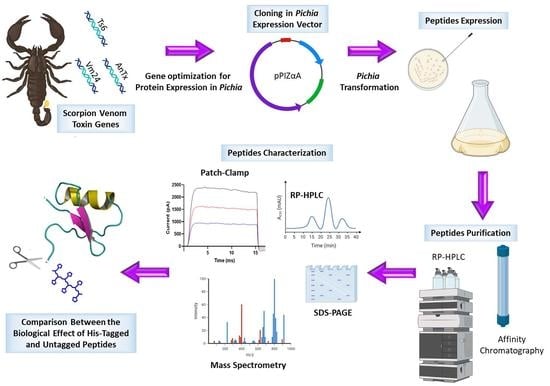
Recombinant Expression in Pichia pastoris System of Three Potent Kv1.3 Channel Blockers: Vm24, Anuroctoxin, and Ts6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plasmid Construction
2.3. Pichia Trasnformation
2.4. Protein Expression and Purification
2.5. Cleavage of the His-Tag
2.6. Mass Spectrometry Analyses
2.7. Cells
2.8. Electrophysiology
2.9. Patch Clamp Data Analyses
2.10. Bioinformatics
2.11. Statistics
3. Results and Discussion
3.1. Plasmid Construction
3.2. Peptide Expression and Purification
3.3. Biological Activity of the Recombinant Peptides on the Kv1.3 Channel
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, F.H.; Catterall, W.A. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE 2004, 2004, re15. [Google Scholar] [CrossRef] [PubMed]
- Perez-Verdaguer, M.; Capera, J.; Serrano-Novillo, C.; Estadella, I.; Sastre, D.; Felipe, A. The voltage-gated potassium channel Kv1.3 is a promising multitherapeutic target against human pathologies. Expert Opin. Ther. Targets 2016, 20, 577–591. [Google Scholar] [CrossRef]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; McKinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stühmer, W.; et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef]
- Teisseyre, A.; Palko-Labuz, A.; Sroda-Pomianek, K.; Michalak, K. Voltage-Gated Potassium Channel Kv1.3 as a Target in Therapy of Cancer. Front. Oncol. 2019, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Panyi, G.; Beeton, C.; Felipe, A. Ion channels and anti-cancer immunity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130106. [Google Scholar] [CrossRef] [Green Version]
- Hofschröer, V.; Najder, K.; Rugi, M.; Bouazzi, R.; Cozzolino, M.; Arcangeli, A.; Panyi, G.; Schwab, A. Ion Channels Orchestrate Pancreatic Ductal Adenocarcinoma Progression and Therapy. Front. Pharmacol. 2020, 11, 586599. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Nerbonne, J.M. Mechanisms contributing to myocardial potassium channel diversity, regulation and remodeling. Trends Cardiovasc. Med. 2016, 26, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Wulff, H.; Zhorov, B.S. K+ Channel Modulators for the Treatment of Neurological Disorders and Autoimmune Diseases. Chem. Rev. 2008, 108, 1744–1773. [Google Scholar] [CrossRef] [Green Version]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef] [Green Version]
- Borrego, J.; Feher, A.; Jost, N.; Panyi, G.; Varga, Z.; Papp, F. Peptide Inhibitors of Kv1.5: An Option for the Treatment of Atrial Fibrillation. Pharmaceuticals 2021, 14, 1303. [Google Scholar] [CrossRef]
- Garcia, M.L.; Garcia-Calvo, M.; Hidalgo, P.; Lee, A.; MacKinnon, R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry 1994, 33, 6834–6839. [Google Scholar] [CrossRef] [PubMed]
- Grissmer, S.; Nguyen, A.N.; Aiyar, J.; Hanson, D.C.; Mather, R.J.; Gutman, G.A.; Karmilowicz, M.J.; Auperin, D.D.; Chandy, K.G. Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol. Pharmacol. 1994, 45, 1227–1234. [Google Scholar] [PubMed]
- Varga, Z.; Gurrola-Briones, G.; Papp, F.; Rodriguez de la Vega, R.C.; Pedraza-Alva, G.; Tajhya, R.B.; Gaspar, R.; Cardenas, L.; Rosenstein, Y.; Beeton, C.; et al. Vm24, a natural immunosuppressive peptide, potently and selectively blocks Kv1.3 potassium channels of human T cells. Mol. Pharmacol. 2012, 82, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennington, M.W.; Byrnes, M.E.; Zaydenberg, I.; Khaytin, I.; de Chastonay, J.; Krafte, D.S.; Hill, R.; Mahnir, V.M.; Volberg, W.A.; Gorczyca, W.; et al. Chemical synthesis and characterization of ShK toxin: A potent potassium channel inhibitor from a sea anemone. Int. J. Pept. Protein Res. 1995, 46, 354–358. [Google Scholar] [CrossRef]
