Soil Type Influences Novel “Milpa” Isolates of Trichoderma virens and Aspergillus tubingensis That Promote Solubilization, Mineralization, and Phytoabsorption of Phosphorus in Capsicum annuum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Area
2.2. Fungal Biodiversity as Measured Macro- and Microscopically in Different Selective Media
2.3. Selection of P-Solubilizing and -Mineralizing Strains
2.4. Biocompatibility among Strains
2.5. In Vitro Effect of the Isolates on Germination of C. annuum L.
2.6. Polyphasic Analysis of Selected Strains
2.7. Effect of Inoculation of Two Selected Strains on the Growth of C. annuum in Greenhouse Conditions
2.8. Experimental Design and Statistical Analysis
3. Results
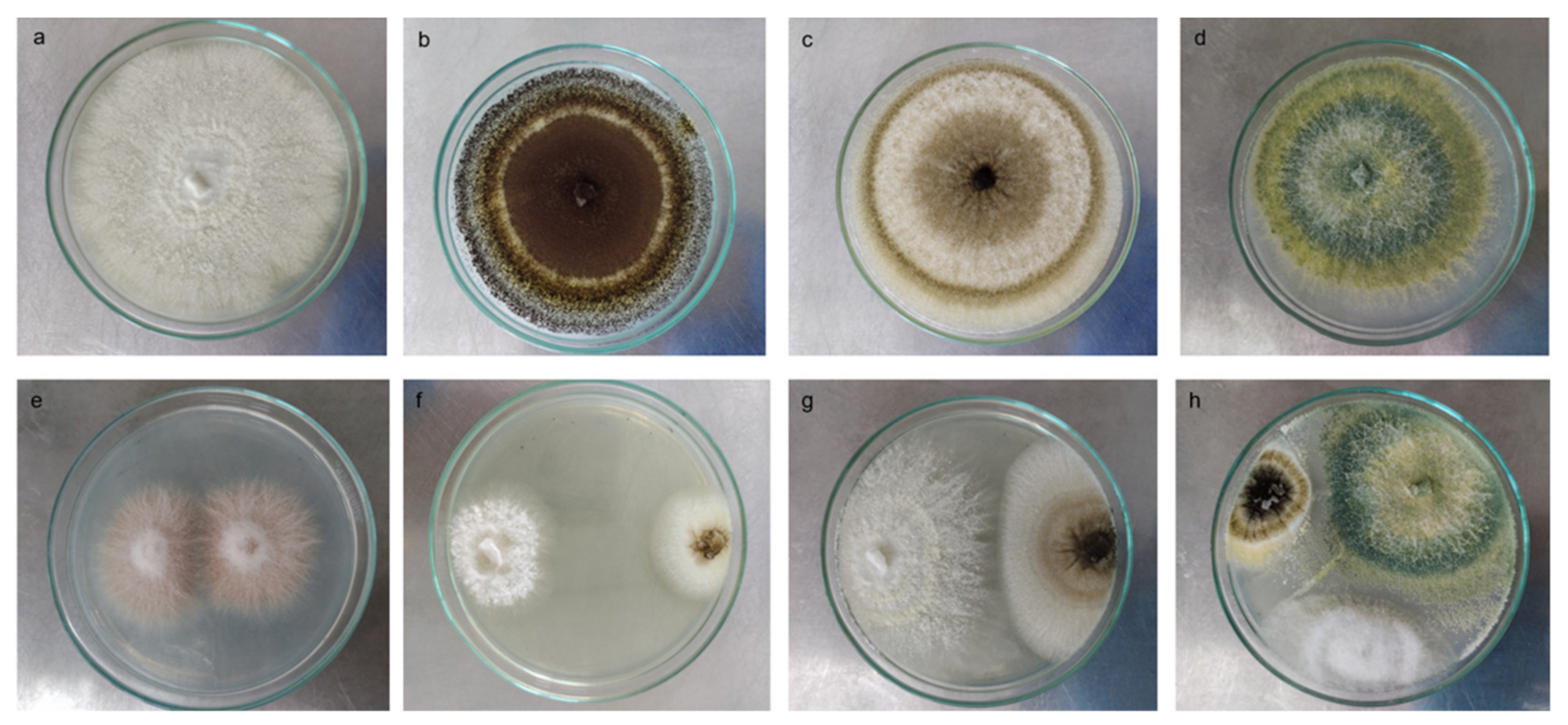
3.1. Fungal Isolates Present in Different Types of Soils and Root Environments
3.2. Biocompatibility among Strains
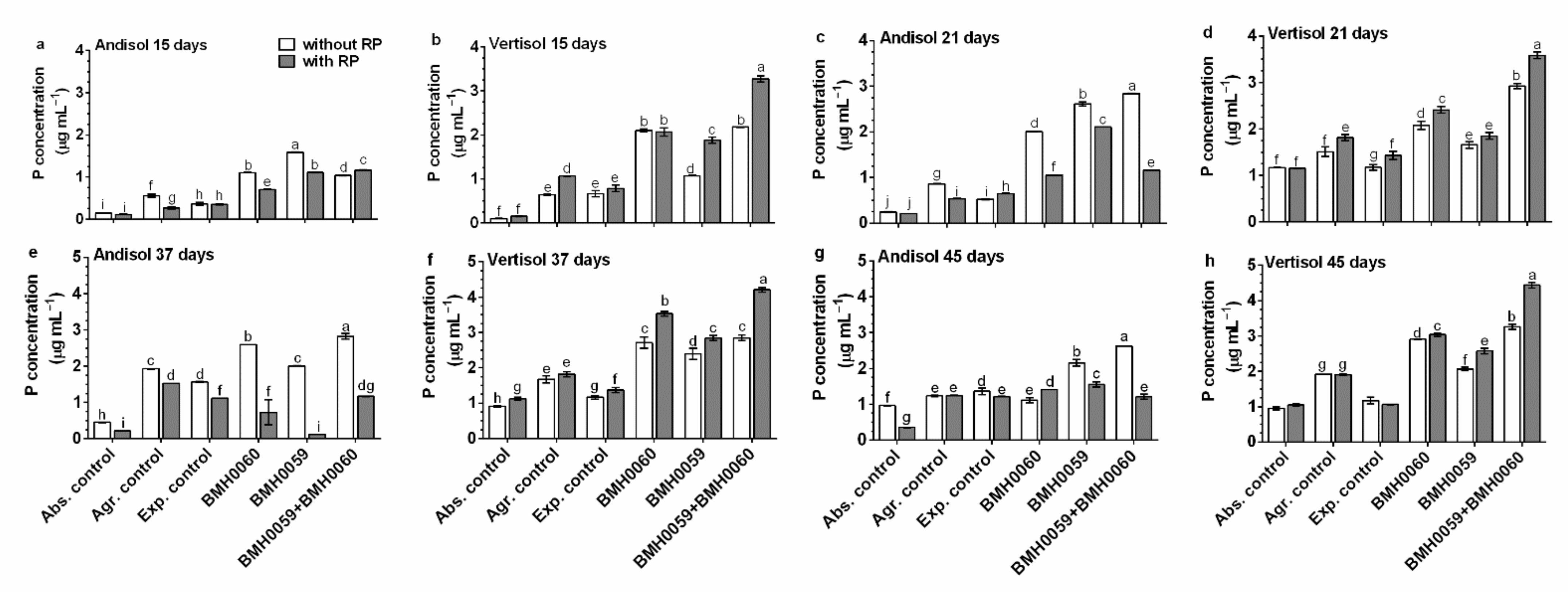
3.3. Selection of P-Solubilizing and -Mineralizing Strains
3.4. In Vitro Germination of Chili Seeds
3.5. Polyphasic Identification of the Best Solubilizer and Mineralizer Strains
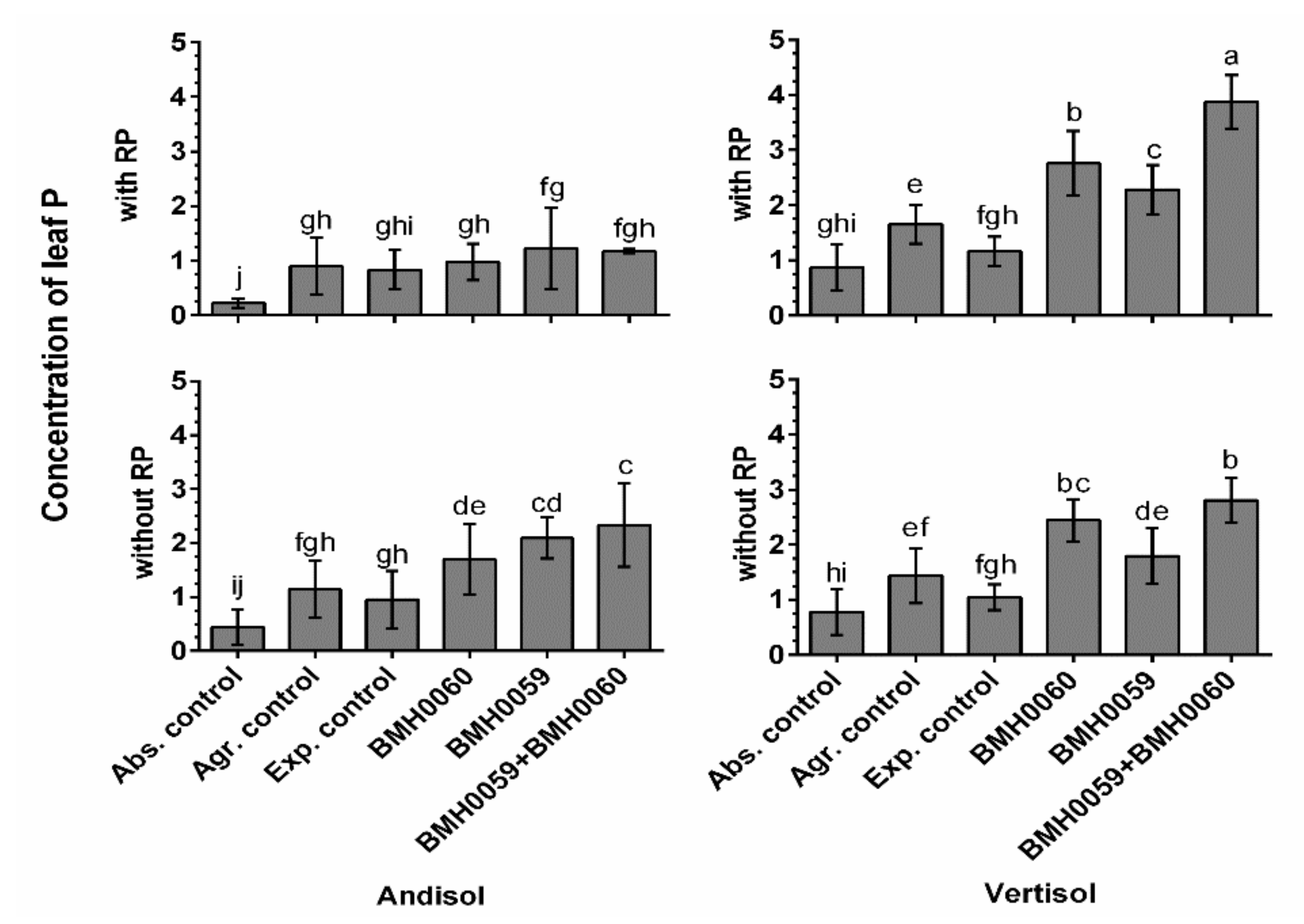
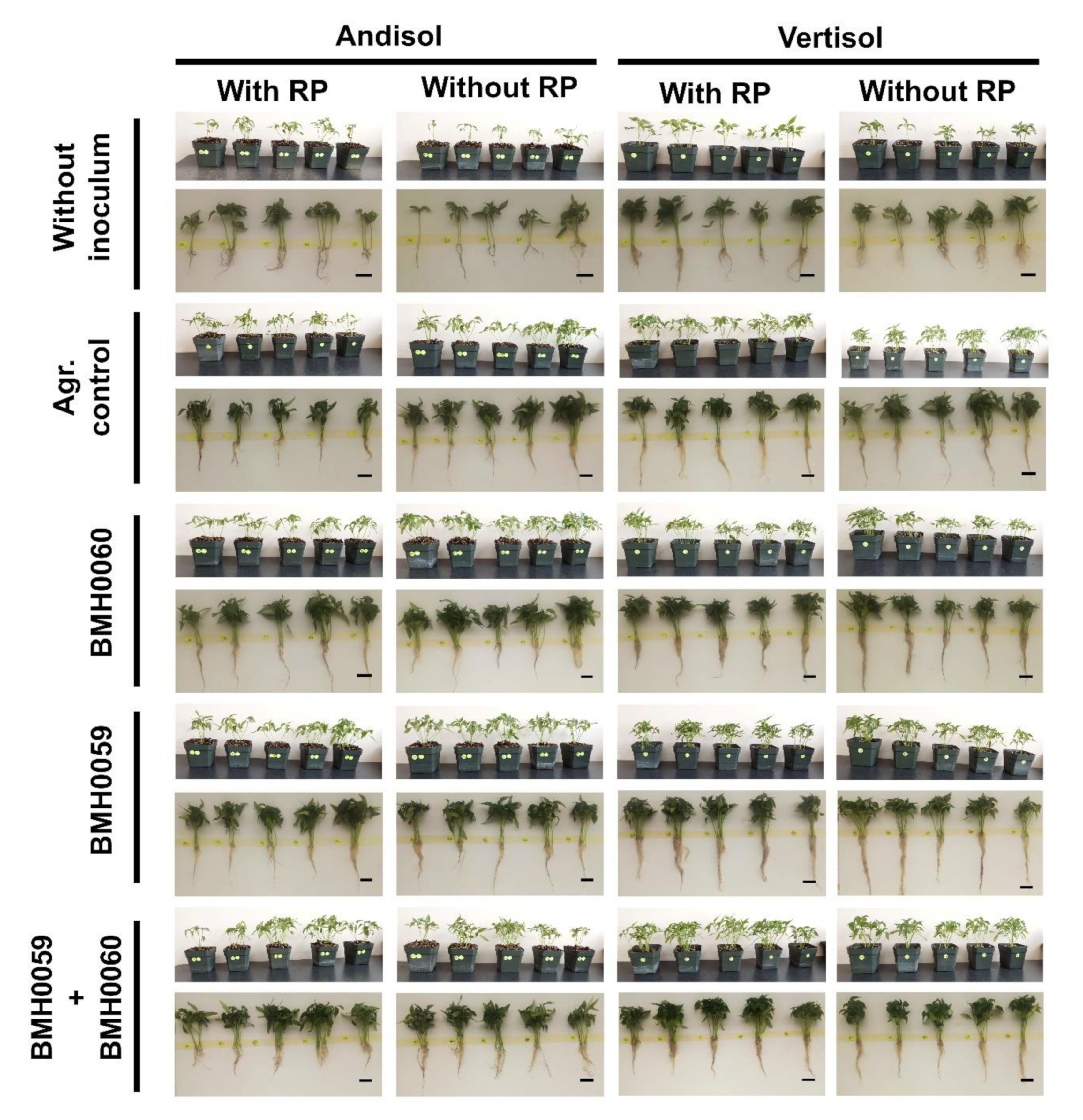
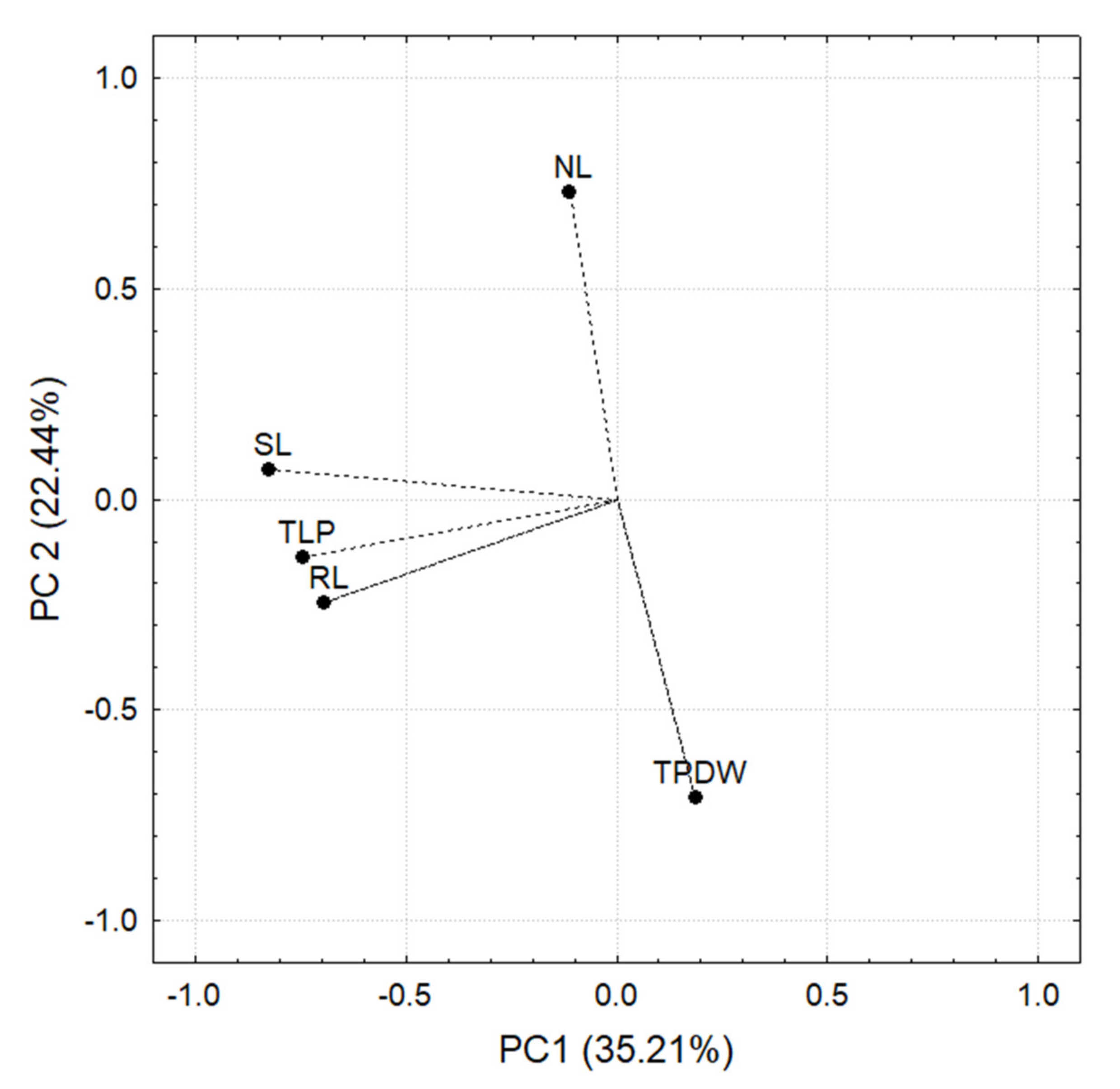
3.6. Greenhouse Evaluation of Chili Plant Performance in Different Kinds of Soils
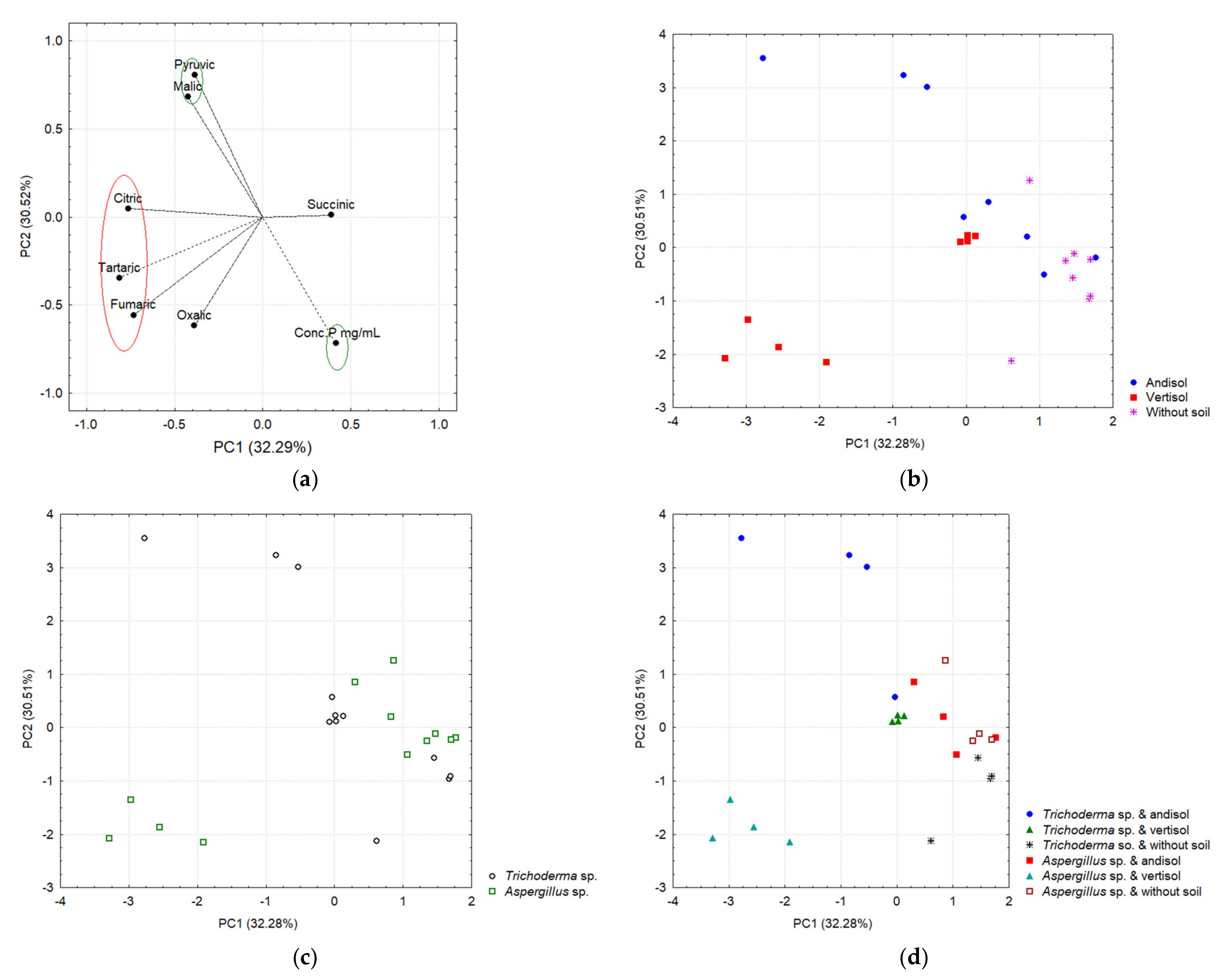
3.7. Profile of Organic Acids (OAs) and Its Correlation in Different Soil Types and P Concentrations in Chili Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrizo Garcia, C.; Barfuss, M.H.; Sehr, E.M.; Barboza, G.E.; Samuel, R.; Moscone, E.A.; Ehrendorfer, F. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann. Bot. 2016, 118, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; de Jesús Luna Ruiz, J.; Coppens d’Eeckenbrugge, G.; Hijmans, R.J.; Gepts, P. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.R.; Tavares, M.T.; Fernandes, T.B.; Parise-Filho, R. Peppers: A “Hot” Natural Source for Antitumor Compounds. Molecules 2021, 26, 1521. [Google Scholar] [CrossRef] [PubMed]
