Flavonoids Modulate Aspergillus flavus Proliferation and Aflatoxin Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results
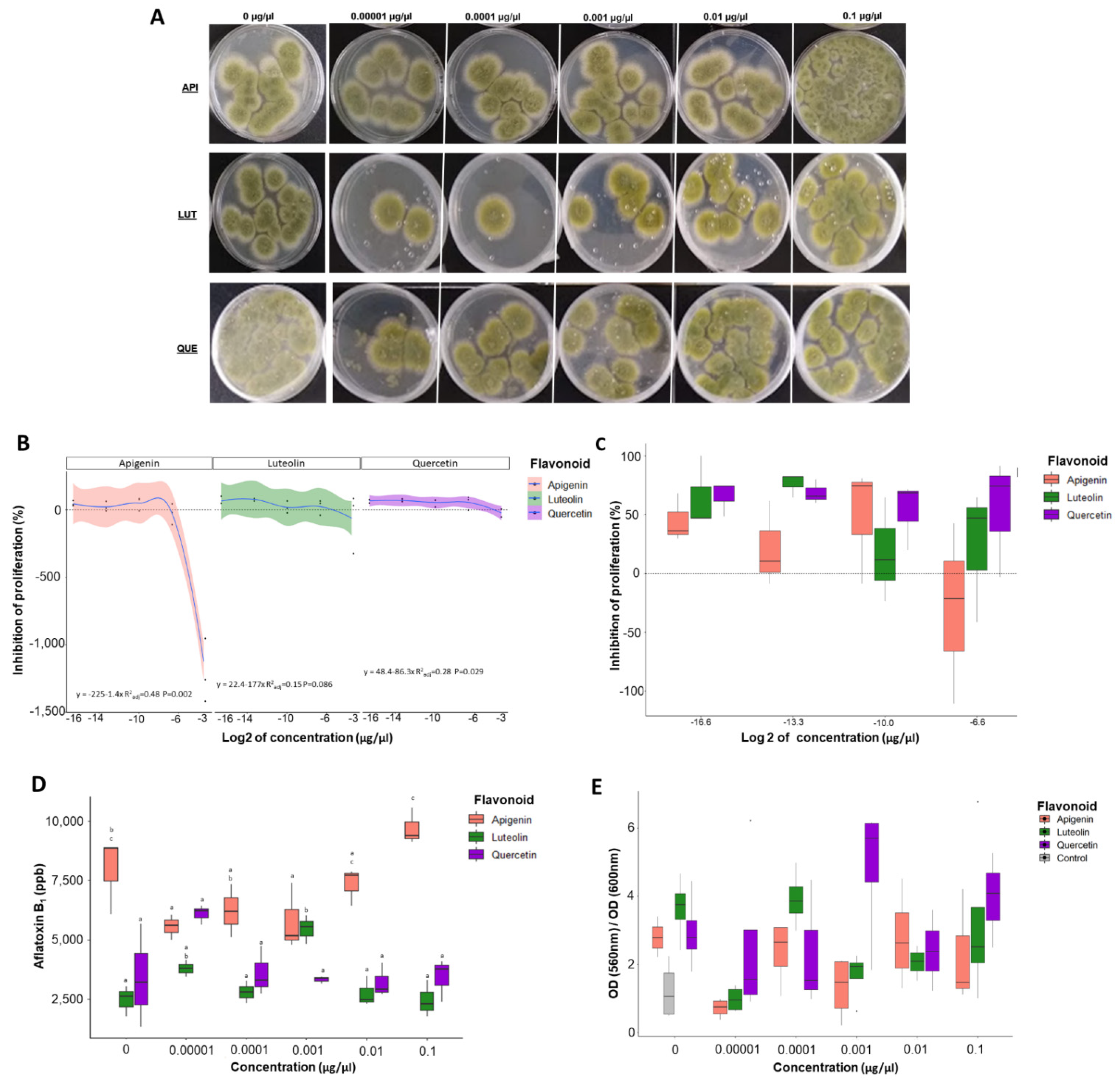
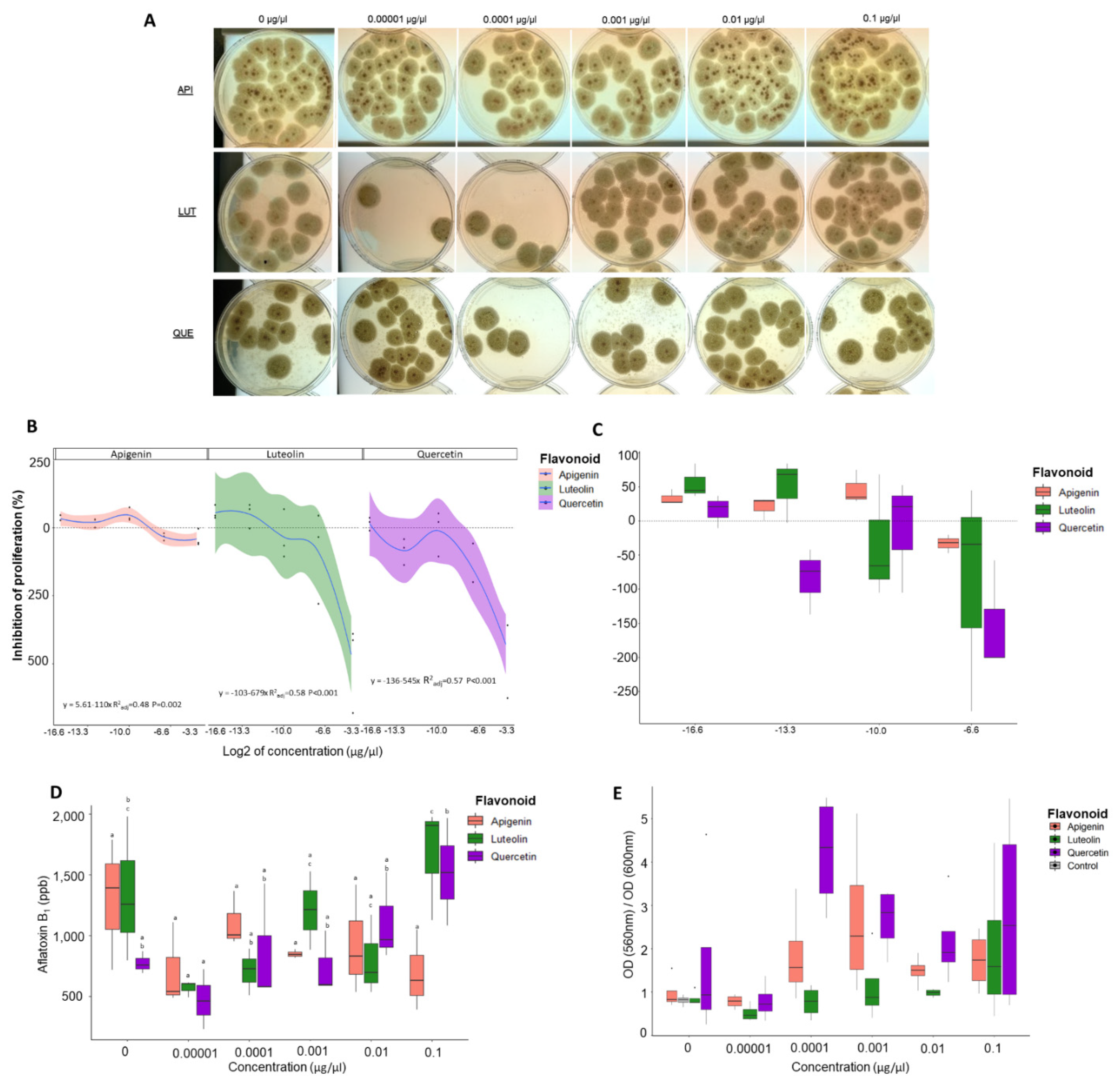
3.1. Proliferation Assays Show That Flavonoids Can Modulate Number of Fungal Colonies
3.2. Flavonoids Significantly Alter Aflatoxin Production in A. flavus
3.3. Luteolin Significantly Affects the Ratio of Metabolic Rate to Fungal Growth Rate
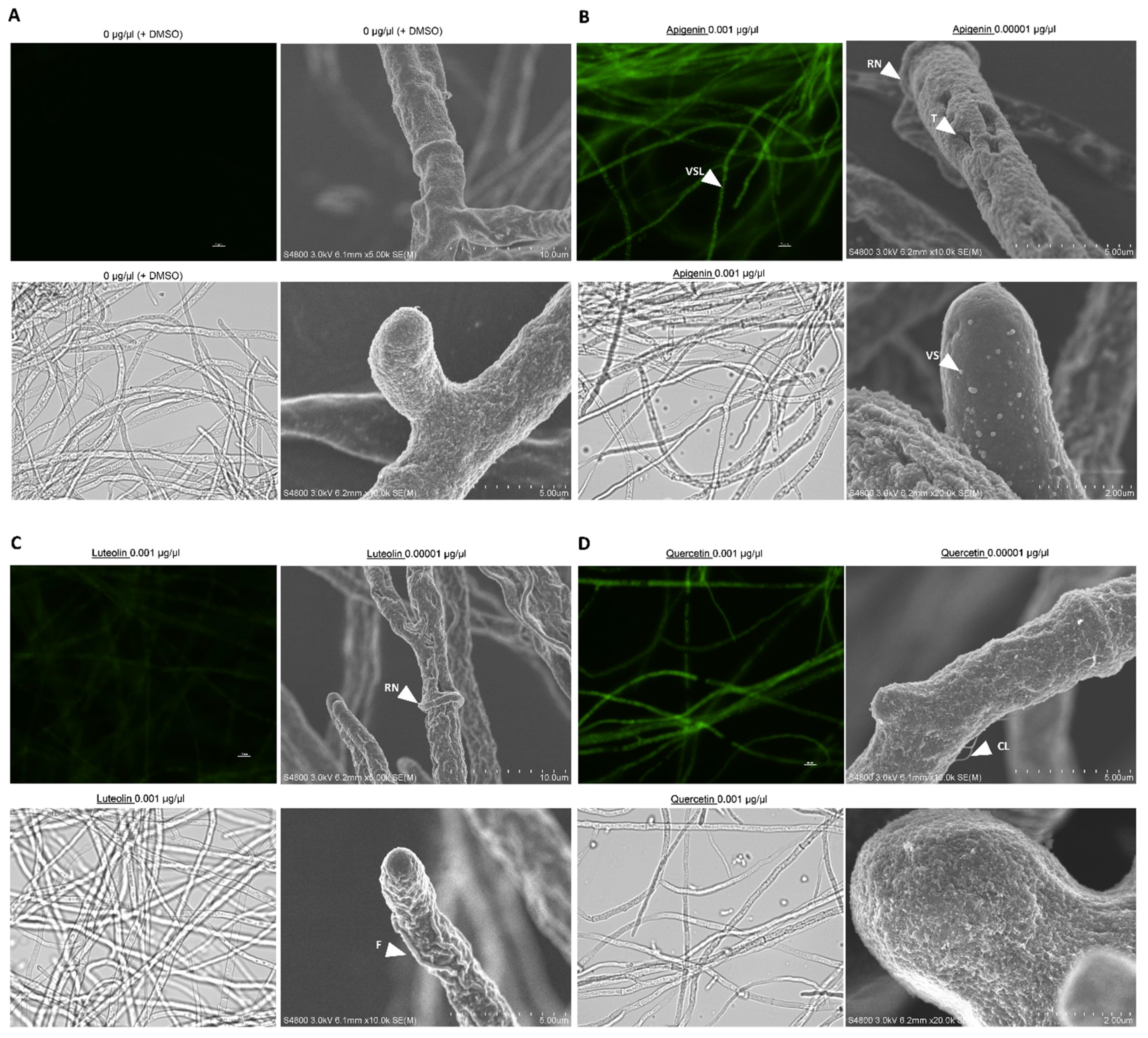
3.4. Flavonoids Affect the Physical Properties of the Fungal Cell Wall
3.5. Flavonoids Are Localized in Vesicle-like Structures on Hyphae
3.6. Low Concentrations of Flavonoids Decrease Antioxidant Capacity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application—A review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubile, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic Profile and Susceptibility to Fusarium Infection of Pigmented Maize Cultivars. Front. Plant Sci. 2018, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Beta, T.; Reyneri, A.; Blandino, M. Changes in the Phenolic Acid Content and Antioxidant Activity During Kernel Development of Corn (Zea mays L.) and Relationship with Mycotoxin Contamination. Cereal Chem. 2017, 94, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Castano-Duque, L.; Gilbert, M.K.; Mack, B.M.; Lebar, M.D.; Carter-Wientjes, C.H.; Sickler, C.M.; Cary, J.W.; Rajasekaran, K. Flavonoids Modulate the Accumulation of Toxins from Aspergillus flavus in Maize Kernels. Front. Plant Sci. 2021, 12, 761446. [Google Scholar] [CrossRef]
- Martucciello, S.; Masullo, M.; Cerulli, A.; Piacente, S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef] [Green Version]
- Pok, P.S.; Londoño, V.A.G.; Vicente, S.; Romero, S.M.; Pacín, A.; Tolaba, M.; Alzamora, S.M.; Resnik, S.L. Evaluation of citrus flavonoids against Aspergillus parasiticus in maize: Aflatoxins reduction and ultrastructure alterations. Food Chem. 2020, 318, 126414. [Google Scholar] [CrossRef]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum Sensu Stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [Green Version]
- Steinkellner, S.; Mammerler, R. Effect of flavonoids on the development of Fusarium oxysporum f. sp. lycopersici. J. Plant Interact. 2007, 2, 17–23. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, R.A. Inhibition of Aflatoxin B1 Biosynthesis in Aspergillus flavus by Anthocyanidins and Related Flavonoids. J. Agric. Food Chem. 1999, 47, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Li, Z.-Y.; Wang, Y.-D.; Wang, J.-Q.; Yang, P.-L. Quercetin Inhibits the Proliferation and Aflatoxins Biosynthesis of Aspergillus flavus. Toxins 2019, 11, 154. [Google Scholar] [CrossRef] [Green Version]
