of Cymbopogon citratus (Lemongrass) Essential Oil on Biofilm Forming by Candida tropicalis Isolates; an in Vitro Study. J. Ethnopharmacol. 2020, 246, 112188. [Google Scholar] [CrossRef] [PubMed]
- Kojic, E.M.; Darouiche, R.O. Candida Infections of Medical Devices. Clin. Microbiol. Rev. 2004, 17, 255–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef] [PubMed]
- Etxaniz, A.; González-Bullón, D.; Martín, C.; Ostolaza, H. Membrane Repair Mechanisms against Permeabilization by Pore-Forming Toxins. Toxins 2018, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Yang, G.; Huang, S.; Li, B.; Li, A.; Kan, J. Chemical Composition of Zanthoxylum schinifolium Siebold & Zucc. Essential Oil and Evaluation of Its Antifungal Activity and Potential Modes of Action on Malassezia restricta. Ind. Crops Prod. 2022, 180, 114698. [Google Scholar] [CrossRef]
- Zore, G.B.; Thakre, A.D.; Rathod, V.; Karuppayil, S.M. Evaluation of Anti-Candida Potential of Geranium Oil Constituents against Clinical Isolates of Candida albicans Differentially Sensitive to Fluconazole: Inhibition of Growth, Dimorphism and Sensitization. Mycoses 2011, 54, e99–e109. [Google Scholar] [CrossRef]
- Ramage, G.; Milligan, S.; Lappin, D.F.; Sherry, L.; Sweeney, P.; Williams, C.; Bagg, J.; Culshaw, S. Antifungal, Cytotoxic, and Immunomodulatory Properties of Tea Tree Oil and Its Derivative Components: Potential Role in Management of Oral Candidosis in Cancer Patients. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, K.; Yin, Y.; Wu, J. D319 Induced Antifungal Effects through ROS-Mediated Apoptosis and Inhibited Isocitrate Lyase in Candida albicans. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130050. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Yusoff, K.; Thomas, W.; Akseer, R.; Alhosani, M.S.; Abushelaibi, A.; Lim, S.-H.-E.; Lai, K.-S. Lavender Essential Oil Induces Oxidative Stress Which Modifies the Bacterial Membrane Permeability of Carbapenemase Producing Klebsiella pneumoniae. Sci. Rep. 2020, 10, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, G.G.; Tan, S.-X.; Dawes, I.W. Reactive Oxygen Species and Yeast Apoptosis. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čáp, M.; Váchová, L.; Palková, Z. Reactive Oxygen Species in the Signaling and Adaptation of Multicellular Microbial Communities. Oxid. Med. Cell. Longev. 2012, 2012, 976753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, I.K.; Pathak, S.; Sharma, M.; Sanwal, H.; Chaudhary, P.; Tupe, S.; Deshpande, M.; Chauhan, V.S.; Prasad, R. Antifungal Activity of Novel Synthetic Peptides by Accumulation of Reactive Oxygen Species (ROS) and Disruption of Cell Wall against Candida albicans. Peptides 2011, 32, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.F.N.; Marques, L.S.M.; Oliveira, J.T.A.; Lima, P.G.; Dias, L.P.; Neto, N.A.S.; Lopes, F.E.S.; Sousa, J.S.; Silva, A.F.B.; Caneiro, R.F.; et al. Synthetic Antimicrobial Peptides: From Choice of the Best Sequences to Action Mechanisms. Biochimie 2020, 175, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.M.F.; Jezler, C.N.; Oliveira, R.A.; Mielke, M.S.E.; Costa, L.C.B. Determinação Do Tempo de Hidrodestilação e Do Horário de Colheita No Óleo Essencial de Menta. Hortic. Brasc 2012, 30, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard; Clinical and Laboratory Standards Institute: online, 2008. [Google Scholar]
- Oliveira, J.T.A.; Souza, P.F.N.; Vasconcelos, I.M.; Dias, L.P.; Martins, T.F.; Van Tilburg, M.F.; Guedes, M.I.F.; Sousa, D.O.B. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII Are Synthetic Antimicrobial Peptides Active against Human Pathogens by Stimulating ROS Generation and Increasing Plasma Membrane Permeability. Biochimie 2019, 157, 10–21. [Google Scholar] [CrossRef]