- Garland, G.; Bünemann, E.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Sixa, J. Phosphorus cycling within soil aggregate fractions of a highly weathered tropical soil: A conceptual model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Moreno, J.; León, J.; Osorio, W. Tree seedling growth promotion by dual inoculation with Rhizoglomus fasciculatum (Thaxt.) Sieverding, Silva & Oehl and Mortierella sp., rhizosphere fungi for reforestation purposes, to promote plant P uptake and growth at the nursery state. Acta Agron. 2016, 65, 239–247. [Google Scholar]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Martínez-Camacho, Y.D.; Negrete-Yankelevich, S.; Maldonado-Mendoza, I.E.; Núñez-de la Mora, A.; Amescua-Villela, G. Agroecological management with intra-and interspecific diversification as an alternative to conventional soil nutrient management in family maize farming. Agroecol. Sustain. Food Syst. 2022, 46, 364–391. [Google Scholar] [CrossRef]
- Restrepo-Llanos, M.; Osorio-Vega, N.; León-Pelaéz, J. Plant growth response of Pinus patula and P. maximinoi seedlings at nursery to three types of ectomycorrhizal inocula. Appl. Environ. Soil Sci. 2018, 2018, 6027351. [Google Scholar]
- Hiruma, K.; Gerlach, N.; Sacristan, S.; Nakano, R.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramírez, D.; Bucher, M.; O’Connell, R.; et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Osorio, W.; Osorno, L.; León, J.; Alvarez, C. Plant-Microbe interactions for phosphate management in tropical soils. In Essential Plant Nutrients; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Swizerland, 2017; pp. 491–512. [Google Scholar]
- Schneider, K.D.; van Straaten, P.; de Orduña, R.M.; Glasauer, S.; Trevors, J.; Fallow, D.; Smith, P.S. Comparing phosphorus mobilization strategies using Aspergillus niger for the mineral dissolution of three phosphate rocks. J. Appl. Microbiol. 2009, 108, 1364–5072. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Pandey, A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. Roots. Rhizosphere 2019, 9, 2–9. [Google Scholar] [CrossRef]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Six, J. Plant-mediated rhizosphere interactions in maize-pigeon pea Intercropping Enhance Soil Aggregation and Organic Phosphorus Storage. Plant Soil 2016, 415, 37–55. [Google Scholar] [CrossRef]