- Garcia-Calvo, M.; Leonard, R.J.; Novick, J.; Stevens, S.P.; Schmalhofer, W.; Kaczorowski, G.J.; Garcia, M.L. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J. Biol. Chem. 1993, 268, 18866–18874. [Google Scholar] [CrossRef]
- Lebrun, B.; Romi-Lebrun, R.; Martin-Eauclaire, M.F.; Yasuda, A.; Ishiguro, M.; Oyama, Y.; Pongs, O.; Nakajima, T. A four-disulphide-bridged toxin, with high affinity towards voltage-gated K+ channels, isolated from Heterometrus spinnifer (Scorpionidae) venom. Biochem. J. 1997, 328 Pt 1, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Boldrini-França, J.; Cologna, C.T.; Pucca, M.B.; Bordon, K.C.F.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cerni, F.A.; Pinheiro-Junior, E.L.; et al. Minor snake venom proteins: Structure, function and potential applications. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 824–838. [Google Scholar] [CrossRef]
- Andersson, L.; Blomberg, L.; Flegel, M.; Lepsa, L.; Nilsson, B.; Verlander, M. Large-scale synthesis of peptides. Biopolym.-Pept. Sci. Sect. 2000, 55, 227–250. [Google Scholar] [CrossRef]
- Jensen, J.E.; Durek, T.; Alewood, P.F.; Adams, D.J.; King, G.F.; Rash, L.D. Chemical synthesis and folding of APETx2, a potent and selective inhibitor of acid sensing ion channel 3. Toxicon 2009, 54, 56–61. [Google Scholar] [CrossRef]
- Geron, M. Production and Purification of Recombinant Toxins. Methods Mol. Biol. 2020, 2068, 73–84. [Google Scholar]
- Klint, J.K.; Senff, S.; Saez, N.J.; Seshadri, R.; Lau, H.Y.; Bende, N.S.; Undheim, E.A.; Rash, L.D.; Mobli, M.; King, G.F. Production of recombinant disulfide-rich venom peptides for structural and functional analysis via expression in the periplasm of E. coli. PLoS ONE 2013, 8, e63865. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.G.; Cordeiro, F.A.; Pinheiro-Junior, E.L.; Boldrini-Franca, J.; Arantes, E.C. Microbial production of toxins from the scorpion venom: Properties and applications. Appl. Microbiol. Biotechnol. 2018, 102, 6319–6331. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Pang, S.-Z.; Oberhaus, S.M.; Rasmussen, J.L.; Knipple, D.C.; Bloomquist, J.R.; Dean, D.H.; Bowman, K.D.; Sanford, J.C. Expression of a gene encoding a scorpion insectotoxin peptide in yeast, bacteria and plants. Gene 1992, 116, 165–172. [Google Scholar] [CrossRef]
- Bagdáany, M.; Batista, C.V.F.; Valdez-Cruz, N.A.; Somodi, S.; de la Vega, R.C.R.; Licea, A.F.; Varga, Z.; Gáspár, R.; Possani, L.D.; Panyi, G. Anuroctoxin, a New Scorpion Toxin of the α-KTx 6 Subfamily, Is Highly Selective for Kv1.3 over IKCa1 Ion Channels of Human T Lymphocytes. Mol. Pharmacol. 2005, 67, 1034–1044. [Google Scholar] [CrossRef]
- Cerni, F.A.; Pucca, M.B.; Peigneur, S.; Cremonez, C.M.; Bordon, K.C.F.; Tytgat, J.; Arantes, E.C. Electrophysiological Characterization of Ts6 and Ts7, K+ Channel Toxins Isolated through an Improved Tityus serrulatus Venom Purification Procedure. Toxins 2014, 6, 892–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurrola, G.B.; Hernandez-Lopez, R.A.; Rodriguez de la Vega, R.C.; Varga, Z.; Batista, C.V.; Salas-Castillo, S.P.; Panyi, G.; del Rio-Portilla, F.; Possani, L.D. Structure, function, and chemical synthesis of Vaejovis mexicanus peptide 24: A novel potent blocker of Kv1.3 potassium channels of human T lymphocytes. Biochemistry 2012, 51, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H. Tricine–SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Alvarado, D.; Cardoso-Arenas, S.; Corrales-García, L.L.; Clement, H.; Arenas, I.; Montero-Dominguez, P.A.; Olamendi-Portugal, T.; Zamudio, F.; Csoti, A.; Borrego, J.; et al. A Novel Insecticidal Spider Peptide that Affects the Mammalian Voltage-Gated Ion Channel hKv1.5. Front. Pharmacol. 2020, 11, 563858. [Google Scholar] [CrossRef]