- Förster, C.; Handrick, V.; Ding, Y.; Nakamura, Y.; Paetz, C.; Schneider, B.; Castro-Falcón, G.; Hughes, C.C.; Luck, K.; Poosapati, S.; et al. Biosynthesis and antifungal activity of fungus-induced O-methylated flavonoids in maize. Plant Physiol. 2022, 188, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Nessa, F.; Ismail, Z.; Mohamed, N. Antimicrobial Activities of Extracts and Flavonoid Glycosides of Corn Silk (Zea mays L.). Int. J. Biotechnol. Wellness Ind. 2012, 1, 115–120. [Google Scholar] [CrossRef]
- Chatterjee, D.; Lesko, T.; Peiffer, M.; Elango, D.; Beuzelin, J.; Felton, G.W.; Chopra, S. Sorghum and maize flavonoids are detrimental to growth and survival of fall armyworm Spodoptera frugiperda. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [Green Version]
- Nierman, W.C.; Yu, J.; Fedorova-Abrams, N.D.; Losada, L.; Cleveland, T.E.; Bhatnagar, D.; Bennett, J.W.; Dean, R.; Payne, G.A. Genome Sequence of Aspergillus flavus NRRL 3357, a Strain That Causes Aflatoxin Contamination of Food and Feed. Genome Announc. 2015, 3, e00168-15. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Cary, J.W.; Cotty, P.J.; Cleveland, T.E. Development of a GFP-Expressing Aspergillus flavus Strain to Study Fungal Invasion, Colonization, and Resistance in Cottonseed. Mycopathologia 2008, 165, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.K.; Horn, B.W.; Abe, K.; Gomi, K. ASPERGILLUS | Introduction. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 77–82. [Google Scholar]
- Commey, L.; Tengey, T.K.; Cobos, C.J.; Dampanaboina, L.; Dhillon, K.K.; Pandey, M.K.; Sudini, H.K.; Falalou, H.; Varshney, R.K.; Burow, M.D.; et al. Peanut Seed Coat Acts as a Physical and Biochemical Barrier against Aspergillus flavus Infection. J. Fungi 2021, 7, 1000. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meer, I.M.; Stam, M.E.; Van Tunen, A.J.; Mol, J.N.; Stuitje, A.R. Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. Plant Cell 1992, 4, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, B.T.; Thompson, E.P. A method for visualizing fluorescence of flavonoid therapeutics in vivo in the model eukaryote Dictyostelium discoideum. BioTechniques 2019, 66, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Bekker, T.F.; Kaiser, C.; Merwe, R.V.D.; Labuschagne, N. In-vitro inhibition of mycelial growth of several phytopathogenic fungi by soluble potassium silicate. S. Afr. J. Plant Soil 2006, 23, 169–172. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 1 November 2019).