- Bononi, L.; Barros-Chiaramonte, J.; Cristiane-Pansa, C.; Alves-Moitinho, M.; Soares-Melo, I. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; Alarcón, A. Trichoderma: Agricultural and Biotechnological Importance, and Fermentation Systems for Producing Biomass and Enzymes Of Industrial Interest. Chilean J. Agric. Anim. Sci. Ex Agro-Cienc. 2019, 35, 98–112. [Google Scholar]
- Alam, S.; Khalil, S.; Ayub, N.; Rashid, M. In vitro solubilization of inorganic phosphate by phosphate solubilizing microorganism (PSM) from maize rhizosphere. Int. J. Agric. Biol. 2002, 4, 454–458. [Google Scholar]
- Gueçaimburu1 Vázquez, J.M.; Tancredi, F.; Reposo, G.P.; Rojo, V.; Martínez, M.; Introcaso, R.M. Evolution of Available Phosphorus At Different Levels Of Compaction By Agricultural Traffic In A Typical Argiudol. Chil. J. Agric. Anim. Sci. Ex Agro-Cienc. 2019, 35, 81–89. [Google Scholar]
- Valdovinos-Nava, W.; Chan-Cupul, W.; Herminia Alejandra Hernández-Ortega, H.A.; Ruíz-Sánchez, E. Effects of biological and mineral fertilization on the growth, nutrition, and yield of Capsicum chinense under greenhouse conditions. J. Plant Nutr. 2020, 43, 2286–2298. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-Induced Acidification Is an Early Trigger for Changes in Arabidopsis Root Growth and Determines Fungal Phytostimulation. Front. Plant Sci. 2017, 8, 822. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil. 2013, 362, 389–417. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. Elements of the Nature and Properties of Soils; Pearson Education International: Hoboen, NJ, USA, 2010. [Google Scholar]
- Shavrukov, Y.; Hirai, Y. Good and bad protons: Genetic aspects of acidity stress responses in plants. J. Exp. Bot. 2016, 67, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lou, H.Q.; Yang, J.L.; Zheng, S.J. The roles of STOP1-like transcription factors in aluminium and proton tolerance. Plant Signal. Behav. 2016, 11, e1131371. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga-Silgado, D.; Rivera-Leyva, J.C.; Coleman, J.J.; Sánchez-Reyes, A.; Valencia-Díaz, S.; Serrano, M.; de-Bashan, L.E.; Folch-Mallol, J.L. Soil Type Affects Organic Acid Production and Phosphorus Solubilization Efficiency Mediated by Several Native Fungal Strains from Mexico. Microorganisms 2020, 8, 1337. [Google Scholar] [CrossRef]
- Osorio, N.W.; Habte, M. Soil phosphate desorption induced by a phosphate-solubilizing fungus. Commun. Soil Sci. Plant. Anal. 2014, 45, 451–460. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; APS Press: St. Paul, MN, USA, 1998; p. 218. [Google Scholar]
- Tapia-Vázquez, I.; Sánchez-Cruz, R.; Arroyo-Domínguez, M.; Lira-Ruan, V.; Sánchez-Reyes, A.; Sánchez-Carbente, M.; Folch-Mallol, J.L. Isolation and characterization of psychrophilic and psychrotolerant plant-growth promoting microorganisms from a high-altitude volcano crater in Mexico. Microbiol. Res. 2020, 232, 126394. [Google Scholar] [CrossRef]
- Brown, R.F.; Mayer, D. Representing cumulative germination. 2. The use of the Weibull function and other empirically derived curves. Ann. Bot. 1988, 57, 49–53. [Google Scholar] [CrossRef]
- García, J.; Monteith, J.L.; Squire, G.R. Time, temperature, and germination of pearl millet (Pennisetum typhoides S. & H.). J. Exp. Bot. 1982, 33, 288–296. [Google Scholar]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K.A. Leaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar]
- Habte, M.; Osorio, N.W. Arbuscular Mycorrhizas: Producing and Applying Arbuscular Mycorrhizal Inoculum; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2001; 47p. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–35. [Google Scholar] [CrossRef]
- Li, Z.; Bai, T.; Dai, L.; Wang, F.; Tao, J.; Meng, S.; Hu, Y.; Wang, S.; Hu, S. A study of organic acid production in contrasts between two phosphate solubilizing fungi: Penicillium oxalicum and Aspergillus niger. Sci. Rep. 2016, 6, 25313. [Google Scholar] [CrossRef]