- Borrego, J.; Clement, H.; Corrales-Garcia, L.L.; Arenas, I.; Corzo, G. Key amino acid residues involved in mammalian and insecticidal activities of Magi4 and Hv1b, cysteine-rich spider peptides from the delta-atracotoxin family. Amino Acids 2020, 52, 465–475. [Google Scholar] [CrossRef]
- Anangi, R.; Koshy, S.; Huq, R.; Beeton, C.; Chuang, W.J.; King, G.F. Recombinant expression of margatoxin and agitoxin-2 in Pichia pastoris: An efficient method for production of KV1.3 channel blockers. PLoS ONE 2012, 7, e52965. [Google Scholar] [CrossRef] [PubMed]
- Booth, W.T.; Schlachter, C.R.; Pote, S.; Ussin, N.; Mank, N.J.; Klapper, V.; Offermann, L.R.; Tang, C.; Hurlburt, B.K.; Chruszcz, M. Impact of an N-terminal Polyhistidine Tag on Protein Thermal Stability. ACS Omega 2018, 3, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.S. Making the most of affinity tags. Trends Biotechnol. 2005, 23, 316–320. [Google Scholar] [CrossRef] [Green Version]
- Bruce, C.; Fitches, E.C.; Chougule, N.; Bell, H.A.; Gatehouse, J.A. Recombinant conotoxin, TxVIA, produced in yeast has insecticidal activity. Toxicon 2011, 58, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, Y. Expression, antiserum preparation and bioactivity assays of insect neurotoxin LqhIT2. Sheng Wu Gong Cheng Xue Bao 2008, 24, 1761–1767. [Google Scholar] [PubMed]
- Turkov, M.; Rashi, S.; Noam, Z.; Gordon, D.; Ben Khalifa, R.; Stankiewicz, M.; Pelhate, M.; Gurevitz, M. In vitro folding and functional analysis of an anti-insect selective scorpion depressant neurotoxin produced in Escherichia coli. Protein Expr. Purif. 1997, 10, 123–131. [Google Scholar] [CrossRef]
- Naseem, M.U.; Tajti, G.; Gaspar, A.; Szanto, T.G.; Borrego, J.; Panyi, G. Optimization of Pichia pastoris Expression System for High-Level Production of Margatoxin. Front. Pharmacol. 2021, 12, 733610. [Google Scholar] [CrossRef]
- Escoubas, P.; Bernard, C.; Lambeau, G.; Lazdunski, M.; Darbon, H. Recombinant production and solution structure of PcTx1, the specific peptide inhibitor of ASIC1a proton-gated cation channels. Protein Sci. 2003, 12, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2012, 30, 1185–1194. [Google Scholar] [CrossRef] [Green Version]
- Ramm, F.; Jack, L.; Kaser, D.; Schloßhauer, J.L.; Zemella, A.; Kubick, S. Cell-Free Systems Enable the Production of AB(5) Toxins for Diagnostic Applications. Toxins 2022, 14, 233. [Google Scholar] [CrossRef]
- Dondapati, S.K.; Stech, M.; Zemella, A.; Kubick, S. Cell-Free Protein Synthesis: A Promising Option for Future Drug Development. BioDrugs 2020, 34, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Khambhati, K.; Bhattacharjee, G.; Gohil, N.; Braddick, D.; Kulkarni, V.; Singh, V. Exploring the Potential of Cell-Free Protein Synthesis for Extending the Abilities of Biological Systems. Front. Bioeng. Biotechnol. 2019, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Miller, C. Mechanism of charybdotoxin block of a voltage-gated K+ channel. Biophys. J. 1993, 65, 1613–1619. [Google Scholar] [CrossRef] [Green Version]