- Dias, L.P.; Souza, P.F.N.; Oliveira, J.T.A.; Vasconcelos, I.M.; Araújo, N.M.S.; Tilburg, M.F.V.; Guedes, M.I.F.; Carneiro, R.F.; Lopes, J.L.S.; Sousa, D.O.B. RcAlb-PepII, a Synthetic Small Peptide Bioinspired in the 2S Albumin from the Seed Cake of Ricinus communis, Is a Potent Antimicrobial Agent against Klebsiella pneumoniae and Candida parapsilosis. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183092. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Harrison, D.G. Methods for Detection of Mitochondrial and Cellular Reactive Oxygen Species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef]
- Staniszewska, M.; Bondaryk, M.; Swoboda-Kopec, E.; Siennicka, K.; Sygitowicz, G.; Kurzatkowski, W. Candida albicans Morphologies Revealed by Scanning Electron Microscopy Analysis. Braz. J. Microbiol. 2013, 44, 813–821. [Google Scholar] [CrossRef] [PubMed]
| Compound | Retention Time | Area (%) |
|---|---|---|
| limonene | 6.02 | 0.61 |
| eucalyptol | 6.14 | 5.62 |
| borneol | 9.84 | 0.64 |
| terpinen-4-ol | 10.13 | 1.32 |
| α-terpineol | 10.52 | 1.16 |
| myrtenol | 10.70 | 0.70 |
| δ-elemene | 14.61 | 0.46 |
| α-ylangene | 15.67 | 0.51 |
| β-bourbonene | 15.92 | 0.95 |
| sativene | 16.15 | 1.99 |
| E-caryophyllene | 16.86 | 1.95 |
| aromadendrene | 17.37 | 0.56 |
| 6,9-guaiadiene | 17.47 | 1.31 |
| α-humulene | 17.75 | 0.73 |
| alloaromadendrene | 17.95 | 1.10 |
| germacrene D | 18.47 | 0.57 |
| γ-himachalene | 18.61 | 0.70 |
| bicyclogermacrene | 18.90 | 0.57 |
| δ-amorphene | 19.54 | 5.92 |
| spathulenol | 21.08 | 1.49 |
| caryophylleneoxide | 21.16 | 20.03 |
| epi- α-muurolol | 22.79 | 5.81 |
| 4.55 | ||
| 2.98 |
| CbEO (50 µg mL−1) | ||||
|---|---|---|---|---|
| Antifungal activity | ||||
| Inhibition of planktonic cells growth (%) | Inhibition of biofilm formation (%) | Biomass reduction of preformed biofilm (%) | Nystatin | |
| C. albicans ATCC 10231 | 78.55 ± 0.3 | 44.12 ± 0.2 | 41.12 ± 0.4 | 85.75 ± 0.1 |
| C. krusei ATCC 6258 | 9.58 ± 0.5 | 00.00 | 00.00 | 75.00 ± 0.6 |
| C. parapsilosis ATCC 22019 | 75.94 ± 0.1 | 74.13 ± 0.7 | 27.75 ± 0.8 | 86.66 ± 0.3 |
| C. tropicalis clinical isolate | 00.00 | 00.00 | 00.00 | 93.85 ± 0.9 |
| C. neoformans ATCC 32045 | 00.00 | 00.00 | 00.00 | 75.02 ± 0.5 |
| Antibacterial activity | Ciprofloxacin | |||
| B. subtilis ATCC 6633 | 23.00 ± 0.3 | 00.00 | 00.00 | 85.42 ± 0.4 |
| E. aerogenes ATCC 13048 | 00.00 | 00.00 | 00.00 | 84.23 ± 0.4 |
| K. pneumoniae ATCC 10031 | 00.00 | 00.00 | 00.00 | 81.68 ± 0.7 |
| P. aeruginosa ATCC 25619 | 27.01 ± 0.7 | 00.00 | 00.00 | 87.39 ± 0.8 |
| S. enterica ATCC 14028 | 28.12 ± 0.5 | 00.00 | 00.00 | 87.74 ± 0.2 |
| Blood Type | Hemolysis (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0.1% Triton | 5% DMSO | CbEO 250 µg mL−1 | CbEO 150 µg mL−1 | CbEO 100 µg mL−1 | CbEO 50 µg mL−1 | CbEO 25 µg mL−1 | |
| Type A | 100 | 0 | 0 | 0 | v | 0 | 0 |
| Type B | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Type O | 100 | 0 | 23 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malveira, E.A.; Souza, P.F.N.; Neto, N.A.S.; Aguiar, T.K.B.; Rodrigues, N.S.; Henrique, C.W.B.; Silva, A.F.B.; Lima, L.B.; Albuquerque, C.C.; Freitas, C.D.T. Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action. J. Fungi 2022, 8, 1147. https://doi.org/10.3390/jof8111147
Malveira EA, Souza PFN, Neto NAS, Aguiar TKB, Rodrigues NS, Henrique CWB, Silva AFB, Lima LB, Albuquerque CC, Freitas CDT. Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action. Journal of Fungi. 2022; 8(11):1147. https://doi.org/10.3390/jof8111147
Chicago/Turabian StyleMalveira, Ellen A., Pedro F. N. Souza, Nilton A. S. Neto, Tawanny K. B. Aguiar, Natanael S. Rodrigues, Carlos W. B. Henrique, Ayrles F. B. Silva, Leandro B. Lima, Cynthia C. Albuquerque, and Cleverson D. T. Freitas. 2022. "Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action" Journal of Fungi 8, no. 11: 1147. https://doi.org/10.3390/jof8111147
APA StyleMalveira, E. A., Souza, P. F. N., Neto, N. A. S., Aguiar, T. K. B., Rodrigues, N. S., Henrique, C. W. B., Silva, A. F. B., Lima, L. B., Albuquerque, C. C., & Freitas, C. D. T. (2022). Essential Oil from Croton blanchetianus Leaves: Anticandidal Potential and Mechanisms of Action. Journal of Fungi, 8(11), 1147. https://doi.org/10.3390/jof8111147