- Lagos, L.M.; Acuña, J.J.; Maruyama, F.; Ogram, A.; de la Luz Mora, M.; Jorquera, M.A. Effect of phosphorus addition on total and alkaline phosphomonoesterase-harboring bacterial populations in ryegrass rhizosphere microsites. Biol. Fertil. Soils 2016, 52, 1007–1019. [Google Scholar] [CrossRef]
- Cade-Menun, B. Characterizing phosphorus forms in cropland soils with solution 31P-NMR: Past studies and future research needs. Chem. Biol. Technol. Agric. 2017, 4, 19. [Google Scholar] [CrossRef]
- Bayuelo Jiménez, J.S.; Ochoa, I.; Cruz Torres, E.; Muraoka, T. Efecto del uso del suelo en las formas y disponibilidad de fósforo de un Andisol de la Meseta P’urhépecha, Michoacán. Terra Latinoam. 2019, 37, 35–44. [Google Scholar] [CrossRef]
- Magallon-Servin, P.; Antoun, H.; Taktek, S.; de-Bashan, L.E. Designing a multi-species inoculant of phosphate rock-solubilizing bacteria compatible with arbuscular mycorrhizae for plant growth promotion in low P soil amended with PR. Biol. Fertil. Soils 2020, 56, 521–536. [Google Scholar] [CrossRef]
- Cubillos-Hinojosa, J.; Valero, N.; Mejía, L. Trichoderma harzianum as a plant growth promoter in yellow passion fruit (Passiflora edulis var. flavicarpa Degener). Agron. Colomb. 2009, 27, 81–86. [Google Scholar]
- Hernández-Leal, T.; Gloria Carrión, G.; Heredia, G. In vitro Phosphate Solubilization by a Strain of Paecilomyces lilacinus (Thom) Samson. Agrociencia 2011, 45, 881–892. [Google Scholar]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [PubMed]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in–chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Arvidsson, J.; Håkansson, I. Response of different crops to soil compaction. Short term effects in Swedish field experiments. Soil Tillage Res. 2014, 138, 56–63. [Google Scholar] [CrossRef]
- Fernández, C.; Mendoza, R. Evaluación del fósforo disponible mediante tres métodos en distintos suelos y manejos productivos. Cienc. Del Suelo 2008, 1, 13–27. [Google Scholar]
- García-López, A.M.; Avilés, M.; Delgado, A. Plant uptake of phosphorus from sparingly available P- sources as affected by Trichoderma asperellum T34. Agric. Food Sci. 2015, 24, 249–260. [Google Scholar] [CrossRef]