- Coelho, V.A.; Cremonez, C.M.; Anjolette, F.A.; Aguiar, J.F.; Varanda, W.A.; Arantes, E.C. Functional and structural study comparing the C-terminal amidated beta-neurotoxin Ts1 with its isoform Ts1-G isolated from Tityus serrulatus venom. Toxicon 2014, 83, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Luna-Ramirez, K.; Csoti, A.; McArthur, J.R.; Chin, Y.K.Y.; Anangi, R.; Najera, R.d.C.; Possani, L.D.; King, G.F.; Panyi, G.; Yu, H.; et al. Structural basis of the potency and selectivity of Urotoxin, a potent Kv1 blocker from scorpion venom. Biochem. Pharmacol. 2020, 174, 113782. [Google Scholar] [CrossRef]
- Estrada, G.; Restano-Cassulini, R.; Ortiz, E.; Possani, L.D.; Corzo, G. Addition of positive charges at the C-terminal peptide region of CssII, a mammalian scorpion peptide toxin, improves its affinity for sodium channels Nav1.6. Peptides 2011, 32, 75–79. [Google Scholar] [CrossRef]
- Kharrat, R.; Mansuelle, P.; Sampieri, F.; Crest, M.; Oughideni, R.; Van Rietschoten, J.; Martin-Eauclaire, M.F.; Rochat, H.; El Ayeb, M. Maurotoxin, a four disulfide bridge toxin from Scorpio maurus venom: Purification, structure and action on potassium channels. FEBS Lett. 1997, 406, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Kharrat, R.; Mabrouk, K.; Crest, M.; Darbon, H.; Oughideni, R.; Martin-Eauclaire, M.F.; Jacquet, G.; el Ayeb, M.; Van Rietschoten, J.; Rochat, H.; et al. Chemical synthesis and characterization of maurotoxin, a short scorpion toxin with four disulfide bridges that acts on K+ channels. Eur. J. Biochem. 1996, 242, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bartok, A.; Panyi, G.; Varga, Z. Potassium Channel Blocking Peptide Toxins from Scorpion Venom. In Scorpion Venoms; Springer: Dordrecht, The Netherlands, 2015; pp. 493–527. [Google Scholar]
- Bartok, A.; Fehér, K.; Bodor, A.; Rákosi, K.; Tóth, G.K.; Kövér, K.E.; Panyi, G.; Varga, Z. An engineered scorpion toxin analogue with improved Kv1.3 selectivity displays reduced conformational flexibility. Sci. Rep. 2015, 5, 18397. [Google Scholar] [CrossRef] [Green Version]
- Castle, N.A.; London, D.O.; Creech, C.; Fajloun, Z.; Stocker, J.W.; Sabatier, J.-M. Maurotoxin: A Potent Inhibitor of Intermediate Conductance Ca2+-Activated Potassium Channels. Mol. Pharmacol. 2003, 63, 409–418. [Google Scholar] [CrossRef]







| Peptide | RT (min) | CH3CN % | TMM (Da) | EMM (Da) | Activity on hKv1.3 |
|---|---|---|---|---|---|
| Tag-Vm24 | 31.8 | 23.4 | 5656.549 | 5656.500 | Positive |
| Tag-AnTx | 32.7 | 21.2 | 6025.615 | 6025.644 | Positive |
| Tag-Ts6 | 36.8 | 25.9 | 6189.546 | 6189.490 | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego, J.; Naseem, M.U.; Sehgal, A.N.A.; Panda, L.R.; Shakeel, K.; Gaspar, A.; Nagy, C.; Varga, Z.; Panyi, G. Recombinant Expression in Pichia pastoris System of Three Potent Kv1.3 Channel Blockers: Vm24, Anuroctoxin, and Ts6. J. Fungi 2022, 8, 1215. https://doi.org/10.3390/jof8111215
Borrego J, Naseem MU, Sehgal ANA, Panda LR, Shakeel K, Gaspar A, Nagy C, Varga Z, Panyi G. Recombinant Expression in Pichia pastoris System of Three Potent Kv1.3 Channel Blockers: Vm24, Anuroctoxin, and Ts6. Journal of Fungi. 2022; 8(11):1215. https://doi.org/10.3390/jof8111215
Chicago/Turabian StyleBorrego, Jesús, Muhammad Umair Naseem, Al Nasar Ahmed Sehgal, Lipsa Rani Panda, Kashmala Shakeel, Attila Gaspar, Cynthia Nagy, Zoltan Varga, and Gyorgy Panyi. 2022. "Recombinant Expression in Pichia pastoris System of Three Potent Kv1.3 Channel Blockers: Vm24, Anuroctoxin, and Ts6" Journal of Fungi 8, no. 11: 1215. https://doi.org/10.3390/jof8111215
APA StyleBorrego, J., Naseem, M. U., Sehgal, A. N. A., Panda, L. R., Shakeel, K., Gaspar, A., Nagy, C., Varga, Z., & Panyi, G. (2022). Recombinant Expression in Pichia pastoris System of Three Potent Kv1.3 Channel Blockers: Vm24, Anuroctoxin, and Ts6. Journal of Fungi, 8(11), 1215. https://doi.org/10.3390/jof8111215