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Fountain, J.C.; Bajaj, P.; Nayak, S.N.; Yang, L.; Pandey, M.K.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Lee, R.D.; Kemerait, R.C.; et al. Responses of Aspergillus flavus to Oxidative Stress Are Related to Fungal Development Regulator, Antioxidant Enzyme, and Secondary Metabolite Biosynthetic Gene Expression. Front. Microbiol. 2016, 7, 2048. [Google Scholar] [CrossRef] [Green Version]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Shantha, T. Fungal degradation of aflatoxin B1. Nat. Toxins 1999, 7, 175–178. [Google Scholar] [CrossRef]
- Maxwell, L.A.; Callicott, K.A.; Bandyopadhyay, R.; Mehl, H.L.; Orbach, M.J.; Cotty, P.J. Degradation of Aflatoxins B1 by Atoxigenic Aspergillus flavus Biocontrol Agents. Plant Dis. 2021, 105, 2343–2350. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellamani, M.; Kalagatur, N.K.; Siddaiah, C.; Mudili, V.; Krishna, K.; Natarajan, G.; Rao Putcha, V.L. Antifungal and Zearalenone Inhibitory Activity of Pediococcus pentosaceus Isolated from Dairy Products on Fusarium graminearum. Front. Microbiol. 2016, 7, 890. [Google Scholar] [CrossRef] [PubMed]
- Da, X.; Nishiyama, Y.; Tie, D.; Hein, K.Z.; Yamamoto, O.; Morita, E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019, 9, 1683. [Google Scholar] [CrossRef]
- Finotti, E.; Parroni, A.; Zaccaria, M.; Domin, M.; Momeni, B.; Fanelli, C.; Reverberi, M. Aflatoxins are natural scavengers of reactive oxygen species. Sci. Rep. 2021, 11, 16024. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; He, X.; Baer, M.; Beirinckx, S.; Tian, T.; Moya, Y.A.T.; Zhang, X.; Deichmann, M.; Frey, F.P.; Bresgen, V.; et al. Plant flavones enrich rhizosphere Oxalobacteraceae to improve maize performance under nitrogen deprivation. Nat. Plants 2021, 7, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Pei, Y.; Huang, W.; Ding, J.; Siemann, E. Increasing flavonoid concentrations in root exudates enhance associations between arbuscular mycorrhizal fungi and an invasive plant. ISME J. 2021, 15, 1919–1930. [Google Scholar] [CrossRef]
- Begum, A.A.; Leibovitch, S.; Migner, P.; Zhang, F. Specific flavonoids induced nod gene expression and pre-activated nod genes of Rhizobium leguminosarum increased pea (Pisum sativum L.) and lentil (Lens culinaris L.) nodulation in controlled growth chamber environments. J. Exp. Bot. 2001, 52, 1537–1543. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castano-Duque, L.; Lebar, M.D.; Carter-Wientjes, C.; Ambrogio, D.; Rajasekaran, K. Flavonoids Modulate Aspergillus flavus Proliferation and Aflatoxin Production. J. Fungi 2022, 8, 1211. https://doi.org/10.3390/jof8111211
Castano-Duque L, Lebar MD, Carter-Wientjes C, Ambrogio D, Rajasekaran K. Flavonoids Modulate Aspergillus flavus Proliferation and Aflatoxin Production. Journal of Fungi. 2022; 8(11):1211. https://doi.org/10.3390/jof8111211
Chicago/Turabian StyleCastano-Duque, Lina, Matthew D. Lebar, Carol Carter-Wientjes, David Ambrogio, and Kanniah Rajasekaran. 2022. "Flavonoids Modulate Aspergillus flavus Proliferation and Aflatoxin Production" Journal of Fungi 8, no. 11: 1211. https://doi.org/10.3390/jof8111211