- García-López, A.M.; Recena, R.; Avilés, M.; Delgado, A. Efect of Bacillus subtilis QST713 and Trichoderma asperellum T34 on P uptake by wheat and how it is modulated by soil properties. J. Soils Sediments 2018, 18, 727–738. [Google Scholar] [CrossRef]
- Redel, Y.; Rubio, R.; Godoy, R.; Borie, F. Phosphorus fractions and phosphatase activity in an Andisol under different forest ecosystems. Geoderma 2008, 145, 216–221. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hadobas, P.A.; Hayes, J.E. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J. 2001, 25, 641–649. [Google Scholar] [CrossRef]
- Graças, J.P.; Ruiz-Romero, R.; Figueiredo, L.D.; Mattiello, L.; Peres, L.E.P.; Vitorello, V.A. Root growth restraint can be an acclimatory response to low pH and is associated with reduced cell mortality: A possible role of class III peroxidases and NADPH oxidases. Plant Biol. 2016, 18, 658–668. [Google Scholar] [CrossRef]
- Gerhart, J.C.; Pardee, A.B. The enzymology of control by feedback inhibition. J. Biol. Chem. 1962, 237, 891–896. [Google Scholar] [CrossRef]
- Aranda, J.S.; Salgado, E. Modelación de catálisis enzimática con enzimas alostéricas. Enzymatic catalysis modelling with allosteric enzymes. Rev. Mex. Ing. Química 2008, 7, 21–27. [Google Scholar]
- Tandon, A.; Fatima, T.; Anshu, A.; Shukla, D. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. J. King Saud Univ.-Sci. 2019, 32, 791–798. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, M.; Braithwaite, M.; de Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed]
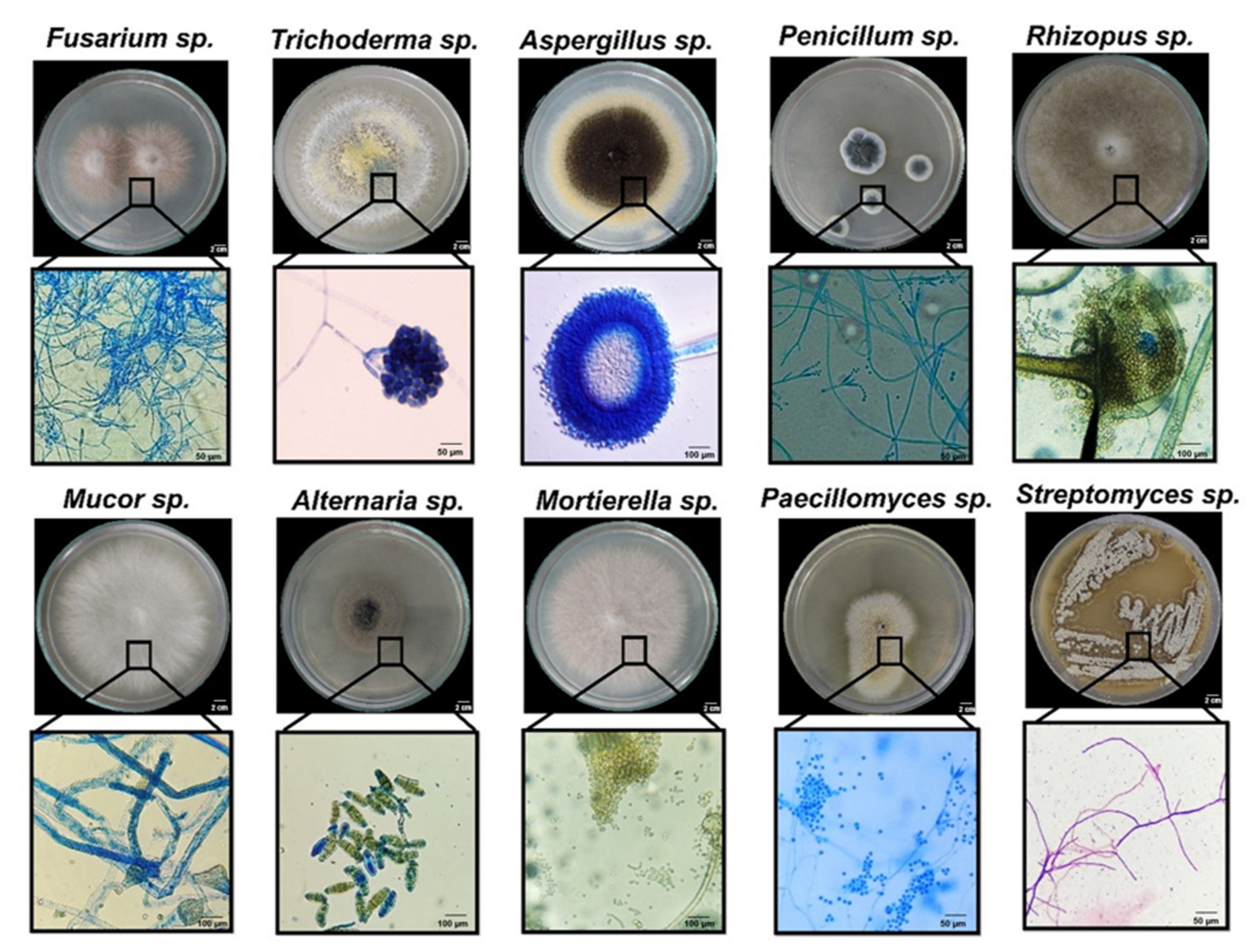




| Substratum | Culture Media | Microbial Genera Present in Vertisol (CFU g−1) | Fungal Genera Present in Andisol (CFU g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trichoderma | Aspergillus | Penicillium | Paecilomyces | Streptomyces | Rhizopus | Fusarium | Trichoderma | Aspergillus | Penicillium | ||
| Rhizosphere | PDA | 6.5 × 104 | 5.4 × 104 | 3.9 × 104 | 3.3 × 104 | 2.4 x104 | 2.5 × 104 | 1.4 × 104 | 2.5 × 104 | 1.4 × 104 | 1.9 × 104 |
| BRA | 7.8 × 104 | 6.0 × 104 | 3.4 × 104 | 3.5 × 104 | 2.0 × 104 | - | 1.5 × 104 | 2.5 × 104 | 1.5 × 104 | 1.4 × 104 | |
| OHM | 4.5 × 104 | 4.5 × 104 | 5.0 × 104 | 4.0 × 103 | 2.5 × 104 | 6.0 × 104 | 1.5 × 104 | 6.0 × 104 | 1.5 × 104 | 1.5 × 104 | |
| PNM | 8.5 × 104 | 7.3 × 104 | 7.5 × 104 | 4.2 × 104 | 2.3 × 104 | 5.5 × 104 | 1.3 × 104 | 5.5 × 104 | 1.3 × 104 | 1.5 × 104 | |
| Rhizoplane | PDA | 1.8 × 104 | 2.5 × 104 | 2.5 × 104 | 1.7 × 104 | 1.5 × 104 | 2.8 × 104 | 4.5 × 104 | 2.8 × 104 | 4.5 × 104 | 4.5 × 104 |
| BRA | 1.0 × 104 | 2.0 × 104 | 2.9 × 104 | 1.3 × 104 | 1.0 × 104 | - | - | 5.5 × 104 | 5.0 × 104 | 4.9 × 104 | |
| OHM | 1.5 × 104 | 2.5 × 104 | 1.2 × 104 | 1.0 × 103 | 1.5 × 104 | 5.0 × 104 | 4.5 × 104 | 5.0 × 104 | 4.5 × 104 | 4.0 × 104 | |
| PNM | 1.8 × 104 | 1.5 × 104 | 1.5 × 104 | 1.7 × 103 | 1.5 × 104 | 5.0 × 104 | 5.0 × 104 | 5.0 × 104 | 1.0 × 104 | 4.0 × 104 | |
| Endophyte | PDA | - | - | - | - | - | - | 1.4 × 104 | - | - | - |
| BRA | 1.8 × 104 | - | - | - | - | - | - | 1.5 × 104 | - | - | |
| OHM | 1.5 × 104 | - | 1.0 × 104 | - | 1.5 × 104 | - | 1.3 × 104 | 1.0 × 104 | - | - | |
| PNM | 1.5 × 104 | - | - | - | 1.3 × 104 | - | 1.0 × 104 | 1.0 × 104 | - | - | |
| Strain(s) | Treatment | GP | GSI | MGT |
|---|---|---|---|---|
| Without Inoculum | Control | 53.4 ± 1.00 d | 0.994 ± 0.003 e | 9.13 ± 0.03 a |
| BMH-0059 T. virens | Low inoculum | 44.1 ± 0.98 e | 1.325 ± 0.10 d | 7.20 ± 0.10 b |
| Medium inoculum | 93.1 ± 1.00 a | 1.962 ± 0.02 c | 5.80 ± 0.15 e | |
| High inoculum | 64.4 ± 1.00 c | 1.725 ± 0.10 c | 6.70 ± 0.10 c | |
| BMH-0061 Trichoderma sp. | Low inoculum | 53.0 ± 1.00 d | 0.990 ± 0.11 e | 9.09 ± 0.06 a |
| Medium inoculum | 93.3 ± 1.00 a | 1.849 ± 0.10 c | 6.17 ± 0.09 d | |
| High inoculum | 52.3 ± 1.17 d | 0.999 ± 0.03 e | 9.00 ± 0.00 a | |
| BMH-0062 T. pubescens | Low inoculum | 60.7 ± 1.00 c | 1.221 ± 0.05 d | 7.40 ± 0.10 b |
| Medium inoculum | 91.7 ± 0,70 a | 2.621 ± 0.11 a | 5.90 ± 0.15 e | |
| High inoculum | 66.7 ± 1.00 b | 1.821 ± 0.10 c | 6.60 ± 0.10 c | |
| BMH-0060 A. tubingensis | Low inoculum | 53.8 ± 0.64 d | 1.294 ± 0.10 d | 9.14 ± 0.03 a |
| Medium inoculum 6 | 91.1 ± 1.00 a | 2.275 ± 0.14 b | 6.90 ± 0.45 c | |
| High inoculum | 65.7 ± 1.00 bc | 1.828 ± 0.10 c | 6.50 ± 0.00 c | |
| BMH-0059 + BMH-0060 | Medium inoculum each | 93.1 ± 1.00 a | 1.662 ± 0.15 c | 4.80 ± 0.10 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zúñiga-Silgado, D.; Sánchez-Reyes, A.; Ortiz-Hernández, M.L.; Otero, M.; Balcázar-López, E.; Valencia-Díaz, S.; Serrano, M.; Coleman, J.J.; Sarmiento-López, L.; De-Bashan, L.E.; et al. Soil Type Influences Novel “Milpa” Isolates of Trichoderma virens and Aspergillus tubingensis That Promote Solubilization, Mineralization, and Phytoabsorption of Phosphorus in Capsicum annuum L. J. Fungi 2022, 8, 1178. https://doi.org/10.3390/jof8111178
Zúñiga-Silgado D, Sánchez-Reyes A, Ortiz-Hernández ML, Otero M, Balcázar-López E, Valencia-Díaz S, Serrano M, Coleman JJ, Sarmiento-López L, De-Bashan LE, et al. Soil Type Influences Novel “Milpa” Isolates of Trichoderma virens and Aspergillus tubingensis That Promote Solubilization, Mineralization, and Phytoabsorption of Phosphorus in Capsicum annuum L. Journal of Fungi. 2022; 8(11):1178. https://doi.org/10.3390/jof8111178
Chicago/Turabian StyleZúñiga-Silgado, Dorcas, Ayixon Sánchez-Reyes, María Laura Ortiz-Hernández, Miranda Otero, Edgar Balcázar-López, Susana Valencia-Díaz, Mario Serrano, Jeffrey J. Coleman, Luis Sarmiento-López, Luz E. De-Bashan, and et al. 2022. "Soil Type Influences Novel “Milpa” Isolates of Trichoderma virens and Aspergillus tubingensis That Promote Solubilization, Mineralization, and Phytoabsorption of Phosphorus in Capsicum annuum L." Journal of Fungi 8, no. 11: 1178. https://doi.org/10.3390/jof8111178
APA StyleZúñiga-Silgado, D., Sánchez-Reyes, A., Ortiz-Hernández, M. L., Otero, M., Balcázar-López, E., Valencia-Díaz, S., Serrano, M., Coleman, J. J., Sarmiento-López, L., De-Bashan, L. E., & Folch-Mallol, J. L. (2022). Soil Type Influences Novel “Milpa” Isolates of Trichoderma virens and Aspergillus tubingensis That Promote Solubilization, Mineralization, and Phytoabsorption of Phosphorus in Capsicum annuum L. Journal of Fungi, 8(11), 1178. https://doi.org/10.3390/jof8111178










